Abstract
Traditional techniques for investigating cultured neural networks, such as the patch clamp and multi-electrode array, are limited by: 1) the number of identified cells which can be simultaneously electrically contacted, 2) the length of time for which cells can be studied, and 3) the lack of one-to-one neuron-to-electrode specificity. Here, we present a new device—the caged neuron multi-electrode array—which overcomes these limitations. This micro-machined device consists of an array of neurocages which mechanically trap a neuron near an extracellular electrode. While the cell body is trapped, the axon and dendrites can freely grow into the surrounding area to form a network. The electrode is bi-directional, capable of both stimulating and recording action potentials. This system is non-invasive, so that all constituent neurons of a network can be studied over its lifetime with stable one-to-one neuron-to-electrode correspondence. Proof-of-concept experiments are described to illustrate that functional networks form in a neurochip system of 16 cages in a 4×4 array, and that suprathreshold connectivity can be fully mapped over several weeks. The neurochip opens a new domain in neurobiology for studying small cultured neural networks.
Keywords: neurochip, multi-electrode array, parylene, connectivity
1 Introduction
Perhaps the brain’s most astonishing feat is its ability, through a combination of nature and nurture, to correctly wire itself. New connections are constantly being formed, changed, broken, and reformed in response to external input and experience. How and why these synapses form and change has been, and continues to be, an area of intense investigation.
To study development and plasticity of small networks of cultured neurons, we desire to establish long-term, specific, bi-directional electrical communication with all the neurons. That is, we desire to simultaneously measure the electrical activity of all identified neurons over timescales ranging from seconds to weeks. We need to be able to stimulate neurons—without damaging them—to map network connectivity, and to apply external inputs to investigate plasticity.
Here we present a caged-neuron multi-electrode array (MEA), a new kind of micro-machined device that meets the above requirements by establishing a stable one-to-one correspondence between neurons and extracellular electrodes over the lifetime of the culture. We refer to the caged neuron MEA as the neurochip.
We also present a software system for analyzing connectivity as well as results from initial experiments. These demonstrate the power of the neurochip for making detailed investigations of network development over the lifetime of a culture.
1.1 Motivation: Previous and Related Work
Our objective was to build a device which establishes a noninvasive, bi-directional electrical interface for stimulation and recording of action potentials (APs) with a stable one-to-one correspondence between neurons and electrodes so that all constituent neurons of a network can be studied over its lifetime (typically several weeks).
The patch clamp technique cannot meet all of these requirements because the seal formed by the glass-pipette–cell-membrane contact wounds the neuron, restricting the the study of a network to a few hours. Additionally, the physical configuration and technical challenges of the patch-clamp system limits the number of neurons that can be simultaneously patched to about three (Fitzsimonds et al., 1997).
To overcome limitations inherent with the patch clamp method the multi-electrode array (MEA) was developed. The MEA consists of an etched pattern of micron-scale metal electrodes with insulated leads deposited on a glass slide (Pine, 1980; Gross et al., 1977). Neurons are cultured atop the array. The primary advantage of the MEA technique is that it is non-destructive. Electrical activity can be recorded and stimulated without harming the nearby neurons, so cultures can be tracked over timescales as long as months. However, the utility of this tool is limited by a lack of one-to-one correspondence between neurons and electrodes. Typically, only a small fraction, on the order of 1%, of neurons are in electrical contact, and stimulation can drive multiple cells at unknown sites. In addition, because neurons are migratory (at least during the first few weeks in culture), the neurons in electrical contact may change over time.
To localize neuron cell bodies atop MEA electrodes, chemical patterning techniques have been developed (Wyart et al., 2002; Jun et al., 2007; Branch et al., 2000). Neural processes grow along the pre-defined geometry of adhesive paths connecting the electrodes. Our ultimate desire, however, is for neurite growth to be unconstrained, so that neurons are not biased or limited in choosing synaptic partners.
In order to maintain a chronic one-to-one neuron-to-electrode correspondence, while allowing the axons and dendrites to grow freely in two dimensions Maher and Wright developed micro-machined neurowells (Maher et al., 1999, 1998). They etched bulk silicon to form wells into which individual neurons were placed, with a grillwork over to prevent the neuron from migrating out of the well. While this neurowell-based design proved to be effective at trapping neurons, and that extracellular stimulation and recording were possible, fabrication was extremely challenging, and the yield was quite low. Their device, therefore, never gained wide-spread popularity. It is, however, the direct predecessor of the neurochip presented here.
1.2 The Neurochip
The neurochip remedies the problems with the techniques discussed above. It is a 16-electrode MEA, arranged in a 4×4 square array, with micro-machined neurocages built over each electrode. It can be scaled up to larger arrays. A neurocage mechanically confines a neuron to a region near an extracellular electrode while still allowing axons and dendrites to grow outside to form synapses with other neurons in the array. It is a silicon and parylene-based device fabricated using conventional micro-machining techniques. Relative to its predecessor, the new device is much simpler to fabricate and the yield is much higher. Additionally, the new neurochip is compatible with fabrication on a glass substrate (instead of silicon) which has significant implications for using optical tweezers to load the neurocages of devices with a large number of electrodes (Chow, 2007).
The neurochip provides for unambiguous, well-isolated access to each neuron in the 4×4 array. Recording and stimulation are highly-specific and non-invasive so that networks can be studied at single-cell resolution for as long as the culture lives. This paper reports on the complete system including: 1) micro-fabrication and assembly, 2) cell-culture technique, 3) electrical stimulation of action potentials (APs), 4) extracellular recording of APs, and 5) results from initial experiments that map the evolution of suprathreshold connectivity in neurochip cultures during the first 3 weeks in vitro. The results presented also demonstrate that development and maturation of neurochip cultures was similar to those observed in standard dissociated hippocampal cultures.
2 Materials and Methods
2.1 Design
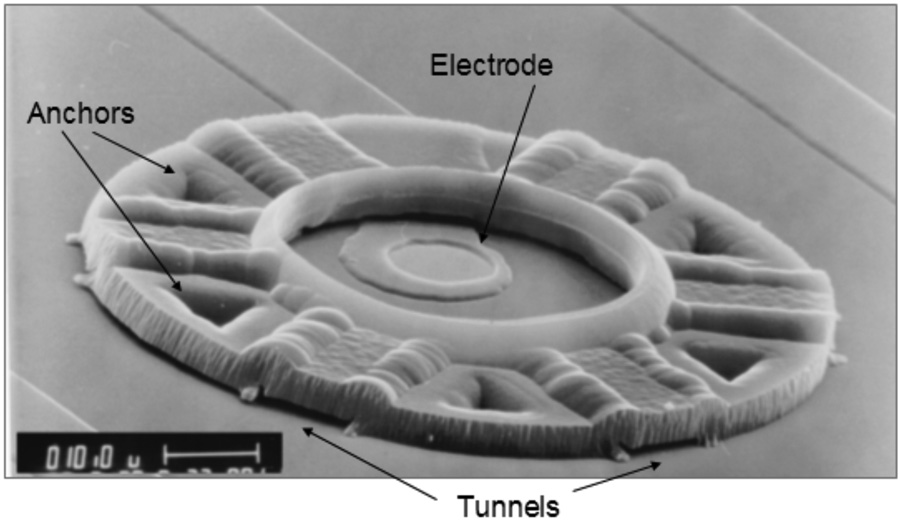
Neurocages (or “cages”, for short) are three-dimensional parylene structures that are fabricated using standard photolithography techniques. As shown in Fig. 1, a neurocage is comprised of four basic elements: a chimney, an electrode, tunnels, and anchors. The chimney is the central region in which the neuron resides. It is 40 µm in diameter and about 9 µm high, with a 30 µm diameter opening at the top for loading the neurons. The 10 µm-diameter electrode at the base of the chimney is used for stimulating the neuron and also recording its electrical activity. It is offset 10 µm from the chimney center to allow for better visibility and to provide a larger effective area for neuron growth. Each neurocage has six tunnels through which neurites can grow out, while preventing the neuron from escaping out of the neurocage. The tunnels are 10 µm wide, 1 µm high, and 25 µm long. Five anchors, interleaved with the tunnels, are deep cavities filled with parylene to firmly secure the cage to the silicon substrate.
Fig. 1.
SEM of the final neurocage design. The major parts of neurocage are labeled. A neuron is placed in the central chimney region, near the electrode. Axons and dendrites are free to grow though the tunnels to synapse with other neurons. The cage is made out of 4 µm parylene, a biocompatible polymer. Low-stress silicon nitride insulates the gold electrode and leads. Scale bar: 10 µm.
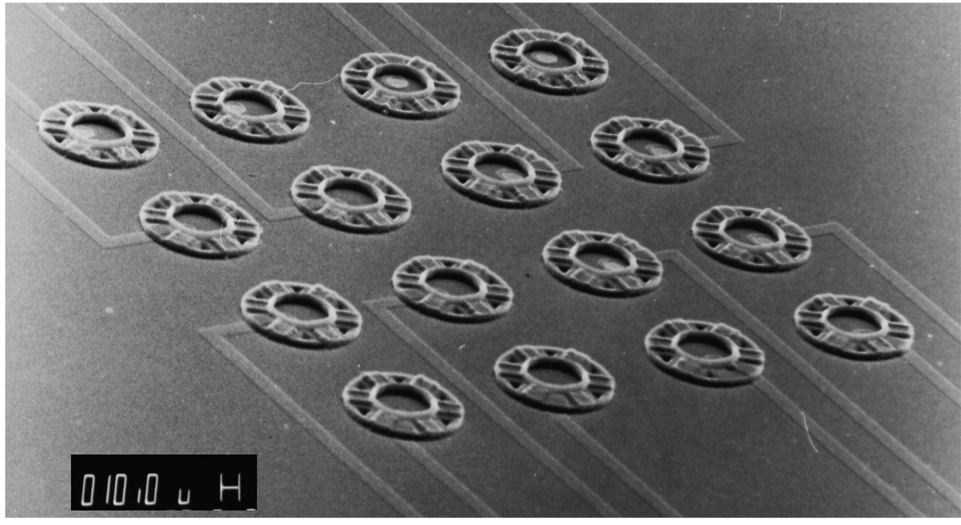
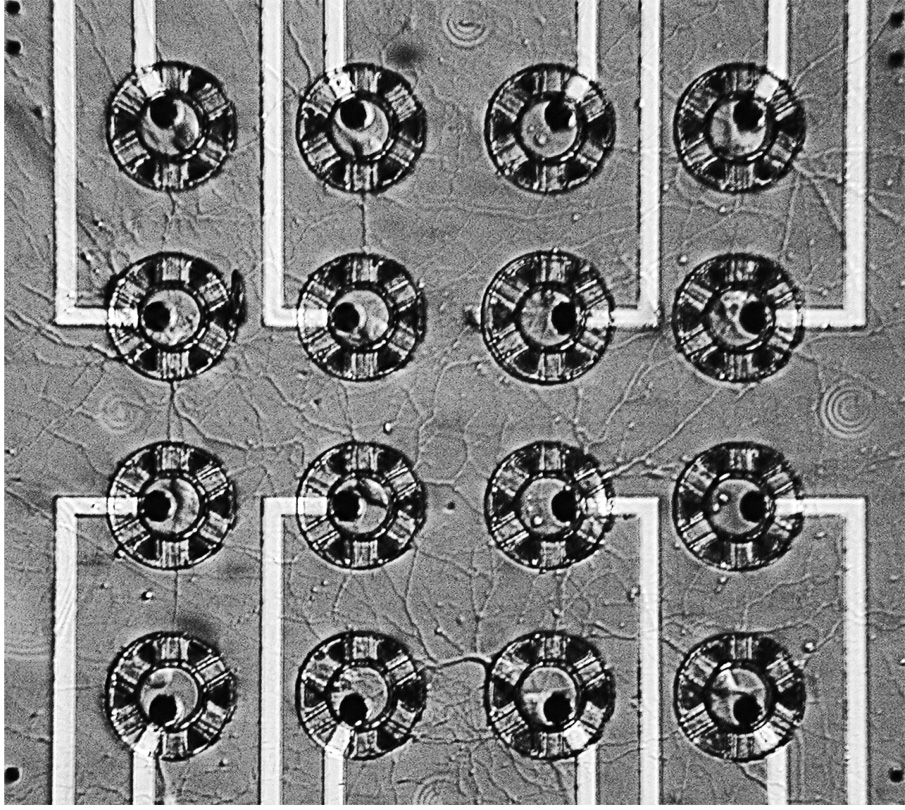
Fig. 2 shows the neurochip design for a 4×4 trial array of cages, spaced 110 µm apart at the center of a 1 cm square chip. This spacing was chosen so that each neuron could (potentially) form synapses with all others.
Fig. 2.
SEM of the 4×4 array of neurocages. The electrical leads run parallel out toward bonding pads. Cages are spaced 110 µm apart. Scale bar: 10 µm.
2.2 Fabrication
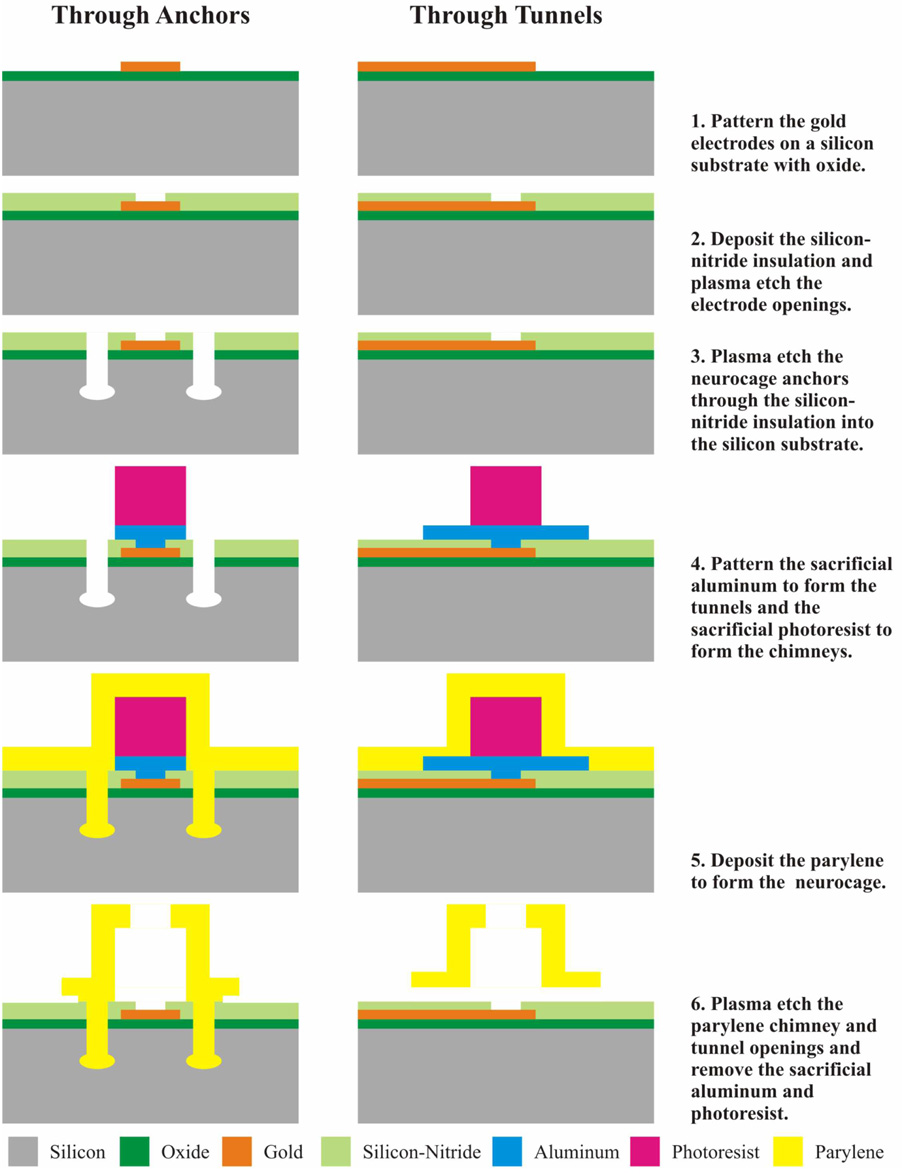
The neurocages were fabricated on silicon wafers during their development, primarily because of the wide variety of standard micro-machining techniques compatible with this substrate. The production process for the cages is shown schematically, not to scale, in Fig. 3. There are two sets of diagrams, showing cross sections through the tunnels and through the anchors.
Fig. 3.
Neurochip fabrication process. Two sets of figures are shown corresponding to the cross-section through the tunnels and anchors. See text for description of individual fabrication steps.
The silicon substrate is a standard 4-inch diameter wafer, which will produce about 20 neurochips when they are cut from it. The first two steps show the creation of the gold electrodes and leads insulated with low-stress silicon-nitride. The third step forms mushroom-shaped cavities, which penetrate the silicon substrate to hold the anchors, so the parylene cannot easily pull out (Tooker, 2007; Tooker et al., 2004). They are necessary because the adhesion of parylene to silicon is not strong enough to firmly hold the cages in place. Step 4 shows the patterning of two sacrificial layers: evaporation of aluminum that will produce the tunnels, and the deposition of a photoresist cylinder that will produce the chimney. In Step 5, the sacrificial layers of photoresist and aluminum are coated with a conformal layer of parylene. The parylene coating is biologically nontoxic, transparent, not soluble in standard organic solvents, and not attacked by acids or bases. It can be selectively removed by oxygen plasma etching through openings in a photoresist coating. Step 6 shows the dissolving of the sacrificial layers to produce the chimney and tunnels, the etching of the opening at the top of the cage (“access hole”), and also the etching of the remaining parylene around the neurocage.
2.3 Assembly
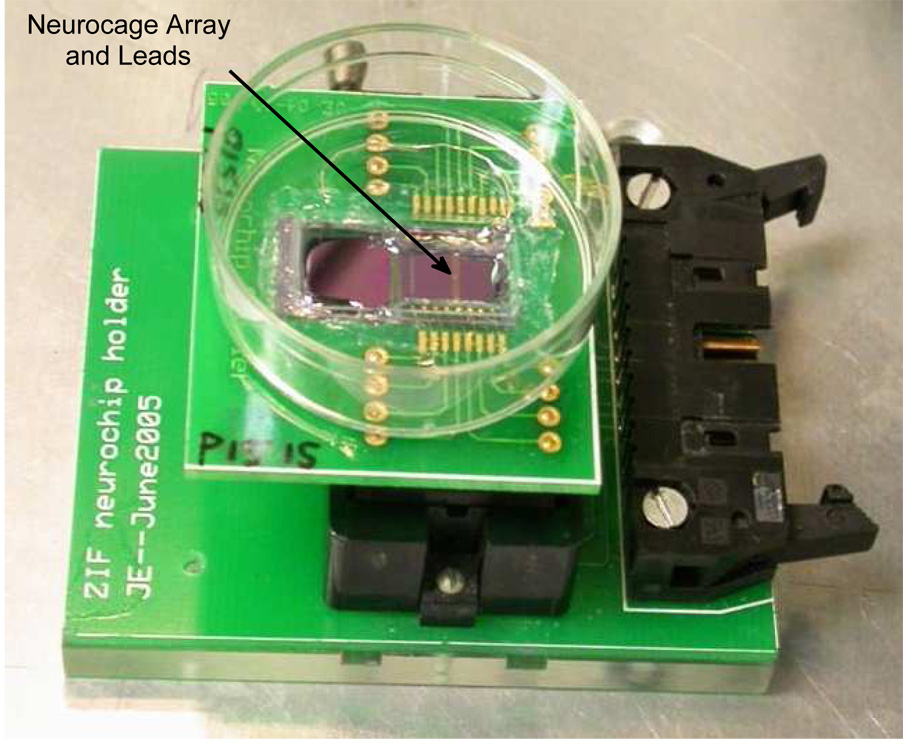
Further assembly is required to interface the neurochip to external electronics and to prepare it for cell-culture. Briefly, a single neurochip is first glued into a custom designed PC carrier board. The board is machined so that the neurochip sits flush in a milled pocket. Ultrasonic gold wire-bonds are made between the neurochip and PC board lead-bonding pads. These bonds are covered in silicone elastomer (Sylgard 184, Dow Corning). The Sylgard serves to electrically insulate the wires, as well as add mechanical protection for the relatively fragile bonds. A 28-pin carrier (Aries Part no. 28-6625-21) (with 11 pins removed) was soldered into the PC board. A platinum wire was electrically connected to the ground pin with silver epoxy (platinum does not solder). A 35-mm cell culture dish with a custom-milled 2 cm × 1 cm window was sealed over the top of the neurochip with silicone elastomer. The fully assembled chip is pictured in Fig. 4.
Fig. 4.
Fully assembled neurochip situated in a ZIF socket. The arrow points to the neurocage array which is visible upon close inspection. Electrical leads on the neurochip are visible as parallel bundles of lines emanating from either side of the array, running roughly from 1 o’clock to 7 o’clock.
The final step is to electroplate platinum black on the gold electrodes to decrease the impedance to bath. As has been discussed previously (Maher et al., 1999; Robinson, 1968), this step is necessary to minimize signal-loss in recording due to parasitic capacitance in the wiring, cables, and pre-amplifier, and to avoid dangerously high electrode voltages during current stimulation.
Electrodes were platinized in Kohlrausch solution (Robinson, 1968) by applying a DC current density of 318 mA/cm2. Electrodes were platinized for 5 seconds once daily, then left to sit overnight in double-distilled water (ddH20). Platinizations were repeated usually about 5–8 times, stopping when the platinum black metallic “bush” started to impinge on the rest of the cage volume. Immediately after the final platinization mean electrode capacitances were measured to be 6600 ± 900 pF, (mean ± standard deviation) measured over 48 electrodes. (Impedance measurements were made at 1 kHz.) This represents a factor of about 150 increase over the unplatinized value. After soaking the neurochips for 2 weeks in cell culture medium, without neurons plated, the mean capacitance decreased to 4300 ± 800 pF, and remained stable thereafter. This capacitance is still sufficiently high that signal attenuation is negligible, and stimulation is safe.
2.4 Cell Culture
We worked with dissociated hippocampal CA1 and CA3 pyramidal cells harvested from embryonic day 18 (E18) Wistar rat embryos. (Dentate gyrus cells are not yet present at this stage in development). The hippocampus is an interesting part of the brain to study because it is known in vivo to serve as a center for consolidation and recall of new memories. CA3 and CA1 pyramidal cells types were chosen because: 1) a large body of literature exists which describes their biophysical properties, 2) they are known to exhibit interesting properties, such as LTP and Hebbian-type learning, in vitro as well as in vivo (Bi and Poo, 2001; Mehta et al., 1997), and 3) the pathway for information flow—the connectivity—in the hippocampus is very well defined (Witter, 1989).
At 18 days gestation, embryos are removed by cesarean section from a pregnant, CO2-asphyxiated Wistar rat. Hippocampi are dissected from the embryonic brains and stored in ice-cold, oxygenated HBSS (Fisher Scientific, SH3001603). The extracellular matrix of the tissue is weakened by incubation at 37 °C in 0.25% trypsin, followed by dilution in tissue culture medium supplemented with 5% equine serum to neutralize the trypsin. The partially digested tissue is centrifuged and re-suspended in tissue culture medium. The cells are then fully dissociated by gentle trituration with a sterile plastic 1 mL disposable pipette tip. This method gives a 80% yield of viable cells after 1 day in culture, with neurons composing about 95% of the population. The other 5% are glial cells.
Prior to plating cells, the neurochip culture dish is sterilized with a combination of 95% ethanol and UV light. Polyethylene-imine (PEI, Sigma P3143) and laminin (Sigma L2020) are applied to the surface of the neurochip to render the surface cytophilic and to promote neurite outgrowth (Lein et al., 1992). The PEI solution is 0.05% w/v, in borate-buffer solution. The laminin solution is 1 µg/mL, dissolved in HBSS. Each deposition is carried out at 37 °C and lasts 8 hours. The dish is rinsed thoroughly with ddH2O and dried after each deposition step.
Making a neurochip culture starts with plating a “mass culture” on the dry PEI/laminin-treated surface. The mass culture consists of 30,000 total neurons split between two 15 µL drops, positioned about 2 mm away from either side of the neurocage array. This isolation is necessary so that results of stimulus-response experiments are not confounded by connections from the neurons in the neurocages out to the mass culture. The mass culture is necessary because 16 neurons alone will not survive; a critical-mass is necessary to condition the medium for neuronal survival. The mass culture is incubated for one hour to allow the cells to anchor. Subsequently, the dish is flooded with 3 mL of plating medium. The plating medium is Neurobasal™ (NB, Invitrogen 21103-049) supplemented with 1 mL B27 (Invitrogen 17504-044), 0.05 mM Glutamax (Invitrogen 35050-061) and 5% equine serum (Hyclone SH30074). It is optimized for survival in low-density cultures (Brewer et al., 1993). The equine serum is added to promote glial cell proliferation.
A 0.75 cm square No. 1 glass cover slip, coated with a non-adhesive substrate, poly(2-hydroxyethyl methacrylate) (polyHEMA, Sigma P3932, 20 mg/mL in EtOH), is put into the culture dish on the side opposite the 4×4 array. A few thousand neurons are gently pipetted onto this surface. Individual neurons were picked-up and carried with a 50-µm-diameter, NB-filled, glass micro-pipette tip mounted on a standard micromanipulator (Leitz) and connected to a manually-controlled fine syringe. After a neuron was captured from the non-adhesive cover slip and carried to the 4×4 array, gentle suction and/or pressure was applied to carefully position it inside a cage. Neurons loaded into cages were rejected or selected based on the following criteria: Cells which were relatively very small or very large were avoided, as were irregularly shaped cells. Otherwise, “typical” cells, round and mid-sized, were selected at random for loading. For a skilled person, loading 16 neurons (one into each neurocage) required about 30 minutes. Loaded neurons were allowed to anchor for 5 minutes before moving the culture into the incubator.
To promote neuron survival and synaptogenesis brain-derived neurotrophic factor (BDNF, Sigma B3795) was added to the culture dish at a concentration of 20 ng/mL at 0 days in vitro (DIV) (just cultured) and again at day 3 DIV (Ip et al., 1993; Scharfman, 1997; Vicario-Abejon et al., 1998; Horch and Katz, 2002; Levine et al., 1995; Lessmann et al., 1994). After about one week, neurons have matured morphologically to include extensive dendritic arborization. By this age, endogenous BDNF may be synthesized by hippocampal cells in the mass culture. (Poo, 2001). A full study of the effect of BDNF on neurochip culture electrical activity and connectivity was not conducted.
Starting at 24 hours in vitro, 1/5 of the Neurobasal-based plating medium was exchanged daily for DMEM-based maintenance medium. The maintenance medium consisted of high-glucose DMEM (Invitrogen 10313-021), supplemented with 10% Ham’s F12 (Invitrogen 31765-035) and 5% equine serum. After a week of exchanging, the culture medium was a mix of about 75% DMEM and 25% NB solutions. (The main difference in the formulation between NB and DMEM is the sodium concentration. In NB the NaCl concentration is 55 mM; in DMEM it is 110 mM.) Subsequent feeding was done weekly with DMEM-based medium. Cultures were started in NB-based media because it is superior for short-term survival in low-density hippocampal cultures. (The low-sodium concentration may aid survival by reducing the excitability of neurons, thereby protecting them from excito-toxic effects.) We gradually replaced NB for DMEM after observing that none of twenty cultures grown for 3 weeks in NB exhibited any spontaneous APs or driven suprathreshold synaptic responses to stimuli. Cultures maintained in DMEM did indeed display functional electrical responsiveness, and were sometimes spontaneously active.
Starting at 7 DIV, osmolarity was maintained at 320 mM by adding sterile ddH20 three times per week over the lifetime of the culture, as it is known to be a crucial factor for long-term neuronal survival (Potter and DeMarse, 2001). Arabinoside-C (Sigma C1768, 1 µL of 1 mM solution) was added to cultures to block replication of glial cells after they formed a monolayer in the mass culture, typically at about 7–10 DIV.
2.5 Hardware and Computer Interface
Electrical measurements were made in differential mode, with the platinum wire in the dish serving as a common reference. High source impedance (≈40 kΩ) electrode signals were transferred to low-noise, 11X non-inverting pre-amplifiers, then through 2-pole low-pass filters(set for 5kHz), and finally to buffer amplifiers with programmable gain. The gain was set to G = 1/2 when the electrode was stimulated, or to G = 63 when it was not. The signals were then passed to 12 bit A/D converters with programmable gain (typically set to G = 50) and a range of ± 10 V (National Instruments PCI-6071E). The resolution of the system at high gain is 0.07 µV/bit.
Pseudo-current stimuli through the cage electrodes were generated by driving biphasic voltage pulses through 500 kΩ resistors located at the input of the preamp board. For an electrode impedance of about 40 kΩ (see Section 3.2), this arrangement approximated a current source. The amplitude of the stimulus was controlled with a digital I/O card (NI 6503) which sends information to a set of 12 bit D/A converters. Stimulation timing was controlled with a high-precision timer board (NI 6602). Current stimulus amplitudes were 0–20 µA and the durations tested were 200–400 µsec per phase.
Neurochip recordings and stimulation were triggered from a 60-Hz sync circuit which generated a trigger-pulse on the up-transitions in the AC line voltage. This technique time-locks every trial to occur at the same phase in the 60 Hz signal so that baseline 60 Hz signals can be easily subtracted off-line. The 60 Hz peak-to-peak noise was usually about 30 µV peak-to-peak, and easily reduced with off-line digital subtraction to undetectably small levels.
The entire data acquisition and stimulation system was controlled with custom drivers implemented in LabView (National Instruments, Austin, TX). A custom LabView application was used for on-line display and data acquisition. Data were digitized and stored at 20 kHz using a 2.3 GHz Intel Pentium IV computer.
2.6 Optical Recording System
Optical measurement using a voltage-sensitive dye (VSD) was employed to study neuron responses to various stimulus waveforms. Electrical recordings cannot be used to assess whether a neuron has been stimulated because the stimulus artifact is much too large (of order volts) for too long (order tens of milliseconds), swamping any extracellular AP recording.
For staining, we used di-4-ANEPPDHQ (Invitrogen, D36802)(Obaid et al., 2004), a membrane-bound, fast response-time potentiometric dye. This dye was found to be superior for the application as it 1) did not internalize (at least over the course of several hours) and 2) did not exhibit any notable photo-toxicity, even after as much as 10 seconds total illumination time. In addition, this dye exhibited approximately −1% change in uorescence intensity per 100 mV change in membrane potential. The stock staining solutions were prepared at a concentration of 1 mg/mL, dissolved in 95% ethanol. For staining, starting with a culture growing in DMEM-based culture medium, the culture was gently rinsed 3X with a HEPES-buffered physiological saline solution (145 mM NaCl, 3 mM KCl, 8 mM glucose, 3 mM CaCl2, 2 mM MgCl2, 10 mM HEPES, in ddH2O). After the third rinse, 7.5 µL stock solution were added to the culture dish already containing 3 mL of saline bath. The staining time was 15 minutes, during which time the culture was placed in the incubator. Following that, the culture was rinsed 3X, leaving the cells in saline.
To measure fluorescence intensity, we used a fast CCD camera, the NeuroCCD (RedShirt Imaging, Decatur, GA). It has 80×80 pixels, a full frame rate of 2 kHz, and low dark noise, which provides the possibility of measuring fluorescence intensity changes on the order of 1 part in 1000. The peak change in fluorescence intensity measured at the soma due to an AP is typically about 1%.
The RedShirt camera is mounted to the top trinocular port of an epi-illumination Olympus BHMJ microscope via a 3:1 demagnifying coupler (Thales-Optem) and custom-machined adapter sleeve. The fluorescence emitted from stained cells (see next section) was imaged through a 40X water-immersion lens with NA = 0.8 (Zeiss). In this configuration each CCD pixel imaged an area approximately 1.5 µm squared.
Stained neurons are illuminated by a mercury arc mounted through the normal illumination port of the microscope. An optical feedback shunt regulator stabilizes the incident illumination to nearly the shot-noise limit (Chien and Pine, 1991a,b). A standard Olympus “G” cube contains filters and a dichroic mirror to steer excitation light (Hg green, λ = 546 nm) to the neuron and to capture fluorescence signals from it (λ > 590 nm). A computer-controlled electromechanical shutter was inserted in the light path; it is normally closed except the short (100 ms) time interval during which a fluorescence signal is measured. The Neuroplex software suite from RedShirt was used to control all camera settings and optical data acquisition. A trigger pulse output from the stimulus generation hardware was used to synchronize the stimulus timing and optical data acquisition.
3 Results
3.1 Cell Culture: Survival and Trapping Efficacy
Figure 5 shows a Nomarski photograph of a neurochip culture at 10 DIV. Eleven of the sixteen originally loaded neurons are growing and trapped in the neurocages. Axons and dendrites have grown out of the tunnels to form a rich network. We know from SEM images that only the thick processes are visible under Nomarski optics; thus, the network is even richer than the figure suggests.
Fig. 5.
Neurochip culture 10 days old. Soma are trapped in cages. Process outgrowth through the tunnels is evident. A rich network has formed. The networking is even richer than shown in the photo as only the thickest processes are visible.
To measure the survival rate versus time, neurons were judged to be alive (or dead) based on visual inspection of neuronal anatomy. A viable neuron is seen to first enlarge, flatten, and then sprout axons and dendrites which elongate for about two weeks. Past two weeks, the tangled web of processes makes identification of disintegrated neural processes and soma—the hallmarks of dying cells—more difficult. At one week the survival rate was measured (from N = 41 cultures) to be about 80%; at two weeks 65% and at three weeks 55%. This survival rate is satisfactory, as survival in low-density (300/mm2) control cultures is comparable (data not shown).
We acknowledge that this neuronal survival assessment method is a subjective and imperfect measure. However, this judgment is easy and unambiguous during the first two weeks, becoming more difficult for older cultures. Optical and electrophysiology experiments with older cultures have confirmed that this visual inspection is accurate.
The neurocages are effective at trapping neurons with nearly 100% efficiency during the first 28 DIV. (Data on trapping for cultures past 4 weeks was not measured, as neurochip cultures were typically terminated at 28 DIV.) Out of the 41 cultures followed here, no escapes were noted. We found that the tunnel height is a critical parameter for preventing migration out of the cages. For cages with a tunnel height of 1.7 µm, 13% of neurons escaped.
After a long-term culture experiment, neurochips were cleaned (3% BM solution for 15 minutes, followed by an overnight soak in ddH20) and have been re-used for 5 or more multi-week sessions. No difference has been noted in the survival rate from the first to the last time the dish was cultured.
3.2 Electrode Properties
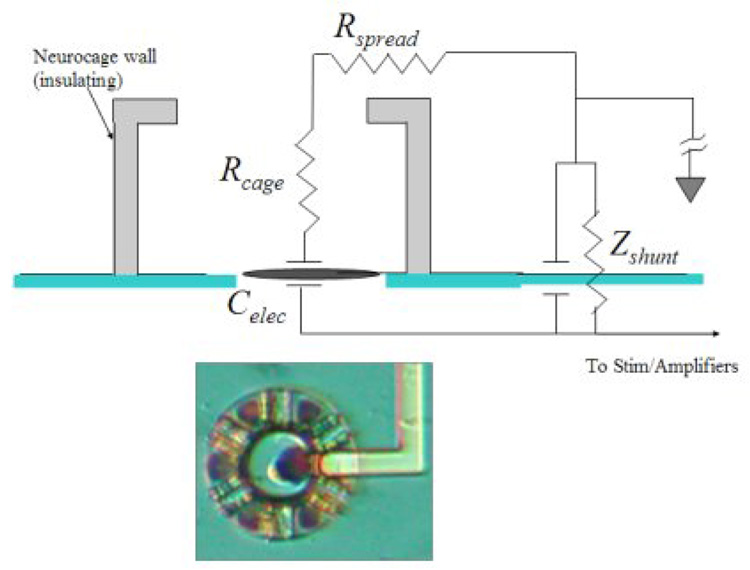
The neurochip electrodes can be represented by a simple model shown in Fig. 6.
Fig. 6.
Cartoon neurocage model illustrating the simplified electrical model of the neurocage electrical connections. An actual neurocage with platinized electrode is shown below. Mean measured values, averaged over 48 electrodes, are: Celec = 4300 pF, Rcage + Rspread = 25 kΩ, Cshunt = 20 pF, Zshunt = 3.5 MΩ. See text for discussion of each electrical element.
The platinum black-saline interface of the electrode is modeled as a lumped capacitor, Celec (Robinson, 1968). Following 5 long-term experiments, during which time a total of about 250 stimuli at current density of 7000 mA/cm2 were applied, the neurochip was cleaned (as described above) one last time. Subsequently, the capacitance was measured to be 6400 ± 800 pF, very nearly the initial value of a virgin, fully platinized electrode—the platinization is robust.
The path in the resistive medium (saline solution) from the electrode to the top of the cage is modeled as a resistor, Rcage, as is the “spreading” resistance, Rspread from the top of the cage out to ground.
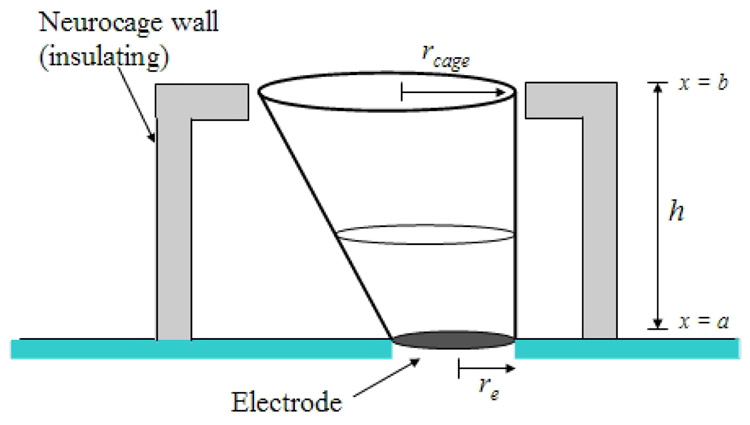
The off-center geometry of the electrode makes exact computation of Rcage difficult. A rough approximation is calculated by considering that current flows roughly through a conical cross section toward the top of the cage, as shown in Fig 7. With this approximation, the total resistance from the path in the saline from the electrode to ground is given by:
| (3.1) |
where h represents the total height of the cage, from the insulation to the top lip of the access hole; re is the radius of the electrode; rcage the radius of the access hole; and the resistivity of the saline, ρ, is assumed to be 70 Ω·cm. The measured real part of the impedance of a fully platinized electrode, averaged over 48 electrodes, was R = 25 ± 3 kΩ, in excellent agreement with the value for the simple model presented above.
Fig. 7.
Geometry of the truncated conical cross section through which current flows from the electrode to the access hole at the top of the cage. The x coordinate points in the vertical direction. The radii of the electrode and access hole at the top of the cage are labeled re and rcage, respectively. The total resistance of this geometry is Rab = ρh/πrercage.
The electrode lead–insulation layer–conductive saline path constitutes a shunt impedance. It was measured by placing a small drop of Sylgard over the 4×4 array of cages, thus blocking the current flow pathway from the electrode to ground. The magnitude of the shunt impedance was measured to be Zshunt = 3.5 MΩ, high enough to avoid any significant signal attenuation.
The theoretical RMS Johnson noise level for an electrode with a resistance to ground of R = 24 kΩ is:
| (3.2) |
for a bandwidth B = 5 kHz and a temperature T = 295 K.
In addition, the pre-amplifier specifications predict an RMS noise level of 1.4 µV. Adding the sources of noise in quadrature, predicts a total noise level of Vrms = 2.0 µV. The measured value of the rms noise was typically 2–3 µV.
3.3 Extracellular Recording
When a neuron in a neurocage fires an AP, roughly speaking, it acts as source/sink of current which flows in the resistive medium (physiological saline) to ground. The Ohmic drop resulting from this current is the signal measured by the neurocage electrode.
The main component of this signal occurs when sodium channels open, driving the fast depolarization of the cell membrane. Measurements in our lab, and by others, show that the membrane potential typically rises at a rate of dVm/dt ≈ 100 mV/ms when an AP is initiated (Spruston and Johnston, 1992; Buchhalter and Dichter, 1991). The secondary component of the signal occurs when potassium channels open to repolarize the cell membrane, typically at a rate of dVm/dt ≈ −30 mV/ms.
An estimate of the current owing is given by:
| (3.3) |
where Clm is the membrane capacitance local to the region of the membrane voltage change—in this case, the soma plus proximal dendrites. The whole-cell capacitance has been measured by others to be Cm ≈ 30–80 pF (Spruston and Johnston, 1992; Offenhausser et al., 1997; Buchhalter and Dichter, 1991). Assuming that the soma and nearby dendrites account for about 1/4 of whole-cell capacitance (Clm ≈ 15 pF), estimates for the sodium and potassium currents are INa+= −1.5 nA and IK+ = 0.5 nA, respectively.
Modeling the neuron as a thin disk of uniform current density during an AP, in the neurocage geometry the neuron-to-ground pathway through which current flows should have a resistance, Rng, similar to that of the electrode-to-ground resistance (given by Equation 3.1). Therefore, we expect to record APs as signals (given by Vsignal = IcRng) containing a −36 µV component lasting ≤ 1 msec, resulting from the sodium current, followed by a +12 µV component lasting about 3 msec, resulting from the potassium current. If current actually flows into the axon hillock a few microns away from the electrode, the result would not be very different.
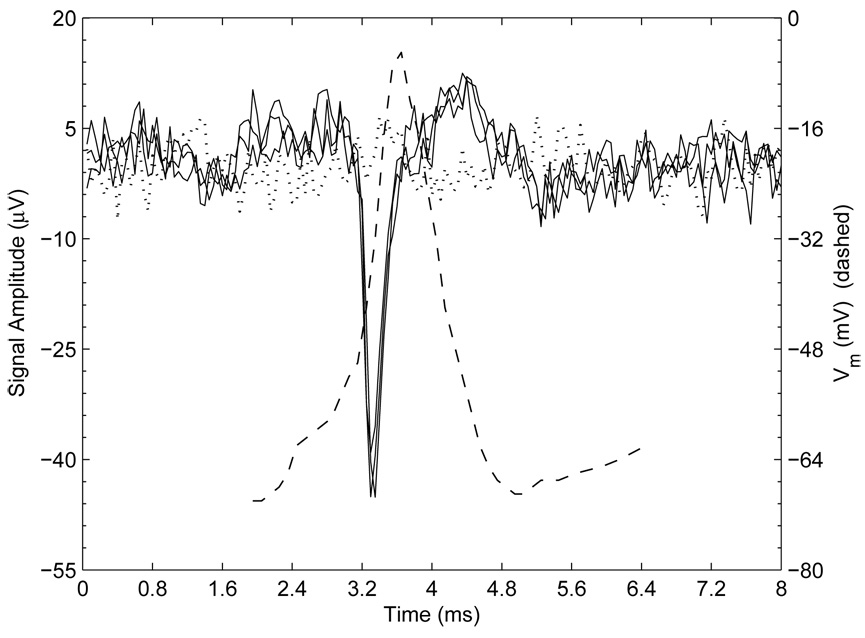
Fig. 8 shows a prototypical extracellular signal of an AP measured with a neurocage electrode. Three successive signals from APs in a 20 day-old neuron are aligned at their maximum negative deflections. Recordings were sampled at 20kHz at a temperature of ≈ 25 °C. Consistent with the theory outlined above, the signals shown contain two main components: 1) a −45 µV component lasting about 0.5 msec due to the fast upward stroke of the AP, followed by 2) a +14 µV upward deflection lasting about 2 msec due to the rectifying potassium current. A typical intracellular recording from a hippocampal neuron in a separate culture is superimposed to help illustrate the origin of the extra-cellular signal components. (Simultaneous neurochip/intracellular recording is possible, but not attempted due to the technical challenge inherent with patching a neuron at the bottom of a neurocage).
Fig. 8.
Prototypical neurochip extracellular recordings of APs. 3 successive APs are aligned at their maximum deflection to demonstrate the stereotypical shape of the signal (solid, black). A typical intracellular recording of membrane potential during an AP is overlaid (dashed) to illustrate the origin of the components of the extracellular signal. A control extracellular trace (dotted) is also shown for reference.
In our system, a variety of signal sizes (typically 15–75 µV) and shapes have been measured, which can explained by the various relative positions of the neuron and electrode as well as the variability of membrane excitability (Na+ channel-density) (Erickson, 2008; Claverol-Tinture and Pine, 2002; Gold, 2007; Rall, 1962; Holt and Koch, 1999). The SNR of AP signals is typically 5–25.
The question naturally arises whether subthreshold signals can be recorded in our system. Given that subthreshold signals change the membrane voltage only about 1/10 as much as an AP, and that excitatory currents (EPSCs) are typically initiated at sites distant from the soma and electrode, the expected size of such a signal is only about 5 µV. Given that the electrode noise level is 3 µV, we do not expect to be able to cleanly measure subthreshold signals.
The signals recorded by a neurocage electrode are believed to be specific (solely due) to the neuron in the same cage based on the following argument. A 10 µm-long segment of a passing axon originating from a neuron in another cage is expected to have a small capacitance of about 0.5 pF. Therefore, the signal component due to the inward sodium current is only expected to be about 2 µV. Clearly this is too small to cleanly record at a Johnson noise level of 3 µV. A similar argument holds for recording from the dendrites of another neuron. For recording, therefore, a one-to-one correspondence between electrodes and caged neurons is firmly established.
3.4 Extracellular Stimulation: Theoretical Considerations
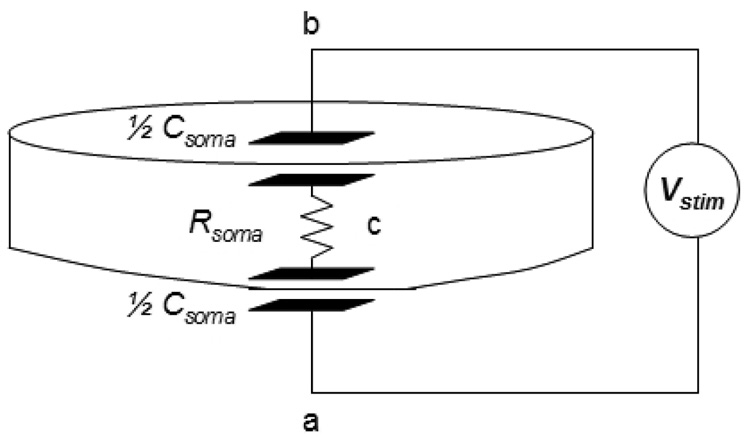
The neuron geometry may be crudely modeled as a cylinder, with the top and bottom membranes approximated as purely capacitive for times much shorter than the membrane time constant. The interior of the cell body is a conductive saline solution, and thus a path across it is modeled as being purely resistive, Rcb ≈ 20 kΩ. This model is illustrated in Fig 9. The current stimulus creates a spatially-dependent voltage difference in the medium between the top and bottom of the neuron given by ΔVstim = IstimRab. For the neurocage geometry and the typical dimensions of a hippocampal cell, the resistance in the saline from point a to point b is Rab ≈ 5 kΩ. Due to the symmetric configuration, the cell’s interior is an isopotential which approximately follows the average of the exterior potential defined by ΔVstim on a timescale of τcb = CsomaRcb ≈ 0.3 µsec. The change in membrane potential, therefore is . In order to initiate an AP, the membrane potential must be raised by ΔVm ≈ 15 mV for a time long enough to open the voltage gated sodium channels (≈200–800 µsec) (Hille, 1992). The threshold current is thus calculated as:
| (3.4) |
Fig. 9.
Electrical model of a hippocampal neuron for stimulation by an extracellular current pulse. Because the time scale of stimulation is short compared to the passive membrane time constant, the top and bottom membranes are modeled as capacitors. The intracellular solution, essentially saline solution, is modeled as a resistor Rsoma. The extracellular current pulse generates a voltage drop in the medium between the top and bottom membranes, Vstim. The capacitance of the top and bottom membrane together is Csoma = 15 pF. The resistance is Rsoma = 20 kΩ. The RC time constant of this arrangement is 0.3 µsec.
Note that this model also predicts that either stimulus polarity should evoke APs because one side of the membrane will always be depolarized (while the other side is hyperpolarized). The model also predicts that the relative configuration of the neuron and electrode will affect the threshold values. These are experimentally confirmed.
3.5 Evoking APs with Extracellular Current Pulses: Experimental Results
Candidate neurons for optical response experiments were selected on the basis of prior visual inspection. Neurons which appeared “healthy” were included in the study. Cultures were stained with VSD (as described in Section 2.6). Biphasic current stimuli (both polarities) were delivered through the cage electrode with amplitude starting at 0 µA, incrementing by 2 µA, up to a maximum value of 20 µA. The amplitude at which an all-or-nothing response was observed was deemed to be the threshold current required to evoke an AP.
Optical data were acquired for 80 msec, with the stimulus being delivered approximately in the middle of the interval. An optical trace was computed as the change in fluorescence intensity over time divided by the resting light intensity (RLI) spatially averaged over all CCD pixels onto which the neuron cell body is projected. Typically the SNR of the optical system was large enough that only one trial for each stimulus was necessary. In instances when this was not the case, multiple trials (3–5) were averaged.
Because optical measurements are an indirect measurement of cell membrane potential, several criteria were used to identify APs based on the optical trace, ΔF/F versus t. If an optical response met all of the following criteria, an AP response was declared to have occurred
Time Delay of Response: An AP is elicited within a few tenths of a millisecond following the end of the stimulus.
Sign and Amplitude of ΔF/F: Given the calibration of the dyes, and that during an AP a cell changes membrane potential by order 100 mV, we expect a peak signal of ΔF/F ≈ −1%.
Width of ΔF/F: Based on patch clamp recordings of APs of hippocampal neurons in control cultures, the (full) width of the optical response should be about 3–5 ms.
All-or-nothing response: Sweeping through stimulus strengths, a discontinuity in the maximum deflection of ΔF/F is expected to occur once the threshold stimulus is reached. For stimuli strengths greater than the threshold, the magnitude of the peak response is expected to remain constant.
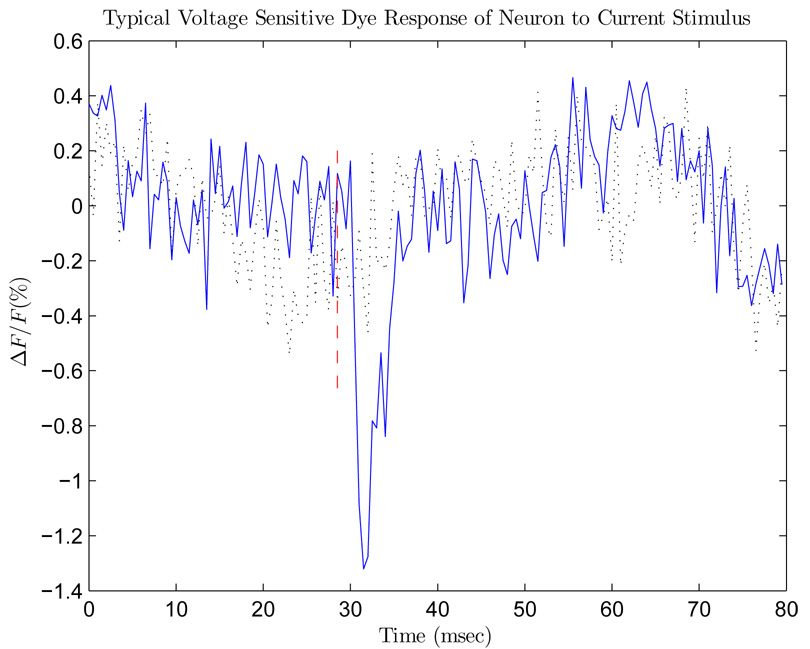
Fig. 10 below shows a sample optical trace at a 2 kHz frame rate. The red dashed line marks the onset of the stimulus, in this case a bipolar current stimulus, negative phase first, 400 µsec per phase. The dotted black trace is a control trace, stimulus of 0 µA. The blue trace shows the response to a biphasic current 12 µA in amplitude. This −1.3% peak ΔF/F response, 5-msec full-width, and < 1-msec response time strongly suggest an AP response.
Fig. 10.
Optical trace in response to current stimuli. The dotted black line is a control optical trace (no stimulus delivered). The solid blue line is the optical response of a neuron to a 12 µA stimulus. The red dashed line marks the onset of the stimulus.
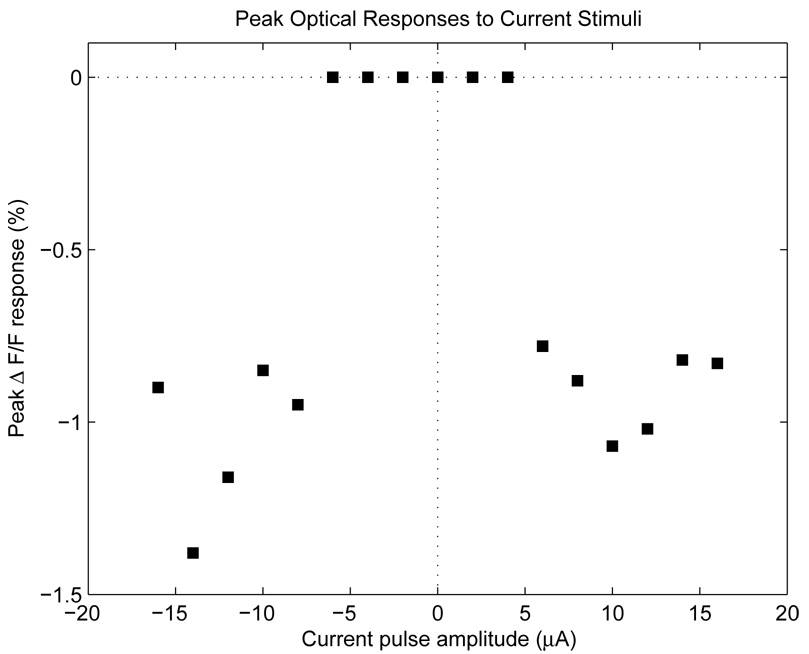
Sweeping through a range of current stimuli yields the data shown in Fig. 11. A positive value for the stimulus current indicates a positive-first stimuli, and vice-versa. Discontinuities occur at currents of +6 µA and −8 µA, which we identified as the thresholds for AP stimulation.
Fig. 11.
Peak ΔF/F responses versus stimulus strength. The sharp discontinuities occurring at +6 and −8 µA are identified as threshold stimulation currents.
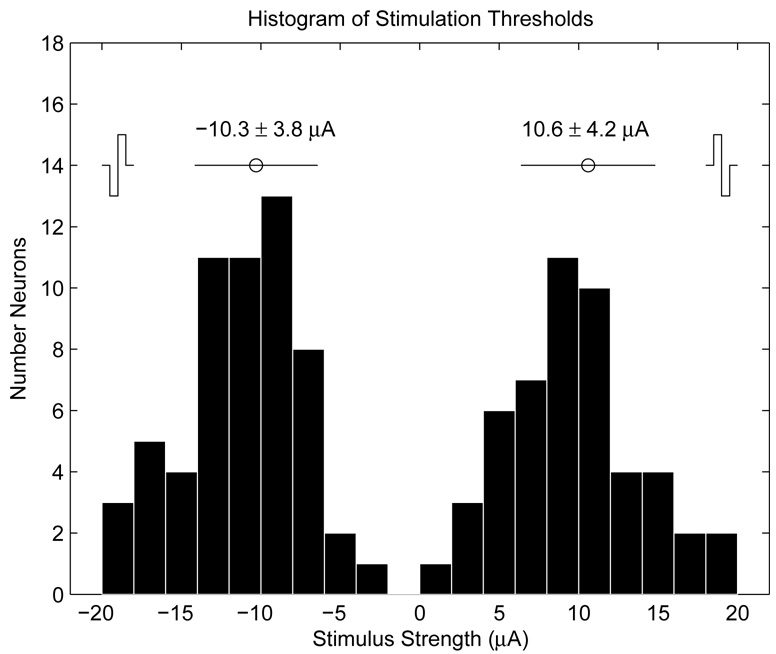
The optical experiment was conducted on 66 neurons in the age range of 6–31 DIV. Fig. 12 shows the distribution of the identified current thresholds. No correlation was noted between the age of the neuron and the threshold stimulus. 59 of the 66 (89%) of neurons tested exhibited AP responses to negative first stimuli, while 50 of 66 (76%) responded to positive first stimuli. The threshold for negative-first stimuli was −10.3 ± 3.8 µA, and 10.6 ± 4.2 µA for positive-first stimuli, in reasonable agreement with the model presented in Section 3.4. The variability in measured thresholds can be explained by the variability in the position of the neuron relative to the electrode, as well as variability in active sodium channel density proximal to the soma.
Fig. 12.
Histogram of bipolar current thresholds for N = 66 neurons. The average threshold values are about 10 µA for both positive and negative first stimuli. Negative-first stimuli were more effective, on average, at evoking APs.
Based on these results, for subsequent network connectivity experiments (see Section 3.6), negative-first, 0.4 msec per phase, 16 µA stimuli were used. This value is appropriate for most cells, while being mindful not to charge the electrode to dangerous voltage levels. Although this stimulus charges the electrode capacitance to about 1.6 V (given by: ΔV = IΔt=Celec), it was deemed to be safe based on the following observations. No gas bubbles which could result from electrolysis of H2O were observed. Neurons noted to be successfully stimulated exhibited the same AP responses when tested again 2 hrs after the original experiment (N = 3). Cultures which were not stained and visually examined before and 24 hours after the presentation of 60 current pulses (1 pulse/sec) exhibited no gross differences in anatomy—soma were still plump and there were no decaying or fragmented membranes.
3.6 Mapping Connectivity
One of the most fundamental properties of a neural circuit is its connectivity, i.e., how it is wired. It is known that the neural network wiring has significant implications for neural information processing (Chklovskii et al., 2004) and for the kinds of computations different circuit structures achieve (Destexhe and Marder, 2004).
With this in mind, we conducted initial stimulus-response experiments to demonstrate the utility of the neurochip for mapping evolving connectivity over the lifetime of a culture, and to demonstrate that development in neurochip cultures is similar to normal dissociated cultures.
To map connectivity in a culture, we stimulated one neuron while recording the synaptic responses from all others. An AP evoked in a neuron other than the one being stimulated with the current pulse is termed a driven response. Repeating this protocol for all neurons in the culture probes all pre- and post- synaptic pairs, fully mapping the culture.
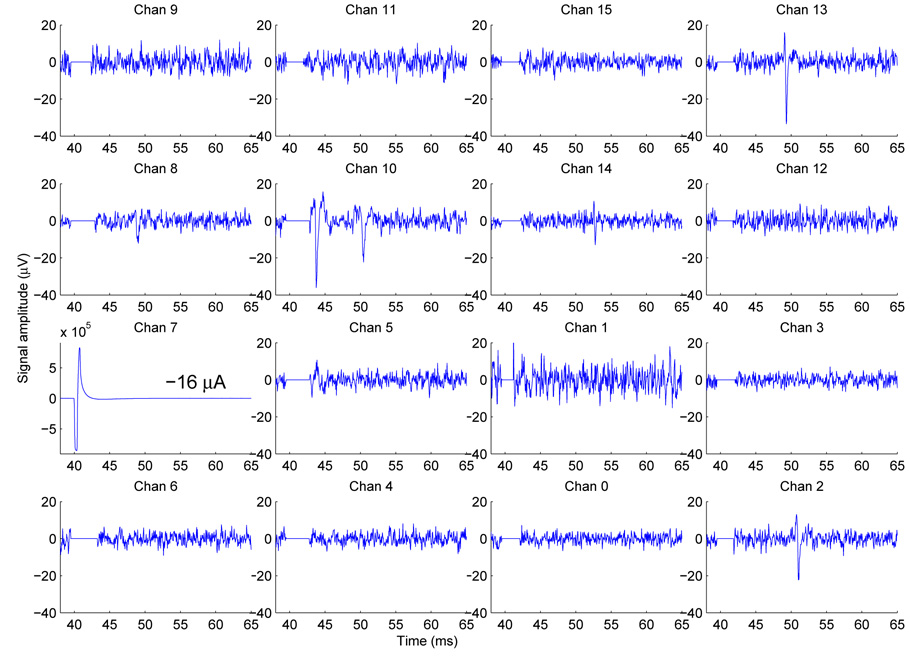
Individual trials lasted 200 msec, with the stimulus presented 40 msec after the start of recording. Raw neurochip signals were processed to subtract a template of the 60 Hz baseline signal, and suppress the stimulus artifact using the SALPA algorithm (Wagenaar and Potter, 2002). Figure 13 shows an example of data traces which have been fully pre-processed. One trace (of ten repeated trials) with the stimulus presented to electrode 7 is shown for clarity. The SALPA algorithm blanked the voltage traces post-stimulus for about 1–2 msec. Spikes were detected using a simple threshold criterion of |V (t)| ≥ 5 × RMS noise level. In Fig. 13 driven responses were detected on five electrodes: 2, 8, 10, 13, 14.
Fig. 13.
Pre-processed “cleaned” traces. The 4×4 layout matches the physical orientation of the neurocages. Only 1 (the 6th) of 10 trials is shown for clarity. A stimulus was presented on electrode 7 at time t = 40 msec, resulting in single spikes on electrodes 2, 8, 13, and 14 at times t ≈ 51, 49, 49, and 53 msec, respectively. Two spikes resulted on electrode 10, at approximately 44 and 51 msec. The total trial lasted for 200 msec, but only the time range of 38–65 msec is shown for clarity.
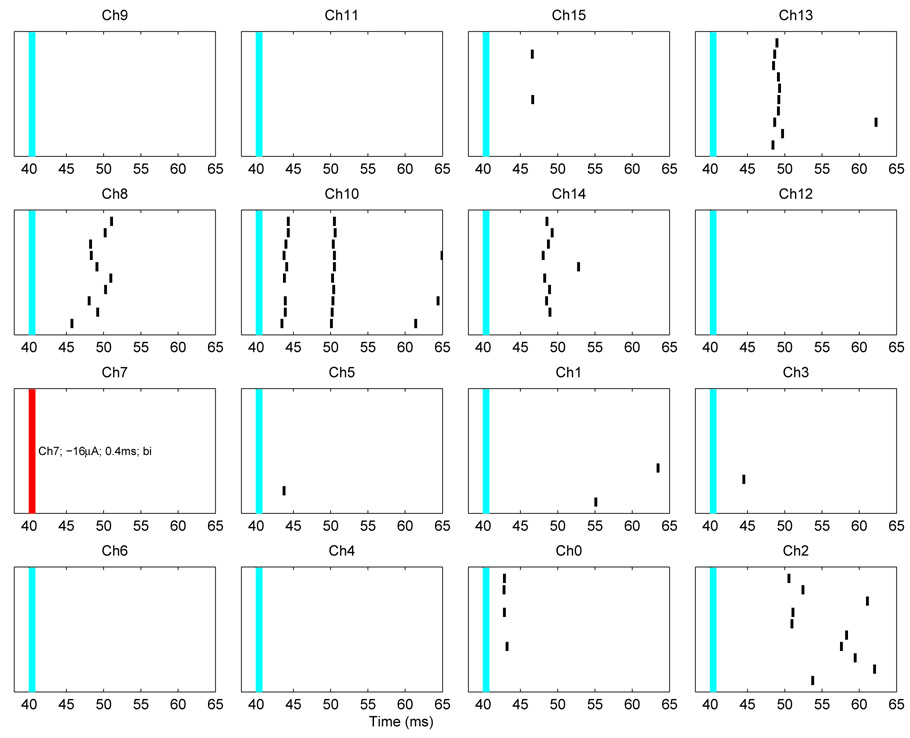
The procedure described above was repeated ten times, generating a raster plot, shown in Fig. 14. Conducting 10 trials allowed for the detection of a significant synaptic response, defined heuristically as 3 or more spikes occurring within a 2-msec window. This definition was reasonable because, for the small cultures ( ≈ 10 neurons) we studied, the spontaneous spike rate was very low (<< 1 spike/sec), so that spikes rarely occurred within a 200 msec test window. The synaptic delay time of a connection, δt, is defined as the average interval between the driven responses and the end of the stimulus. In Fig 14, significant synaptic responses occurred on electrodes 0, 2, 8, 13, and 14 at times t ≈ 43, 51, 49, 49, and 53 msec, respectively. Two distinct synaptic responses (termed “first generation” and “second generation”) are also seen on electrode 10, at approximately 44 and 51 msec, respectively.
Fig. 14.
Raster plot showing the spike times from all 10 trials with stimulation on electrode 7. Individual spike times are denoted by black tick-marks. Consecutive trials are stacked vertically with trial 1 at the bottom, and trial 10 at the top of each electrode’s display. The 4×4 layout matches the physical orientation of the neurocages. Significant synaptic responses (see text) occurred on electrodes 0, 2, 8, 10, 13 and 14. Two distinct responses occurred on electrode 10. Some responses are tightly timed (electrodes 0, 10, 13, and 14), while others are more diffuse (electrodes 2 and 8).
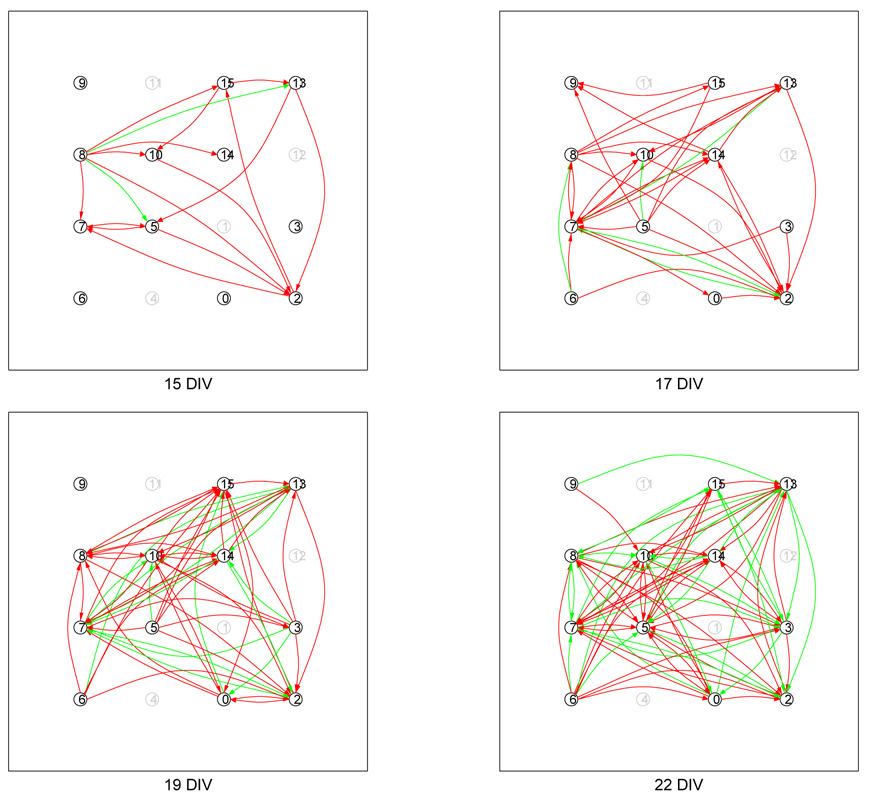
Having the ability to map connectivity noninvasively, we mapped cultures at 2 or 3 day intervals, starting at about 7 DIV continuing up to 21 DIV, or longer. As an example, the evolving connectivity of a culture, mapped at 15, 17, 19 and 22 DIV, is shown in Fig. 15. A connection is shown as an arrow emanating from the pre-synaptic neuron, A, with the arrowhead terminating at the post-synaptic neuron, B. The arrows are color-coded according to the whether the pathway has been determined to be mono- or poly- synaptic (see Section 3.7). A monosynaptic pathway is colored red; a polysynaptic pathway is colored green. (Note that an polysynaptic arrow does not specify any of the intermediate neuron(s) in the pathway from A to B.) This figure illustrates the unprecedented capability that the neurochip offers.
Fig. 15.
Evolution of a cultured neural network’s connectivity from 15–22 DIV. The color of an arrow encodes whether the response pathway is monosynaptic (red), or polysynaptic (green). In the case that both mono- and polysynaptic responses were observed, only the monosynaptic response is indicated with an arrow. Connections for which the delay time is longer than 20 msec are not drawn (see Section 3.7). A grayed-out circle indicates an electrode which was not probed (no neuron present). The culture undergoes a rapid maturation period. At 15 DIV modest levels of connectivity are present. The culture evolves rapidly over the next 7 days—much richer connectivity is observed at 22 DIV.
3.7 A More Detailed View of Connectivity
To more fully analyze connectivity—to determine whether a connection resulted from a mono- or polysynaptic pathway—putative intermediate neurons were detected by applying the following two criteria:
For a connection from neuron A to neuron B, responses also exist from A to neuron C and also from C to B—i.e., C is the intermediate neuron in the A-C-B pathway.
- The delay times for the responses from A to C and from C to B approximately sum to the measured delay time for the connection from A to B:
where ∈ is chosen here to be 2 msec. The polysynaptic delay time is restricted to δtAB ≤ 20 msec.
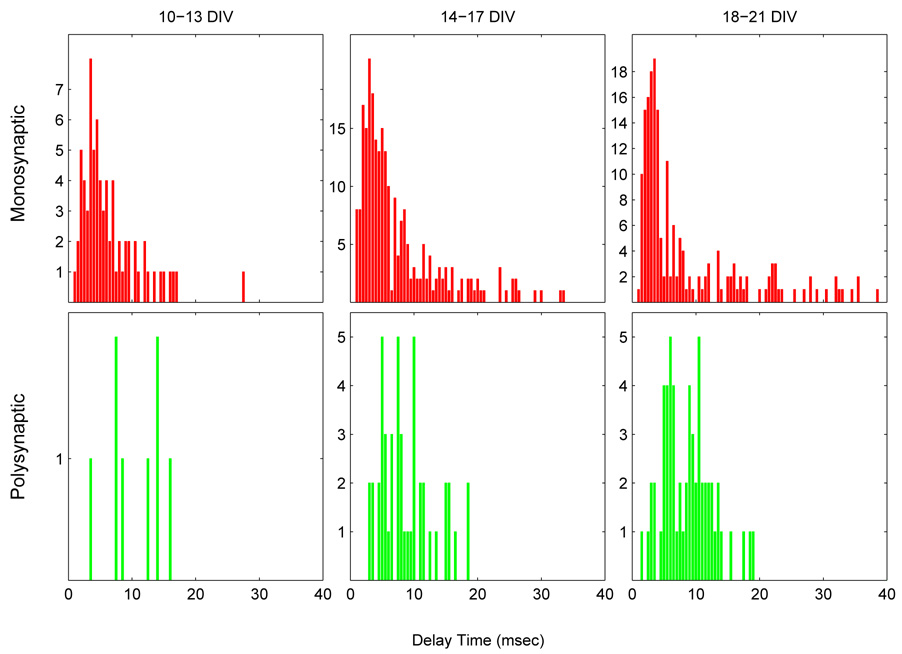
If all three criteria above were met, then neuron C was tagged as an intermediate neuron, and the pathway was deemed to be polysynaptic. If no intermediate neuron was found, then the pathway was termed monosynaptic. Note, however, that, according to the criterion 3) above, a connection for which δt > 20 msec is automatically labeled as monosynaptic, probably a misnomer for the following reason. The third criteria was imposed based on the finding that the distribution of delay times had a small, but growing tail for δt > 20 msec (Fig. 18). These long delay times were hypothesized to result from long-distance (≥ 2 mm) connections between caged neurons and the mass-culture. Such a pathway would include a neuron residing far from the 4×4 array, not in electrical communication with a neurocage electrode. Therefore, such a pathway can not be identified as polysynaptic because the delay times δtAC and δtCB would not measured or known.
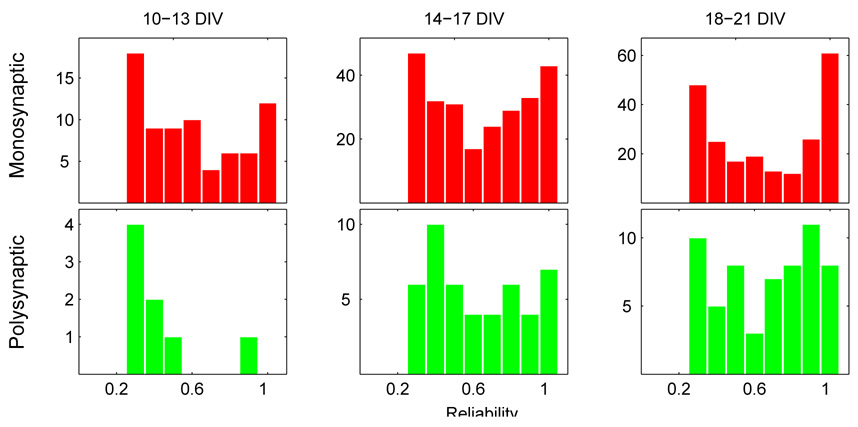
Fig. 18.
Histogram of monosynaptic (top row) and polysynaptic (bottom row) connection delay times at various developmental stages (bin width: 0.5 msec). Most monosynaptic delay times fall in the range of 2–10 msec, while polysynaptic connections exhibit longer delay times of, on average, about 9 msec. The distribution of delay times for δt < 2 msec shown here is an imperfect measure—some are lost in the stimulus artifact blanking by SALPA, which is typically about 1–2 msec long. The increasing frequency of long delays (δt > 20 msec) in older cultures is consistent with the hypothesis that they are generated by connections to the distant mass-culture. Overall, with the exception of an increasing frequency of long delays, the monosynaptic delay distribution does not change appreciably from 10–21 DIV.
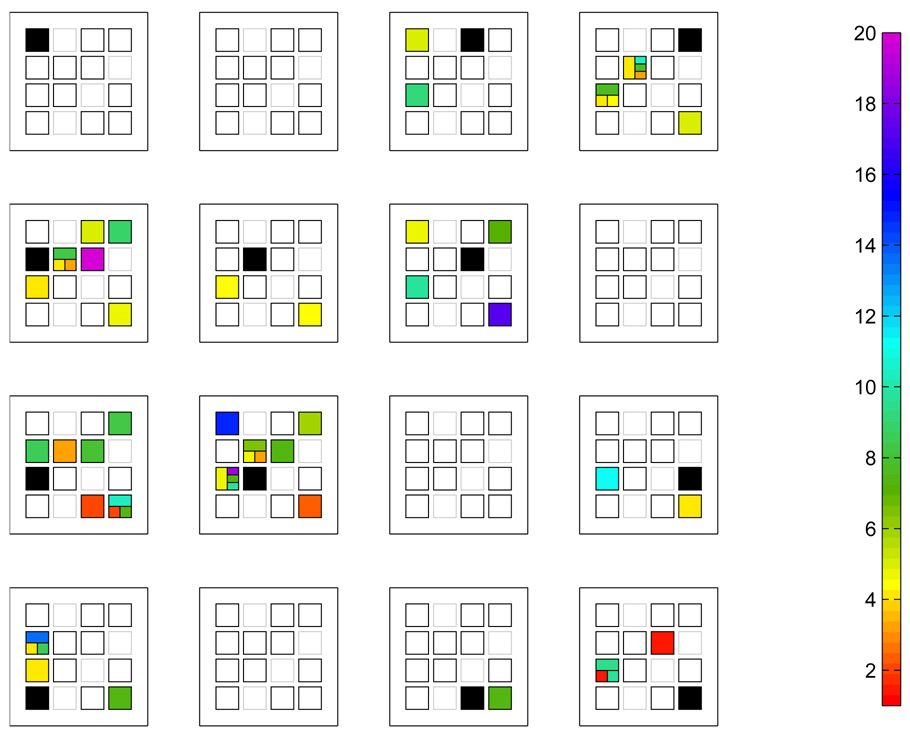
The results of partitioning connections into mono- or polysynaptic pathways, or both, can be visualized as in Fig. 16, referred to as a CultureState figure. The colors of the boxes encode the connection delay time. In this instance, 8 out of 37 connections had polysynaptic pathways; 6 of these were exclusively polysynaptic, and 2 of the connections had both mono- and polysynaptic path-ways.
Fig. 16.
CultureState view of connectivity (corresponding to connections shown as red and green arrows in Fig 15 at 17 DIV). Each sub-panel in the 4×4 display shows the responses to the cell being stimulated. Inside each sub-panel is a 4×4 grid of squares which is isomorphic to the physical geometry of the neurocages. Boxes outlined in black indicate the neuron was tested, while a grayed-out box indicates that no neuron was present/tested. The solid black square represents the stimulus electrode. The other boxes are colored according to the observed mono-and/or polysynaptic response times. If a connection is solely monosynaptic (i.e., no polysynaptic connection was found) the entire box is colored according to the monosynaptic delay, δtAB. If the connection is solely polysynaptic the box is broken into sub-boxes: The top-half is the measured polysynaptic pathway response time, δtAB. The colors of the two 1/4 boxes covering the bottom-half correspond to δtAC and δtCB. Finally, in the case that both mono- and polysynaptic pathways were detected, the box is broken into a total of 4 sub-boxes as follows: The box covering the left-half is color-coded according to the monosynaptic delay. The right half is broken into thirds: the top represents the polysynaptic pathway delay time, and the bottom two 1/3-boxes colored according to the two intermediate pathways which are linked to form the polysynaptic response. The color bar at right encodes the response time delay in msec.
3.8 Basic Network Metrics
The results from connectivity mapping experiments and subsequent partitioning of mono- and polysynaptic connections, were used to compute some basic metrics that describe neurochip networks. Data were gathered from 10 different neurochip cultures. The following results are intended to highlight the utility of the neurochip for studying the average evolution of cultured neural networks over time.
The connectivity fraction (CF) describes how fully connected a culture is—i.e., what fraction of its potential connections actually exist. Since autapses cannot be detected, we defined
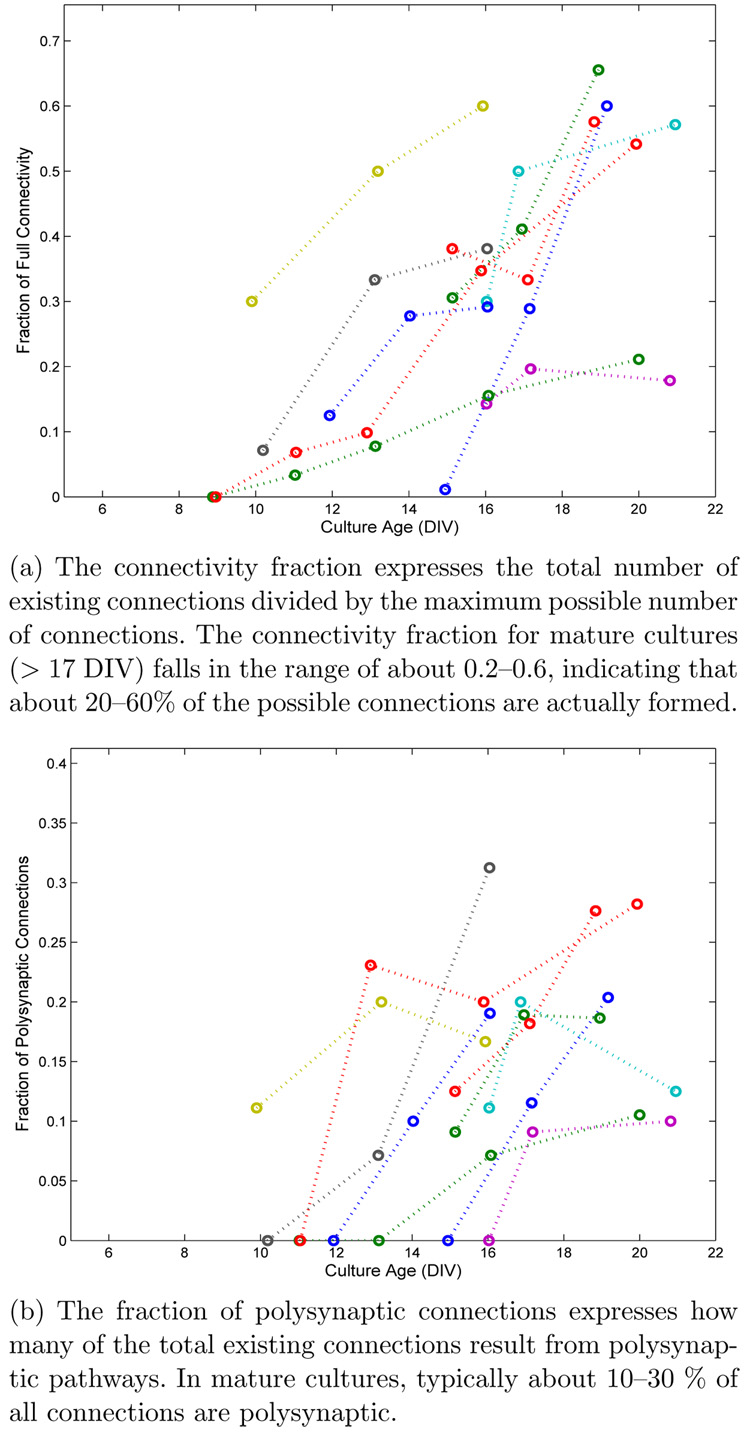
where nneurons represents the number of neurons in the culture, and nconnections represents the total number of connections in the culture. When both mono-and polysynaptic pathways existed between neurons A and B, both were tallied. The connectivity fraction versus time is shown in Fig. 17(a). Suprathresh-old connections are first observed at about 10 DIV. Mature networks (> 17 DIV) were typically 20–50% connected, meaning that, on average, a neuron connected to 20–50% of its potential targets. Also, the fraction of connections which were polysynaptic, FP, was computed as
where npoly is the number of identified polysynaptic connections. The fraction of polysynaptic connections versus time is shown in Fig. 17(b). Typically, between 10–30% of existing connections in mature networks were polysynaptic. The increase in both CF and FP over time indicates that connectivity became richer as cultures matured.
Fig. 17.
The richness of connectivity increases over time. Each color (circles and dashed lines) represents an individual culture. Error bars are not shown to aid visual clarity.
(a) The connectivity fraction expresses the total number of existing connections divided by the maximum possible number of connections. The connectivity fraction for mature cultures (> 17 DIV) falls in the range of about 0.2–0.6, indicating that about 20–60% of the possible connections are actually formed.
(b) The fraction of polysynaptic connections expresses how many of the total existing connections result from polysynaptic pathways. In mature cultures, typically about 10–30 % of all connections are polysynaptic.
The distribution of mono- and polysynaptic delay times for three different periods (10–13, 14–17, and 18–21 DIV) is shown in Fig 18. The majority of monosynaptic delay times observed were between 2–10 msec. The distribution of short (δt < 20 msec) monosynaptic delays did not change appreciably over this timespan. The median remains nearly constant at about 5 msec; the peak of the distribution remains nearly constant at about 3–3.5 msec. However, an increasing number of connections with long delay times (δt > 20 msec) is observed in older cultures. These long delay connections are believed to result from long-distance (≥ 2 mm) contacts between the mass-culture and caged neurons which form after several weeks, when long axons are sometimes observed to grow near, or into, the 4×4 array.
The distribution of polysynaptic delays is relatively atter and shifted to the right of the monosynaptic distribution—as expected, on average, longer delay times are observed. Polysynaptic delay times for cultures 14–17 DIV typically fell in the range 9.0 ± 4.1 msec (mean ± s.d.); for cultures 18–21 DIV, delay times typically fell in the range of 8.9 ± 3.9 msec.
The connection reliability, defined as the fraction out of ten trials for which a driven response was observed in a particular synaptic pair, was also tracked over time. Fig. 19 shows the distribution of reliabilities for three developmental periods, for both mono- and polysynaptic connections. For both types, a mix of relatively reliable and unreliable connections was always present. (The 10–13 DIV period does not contain enough polysynaptic connections to deem the subsequent apparent strengthening significant). It is known that the long-delay connections—labeled monosynaptic—are always low reliability (data not shown). This fact may, in part, explain the relatively high frequency of low-reliability connections in older cultures.
Fig. 19.
Histogram of connection “reliability” for monosynaptic (top row) and polysynaptic (bottom row) connections, broken down into three developmental periods. Both connection types exhibit a mix of reliable and unreliable connections.
4 Discussion
4.1 Basic Conclusions
Cultured networks can be grown with neurons trapped in neurocages optimized for hippocampal CA3/CA1 cell types, with process growth unconfined to any predefined geometry. Electrodes in 1:1 correspondence with neurons can safely and effectively stimulate APs, as well as record them with high fidelity. Connectivity can be mapped over the lifetime of a culture at single cell resolution.
4.2 Network Analysis
Neurochip cultures typically exhibited suprathreshold activity—spontaneous and/or driven—starting around 10–14 DIV. This time range for formation of functional synapses and network maturation is consistent with previous studies of dissociated hippocampal cultures (Renger et al., 2001; Verderio et al., 1999; Bading et al., 1995; Arnold et al., 2005). As a general rule, cultures exhibiting spontaneous activity also exhibited driven network responses. The converse, however, was not always true: cultures not exhibiting spontaneous activity often exhibited driven network responses. For unknown reasons, not all cultures developed suprathreshold synaptic connectivity. 29 out of 41 cultures tested did develop suprathreshold connectivity. Also, the richness of connectivity observed in neurochip networks (20–60% connected) is similar to levels noted in low-density (300/mm2) hippocampal control cultures (G. Bi, personal communication).
We investigated whether the observed responses in neurochip cultures result from mono- or polysynaptic connections. A large proportion of monosynaptic delays observed in neurochip cultures fell in the range of 2–10 msec, while the majority of polysynaptic delays fell in the range of about 3–15 msec. Working with voltage-clamped cell triplets in dissociated hippocampal cultures, Fitzsi-monds and Poo measured monosynaptic latency times in the range of 1.5–2.6 msec, and polysynaptic latencies in the range of 5–15 msec (Bi and Poo, 1999). The spectrum of monosynaptic delay times measured in neurochip cultures is relatively broad, and, on average, longer than synaptic latencies measured by Fitzsimonds and Poo, by about 1–2 msec. However, the neurochip stimulus-response timing measures the sum of delays from axonal conduction, synaptic transmission, and spike initiation, whereas the synaptic latency measured in voltage-clamp mode is the sum of only the first two components. Thus, it is reasonable to expect that monosynaptic delays measured in neurochip cultures are longer than latencies measured in voltage-clamp mode by about 1–2 msec (Fricker and Miles, 2000). The use of high-KCl medium by Fitzsimonds, or earlier contact with astrocytes may also help explain further discrepancy in average monosynaptic delay times (Ziv and Garner, 2001). The range of polysynaptic delays measured in neurochip cultures is in reasonable accord with normal dissociated cultures.
The spectrum of reliabilities of driven network responses was relatively at. This broad range of connection reliabilities was expected owing to: 1) the intrinsic stochastic nature of synaptic vesicle release and signal transmission (Rosenmund et al., 1993), 2) trial-to-trial uctuations in the amplitude of transmitter release (Savtchenko et al., 2001), and 3) the dynamic nature of cultures which contain synapses at different stages of maturity and with variable strengths (Verderio et al., 1999). It is also tempting to speculate that neurochip cultures reach a synaptic satiety level—i.e., there is a maximum number of synapses which can be developed and maintained, therefore a maximum level of average reliability. The observed increase in the number of connections with long-delays (δt > 20 msec) is very likely due to long-range (≈ 2 mm) connections formed by neurons in the mass culture to neurons in the 4×4 array. Perfect isolation from the mass culture could be achieved by deposition of a non-adhesive substrate (agarose) around the 4×4 array.
4.3 Limitations
One limitation with the current system is the maximum number of neurons in the neurochip cultures. Significant progress has already been made toward scaling the design up to 60 cages (Chow, 2007; Tooker, 2007). Another inherent limitation is the inability to reliably detect connections with delay times δt ≤ 2 msec due to the stimulus artifact suppression algorithm. The observed performance of the SALPA algorithm indicated that driven responses with very short delay times (δt < 2 msec) went undetected about 50% of the time (Erickson, 2008). Currently, this problem is difficult, if not impossible to easily remedy. A significant limitation is that only excitatory, suprathreshold connectivity can be detected. We expect from immunostaining experiments that about 10% of E18 dissociated hippocampal cells may be GABAergic, so one should not expect to lose much connectivity information. Regarding the latter, one possible remedy is to incorporate measurement elements (e.g. nanowires (Patolsky et al., 2006)) that are capable of measuring subthreshold potential differences extracellularly.
In spite of these limitations, the current neurochip system offers new and powerful ways to study neural networks.
4.4 Future experiments
The neurochip system can be used to study the effects of various pharmacological agents on network formation and dynamics (Murrey et al., 2006; Kalovidouris et al., 2005).
Importantly, the neurochip can be also used to perform chronic plasticity experiments to elucidate activity-dependent mechanisms. Previous experiments, in which a small number of neurons were manipulated, have revealed that in vitro network function is readily modified in response to imposed electrical activity (Bi and Poo, 2001; Jimbo et al., 1999; Eytan et al., 2003; Shahaf and Marom, 2001). Activity-dependent dynamics in the hippocampus are crucial for learning and storing new memories. In addition, many in vivo sensory systems demonstrate that use-dependent and experience-driven activity regulate the development of neural circuits, modulating neurite branch stability and synaptogenesis (Hua and Smith, 2004; Zhang and Poo, 2001).
The neurochip could be used to apply various patterns of stimulated activity on the network to study plasticity mechanisms, and “learned” responses on different time scales, ranging from minutes to days, could be investigated. Stimuli could be either acute or chronic. Comparing activity between neurochip networks which are subject to stimulation versus those that are not, or comparing a single network’s activity before and after stimulation, will reveal how activity-dependent effects shape the network’s input-output responses. These studies could help elucidate how normal circuit formation and plasticity mechanisms give rise to network function for both in vitro and in vivo systems.
Acknowledgements
We wish to express our sincerest gratitude to Sheri McKinney for expert assistance with cell-culture; Pat Koen and Jean Edens for masterful production of SEMs; machine shop wizards Mike Roy and Steven Olson; Trevor Roper for assistance in the clean-room; John Rolston for assisting the implemention of the SALPA algorithm in MATLAB; Daniel Wagenaar and Gary Chow for helpful suggestions and discussions relating to this work. Funding for this work was provided by NIH grant NS044134.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold F, Hofmann F, Bengtson C, Wittmann M, Vanhoutte P, Bading H. Microelectrode array recordings of cultured hippocampal networks reveal a simple model for transcription and protein synthesis-dependent plasticity. J. Physiol. (Lond.) 2005 Apr;564:3–19. doi: 10.1113/jphysiol.2004.077446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading H, Segal M, Sucher N, Dudek H, Lipton S, Greenberg M. N-methyl-D-aspartate receptors are critical for mediating the effects of glutamate on intracellular calcium concentration and immediate early gene expression in cultured hippocampal neurons. Neuroscience. 1995 Feb;64:653–664. doi: 10.1016/0306-4522(94)00462-e. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M. Distributed synaptic modification in neural networks induced by patterned stimulation. Nature. 1999 Oct;401(6755):792–796. doi: 10.1038/44573. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo MM. Synaptic modification by correlated activity: Hebb’s postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Branch D, Wheeler B, Brewer G, Leckband D. Long-term maintenance of patterns of hippocampal pyramidal cells on substrates of polyethylene glycol and microstamped polylysine. IEEE Trans. Biomed. Eng. 2000;47(3):290–300. doi: 10.1109/10.827289. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35(5):567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Buchhalter J, Dichter M. Electrophysiological comparison of pyramidal and stellate nonpyramidal neurons in dissociated cell culture of rat hippocampus. Brain Res. Bull. 1991 Mar;26:333–338. doi: 10.1016/0361-9230(91)90003-3. [DOI] [PubMed] [Google Scholar]
- Chien CB, Pine J. An apparatus for recording synaptic potentials from neuronal cultures using voltage-sensitive fluorescent dyes. J Neurosci Methods. 1991a;38:93–105. doi: 10.1016/0165-0270(91)90159-w. [DOI] [PubMed] [Google Scholar]
- Chien CB, Pine J. Voltage-sensitive dye recording of action potentials and synaptic potentials from sympathetic microcultures. Biophys J. 1991b;60(3):697–711. doi: 10.1016/S0006-3495(91)82099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii D, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- Chow G. Ph.D. thesis, Caltech. 2007. Laser Tweezers for Moving Live Dissociated Neurons. [Google Scholar]
- Claverol-Tinture E, Pine J. Extracellular potentials in low-density dissociated neuronal cultures. J Neurosci Methods. 2002 May;117(1):13–21. doi: 10.1016/s0165-0270(02)00043-2. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Marder E. Plasticity in single neuron and circuit computations. Nature. 2004;431:789–795. doi: 10.1038/nature03011. [DOI] [PubMed] [Google Scholar]
- Erickson J. Ph.D. thesis, Caltech. 2008. The Neurochip: A Complete System for Studying Cultured Neural Network Connectivity. [Google Scholar]
- Eytan D, Brenner N, Marom S. Selective adaptation in networks of cortical neurons. J Neurosci. 2003 Oct;23(28):9349–9356. doi: 10.1523/JNEUROSCI.23-28-09349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimonds RM, Song HJ, Poo MM. Propagation of activity-dependent synaptic depression in simple neural networks. Nature. 1997 Jul;388(6641):439–448. doi: 10.1038/41267. [DOI] [PubMed] [Google Scholar]
- Fricker D, Miles R. EPSP amplification and the precision of spike timing in hippocampal neurons. Neuron. 2000 Nov;28:559–569. doi: 10.1016/s0896-6273(00)00133-1. [DOI] [PubMed] [Google Scholar]
- Gold C. Ph.D. thesis, Caltech. 2007. Biophysics of extracellular action potentials. [Google Scholar]
- Gross GW, Rieske E, Kruetzbert GW, A M. A New fixed-array multi-microelectrode system designed for long-term monitoring of extracellular single unit neuronal activity in vitro. Neuroscience Letters. 1977;6:101–105. doi: 10.1016/0304-3940(77)90003-9. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates; 1992. [Google Scholar]
- Holt GR, Koch C. Electrical interactions via the extracellular potential near cell bodies. J Comput Neurosci. 1999 Mar;6(2):169–184. doi: 10.1023/a:1008832702585. [DOI] [PubMed] [Google Scholar]
- Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat. Neurosci. 2002 Nov;5:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smith S. Neural activity and the dynamics of central nervous system development. Nat. Neurosci. 2004;7(4):327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Ip NY, Li Y, Yancopoulos GD, Lindsay RM. Cultured hippocampal neurons show responses to BDNF, NT-3, and NT-4, but not NGF. J Neurosci. 1993 Aug;13(8):3394–3405. doi: 10.1523/JNEUROSCI.13-08-03394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimbo Y, Tateno T, Robinson HP. Simultaneous induction of pathway-specific potentiation and depression in networks of cortical neurons. Biophys J. 1999 Feb;76(2):670–678. doi: 10.1016/S0006-3495(99)77234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun SB, Hynd MR, Dowell-Mesfin N, Smith KL, Turner JN, Shain W, Kim SJ. Low-density neuronal networks cultured using patterned poly-l-lysine on microelectrode arrays. J Neurosci Methods. 2007 Mar;160(2):317–326. doi: 10.1016/j.jneumeth.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalovidouris S, Gama C, Lee L, Hsieh-Wilson L. A role for fucose alpha(1–2) galactose carbohydrates in neuronal growth. J. Am. Chem. Soc. 2005 Feb;127:1340–1341. doi: 10.1021/ja044631v. [DOI] [PubMed] [Google Scholar]
- Lein PJ, Banker GA, Higgins D. Laminin selectively enhances axonal growth and accelerates the development of polarity by hippocampal neurons in culture. Dev Brain Res. 1992 Oct;69(2):191–197. doi: 10.1016/0165-3806(92)90159-t. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Heumann R. BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurones. Neuroreport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- Levine E, Dreyfus C, Black I, Plummer M. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc. Natl. Acad. Sci. U.S.A. 1995 Aug;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher MP, Pine J, Wright J, Tai Y-C. The neurochip: a new multielectrode device for stimulating and recording from cultured neurons. J Neurosci Methods. 1999 Feb;87(1):45–56. doi: 10.1016/s0165-0270(98)00156-3. [DOI] [PubMed] [Google Scholar]
- Maher MP, Wright J, Pine J, Tai YC. A microstructrue for interfacing with neurons: the Neurochip. Proc. IEEE Med. Bio. 1998;20(4):1698–1702. [Google Scholar]
- Mehta M, Barnes C, McNaughton B. Experience-dependent, asymmetric expansion of hippocampal place fields. Proc. Natl. Acad. Sci. U.S.A. 1997 Aug;94:8918–8921. doi: 10.1073/pnas.94.16.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrey H, Gama C, Kalovidouris S, Luo W, Driggers E, Porton B, Hsieh-Wilson L. Protein fucosylation regulates synapsin Ia/Ib expression and neuronal morphology in primary hippocampal neurons. Proc. Natl. Acad. Sci. U.S.A. 2006 Jan;103:21–26. doi: 10.1073/pnas.0503381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid AL, Loew LM, Wuskell JP, Salzberg BM. Novel naphthylstyryl-pyridium potentiometric dyes offer advantages for neural network analysis. J Neurosci Methods. 2004;134(2):179–190. doi: 10.1016/j.jneumeth.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Offenhausser A, Sprossler C, Matsuzawa M, Knoll W. Electro-physiological development of embryonic hippocampal neurons from the rat grown on synthetic thin films. Neurosci Lett. 1997 Feb;223(1):9–12. doi: 10.1016/s0304-3940(97)13372-9. [DOI] [PubMed] [Google Scholar]
- Patolsky F, Timko BP, Yu G, Fang Y, Greytak AB, Zheng G, M LC. Detection, Stimulation, and Inhibition of Neuronal Signals with High-Density Nanowire Transistor Arrays. Science. 2006;313:1100–1104. doi: 10.1126/science.1128640. [DOI] [PubMed] [Google Scholar]
- Pine J. Recording action potentials from cultured neurons with extracellular microcircuit electrodes. J Neurosci Methods. 1980 Feb;2(1):19–31. doi: 10.1016/0165-0270(80)90042-4. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as Synaptic Modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Potter S, DeMarse T. A new approach to neural cell culture for long-term studies. J. Neurosci. Methods. 2001 Sep;110:17–24. doi: 10.1016/s0165-0270(01)00412-5. [DOI] [PubMed] [Google Scholar]
- Rall W. Electrophysiology of a dendritic neuron model. Biophys J. 1962 Mar;2:145–167. doi: 10.1016/s0006-3495(62)86953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger J, Egles C, Liu G. A developmental switch in neurotransmitter flux enhances synaptic efficacy by affecting AMPA receptor activation. Neuron. 2001 Feb;29:469–484. doi: 10.1016/s0896-6273(01)00219-7. [DOI] [PubMed] [Google Scholar]
- Robinson DA. The electrical properties of metal microelectrodes. Proc. IEEE. 1968 June;56(6):1065–1072. [Google Scholar]
- Rosenmund C, Clements J, Westbrook G. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993 Oct;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- Savtchenko L, Gogan P, Tyc-Dumont S. Dendritic spatial flicker of local membrane potential due to channel noise and probabilistic firing of hippocampal neurons in culture. Neurosci. Res. 2001 Oct;41:161–183. doi: 10.1016/s0168-0102(01)00274-7. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Hyperexcitability in combined entorhinal/hippocampal slices of adult rat after exposure to brain-derived neurotrophic factor. J Neurophysiol. 1997 Aug;78(2):1082–1095. doi: 10.1152/jn.1997.78.2.1082. [DOI] [PubMed] [Google Scholar]
- Shahaf G, Marom S. Learning in networks of cortical neurons. J Neurosci. 2001 Nov;21(22):8782–8788. doi: 10.1523/JNEUROSCI.21-22-08782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, Johnston D. Perforated patch-clamp analysis of the passive membrane properties of three classes of hippocampal neurons. J Neurophysiol. 1992 Mar;67(3):508–529. doi: 10.1152/jn.1992.67.3.508. in Vitro. [DOI] [PubMed] [Google Scholar]
- Tooker A. Ph.D. thesis, Caltech. 2007. Development of Biocompatible Parylene Neurocages for Action Potential Stimulation and Recording. [Google Scholar]
- Tooker A, Meng E, Erickson J, Tai Y-C, Pine J. Proc. IEEE-EMBS. San Francisco, CA, USA: 2004. Sep, Development of biocompatible parylene neurocages; pp. 2542–2545. [DOI] [PubMed] [Google Scholar]
- Verderio C, Coco S, Pravettoni E, Bacci A, Matteoli M. Synaptogenesis in hippocampal cultures. Cell. Mol. Life Sci. 1999 Aug;55:1448–1462. doi: 10.1007/s000180050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario-Abejon C, Collin C, McKay RD, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J. Neurosci. 1998 Sep;18:7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar DA, Potter SM. Real-time multi-channel stimulus artifact suppression by local curve fitting. J Neurosci Methods. 2002 Oct;120(2):113–120. doi: 10.1016/s0165-0270(02)00149-8. [DOI] [PubMed] [Google Scholar]
- Witter MP. Connectivity of the rat hippocampus. NY: Alan R. Liss Inc; 1989. pp. 53–69. [Google Scholar]
- Wyart C, Ybert C, Bourdieu L, Herr C, Prinz C, Chatenay D. Constrained synaptic connectivity in functional mammalian neuronal networks grown on patterned surfaces. J Neurosci Methods. 2002 Jun;117(2):123–131. doi: 10.1016/s0165-0270(02)00077-8. [DOI] [PubMed] [Google Scholar]
- Zhang L, Poo MM. Electrical activity and development of neural circuits. Nat. Neurosci. Suppl. 2001;4:1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Ziv N, Garner C. Principles of glutamatergic synapse formation: seeing the forest for the trees. Curr. Opin. Neurobiol. 2001 Oct;11:536–543. doi: 10.1016/s0959-4388(00)00246-4. [DOI] [PubMed] [Google Scholar]