Abstract
Recent advances in nanotechnology and molecular self-assembly may provide novel solutions to current cell transplantation deficiencies. Heparin-binding peptide amphiphiles (HBPAs) self-assemble from aqueous media into nanofibers that bind growth factors through interactions with the bioactive polymer heparin. In this report, we demonstrate that delivery of vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2) from HBPA scaffolds significantly increases blood vessel density in the mouse omentum over control scaffolds without growth factors (P<0.0005) and significantly enhances islet engraftment. Diabetic recipients transplanted with 250 isologous islets and HBPA scaffolds containing VEGF/FGF-2 achieved normoglycemia at a higher rate (78%) than control animals receiving identical scaffolds without growth factors (30%; P<0.05) or growth factors alone (20%). These data indicate that the enhanced engraftment can be attributed to specific growth factor effects that were made possible by the delivery mechanism of HBPA nanostructures.
Keywords: islet transplantation, omentum, peptide amphiphile nanofibers, fibroblast growth factor-2, vascular endothelial growth factor
Clinical islet transplantation trials have resulted in promising therapeutic benefits for select patients with type 1 diabetes(1, 2). Despite many advances, the procedure’s clinical potential remains limited by suboptimal engraftment and lingering concerns about the intrahepatic transplant site(3–6). Recent advances in nanotechnology and molecular self-assembly may provide novel solutions to address these issues. We recently developed a specific family of molecules known as peptide amphiphiles (PAs) that self-assemble from aqueous media into gels consisting of nanofibers that can be customized to present biological signals to cells in high density(7–9). By presenting appropriate biological signals, PA nanofibers have the potential to create more favorable environments for transplanted islets and facilitate functional engraftment in extrahepatic locations that have been previously plagued by failure.
In this study, we used heparin-binding PAs (HBPAs)(10) to deliver angiogenic growth factors to extrahepatic islet isografts in diabetic mice. Heparin-binding PAs (HBPAs) form nanofibers that bind strongly to heparin and related glycosaminoglycans(10). These matrix components play critical roles in growth factor binding, storage, and activation(11). We used the heparin-binding nanostructures to attach vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2), two growth factors which are known to play critical roles in islet revascularization, survival, and function(12–15). Attachment to the HBPA nanostructures via heparin is intended to protect the growth factors from proteolysis and activate them for signaling through exposure of their receptor binding domains(10). The effectiveness of the HBPA delivery strategy is confirmed by recent work showing extended growth factor release in vitro and significant angiogenesis in vivo using a rat cornea model(10).
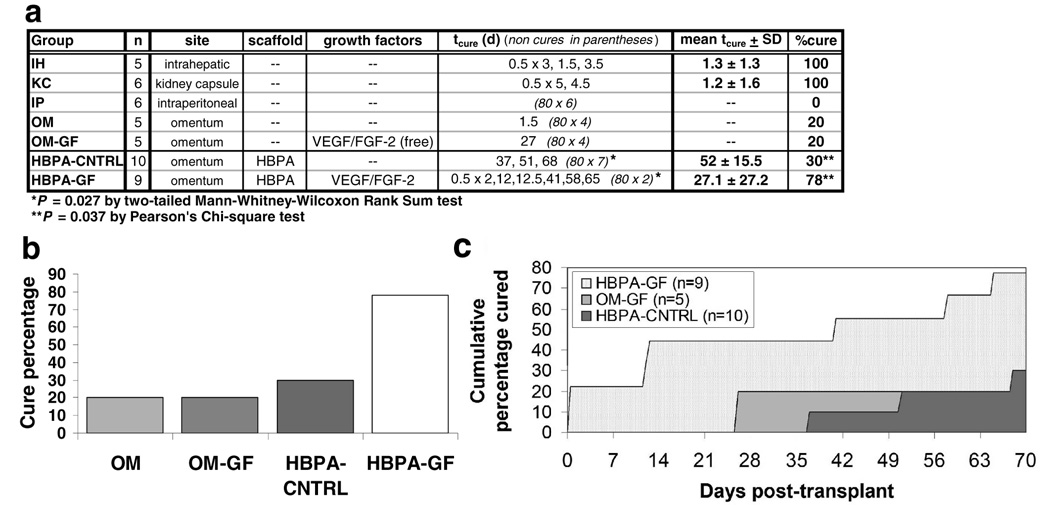
The purpose of this model study was twofold: (a) to assess the bioactivity of VEGF/FGF-2-binding HBPA scaffolds implanted in the mouse omentum, and (b) to evaluate the impact of VEGF/FGF-2-binding HBPA scaffolds in a functional islet isograft model. The experimental design incorporated 46 transplant recipients and six control groups, as outlined in Figure 3a. Diabetes was induced in 8–12 week male FVB/N mice (25.5–30 g) with intraperitoneal streptozotocin and islets were isolated from adult male FVB/N strain mice (Jackson Laboratories) as previously described(16). Islets were quantified by hand-picking in a double-blinded manner and each islet preparation (16 total) was distributed over multiple experimental groups during transplantation.
Figure 3. Effects on transplant outcome of VEGF and FGF-2 delivered via HBPA nanostructures.
(a) Group outcomes for transplants of 250 islets. tcure represents the post-transplant time (days) in which a streptozotocin-induced diabetic recipient achieved sustained normoglycemia. (b) Comparison of the proportions of islet isograft recipients that ever achieved normoglycemia using the omental transplantation site. (c) Comparison of the proportions of islet isograft recipients that achieved normoglycemia according to time post-transplant. The area under each curve (AUC) provides a visual metric of transplant effectiveness. Cure percentages and tcures for omental transplants improved considerably when islets were delivered with HBPA scaffolds containing growth factors (HBPA-GF), as indicated by the large AUC. Islets delivered with either unbound growth factors (OM-GF) or plain HBPA scaffolds without growth factors (HBPA-CNTRL) had substantially smaller AUCs and showed little difference in performance over the OM and IP controls.
Scaffolds consisting of VEGF/FGF-2-binding HBPA gels in fibrous poly(l-lactic acid) (PLLA) matrices were prepared as follows. For each recipient, 7 µL of aqueous HBPA (30 mg/mL; synthesized as described previously(7, 10)) was added to 1.5 mm2 sections of PLLA. A heparin/growth factor solution containing 1 µL 1.0 mg/mL recombinant murine VEGF-165 (PeproTech), 1 µL 1.0 mg/mL recombinant human FGF-2 (PeproTech), and 4 µL 20 mg/mL aqueous heparin (Sigma) was then added to gel the HBPA solution. Lastly, 1 µL aqueous HBPA was added to the top of scaffolds (scaffold microstructure shown in Supplementary Figure S1 online).
Gelled scaffolds were placed on the omentum of each transplant recipient. Islets (250/recipient) were delivered adjacently to scaffolds, and the omentum was folded over the scaffolds and islets. Test group recipients received islets and HBPA scaffolds containing growth factors as described above (HBPA-GF; n=9). Control recipients received an equivalent number of islets (250) under the following conditions: (a) HBPA-CNTRL (n=10): HBPA scaffolds gelled with heparin (no GFs), placed adjacent to omental islet transplants; (b) OM (n=5): omental islet transplants without scaffolds; (c) OM-GF (n=5): omental islet transplants with local administration of free growth factors (no scaffolds); (d) IP (n=6): free islets placed in the peritoneal cavity; (e) IH (n=5): intrahepatic islet implantation via portal venous delivery (no scaffolds, no GFs); (f) KC (n=6): islets implanted in the subcapsular space of the kidney (no scaffolds, no GFs). In a separate study to assess transplant site vascularization, HBPA-GF (n=6) and HBPA-CNTRL (n=6) scaffolds were implanted separately into the omenta of male FVB/N mice and analyzed via histochemical procedures for the presence of CD31, an endothelial-specific marker. Another set of HBPA-GF (n=3) and HBPA-CNTRL (n=3) scaffolds were implanted into omenta of FVB/N-Tg(Vegfr2-luc) transgenic mice (Xenogen Corp.)(17) which have a modified VEGFR2 (VEGF receptor 2) promoter to couple VEGFR2 gene expression to a luciferase reporter. Potential post-implantation angiogenic activity related to VEGFR2 up-regulation in existing or proliferating cells was monitored noninvasively by injecting subjects with 150 mg/kg intraperitoneal luciferin and viewing them with an IVIS imaging system (Xenogen).
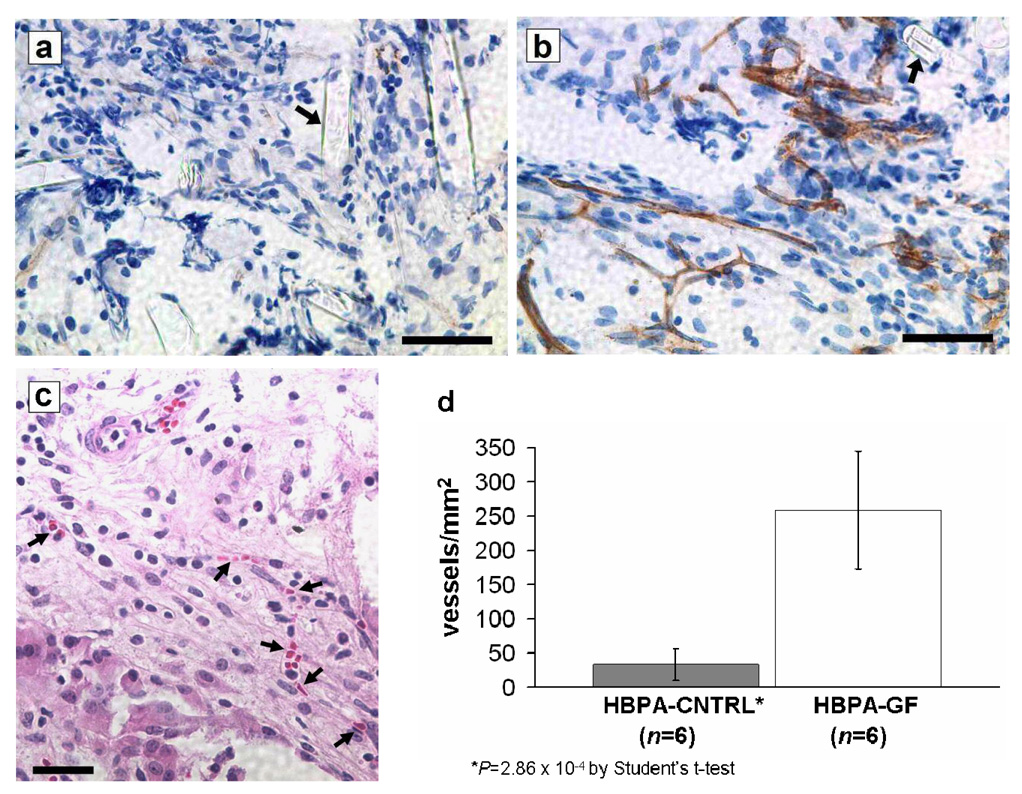
Implantation studies indicated that HBPA-GF scaffolds induced significant biological responses in the surrounding tissue (Figure 1; see Supplementary Figure S2 online for images of the response at an alternative site). Mean densities of CD31-positive neovessels were nearly eight times greater in histochemical sections of HBPA-GF scaffolds than in sections of HBPA-CNTRL scaffolds, which did not contain growth factors (Figure 1d; P=2.86×10−4). The distribution of neovessels throughout the scaffolds and immediately surrounding omental tissue suggested that omental islets in HBPA-GF transplants were within close proximity to newly formed vessels. The presence of intralumenal erythrocytes (Figure 1c) in these vessels is consistent with early stage angiogenesis and implies basic vessel functionality(18). These outcomes are consistent with our previous findings pertaining to HBPA-delivered VEGF/FGF-2 in a rat cornea angiogenesis model(10).
Figure 1. VEGF and FGF-2 delivered via HBPA nanostructures significantly increase vascular density at the omental transplant site.
(a,b) CD31 staining of (a) HBPA-CNTRL and (b) HBPA-GF scaffolds retrieved from omenta on post-transplant day 14. CD31 positive cells are stained brown by DAB chromogen; cell nuclei are stained blue by hematoxylin. Arrows denote sections of PLLA filaments amongst the infiltrating cellular tissue (scale bars represent 25 µm). (c) Hematoxylin and eosin staining of an HBPA-GF scaffold retrieved on day 14. Erythrocytes in the lumens (see arrows) suggest that neovessels have functional characteristics (scale bar represents 30 µm). (d) Density of CD31-positive neovessels in HBPA scaffold specimens retrieved between days 11 and 14. Neovessel densities in HBPA-GF specimens were nearly 8 times greater than those in HBPA-CNTRL specimens (error bars represent 95% confidence intervals).
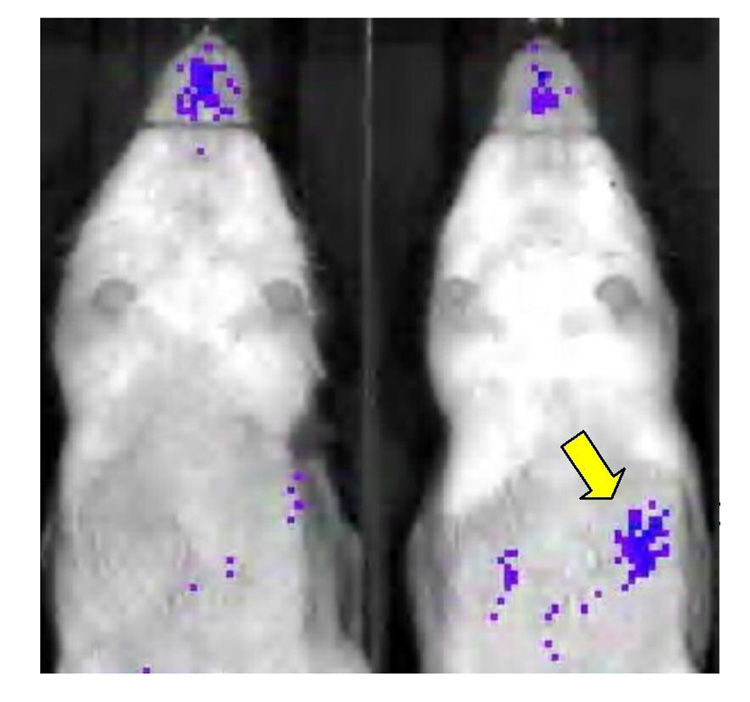
Bioluminescent images of HBPA scaffolds implanted in Vegfr2-luc transgenic mice provided additional evidence of the biological activity of scaffold-derived growth factors. HBPA-GF scaffold recipients generally showed stronger bioluminescent signals than HBPA-CNTRL recipients (Figure 2). Signal intensities in these images are spatially and temporally correlated to the up-regulation of VEGFR2, a primary VEGF receptor on endothelial cell surfaces that mediates the growth factor's mitogenic, survival, and permeability effects that are essential to angiogenesis(19). Signals in HBPA-GF recipients appeared 2–3 days post-implantation and diminished over the course of 10–14 days, thus indicating the kinetics of the biological response to growth factors released from HBPA-GF scaffolds.
Figure 2. Bioluminescent signals in vegfr2-luc transgenic mice provide additional, noninvasive evidence of the biological activity of HBPA-GF scaffolds.
The HBPA-GF recipient (right) shows considerably greater bioluminescence due to growth factor-induced VEGFR2 expression than the HBPA-CNTRL recipient (left). The yellow arrow indicates the location of the omental bioluminescent signal in the HBPA-GF recipient.
The increased vascular density in HBPA-GF scaffolds was associated with a significant improvement in islet functional outcomes (Figure 3; see Supplementary Figure S3 online for a sample recipient blood glucose profile). Specifically, the proportion of HBPA-GF recipients which achieved normoglycemia (78%) was over 2.5 times greater than that of controls which received identical scaffolds without growth factors (HBPA-CNTRL; 30%)(P=0.037) or free growth factors without scaffolds (OM-GF; 20%). HBPA-GF recipients also achieved normoglycemia in significantly shorter times than HBPA-CNTRL recipients (P=0.027). Collectively, these findings confirm the bioactivity of the HBPA-GF scaffolds and suggest that their positive impact on transplant outcomes relates—at least in part—to their ability to modify the omental microenvironment in ways that make it more suitable for islet engraftment.
The critical role of the HBPA-delivered VEGF and FGF-2 in facilitating islet function is evident in the low rates of normoglycemia in the HBPA-CNTRL and OM groups, where growth factors were not used. These outcomes indicate that the unmodified omentum is marginally suited for islet transplantation and that HBPA scaffolds had relatively little effect on transplanted islets in the absence of the growth factors. On the other hand, the low cure percentages of the OM-GF group show that bolus growth factor delivery without scaffolds is also ineffective. This indicates that HBPA scaffolds play an important role in growth factor storage and delivery. Thus, the enhanced engraftment in HBPA-GF transplants can be attributed to specific growth factor effects that were made possible by the growth factor delivery mechanism of the HBPA nanostructures. This outcome is consistent with previous findings in a rat cornea angiogenesis assay, where it was shown that HBPA-GF scaffolds produced significantly stronger angiogenic responses than either direct growth factor administration or delivery by other carriers such as collagen or heparin(10).
The transplant outcomes were observed in three ways: 1) some HBPA-GF recipients achieved immediate islet function that was not observed in HBPA-CNTRL or OM-GF recipients, 2) some HBPA-GF recipients achieved islet function one-to-two weeks earlier than HBPA-CNTRL or OM-GF recipients, and 3) some HBPA-GF recipients achieved islet function at later intervals post-transplant. This was an interesting observation that will require further evaluation to discern whether there are multiple mechanisms to the engraftment process. Considering that post-transplant islet revascularization requires 7–14 days, these results raise the possibility that the HBPA-delivered VEGF and FGF-2 may have had additional beneficial effects beyond transplant site revascularization. Support for this hypothesis can be found in the fact that VEGF, FGF-2, and their receptors are expressed in both developing and mature islets(12–14, 20, 21), even though angiogenic activity diminishes greatly after initial development. VEGF is present in elevated levels inside healthy islets and has been implicated in post-transplant preservation of β-cell mass(18, 22, 23) and the maintenance of vascular features required for proper function(12, 15). Similarly, FGF signaling has been shown to play important roles in islet morphogenesis(13), insulin processing(14), glucose sensing(14), and β-cell differentiation(13) and proliferation(14). This hypothesis is further supported by recent evidence suggesting important relationships between β-cells and intraislet endothelial cells(24).
In conclusion, this study provides the first demonstration of HBPA nanofiber signaling efficacy in a functional transplant model. HBPA-based delivery of VEGF and FGF-2 to the omental transplant site significantly increased vascular density (P<0.0005) and improved islet engraftment, as evidenced by significantly higher cure percentages (P<0.05) and significantly shorter times to achieve normoglycemia (P<0.05). While the HBPA-GF cure percentage did not surpass that of the IH group, the results illustrate the potential utility of HBPA scaffolds in developing alternatives to intrahepatic implantation. Alternative islet engraftment sites can potentially address concerns associated with portal venous puncture and post-engraftment liver damage(25–27), and may offer additional advantages, such as the accommodation of larger volumes of tissue(4) and insulin delivery via the portal venous circulation(28).
Supplementary Material
ACKNOWLEDGEMENTS
HBPAs were synthesized by Chung-Yan Koh, bioluminescent imaging was performed by Courtney Larson, and histological sections were prepared by Bob Meyer (Robert H. Lurie Comprehensive Cancer Center, Northwestern University). Scanning electron microscopy (SEM) specimens were prepared in the Biological Imaging Facility and imaged in the Electron Probe Instrumentation Center at Northwestern University.
This work was funded by the Juvenile Diabetes Research Foundation International (Award No. 4-2004-781), the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health (Award No. 1 R01EB003806), and the Institute for BioNanotechnology in Medicine (IBNAM) at Northwestern University. All animal procedures were approved by the Center for Comparative Medicine at Northwestern University and followed guidelines set by the American Veterinary Medical Association. The authors declare that they have no competing financial interests.
Glossary
Abbreviations
- PA
peptide amphiphile
- HBPA
heparin-binding peptide amphiphile
- ECM
extracellular matrix
- VEGF
vascular endothelial growth factor
- FGF-2
fibroblast growth factor 2
- PLLA
poly(l-lactic acid)
- CD31
cluster determinant 31 (also called platelet endothelial cell adhesion molecule-1, PECAM-1)
- tcure
cure time; duration needed to achieve sustained normoglycemia, measured in days post-transplant
- VEGFR2
vascular endothelial growth factor receptor 2 (also KDR/FLK-1)
- AUC
area under curve for plot of cumulative percentage cured vs. time
Transplant designations (see Fig. 3a)
- HBPA-GF
islets wrapped in the omentum adjacent to HBPA scaffolds containing growth factors (VEGF, FGF-2)
- HBPA-CNTRL
islets wrapped in the omentum adjacent to HBPA scaffolds without growth factors
- OM
islets wrapped in the omentum without scaffolds
- OM-GF
islets wrapped in the omentum with free growth factors (no scaffolds)
- IP
free islets introduced into the peritoneal cavity via Pasteur pipette
- IH
free islets injected into the hepatic portal vein (single injection)
- KC
centrifuged islet pellets introduced into the subcapsular space of the kidney
REFERENCES
- 1.Shapiro AMJ, Ricordi C, Hering BJ, et al. International trial of Edmonton Protocol for islet transplantation. N Engl J Med. 2006;355:1318. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 2.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal dose islet transplantation in patients with type 1 diabetes. J Am Med Assoc. 2005;293:830. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 3.Bhargava R, Senior PA, Ackerman TE, et al. Prevalence of hepatic steatosis after islet transplantation and its relation to graft function. Diabetes. 2004;53:1311. doi: 10.2337/diabetes.53.5.1311. [DOI] [PubMed] [Google Scholar]
- 4.Robertson RP. Islet transplantation as a treatment for diabetes- a work in progress. N Engl J Med. 2004;350:694. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 5.Oberholzer J, Triponez F, Lou J, Morel P. Clinical islet transplantation: a review. Ann NY Acad Sci. 1999;875:189. doi: 10.1111/j.1749-6632.1999.tb08503.x. [DOI] [PubMed] [Google Scholar]
- 6.Kenyon NS, Harlan DM, Alejandro R, Ricordi C, Mintz DH. Islet Cell Transplantation. In: LeRoith D, Taylor SI, Olefsky JM, editors. Diabetes mellitus: a fundamental and clinical text. Philadelphia: Lippincott, Williams, & Wilkins; 2000. p. 507. [Google Scholar]
- 7.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 8.Hartgerink JD, Beniash E, Stupp SI. Peptide amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci USA. 2002;99:5133. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva GA, Czeisler CA, Niece KL, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 10.Rajangam K, Behanna HA, Hui MJ, et al. Heparin binding nanostructures to promote growth of blood vessels. Nano Lett. 2006;6:2086. doi: 10.1021/nl0613555. [DOI] [PubMed] [Google Scholar]
- 11.Nurcombe V, Kumarasuriyar A, Cool SM. The development of heparan sulfate sugars as therapeutics: versatility that couples stem cells, tissue engineering, and wound repair. Drug Devel Res. 2004;62:303. [Google Scholar]
- 12.Brissova M, Shostak A, Shiota M, et al. Pancreatic Islet Production of Vascular Endothelial Growth Factor-A is Essential for Islet Vascularization, Revascularization, and Function. Diabetes. 2006;55:2974. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 13.Hardikar AA, Marcus-Samuels B, Geras-Raaka E, Raaka BM, Gershengorn MC. Human pancreatic precursor cells secrete FGF2 to stimulate clustering into hormone-expressing islet-like cell aggregates. Proc Natl Acad Sci USA. 2003;100:7117. doi: 10.1073/pnas.1232230100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart AW, Baeza N, Apelqvist A, Edlund H. Attenuation of FGF signaling in mouse β-cells leads to diabetes. Nature. 2000;408:864. doi: 10.1038/35048589. [DOI] [PubMed] [Google Scholar]
- 15.Lammert E, Gu G, McLaughlin M, et al. Role of VEGF-A in vascularization of pancreatic islets. Curr Bio. 2003;13:1070. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Zhang X, Larson CS, Baker MS, Kaufman DB. In vivo bioluminescence of transplanted islets and early detection of graft rejection. Transplantation. 2006;81:1421. doi: 10.1097/01.tp.0000206109.71181.bf. [DOI] [PubMed] [Google Scholar]
- 17.Zhang N, Fang Z, Contag PR, Purchio AF, West DB. Tracking angiogenesis induced by skin wounding and contact hypersensitivity using a VEGFR2-luciferase transgenic mouse. Blood. 2004;103:617. doi: 10.1182/blood-2003-06-1820. [DOI] [PubMed] [Google Scholar]
- 18.Oberg-Welsh C, Sandler S, Andersson A, Welsh M. Effects of vascular endothelial growth factor on pancreatic duct cell replication and the insulin production of fetal islet-like cell clusters in vitro. Mol Cell Endocrinol. 1997;126:125. doi: 10.1016/s0303-7207(96)03977-9. [DOI] [PubMed] [Google Scholar]
- 19.Millauer B, Wizigmann-Voos S, Schnurch H, et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 20.Rooman I, Schuit F, Bouwens L. Effect of vascular endothelial growth factor on growth and differentiation of pancreatic duct epithelium. Lab Invest. 1997;76:225. [PubMed] [Google Scholar]
- 21.Kuroda M, Oka T, Oka Y, et al. Colocalization of vascular endothelial growth factor (vascular permeability factor) and insulin in pancreatic islet cells. J Clin Endocrinol Metab. 1995;8:3196. doi: 10.1210/jcem.80.11.7593426. [DOI] [PubMed] [Google Scholar]
- 22.Stagner J, Mokshagundam S, Wyler K, et al. Beta-cell sparing in transplanted islets by vascular endothelial growth factor. Transplantation Proc. 2004;36:1178. doi: 10.1016/j.transproceed.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Reimer MK, Mokshagundam SP, Wyler K, Sundler F, Ahren B, Stagner J. Local growth factors are beneficial for the autonomic reinnervation of transplanted islets in rats. Pancreas. 2003;26:392. doi: 10.1097/00006676-200305000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Brissova M, Fowler M, Wiebe P, et al. Intraislet Endothelial Cells Contribute to Revascularization of Transplanted Pancreatic Islets. Diabetes. 2004;53:1318. doi: 10.2337/diabetes.53.5.1318. [DOI] [PubMed] [Google Scholar]
- 25.Bhargava R, Senior PA, Ackerman TE, et al. Prevalence of hepatic steatosis after islet transplantation and its relation to graft function. Diabetes. 2004;53:1311. doi: 10.2337/diabetes.53.5.1311. [DOI] [PubMed] [Google Scholar]
- 26.Robertson RP. Islet transplantation as a treatment for diabetes- a work in progress. N Engl J Med. 2004;350:694. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 27.Oberholzer J, Triponez F, Lou J, Morel P. Clinical islet transplantation: a review. Ann NY Acad Sci. 1999;875:189. doi: 10.1111/j.1749-6632.1999.tb08503.x. [DOI] [PubMed] [Google Scholar]
- 28.Kin T, Korbutt GS, Rajotte RV. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am J Transpl. 2003;3:281. doi: 10.1034/j.1600-6143.2003.00049.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.