Abstract
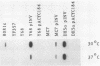
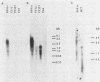
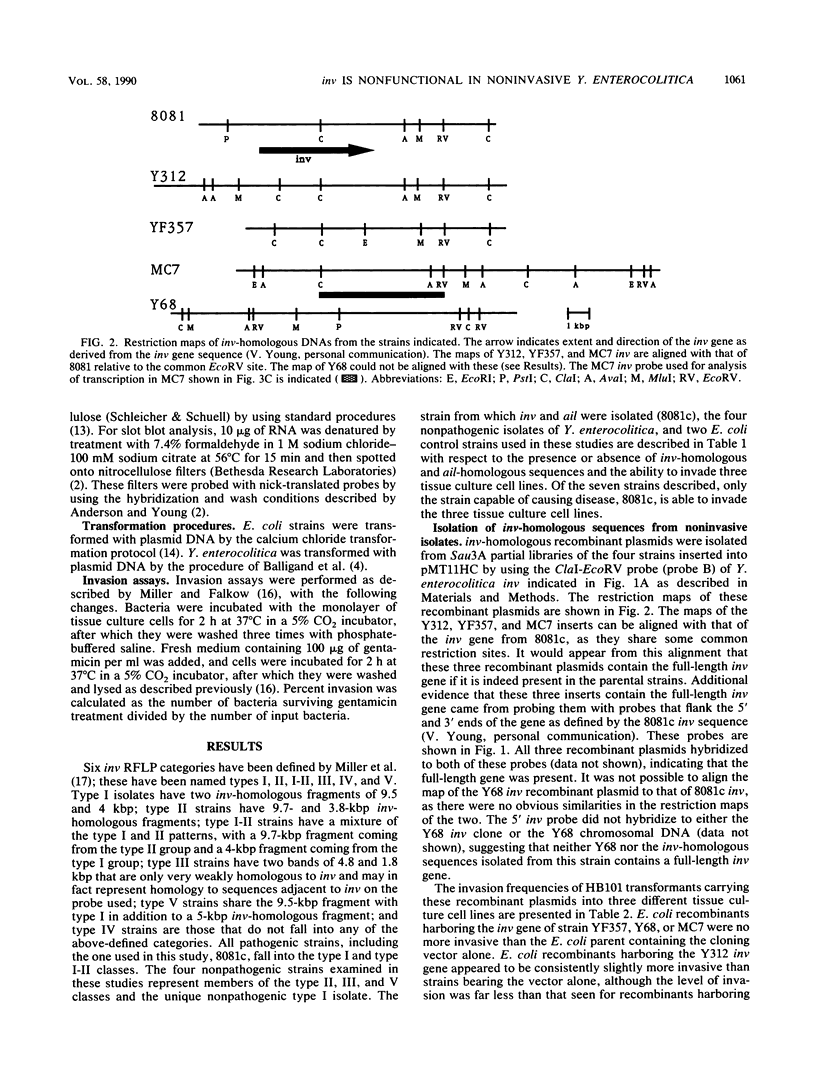
Previous studies have demonstrated a correlation between the ability of isolates of Yersinia enterocolitica to cause disease and to invade tissue culture cells in vitro. Two genes, inv and ail, isolated from a pathogenic strain of Y. enterocolitica have each been shown to confer this invasive phenotype upon Escherichia coli. Eighty pathogenic, invasive isolates studied by Miller et al. (Infect. Immun. 57:121-131, 1989) contained sequences homologous to both of these genes. Thirty-five nonpathogenic, noninvasive isolates similarly studied had no ail homology but carried inv-homologous sequences. We investigated inv-homologous sequences from four nonpathogenic isolates. Recombinant clones of these inv-homologous sequences did not confer the invasive phenotype upon E. coli. No RNA transcripts capable of encoding a full-length Inv protein were detected in the four noninvasive Yersinia strains. When the inv gene from a pathogenic isolate was introduced into two of these strains, the resulting transformants invaded tissue culture cells in vitro. The inv gene was transcribed in a pathogenic Yersinia isolate grown at 30 degrees C but not at all in these cells grown at 37 degrees C. The production of RNA transcripts homologous to inv in transformants was not regulated by temperature to the same degree as was seen for pathogenic isolates. We conclude that the inv gene in nonpathogenic strains of Y. enterocolitica is nonfunctional. Y. enterocolitica isolates epidemiologically linked to disease contain both a functional inv gene and a functional ail gene. Environmental isolates not associated with disease have a nonfunctional inv gene and no ail gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aho K., Leirisalo-Repo M., Repo H. Reactive arthritis. Clin Rheum Dis. 1985 Apr;11(1):25–40. [PubMed] [Google Scholar]
- Aricò B., Rappuoli R. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J Bacteriol. 1987 Jun;169(6):2847–2853. doi: 10.1128/jb.169.6.2847-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottone E. J. Yersinia enterocolitica: a panoramic view of a charismatic microorganism. CRC Crit Rev Microbiol. 1977;5(2):211–241. doi: 10.3109/10408417709102312. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G., Laroche Y., Balligand G., Sory M. P., Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987 Jan-Feb;9(1):64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Swain A., Falkow S. Analysis of expression and thermoregulation of the Yersinia pseudotuberculosis inv gene with hybrid proteins. Infect Immun. 1988 Aug;56(8):2133–2138. doi: 10.1128/iai.56.8.2133-2138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Voorhis D. L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987 Aug 28;50(5):769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- Lee W. H., McGrath P. P., Carter P. H., Eide E. L. The ability of some Yersinia enterocolitica strains to invade HeLa cells. Can J Microbiol. 1977 Dec;23(12):1714–1722. doi: 10.1139/m77-247. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Farmer J. J., 3rd, Hill W. E., Falkow S. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect Immun. 1989 Jan;57(1):121–131. doi: 10.1128/iai.57.1.121-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfield R. J., Campbell A. M. Origin of cryptic lambda prophages in Escherichia coli K-12. Cold Spring Harb Symp Quant Biol. 1984;49:199–206. doi: 10.1101/sqb.1984.049.01.023. [DOI] [PubMed] [Google Scholar]
- Robins-Browne R. M., Miliotis M. D., Cianciosi S., Miller V. L., Falkow S., Morris J. G., Jr Evaluation of DNA colony hybridization and other techniques for detection of virulence in Yersinia species. J Clin Microbiol. 1989 Apr;27(4):644–650. doi: 10.1128/jcm.27.4.644-650.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Skurnik M., Wolf-Watz H. Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature. 1988 Aug 11;334(6182):522–524. doi: 10.1038/334522a0. [DOI] [PubMed] [Google Scholar]
- Schiemann D. A., Devenish J. A. Relationship of HeLa cell infectivity to biochemical, serological, and virulence characteristics of Yersinia enterocolitica. Infect Immun. 1982 Feb;35(2):497–506. doi: 10.1128/iai.35.2.497-506.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayegani M., DeForge I., McGlynn D. M., Root T. Characteristics of Yersinia enterocolitica and related species isolated from human, animal, and environmental sources. J Clin Microbiol. 1981 Sep;14(3):304–312. doi: 10.1128/jcm.14.3.304-312.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T. Studies on the pathogenicity of Yersinia enterocolitica. I. Experimental infection in rabbits. Microbiol Immunol. 1977;21(7):341–363. [PubMed] [Google Scholar]
- Une T. Studies on the pathogenicity of Yersinia enterocolitica. II. Interaction with cultured cells in vitro. Microbiol Immunol. 1977;21(7):365–377. doi: 10.1111/j.1348-0421.1977.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Winberg G., Hammarskjöld M. L. Isolation of DNA from agarose gels using DEAE-paper. Application to restriction site mapping of adenovirus type 16 DNA. Nucleic Acids Res. 1980 Jan 25;8(2):253–264. doi: 10.1093/nar/8.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]