Abstract
BACKGROUND
It remains unknown whether local anesthetic concentration, or simply total drug dose, is the primary determinant of continuous peripheral nerve block effects. We therefore tested the null hypothesis that providing different concentrations and rates of ropivacaine, but at equal total doses, produces comparable effects when used in a continuous sciatic nerve block in the popliteal fossa.
METHODS
Preoperatively, a perineural catheter was inserted adjacent to the sciatic nerve using a posterior popliteal approach in patients undergoing moderately painful orthopedic surgery at or distal to the ankle. Postoperatively, patients were randomly assigned to receive a perineural ropivacaine infusion of either 0.2% (basal 8 mL/h, bolus 4 mL) or 0.4% (basal 4 mL/h, bolus 2 mL) through the second postoperative day. Therefore, both groups received 16 mg of ropivacaine each hour with a possible addition of 8 mg every 30 min via a patient-controlled bolus dose. The primary end point was the incidence of an insensate limb, considered undesirable, during the 24-h period beginning the morning after surgery. Secondary end points included analgesia and patient satisfaction.
RESULTS
Patients given 0.2% ropivacaine (n = 25) experienced an insensate limb with a mean (sd) of 1.8 (1.8) times, compared with 0.6 (1.1) times for subjects receiving 0.4% ropivacaine (n = 25; estimated difference = 1.2 episodes, 95% confidence interval, 0.3–2.0 episodes; P = 0.009). In contrast, analgesia and satisfaction were similar in each group.
CONCLUSIONS
For continuous popliteal-sciatic nerve blocks, local anesthetic concentration and volume influence block characteristics. Insensate limbs were far more common with larger volumes of relatively dilute ropivacaine. During continuous sciatic nerve block in the popliteal fossa, a relatively concentrated solution in smaller volume thus appears preferable.
Continuous peripheral nerve blockade involves the percutaneous insertion of a catheter directly adjacent to a peripheral nerve. The catheter is then infused with local anesthetic, resulting in potent, site-specific analgesia that lasts well beyond the normal duration of a single-injection nerve block.1,2
However, a transiently insensate limb is a well-recognized effect of perineural local anesthetic infusion.1-5 It is postulated that an insensate extremity is best minimized during continuous peripheral nerve blocks because insensate limbs may be prone to accidental injury.4-8 This concern of injury has resulted in recommendations to protect the surgical extremity in a sling and/or brace and use crutches or walkers (lower extremity surgery) for the duration of infusion.2,3,7,9 Some have suggested delaying hospital discharge until sensation returns.5
Local anesthetic pharmacodynamics varies considerably among introduction techniques. For example, during subarachnoid block, the total dose is the primary determinant of clinical effects, even when the concentration and volume of local anesthetic are varied over a large range.10 In contrast, the effects are mixed for epidural local anesthetic infusions; total dose is the primary determinant of analgesia quality and dermatomal spread, whereas concentration is the primary determinant of motor block and sympathectomy/hypotension.11 At a constant total dose, local anesthetic volume is the primary determinant of efficacy for single-injection axillary blocks.12 However, the relative importance of local anesthetic concentration and/or volume versus dose remains unexamined for continuous peripheral nerve blocks.
We therefore tested the null hypothesis that providing ropivacaine at different concentrations and rates (0.2% at 8 mL/h vs 0.4% at 4 mL/h), but at an equal total basal dose (16 mg/h), produces comparable effects when used in a continuous sciatic nerve block. Our primary end point was the incidence of an insensate limb (e.g., inability to perceive touch on any aspect of the foot) during the 24-h period beginning the morning after surgery.
METHODS
Enrollment
The Institutional Review Board at each participating clinical center approved all study procedures (University of FL, Gainesville, FL; University of CA San Diego, San Diego, CA). All subjects provided written, informed consent; because this was a multicenter trial, a Data Safety Monitoring Board (University of FL, Gainesville, FL) reviewed combined data and adverse events.
Patients offered enrollment included adults (18–75 years) scheduled for moderately painful, ambulatory, unilateral, orthopedic surgery of the lower extremity at or distal to the ankle who desired a continuous sciatic nerve block for postoperative analgesia. Exclusion criteria included weight <40 kg, a history of opioid dependence or current chronic opioid use (defined as frequent use for more than 1 wk before surgery), known contraindication to any study medication, known hepatic or renal insufficiency/disease, insulin-dependent diabetes mellitus, known neuropathy of any etiology in the surgical extremity, pregnancy, incarceration, difficulty understanding the study protocol or caring for the infusion pump/catheter system, American Society of Anesthesiologists physical status 4–6, and any major incision outside of the sciatic nerve distribution of the lower leg (e.g., a planned incision into the saphenous nerve distribution).
Protocol
A stimulating catheter (StimuCath, Arrow International, Reading, PA) was inserted adjacent to the sciatic nerve via the posterior popliteal intertendonous approach13 using a previously described technique.3,14 Fifty milliliters of mepivacaine 1.5%, with epinephrine, 5 μg/mL, was injected via the catheter with gentle aspiration every 3 mL. The popliteal sciatic nerve block was evaluated 15 min later and considered successful when patients demonstrated muscle weakness upon plantar flexion and a decreased sensation to cold of the skin on the plantar aspect of their foot. Subject demographic and catheter placement data were uploaded via the Internet to a secure,15 password-protected, encrypted central server (www. PAINfRE.com, General Clinical Research Center, Gainesville, FL).16
Patients with a successful catheter placement per protocol and nerve block onset were retained in the study. Patients were randomized to one of two groups, ropivacaine 0.2% or 0.4%, stratified by institution using computer-generated tables and provided to study centers via the PAINfRE.com Web site. Placement of a femoral or saphenous single-injection nerve block with 20 mL of mepivacaine 1.5%, with epinephrine 5 μg/mL, was left to the discretion of the attending anesthesiologist.
After surgery, the ropivacaine infusion was initiated using a portable, programmable, disposable, electronic infusion pump (ambIT PCA, Sorenson Medical, West Jordan, UT). The pumps were programmed by investigators and the infusion basal rate and patient-controlled bolus dose volume depended upon treatment group (Table 1). Although patients were not specifically informed of their ropivacaine concentration, the infusion pump and local anesthetic reservoir accessible to subjects revealed enough information that subjects should not be considered masked to treatment group. At the discretion of investigators, a 20-mL bolus of mepivacaine 1.5% (with epinephrine, 5 μg/mL) could be injected via the popliteal catheter to prolong the initial surgical block in the case of an unexpected delay in the surgical start (perineural catheters were placed in preoperative holding areas, or “block rooms,” before entering the operating room).
Table 1.
Perineural Ropivacaine Infusion Profile by Treatment Group
| Ropivacaine concentration |
Basal rate (mL/h) |
Basal dose (mg/h) |
Bolus volume (mL) |
Bolus dose (mg) |
Lockout duration (min) |
Maximum dose (mg/h) |
|---|---|---|---|---|---|---|
| 0.2% (2 mg/mL) | 8 | 16 | 4 | 8 | 30 | 24 |
| 0.4% (4 mg/mL) | 4 | 16 | 2 | 8 | 30 | 24 |
Patient Education
Patients were discharged home with their infusion pump and perineural catheter in situ. Patients were instructed on care of the perineural catheter, the infusion pump, and signs and symptoms of local anesthetic toxicity; they were also given contact details for a continuously available local physician. For break-through pain, patients were instructed to depress the bolus button on their infusion pump, wait 15 min, and then take 5–10 mg of the oral opioid oxycodone if necessary.
Patients were also informed that an insensate extremity is expected after surgery because of the dense surgical block (reinforced with the ropivacaine infusion). However, if any part of their surgical extremity was completely insensate after 09:00 am the morning after surgery, patients were to pause their infusion until they regained feeling in their extremity, and then restart the infusion. “Completely insensate” was defined as being unable to determine with eyes closed that another individual was touching various parts of the foot/toes. Patients were instructed to perform this examination during telephone calls in both the morning and afternoon of postoperative day (POD) 1–3. They were also encouraged to perform the examination throughout the infusion period, beginning the morning of POD 1.
Patients were contacted by health care providers beginning the night of surgery, and each afternoon thereafter through POD 3. Patients were questioned about symptoms of local anesthetic toxicity, catheter migration, and infection, gross sensory and motor function, and the appearance of the catheter site. In the afternoon of POD 2, patients’ caretakers removed the catheters with a physician in telephone contact. The presence of a metallic catheter tip confirmed complete removal.
Measurements
Subjects were contacted by telephone in the mornings of POD 1–3 by a clinical research nurse at the University of Florida General Clinical Research Center. Nurses were masked to treatment group. Pain severity and oral oxycodone use for the previous 12 h (POD 1) or 24 h (PODs 2 and 3) were recorded. Pain severity was evaluated using a numeric rating scale of 0–10, with 0 equal to no pain and 10 being the worst imaginable pain.17 The number of awakenings resulting from pain the previous night was also recorded, as were the number of times the infusion pump was paused because of an insensate extremity. Patient satisfaction with postoperative analgesia was recorded on POD 2 using a 0–10 scale, 0 equal to “very unsatisfied” and 10 equal to “very satisfied.” All data were recorded on case report forms and then uploaded to the PAINfRE.com Web site. The case report forms data were subsequently entered into a separate database which were, upon study completion, compared with the Web site data to identify and correct any data entry errors. Of note, the number of patient-administered bolus doses and total infusion volume were not available to investigators.
Statistical Analysis
The study was powered for one primary end point related to the primary null hypothesis that differing the concentration but providing an equal total dose of ropivacaine has no impact on the incident number of numbness events. The primary end point was incidence of an insensate extremity in the 24-h period beginning at 09:00 am on POD 1. Based on previously published data,2,3 the planning distribution for the number of events for the two groups (0.2% vs 0.4%) was: 0 (60% vs 24%), 1 (30% vs 48%), 2 (10% vs 22%), and 3 (0% vs 6%). Based on a two-sample, two-sided t-test, to obtain 80% power at P = 0.05, a sample size of 25 patients per group was required. The calculation used large sample methods, but simulation results agreed well for both Type I error (0.05) and power (79.0%).
Because the number of events is a quantitative end point, we used the two-sample two-sided t-test which is virtually identical to the two-sided Z-test when sample sizes are approximately equal.18 All other outcome variables (secondary, ordinal) were analyzed by the two-sided Wilcoxon’s ranked sum test, which provides distribution-free P values and is highly robust against outliers. A two-sided P < 0.05 was considered statistically significant for the primary end point. Because each comparison dilutes all other P values, we restricted our analysis to four comparisons among secondary end points.19 P < 0.05 was again considered significant. Significant findings in secondary outcomes should be viewed as suggestive, requiring confirmation in a future trial before considering them as definitive.
RESULTS
Fifty-two patients enrolled and all but one had a perineural catheter successfully positioned per protocol. Two subjects exhibited no sensory or motor block 15 min after being given a local anesthetic bolus via the catheter. One of these two subjects had a catheter placed using ultrasound assistance with a subsequent dense sensory and motor block (see Protocol Violations below). The other of the two was not randomized per protocol. The 50 remaining subjects (96% of those enrolled) were randomized to one of the two treatment groups. The demographic, morphometric, and surgical characteristics were similar between groups (Tables 2 and 3). However, applying statistics to preintervention variables for subjects randomized to treatment groups is inappropriate. For this reason, no statistical comparisons were applied to the data of these two tables.
Table 2.
Population Data and Surgical Information
| Group 0.2% (n = 25) |
Group 0.4% (n = 25) |
|
|---|---|---|
| Age (yr) | 51 (35–60) | 55 (38–66) |
| Sex (female/male) | 19/6 | 19/6 |
| Height (cm) | 163 (157–170) | 170 (163–175) |
| Weight (kg) | 73 (61–82) | 73 (68–92) |
| Minimum current via needle (mA) |
0.48 (0.44–0.48) | 0.46 (0.34–0.48) |
| Minimum current via catheter (mA) |
0.40 (0.30–0.52) | 0.46 (0.64–0.58) |
| Subjects receiving an additional 20-mL mepivacaine bolus (#) |
14 | 15 |
| Intraoperative midazolam (mg) |
4 (2–4) | 4 (2–4) |
| Intraoperative fentanyl (μg) |
150 (100–200) | 100 (100–200) |
| Intraoperative morphine (mg) |
0 (0–0) | 0 (0–0) |
| Surgery duration (min) | 60 (40–105) | 50 (35–70) |
| Subjects from site A/B (#) |
8/17 | 8/17 |
Values are reported as median (25th-75th percentiles) or number of subjects, as indicated. Applying statistics to preintervention variables for subjects randomized to treatment groups is inappropriate. For this reason, no statistical comparisons were applied to the data of this table.
Table 3.
Primary Surgical Procedures
| Group 0.2% (n = 25) |
Group 0.4% (n = 25) |
|
|---|---|---|
| Achilles tendon repair | 1 | 1 |
| Calcaneal osteotomy | 6 | 5 |
| Claw-/Hammer-toes correction |
1 | 2 |
| Hallux valgus correction | 3 | 3 |
| Metatarsal osteotomy | 5 | 8 |
| Subtalar arthrodesis | 3 | 1 |
| Other | 6 | 5 |
Applying statistics to preintervention variables for subjects randomized to treatment groups is inappropriate. For this reason, no statistical comparisons were applied to the data of this table.
Primary End Point
Patients given 0.2% ropivacaine (n = 25) experienced an insensate limb a mean (sd) of 1.8 (1.8) times, compared with 0.6 (1.1) times for subjects receiving 0.4% ropivacaine (n = 25; estimated difference = 1.2 episodes, 95% confidence interval, 0.3–2.0 episodes; P = 0.009). Among patients assigned to 0.2% ropivacaine, 64% experienced at least one instance of an insensate extremity; in contrast, only 36% of the patients receiving 0.4% ropivacaine had an insensate extremity even once.
Secondary End Points
There were minimal differences between the two treatment groups for average (Fig. 1a) and worst daily pain scores (Fig. 1b), and both groups required similar doses of supplemental oral opioids (Table 4). However, patients assigned to 0.2% ropivacaine were awakened at night by pain slightly more often (Table 4). Satisfaction with postoperative analgesia was scored a median (25th—75th percentiles) of 10.0 (8.0–10.0) in Group 0.2% and 10.0 (9.8–10.0) in Group 0.4% (P = 0.13). There were no infusion pump malfunctions.
Figure 1.
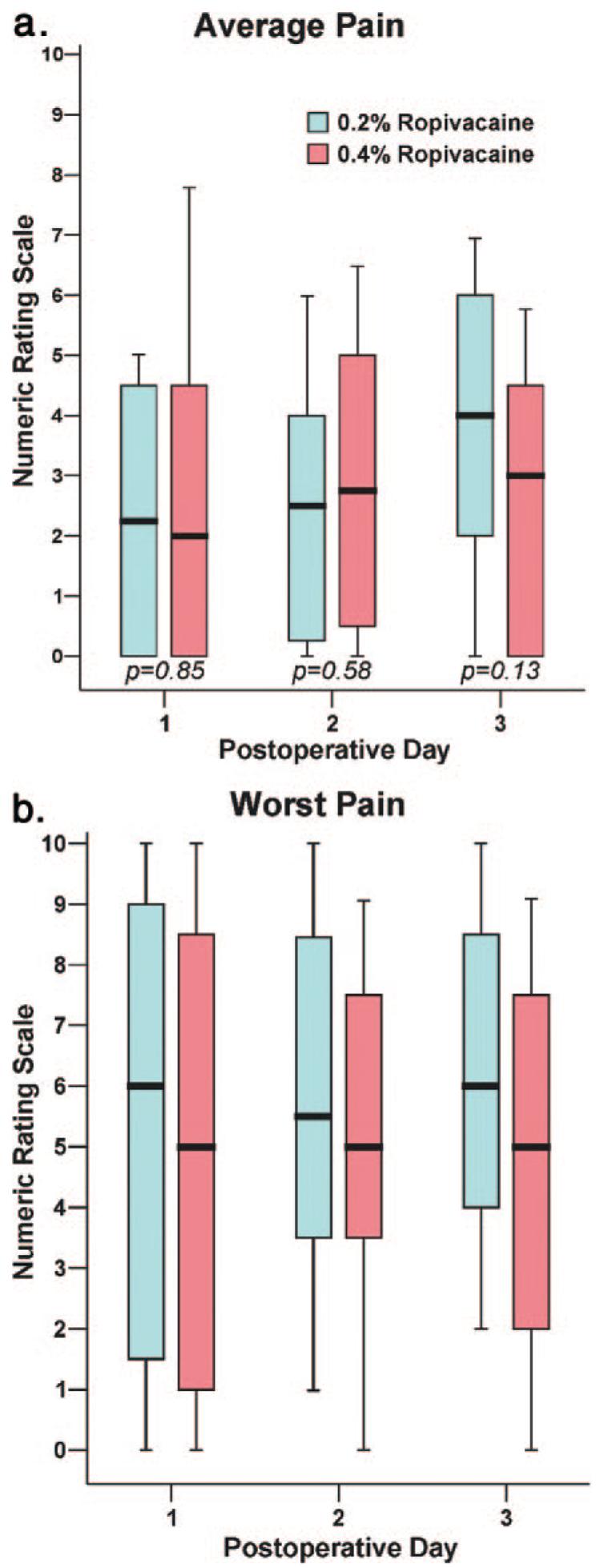
Effects of popliteal sciatic perineural ropivacaine concentration on postoperative pain after moderately painful surgery at or distal to the ankle. Pain severity indicated using a numeric rating scale of 0–10, with 0 equal to no pain and 10 being the worst imaginable pain. Data are expressed as median (horizontal bar) with 25th—75th (box) and 10th—90th (whiskers) percentiles for patients randomly assigned to Group 0.2% (0.2% ropivacaine, 8 mL/h basal, 4 mL bolus) or Group 0.4% (0.4% ropivacaine, 4 mL/h basal, 2 mL bolus). Because each comparison dilutes all other P values, we restricted our analysis to four comparisons among secondary end points. P values are provided where statistical comparisons were applied.
Table 4.
Secondary End Points
| Group 0.2% (n = 25) |
Group 0.4% (n = 25) |
|
|---|---|---|
| Home oral opioid consumption (mg)a |
||
| Postoperative day 1 | 0 (0–10) | 0 (0–10) |
| Postoperative day 2 | 5 (0–17.5) | 0 (0–20) |
| Postoperative day 3 | 20 (12.5–30) | 10 (2.5–20) |
| Awakenings because of pain (#) |
||
| Postoperative day/ night 0 |
1.7 (0–2.4) | 0 (0–1) |
| Postoperative day/ night 1 |
0.3 (0–2) | 0 (0–1) |
| Postoperative day/ night 2 |
1.7 (0–4) | 0 (0–1) |
Values are reported as median (25th—75th percentiles). Because each comparison dilutes all other P values, we restricted our analysis to four comparisons among secondary end points. For this reason, no statistical comparisons were applied to the data of this table.
Oral opioid provided as 5 mg oxycodone tablets. Values include home opioid consumption in the 24 h previous to the daily data collection phone calls.
Protocol Violations and Adverse Events
One subject had a perineural catheter placed per protocol, but exhibited no sensory or motor block 15 min after the initial local anesthetic bolus. The catheter was removed and replaced using ultrasound guidance and not solely nerve stimulation. Local anesthetic (20 mL) was administered and a sensory and motor block evolved within 15 min. The patient was randomized although the catheter had not been placed per protocol.
One subject from Group 0.2% without comorbidities had an unremarkable perioperative course, but reported a moderate sensory and motor deficit in the sciatic nerve distribution after infusion discontinuation on POD 2. A neurology consultant believed the patient had a sciatic nerve deficit just caudal to the gluteus maximus muscle of unknown etiology (greatly cephalad to the perineural catheter insertion and surgical site). The deficit fully resolved within 2 mo.
DISCUSSION
This investigation provides evidence that, for continuous popliteal-sciatic nerve blocks, local anesthetic concentration and volume influence perineural infusion effects in addition to the total mass of local anesthetic administered. In a 24-h period, patients given 0.2% ropivacaine at 8 mL/h experienced an insensate limb three times more often than patients given the same basal dose (16 mg/h), but as 0.4% ropivacaine at 4 mL/h.
The relative importance of local anesthetic concentration versus dose has clinical consequence given the wide range of local anesthetic concentrations investigators have used during perineural infusion: for ropivacaine alone, concentrations have included 0.1%,20 0.15%,21 0.2%,22 0.3%,23 and 0.4%.24 The issue has particular importance for ambulatory infusion where the local anesthetic reservoir volume and patient monitoring are limited.6 In this case, reducing the volume of local anesthetic delivered has the advantage of prolonging infusion duration.25 Unfortunately, simply decreasing the basal infusion rate, and therefore total drug dose, may result in a concomitant decrease in analgesia and other infusion benefits.22 Therefore, using a relatively high concentration of local anesthetic at a low infusion rate is an attractive possibility and has been reported.24
Insensate Extremity
The reported incidence of an insensate extremity for continuous sciatic nerve block when infusing ropivacaine 0.2% at 8 mL/h is 20%–25%.2,3 But among our patients who were given this ropivacaine concentration and basal rate, nearly three times this many (64%) reported an insensate extremity at least once during the 24-h study period. There is limited evidence that a stimulating catheter may decrease the catheter-to-nerve distance,26 theoretically increasing the incidence of an insensate extremity, and partially explaining the lower incidence in one of the previous studies that used nonstimulating catheters.2 However, the second study was completed with a nearly identical catheter/infusion protocol, as well as identical patient instructions and definition of an insensate extremity.3 We speculate that particular attention to this end point in the present study increased the reported incidence. Our results suggest that the insensate limbs during ambulatory continuous peripheral nerve blocks may be far more common than generally appreciated. Because there are no definitive studies showing that insensate extremities have more morbidity, the clinical relevance of our findings remains unknown.
Dose—Response
This is not the first dose—response investigation involving perineural infusion.5,22,27–30 However, previous studies varied either local anesthetic concentration or rate/volume while holding the other constant, resulting in differing drug doses.5,22,28–30 When both variables were allowed to vary, an equal mass among groups was not required.27 Our study is thus unique in that it varied both concentration and infusion rate in a static ratio so that the total dose from the basal infusion was comparable in each treatment group.
We can only speculate on why 0.2% ropivacaine at 8 mL/h resulted in a higher incidence of an insensate extremity compared with a concentration of 0.4% at 4 mL/h. Anatomic relationships of the perineural space and target nerve/plexus may play a significant role in determining the relative effects of volume and concentration for perineural infusions. For example, the relatively confined perineural space, such as found for the brachial plexus between the anterior and middle interscalene muscles, might influence the relative concentration/volume effects differently than the perineural space of the femoral nerve at the level of the inguinal ligament at which local anesthetic may more easily spread medially and laterally beneath the fascia iliaca.31 Based on this possibility, we propose that the difference between groups in the present study may have been due primarily to the greater basal infusion rate rather than the lower concentration.
Study Limitations
Subjects and investigators were not masked to treatment group, although it is improbable that patients had a bias toward one concentration and data collection was performed by clinical research nurses masked to treatment group assignments. In addition, the primary end point used in this study was somewhat subjective in that patients and their caretakers evaluated extremity sensation and reported the results without a clinical examination by an investigator. Furthermore, although each patient-controlled bolus dose delivered the same ropivacaine dose for both treatment groups (8 mg available every 30 min), the actual delivered doses for each group are unavailable. Therefore, it is possible that patients assigned to 0.2% ropivacaine self-administered more bolus doses resulting in a higher total dose of delivered ropivacaine.
Clinicians must be cognizant of the fact that our results hold only for the concentration/rate combination examined in this study. The present study provides evidence that concentration cannot be ignored in lieu of anesthetic mass, but perhaps there is a superior concentration/rate combination to those used in this investigation; only additional dose—response studies can provide practitioners with the optimal ropivacaine concentration and infusion rate combination.27
ACKNOWLEDGMENTS
The authors gratefully acknowledge the invaluable assistance of Joanne Ramjohn, MD, and Linda Le, MD, Regional Anesthesia Fellows, Department of Anesthesiology, University of Florida (Gainesville, FL); Steven Back, MD, Regional Anesthesia Fellow, Department of Anesthesiology, University of California San Diego (San Diego, CA); Jennifer Woodard, BS, Research Coordinator, Department of Anesthesiology, University of Florida (Gainesville, FL); Cindy Wang, MS, programmer, University of Florida General Clinical Research Center (Gainesville, FL); and the entire staffs of the University of Florida General Clinical Research Center (Gainesville, FL), Florida Surgical Center (Gainesville, FL), UCSD Hillcrest Outpatient Surgery Center (San Diego, CA), and Thornton Hospital (LA Jolla, CA).
Funding for this project provided by NIH grant GM077026 from the National Institute of General Medical Sciences (Bethesda, MD); NIH grant RR00082 from the National Center for Research Resources (Bethesda, MD); the Departments of Anesthesiology, University of CA San Diego (San Diego, CA), Wake Forest Medical Center (Wake Forest, NC), University of Louisville (Louisville, KY), University of Ottawa (Ottawa, Ontario, Canada), Cleveland Clinic (Cleveland, OH); and the University of FL (Gainesville, FL); and Sorenson Medical (West Jordan, UT). Supported by NIH grant GM061655 from the National Institute of General Medical Sciences (Bethesda, MD) and the Joseph Drown Foundation (Los Angeles, CA) (to D.I.S.).
Sorenson Medical (West Jordan, UT) provided funding and donated portable infusion pumps for this investigation. This company had no input into any aspect of study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation. None of the authors has a personal financial interest in this research.
Footnotes
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of these entities.
Abbreviated, preliminary results of this investigation were presented at the Annual Meeting of the American Society of Regional Anesthesia and Pain Medicine, Playa del Carmen, Mexico, May 1–4, 2008.
Reprints will not be available from the authors. On the world wide web: www.or.org.
REFERENCES
- 1.White PF, Issioui T, Skrivanek GD, Early JS, Wakefield C. The use of a continuous popliteal sciatic nerve block after surgery involving the foot and ankle: does it improve the quality of recovery? Anesth Analg. 2003;97:1303–9. doi: 10.1213/01.ANE.0000082242.84015.D4. [DOI] [PubMed] [Google Scholar]
- 2.Ilfeld BM, Morey TE, Wang RD, Enneking FK. Continuous popliteal sciatic nerve block for postoperative pain control at home: a randomized, double-blinded, placebo-controlled study. Anesthesiology. 2002;97:959–65. doi: 10.1097/00000542-200210000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Ilfeld BM, Thannikary LJ, Morey TE, Vander Griend RA, Enneking FK. Popliteal sciatic perineural local anesthetic infusion: a comparison of three dosing regimens for postoperative analgesia. Anesthesiology. 2004;101:970–7. doi: 10.1097/00000542-200410000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Capdevila X, Dadure C, Bringuier S, Bernard N, Biboulet P, Gaertner E, Macaire P. Effect of patient-controlled perineural analgesia on rehabilitation and pain after ambulatory orthopedic surgery: a multicenter randomized trial. Anesthesiology. 2006;105:566–73. doi: 10.1097/00000542-200609000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez J, Taboada M, Carceller J, Lagunilla J, Barcena M, Alvarez J. Stimulating popliteal catheters for postoperative analgesia after hallux valgus repair. Anesth Analg. 2006;102:258–62. doi: 10.1213/01.ane.0000189219.00096.0c. [DOI] [PubMed] [Google Scholar]
- 6.Ilfeld BM, Enneking FK. Continuous peripheral nerve blocks at home: a review. Anesth Analg. 2005;100:1822–33. doi: 10.1213/01.ANE.0000151719.26785.86. [DOI] [PubMed] [Google Scholar]
- 7.Corda DM, Enneking FK. A unique approach to postoperative analgesia for ambulatory surgery. J Clin Anesth. 2000;12:595–9. doi: 10.1016/s0952-8180(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 8.Cohen DE, Van Duker B, Siegel S, Keon TP. Common peroneal nerve palsy associated with epidural analgesia. Anesth Analg. 1993;76:429–31. [PubMed] [Google Scholar]
- 9.Boezaart AP. Perineural infusion of local anesthetics. Anesthesiology. 2006;104:872–80. doi: 10.1097/00000542-200604000-00033. [DOI] [PubMed] [Google Scholar]
- 10.Van Zundert AA, Grouls RJ, Korsten HH, Lambert DH. Spinal anesthesia. Volume or concentration—what matters? Reg Anesth. 1996;21:112–18. [PubMed] [Google Scholar]
- 11.Dernedde M, Stadler M, Bardiau F, Boogaerts JG. Continuous epidural infusion of large concentration/small volume versus small concentration/large volume of levobupivacaine for postoperative analgesia. Anesth Analg. 2003;96:796–801. doi: 10.1213/01.ANE.0000048977.66133.D5. [DOI] [PubMed] [Google Scholar]
- 12.Vester-Andersen T, Christiansen C, Sorensen M, Kaalund-Jorgensen HO, Saugbjerg P, Schultz-Moller K. Perivascular axillary block II: influence of injected volume of local anaesthetic on neural blockade. Acta Anaesthesiol Scand. 1983;27:95–8. doi: 10.1111/j.1399-6576.1983.tb01913.x. [DOI] [PubMed] [Google Scholar]
- 13.Hadzic A, Vloka JD, Singson R, Santos AC, Thys DM. A comparison of intertendinous and classical approaches to popliteal nerve block using magnetic resonance imaging simulation. Anesth Analg. 2002;94:1321–4. doi: 10.1097/00000539-200205000-00051. [DOI] [PubMed] [Google Scholar]
- 14.Ilfeld BM, Enneking FK. Perineural catheter placement for a continuous nerve block: a single operator technique. Reg Anesth Pain Med. 2003;28:154–5. doi: 10.1053/rapm.2003.50126. [DOI] [PubMed] [Google Scholar]
- 15.van Oostrom JH. Web-based data collection: security is only as good as the weakest link. Anesth Analg. 2005;101:1888. doi: 10.1213/01.ANE.0000180265.58307.52. [DOI] [PubMed] [Google Scholar]
- 16.Avidan A, Weissman C, Sprung CL. An internet web site as a data collection platform for multicenter research. Anesth Analg. 2005;100:506–11. doi: 10.1213/01.ANE.0000142124.62227.0F. [DOI] [PubMed] [Google Scholar]
- 17.Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003;105:151–7. doi: 10.1016/s0304-3959(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 18.Shuster JJ. Diagnostics for assumptions in moderate to large simple clinical trials: do they really help? Stat Med. 2005;24:2431–8. doi: 10.1002/sim.2175. [DOI] [PubMed] [Google Scholar]
- 19.Mariano ER, Ilfeld BM, Neal JM. “Going fishing”-the practice of reporting secondary outcomes as separate studies. Reg Anesth Pain Med. 2007;32:183–5. doi: 10.1016/j.rapm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Sandefo I, Bernard JM, Elstraete V, Lebrun T, Polin B, Alla F, Poey C, Savorit L. Patient-controlled interscalene analgesia after shoulder surgery: catheter insertion by the posterior approach. Anesth Analg. 2005;100:1496–8. doi: 10.1213/01.ANE.0000149901.42804.92. [DOI] [PubMed] [Google Scholar]
- 21.Seet E, Leong WL, Yeo AS, Fook-Chong S. Effectiveness of 3-in-1 continuous femoral block of differing concentrations compared to patient controlled intravenous morphine for post total knee arthroplasty analgesia and knee rehabilitation. Anaesth Intensive Care. 2006;34:25–30. doi: 10.1177/0310057X0603400110. [DOI] [PubMed] [Google Scholar]
- 22.Ilfeld BM, Morey TE, Wright TW, Chidgey LK, Enneking FK. Interscalene perineural ropivacaine infusion: a comparison of two dosing regimens for postoperative analgesia. Reg Anesth Pain Med. 2004;29:9–16. doi: 10.1016/j.rapm.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Borgeat A, Blumenthal S, Lambert M, Theodorou P, Vienne P. The feasibility and complications of the continuous popliteal nerve block: a 1001-case survey. Anesth Analg. 2006;103:229–33. doi: 10.1213/01.ane.0000221462.87951.8d. [DOI] [PubMed] [Google Scholar]
- 24.van Oven H, Agnoletti V, Borghi B, Montone N, Stagni F. Patient controlled regional analgesia (PCRA) in surgery of stiff elbow: elastomeric vs electronic pump. Minerva Anestesiol. 2001;67:117–20. [PubMed] [Google Scholar]
- 25.Ilfeld BM, Morey TE, Enneking FK. Infraclavicular perineural local anesthetic infusion: a comparison of three dosing regimens for postoperative analgesia. Anesthesiology. 2004;100:395–402. doi: 10.1097/00000542-200402000-00032. [DOI] [PubMed] [Google Scholar]
- 26.Casati A, Fanelli G, Koscielniak-Nielsen Z, Cappelleri G, Aldegheri G, Danelli G, Fuzier R, Singelyn F. Using stimulating catheters for continuous sciatic nerve block shortens onset time of surgical block and minimizes postoperative consumption of pain medication after halux valgus repair as compared with conventional nonstimulating catheters. Anesth Analg. 2005;101:1192–7. doi: 10.1213/01.ane.0000167232.10305.cd. [DOI] [PubMed] [Google Scholar]
- 27.Brodner G, Buerkle H, Van Aken H, Lambert R, Schweppe-Hartenauer ML, Wempe C, Gogarten W. Postoperative analgesia after knee surgery: a comparison of three different concentrations of ropivacaine for continuous femoral nerve blockade. Anesth Analg. 2007;105:256–62. doi: 10.1213/01.ane.0000265552.43299.2b. [DOI] [PubMed] [Google Scholar]
- 28.Singelyn FJ, Vanderelst PE, Gouverneur JM. Extended femoral nerve sheath block after total hip arthroplasty: continuous versus patient-controlled techniques. Anesth Analg. 2001;92:455–9. doi: 10.1097/00000539-200102000-00033. [DOI] [PubMed] [Google Scholar]
- 29.Ganapathy S, Wasserman RA, Watson JT, Bennett J, Armstrong KP, Stockall CA, Chess DG, MacDonald C. Modified continuous femoral three-in-one block for postoperative pain after total knee arthroplasty. Anesth Analg. 1999;89:1197–202. [PubMed] [Google Scholar]
- 30.Anker-Moller E, Spangsberg N, Dahl JB, Christensen EF, Schultz P, Carlsson P. Continuous blockade of the lumbar plexus after knee surgery: a comparison of the plasma concentrations and analgesic effect of bupivacaine 0.250% and 0.125% Acta Anaesthesiol Scand. 1990;34:468–72. doi: 10.1111/j.1399-6576.1990.tb03125.x. [DOI] [PubMed] [Google Scholar]
- 31.Marhofer P, Nasel C, Sitzwohl C, Kapral S. Magnetic resonance imaging of the distribution of local anesthetic during the three-in-one block. Anesth Analg. 2000;90:119–24. doi: 10.1097/00000539-200001000-00027. [DOI] [PubMed] [Google Scholar]


