Abstract
Observations that dendritic cells (DCs) constitutively enter afferent lymphatic vessels in many organs and that DCs in some tissues, such as the lung, turnover rapidly in the steady state have led to the concept that a major fraction of lymph node DCs are derived from migratory DCs that enter the lymph node through upstream afferent lymphatic vessels. We used the lysozyme M–Cre reporter mouse strain to assess the relationship of lymph node and nonlymphoid organ DCs. Our findings challenge the idea that a substantial proportion of lymph node DCs derive from the upstream tissue during homeostasis. Instead, our analysis suggests that nonlymphoid organ DCs comprise a major population of DCs within lymph nodes only after introduction of an inflammatory stimulus.
Afferent lymphatic vessels transport interstitial fluid and cells from tissues before terminating in draining lymph nodes. DCs and T cells are the major cellular components of afferent lymph (1–3), but very few DCs enter efferent lymph (3, 4). Inflammatory stimuli increase the rate of afferent lymphatic DC output by approximately an order of magnitude (5). However, inflammatory stimuli are not required for DC and T cell entry into afferent lymphatics, because the recovery of these cells from lymph in the steady state is still substantial (5). Indeed, the projected afferent lymphatic DC output is high enough to fully supply all lymph node DCs, even considering a short DC lifespan (4). Thus, from these early observations, it seemed quite plausible that lymph was the major source of lymph node DCs in general. Reinforcing this possibility were other early studies revealing that DCs purified from the spleen can, upon adoptive transfer, immigrate to lymph nodes through lymphatic vessels but not through the bloodstream (6). Furthermore, revelations that the steady-state turnover of pulmonary DCs is very rapid fit well with the interpretation that this fast turnover was linked to a high rate of DC entry into afferent lymphatics (7). Functionally, DCs that migrate through afferent lymph to lymph nodes in the steady state transport parenchymal antigens from various organs (8–10), and such transport may facilitate the initiation of peripheral immune tolerance (11), possibly working in concert with other mechanisms that give rise to tolerance within lymph nodes (12).
More recently, our understanding of DC homeostasis in lymph nodes has evolved to include not only lymph-migrating DCs but also a role for DCs that enter from blood vessels, the specialized high endothelial venules, to maintain specific DC subsets, notably plasmacytoid DCs (13, 14) and mouse CD8α+ DCs (15, 16). Accordingly, a recent review of DC biology suggested that half of the DCs within lymph nodes enter from the bloodstream, including the full complement of these specialized subsets, but the other half of DCs were still believed to arise from lymph-migrating tissue DCs (for review see reference 17). This conclusion was based in part on the notion that it is possible to readily identify which DCs traveled to lymph nodes through afferent lymph based on combinations of various markers, such as higher expression of MHC II in the steady state (18, 19). However, the relationship between DCs bearing such markers and their presumed precursors derived from upstream tissue has not been established.
Our past studies on the role of monocytes as a potential source of lymph-trafficking DCs (20–22) fueled our interest in the relationship between nonlymphoid organ DCs and lymph node DCs. Several recent studies have concluded that spleen DCs do not arise from monocytes using adoptive transfer approaches (23–26). In addition, a study in parabiotic mice suggested that monocytes are also not precursors for lymph node DCs, because blood monocytes and lymph node DCs exchanged between parabionts to differing extents (27). Strikingly, one of these studies indicated that, in strong contrast to spleen DCs, monocytes are precursors of peripheral nonlymphoid organ DCs (25), implying for the first time that DCs in lymphoid organs may have a distinct origin from DCs in nonlymphoid organs. However, little lineage analysis has been conducted in lymph nodes, which in contrast to the spleen, are positioned to serve as a nexus wherein lymphoid organ–resident DCs and nonlymphoid organ DCs would converge, presumably in equal proportions (17), to interact with lymphocytes and drive immune responses.
Indications that nonlymphoid organ DCs may arise from monocytes (22, 25, 28), in contrast to other DCs, suggested to us an approach to analyze the proportion of lymph node DCs that might arise from DCs originating in upstream tissues. In this study, we used a popular reporter mouse line that uses the lysozyme promoter, active in many monocytes, to drive Cre recombinase (29) that subsequently removes a stop cassette upstream of enhanced GFP (EGFP) (30). Studies in this model fit well with the evidence that nonlymphoid organ and spleen DCs may be unrelated in origin and, most importantly, reveal that, against expectations and prevailing models in the field, only a minority of lymph node DCs in the steady state arises from DCs residing in upstream nonlymphoid organs. Instead, DCs that migrate through lymphatics from upstream tissues contribute substantially to the pool of lymph node DCs, mainly during inflammatory states.
RESULTS
Illustration of the utility of lysozyme M (LysM)–Cre × Rosa26-stopfloxEGFP mice to assess DC relationships with monocytes or other DC populations
LysM is expressed by neutrophils and macrophages. Accordingly, LysM-Cre mice (29, 31) have been widely used to generate cell type–selective deletions of floxed genes or expression of floxed reporter markers, especially Rosa26 promoter-driven reporter genes. In these mice, Cre activity removes a stop cassette upstream of the floxed reporter in neutrophils and monocyte-derived macrophages. Neutrophils are not considered candidates for DC precursors, but monocytes may be precursors for at least some DC populations (20–22, 25). If only some DCs are derived from monocytes, and if the lysozyme promoter has low activity in other DC precursors, then the LysM-Cre reporter mice would be a useful tool to trace relationships between monocytes and DCs and possibly between different DC populations in vivo.
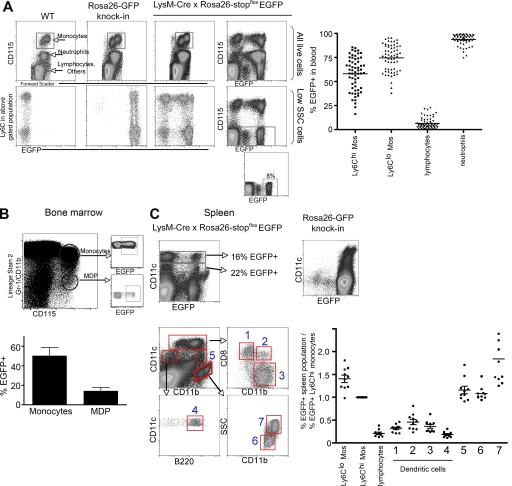
When we analyzed blood monocytes in LysM-Cre × Rosa26-stopfloxEGFP mice in the steady state, the two subsets of circulating CD115hi blood monocytes (32–34) differed in the percentage that was EGFP+. Ly-6Chi monocytes (sometimes called Gr-1hi monocytes) were on average 55% EGFP+, whereas the Ly-6Clo monocyte subset (sometimes called Gr-1lo monocytes) averaged 75% EGFP+ (Fig. 1 A). Since their discovery in mice, these two subsets have not been further subdivided, and accordingly, we found no obvious differences in cell-surface markers between EGFP− and EGFP+ monocyte subpopulations. Furthermore, transfer of EGFP− monocytes into congenic recipient mice revealed that, 1 d later, many of the transferred EGFP− monocytes had become EGFP+ (unpublished data), indicating that EGFP− monocytes do not maintain a distinct population from EGFP+ monocytes. Instead, the failure of EGFP to be uniformly expressed by a given monocyte subset in blood is more likely caused by the stochastic failure of strong lysozyme promoter usage or Cre-mediated excision of the stop cassette in the reporter construct.
Figure 1.
EGFP reporter expression in LysM-Cre × Rosa26-stopfloxEGFP mice by blood monocytes and spleen macrophages or DCs. (A) EGFP frequency in blood populations was analyzed. CD115 and Gr-1/Ly-6C stainings identified blood monocyte subsets in wild-type C57BL/6 mice, Rosa26-EGFP knock-in mice, or LysM-Cre × Rosa26-stopfloxEGFP mice. The percentage of each subset that expressed EGFP was analyzed. High side scatter (SSC) and lower levels of CD115 expression were used to discern neutrophils. Low SSC CD115− cells, which are mostly lymphocytes, were evaluated to measure the extent of promiscuous usage of the lysozyme promoter (reference 31). (right) Plot shows the percentage of EGFP of various blood populations in all mice enrolled in this study (n = 60). (B) The percentage of EGFP+ cells in CD115hi bone marrow cells in LysM-Cre × Rosa26-stopfloxEGFP mice was analyzed and plotted. A panel of lineage markers was first used to narrow down bone marrow cells that might be monocytes or MDPs. Bone marrow monocytes were CD115hi cells that coexpressed the lineage markers Gr-1 and/or CD11b (lineage stain 2), whereas MDPs were lineage− CD115hi cells (reference 37). Data represent the mean ± SEM. (C) Stainings for CD11c, CD11b, CD8α, and B220 defined conventional DC subsets and other populations in steady-state spleens of LysM-Cre × Rosa26-stopfloxEGFP mice or Rosa26-GFP knock-in mice. Conventional DCs correspond to populations 1 (CD8α+ DCs), 3 (CD8α− DCs), and 4 (plasmacytoid DCs). Macrophages comprise gates 6 and 7, and gate 5 includes monocytes (reference 37). The percentage of EGFP in these and other designated populations was normalized to the percentage of EGFP+ Ly-6Chi monocytes in the same mouse. In the graphs, all mice studied are compiled, and each data point corresponds to one mouse. Two to three mice per group were handled per experiment. Horizontal bars on scatter plots represent means. In B, two experiments were performed with three mice in each.
In blood, CD115lo cells are granulocytes, mostly high-scatter neutrophils, and 93% of these cells were EGFP+ on average, consistent with the expectation that the lysozyme promoter is most strongly transcribed in these cells (Fig. 1 A). Because of a low level of utilization of the lysozyme promoter by hematopoietic stem cells (31), populations other than neutrophils and monocyte-derived cells, such as lymphocytes, were EGFP+ at a low frequency, but this frequency (6% EGFP+ on average; Fig. 1 A) was substantially less than that of neutrophils or monocytes. As expected, control WT naive mice had no EGFP expression, whereas mice that have EGFP knocked into the Rosa26 locus for constitutive expression (35) showed that >98% of monocytes were EGFP+, thus indicating that the Rosa26 promoter is constitutively active in these cells (Fig. 1 A).
In the bone marrow, monocytes are mainly Ly-6Chi monocytes (36), and these monocytes were EGFP+ at a frequency similar to their blood counterparts, averaging 50% EGFP+ (Fig. 1 B). In contrast, the recently described macrophage DC progenitors (MDPs) (24), identified as CD115+ lineage− cells (37), were EGFP+ to a lower frequency (14% on average; Fig. 1 B).
Although not all monocytes were EGFP+, we reasoned that populations of DC subsets derived from monocytes should express EGFP at a percentage at least equal to the percentage of monocytes that scored positive. If the lysozyme promoter remained or became active during the differentiation of DCs after the monocyte stage, the percentage of DCs positive for EGFP might exceed that of monocytes. However, DCs positive for EGFP at a percentage sufficiently high to meet the criterion for potentially being derived from monocytes would not prove that they originated from monocytes. Nonetheless, as long as the Rosa26 promoter upstream of the EGFP reporter remained active, DC populations expressing a much lower fraction of EGFP than blood monocytes certainly could not be derived primarily from monocytes, and most importantly, EGFP− DC populations could not be descendents of EGFP+ DCs or monocytes.
Thus, we next analyzed DC subsets in the spleens of LysM-Cre × Rosa26-stopfloxEGFP mice to determine if their propensity to express EGFP may differ from that of monocytes, as might be expected from previous work (23–26). When total CD11c+ cells were considered, EGFP+ frequency more closely matched that of MDPs than monocytes (Fig. 1 C). To assess spleen DCs more carefully, we analyzed various DC subsets by further flow cytometric staining and gating. Conventional DCs in the spleen are CD11chi cells that differentially express CD8α and CD11b, and based on these gates, we separately evaluated EGFP expression in these subsets, labeled as populations 1–3 (Fig. 1 C). CD11cintCD11b−B220+ cells were plasmacytoid DCs (Fig. 1 C, population 4). Other CD11cint or CD11c− populations that expressed high CD11b were likely splenic monocytes and macrophages (37), and these could be divided in three populations (Fig. 1 C, labeled populations 5–7). In our analyses, we used monocytes as a reference population to normalize the data and draw a direct comparison to monocytes. In every mouse analyzed, we calculated the percentage of EGFP expressed in a given spleen DC or macrophage population and divided this value by the percentage of Ly-6Chi monocytes that were EGFP+ in the same mouse, because DCs might arise from Ly-6Chi monocytes in some tissues (20–22, 25). If the percentage of a given DC subset that was EGFP+ was similar to Ly-6Chi monocytes, then the product of this division would produce a value of 1. Ratios substantially <1 would indicate populations that could not have received a majority of precursor input from monocytes or any other EGFP+ population. In the spleen, all DC subsets (Fig. 1 C, gated populations 1–4) (37) exhibited a low level of EGFP expression and, fitting with previous conclusions (23–26), did not meet the criterion for a potentially close relationship with monocytes. Failure to meet this criterion was not caused by inactivity at the Rosa26 promoter, because CD11chi and CD11cint cells were uniformly EGFP+ in control Rosa26-EGFP knock-in mice (Fig. 1 C). In contrast to DCs, monocytes and macrophages in the spleen (gated populations 5–7) (37) met or exceeded the minimal criterion for possible derivation from monocytes (Fig. 1 C), serving as a positive control for the approach taken.
Reporter expression by nonlymphoid organ DCs is distinct from spleen DCs in the steady state
Next, we examined peripheral organ DCs in skin, lung, and small intestine in the steady state. Skin DCs were separated into epidermal DCs (Langerhans cells; Fig. 2 A) and dermal DCs that, as a whole, are CD11c+ and MHC II+ (Fig. 2 A, Dermal 1) (38). These tissue DC populations showed a much higher frequency of EGFP+ cells than that observed for spleen DCs, and overall, the frequency of EGFP+ skin DCs resembled the frequency seen in Ly-6Chi blood monocytes (Fig. 2 A). As noted previously (38), CD11bhiCD11c− cells in the dermis could be separated into MHC II+ and MHC II− populations (Fig. 2 A, Dermal 2 and 3, respectively). These cells likely include macrophages (38), with considerable heterogeneity in CD11bhi MHC IIlo cells. Overall, these populations also showed a frequency of EGFP expression that resembled Ly-6Chi monocytes (Fig. 2 A).
Figure 2.
EGFP reporter expression in LysM-Cre × Rosa26-stopfloxEGFP mice by DCs and macrophages from skin, lung, and small intestine. (A) Single-cell suspensions were prepared from the epidermis and dermis. Epidermal DCs were CD11c+ langerin+, and dermal DCs were gated as CD11c+ CD11bhi MHC IIhi (dermal 1), CD11c− CD11bhi MHC IIhi (dermal 2), or CD11c− CD11bhi MHC IIlo (dermal 3). The percentage of EGFP+ cells in these populations was normalized to the percentage of EGFP+ Ly-6Chi monocytes in the same mouse. (B) The percentage of EGFP in the BAL and lung of the two DC subsets (CD103−CD11bhi and CD103+CD11blo) and macrophages was normalized to the percentage of EGFP+ Ly-6Chi monocytes in the same mouse. (C) Gated live cells derived from the small intestine LP (or LP associated with the villous tips during removal of the epithelium) were stained and analyzed to reveal populations expressing CD11c, CD11b, CD8α, and MHC II. The LP contained lymphocytes and three populations that resembled DCs and/or macrophages, including CD11chi MHC IIint cells that differed with respect to CD11b expression (LP1 and LP2). Population LP2 was also associated with villous tips processed along with the intestinal epithelium (Villi DC). The third population was CD11cint MHC IIhi CD11bhi (LP 3). The percentage of these LP populations that were EGFP+ was normalized to the percentage of EGFP+ Ly-6Chi monocytes in the same mouse. In each graph, all mice studied are compiled, and each data point corresponds to one mouse. Two to three mice per group were handled per experiment. Horizontal bars represent means.
Pulmonary DCs are identified as CD11c+ cells with low autofluorescence, whereas CD11c+ cells with high autofluorescence are macrophages (22). Two subsets of pulmonary DCs exist, displaying differential expression of CD11b and CD103: CD103+CD11blo and CD103−CD11bhi DCs (22, 39, 40). Both DC subsets, whether recovered from the lung interstitium or bronchoalveolar lavage (BAL), were EGFP+ to a similar extent as Ly-6Chi blood monocytes (Fig. 2 B). Macrophages in the BAL were nearly 100% EGFP+ (Fig. 2 B), yielding a mean ratio of ∼2. Thus, as in the skin, DCs in the lung displayed considerable expression of EGFP, in contrast to spleen DCs and consistent with previous suggestions that blood monocytes may serve as a major precursor source for these cells (22, 25, 28).
In the small intestine, we analyzed DCs and/or macrophages in the lamina propria (LP). Two thirds of MHC II+ cells expressed high levels of CD11b and intermediate levels of CD11c (Fig. 2 C, gate 3/LP3). Most of these cells were EGFP+ in LysM-Cre × Rosa26-stopfloxEGFP mice. CD11chi cells with lower levels of MHC II could be separated into two populations based on differential surface expression of CD11b. One of these populations, representing from 4 to 10% of MHC II+ CD11c+ cells and expressing low levels of CD11b (Fig. 2 C, gate 1/LP1), was mainly EGFP− and stood out as the only potential DC population in any of the nonlymphoid organs examined that expressed EGFP at percentages as low as those observed for spleen DCs. However, this population was also CD8α+ (Fig. 2 C), resembling a subset of CD11chiCD11bloCD8α+ DCs in nearby Peyer's patches (41) and CD8α+ lymph node DCs in general. Indeed, this population may not be part of the LP proper but instead may be a contaminant from Peyer's patches, as we noticed that the population became progressively reduced in frequency (down to 4% of recovered DCs) as our skills in the dissection of the Peyer's patches away from the LP improved. Another population (Fig. 2 C, gate 2/LP2) expressed intermediate levels of EGFP that did not reach the frequency of EGFP+ cells observed in monocytes but that maintained a frequency higher than spleen DCs. These cells (Fig. 2 C, gate 2/LP2) are likely the DCs that populate the villi of the intestine and upper LP layers, because in some mice, we recovered DCs from villous tips that were removed while stripping the intestinal epithelium (Fig. 2 C, Villi DC), and the LP DCs recovered from these villi appeared identical in phenotype (not depicted) and EGFP+ frequency to LP2 (Fig. 2 C).
Considering data from all of these organs together, we conclude that most nonlymphoid organ DCs or their precursors use the lysozyme promoter in the steady state. This feature distinguishes them from spleen DCs and is consistent with, but does not prove, that the origins of nonlymphoid organ DCs may be distinct from spleen DCs, as previously proposed (25).
Nonlymphoid organ–derived DCs are scarce in lymph nodes
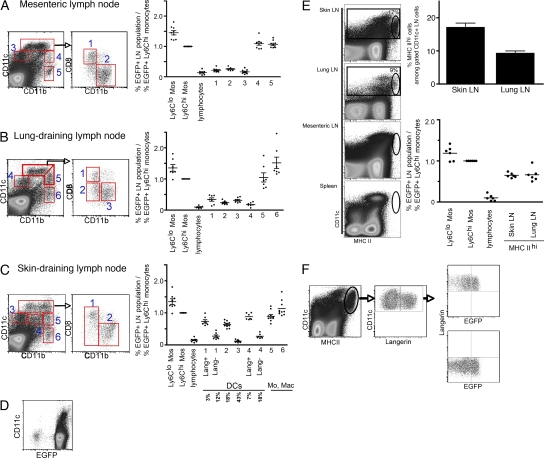
Because it is thought that lymph nodes receive substantial seeding by DCs that emigrate from upstream nonlymphoid organs through the afferent lymphatics (see Introduction), we expected to find that lymph node DCs, and discrete subpopulations of DCs in particular, would express a higher frequency of EGFP than observed in spleen DCs. However, the frequency of EGFP+ cells within lymph node DC subpopulations was much like that of spleen DCs, with the exception of skin-draining lymph nodes (Fig. 3, A–C). This could not be attributed to loss of reporter expression through failure to use the Rosa26 promoter, because all lymph node cells and all CD11cint and CD11chi cells that would be candidate DCs were EGFP+ in Rosa26-EGFP knock-in mice (Fig. 3 D).
Figure 3.
EGFP reporter expression in LysM-Cre × Rosa26-stopfloxEGFP mice by DCs within lymph nodes that drain intestine, lung, or skin. Stainings for CD11c, CD11b, B220, and CD8α were used to delineate various DC subsets within lymph nodes. Conventional CD11chi cells comprised populations in gates 1 and 2 for all lymph nodes (A–C), and also gate 3 for lung-draining lymph nodes (B). Plasmacytoid DCs were in gate 3 (A and C) or gate 4 (B). In skin-draining lymph nodes, gate 4 was considered a DC population because many of the langerin+ cells were found within it (C). The lower flow cytometry plot in C is an overlay of langerin+ cells (black) over all live cells (gray). Monocytes and macrophages were within populations 4 and 5 (A) or 5 and 6 (B and C). The percentage of EGFP within these gated lymph node populations was normalized to the percentage of EGFP+ Ly-6Chi monocytes in the same mouse. In pooled skin-draining lymph nodes, the mean percentage of each DC population relative to the DC gates overall is shown beneath the bar graph. (D) Flow cytometry of a representative lymph node (a skin-draining lymph node) from Rosa26-GFP knock-in mice. Live cells were gated on and plotted to show CD11c versus EGFP. (E) Various lymph nodes and spleens from LysM-Cre × Rosa26-stopfloxEGFP mice were stained with anti–MHC II mAb to identify CD11c+ cells with very high MHC II expression. Plots of these stains are shown on the left, with gated populations of MHC IIhi cells (oval gates) within overall CD11c+ cells (rectangular gates). The mean frequencies of this population in skin- and lung-draining lymph nodes are shown on the top right and are also indicated adjacent to the gate within the dot plots. Data represent the mean ± SEM. The bottom right plot shows the normalized frequency of EGFP+ cells in these gated MHC IIhi DCs. (F) Analysis of EGFP frequency in MHC IIhi CD11c+ lymph node cells that were langerin+ or langerin−. In each graph, all mice studied are compiled, and each data point corresponds to one mouse. Two to three mice per group were handled per experiment. In E, two experiments were performed with three mice in each. Horizontal bars represent means.
None of the classical DC populations in the mesenteric or lung-draining lymph nodes mirrored the percentage of EGFP+ DC populations of the upstream organs (Fig. 3 A, gates 1–3; and Fig. 3 B, gates 1–4; compare with Fig. 2, B and C). As in the spleen, the frequency of EGFP+ cells was elevated in CD11bhiCD11cint and CD11bhiCD11c− lymph node populations, but these are not typically considered classical DC populations (Fig. 3 A, gates 4 and 5; Fig. 3, B and C, gates 5 and 6). Instead, these gates have been suggested to mainly include macrophages and newly recruited monocytes (37). The lack of EGFP expression in most lymph node DCs was not likely caused by dilution of EGFP during the proliferation of EGFP+ DCs that migrated in from the upstream organs, because if the proliferation of DCs accounted for loss of EGFP, we would expect to find DCs with a range of reduced EGFP intensities in the draining lymph node representing DCs that had divided different numbers of times since leaving the upstream organ; instead, EGFP intensity was not different between nonlymphoid organ EGFP+ DCs and draining lymph node EGFP+ DCs, even in the lung, where it has been shown that DC turnover is especially rapid (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20081430/DC1).
Like the other lymph nodes, most skin-draining lymph node DCs were EGFP−, but certain populations of DCs within skin-draining lymph nodes, especially those that were langerin+, were EGFP+. Expression of langerin, found on all epidermal DCs and a small fraction of dermal DCs (21, 38), is considered a long-lasting marker of DCs that survey the skin and enter skin-draining lymph nodes through lymphatics (42). Langerin+ cells in resting LysM-Cre × Rosa26-stopfloxEGFP mice represented, on average, 10% of all CD11c+ cells in skin-draining lymph nodes and fell in gates 1 and 4 according to the intensity of CD11c, CD11b, and CD8α staining used to divide the subsets (Fig. 3 C). These langerin+ lymph node DCs and CD11bhi CD11chi CD8α− langerin− DCs (Fig. 3 C, gate 2) expressed a percentage of EGFP that was elevated above the fraction seen in background populations such as lymphocytes, but the EGFP levels were somewhat lower than any of the upstream populations of skin DCs (Fig. 2 A). Collectively, skin-draining lymph nodes contrasted with other lymph nodes examined, as they showed evidence of containing tissue-derived DCs, with up to 20% of the total lymph node DCs (∼10% langerin+ cells plus half of the DCs in gate 2 equals ∼20%; Fig. 3 C) possibly arriving from skin lymphatics.
DCs highly enriched in MHC II were observed in skin- and lung-draining lymph nodes, present on average in 17 and 9% of total CD11c+ cells, respectively. MHC IIhi DCs were not readily identified in mesenteric lymph nodes or the spleen (Fig. 3 E), although in other studies MHC IIhi cells were found in the spleen (43), indicating that even under specific pathogen-free conditions the phenotype of DCs in the steady state can vary between different mouse colonies. Because migrating DCs have sometimes been defined by being MHC IIhi to a level elevated over other DCs (18, 19), we assessed the frequency of EGFP+ cells within gated MHC IIhi lymph node DCs. The frequency of EGFP+ MHC IIhi DCs was, like langerin+ DCs, elevated in this gate relative to lymph node DCs overall. However, the percentage of MHC IIhi CD11c+ cells that were EGFP+ (Fig. 3 E) fell short of that observed in monocytes and in upstream tissue DCs (compare Fig. 3 E with Fig. 2 A), suggesting that many but not all of the MHC IIhi DCs might arise from defined, upstream tissue DC populations. As expected (18), the MHC IIhi DC gate was partially redundant with the langerin+ gated DCs, because in our analysis 31 ± 2% of MHC IIhi DCs were also langerin+ (Fig. 3 F). There was no difference in the EGFP frequency in either langerin+ or langerin− MHC IIhi cells (Fig. 3 F), suggesting that some of the MHC IIhi DCs may be migratory dermal DCs that are langerin− and that overall EGFP+ MHC IIhi DCs were equivalent to the sum of langerin+ cells in gates 1 and 4 and DCs in gate 2, as shown in Fig. 3 C.
Given that EGFP+ DCs could not give rise to EGFP− DCs, the failure to observe substantial EGFP expression in the vast majority of lymph node DC populations suggests that upstream organ DCs do not substantially populate lymph nodes in the steady state and certainly cannot account for as many as half of lymph node DCs (17). Clearly, some subpopulations of DCs accumulated in steady-state lymph nodes, most notably langerin+ and MHC IIhi DCs within skin-draining lymph nodes, but these subpopulations maintained a relatively low overall frequency.
Contrasting with the steady state, DCs from upstream organs are populous in lymph nodes that drain sites of inflammation and immune sensitization
One caveat to the conclusion drawn from Fig. 3 is the possibility that EGFP− organ DCs preferentially migrate to draining lymph nodes over their EGFP+ counterparts. To consider this possibility and to be certain that migratory DCs could be detected as EGFP+ cells in lymph nodes if they were present, we experimentally induced large-scale DC migration by applying a fluorescent contact sensitizer to the skin, thereby using one of the most established DC migration assays (3, 44). Tetramethylrhodoamine-5-(and-6)-isothiocyanate (TRITC) was mixed with dibutylphthalate and acetone and applied epicutaneously to trace DC migration (15, 45). As previously reported (45), the migrating population observed in lymph nodes 1 d after sensitization were mainly langerin− cells (not depicted), which are thought to arise from dermal langerin− DCs (45), such as the CD11bhi populations observed in Fig. 2 A. In the draining lymph node, TRITC+ DCs represented >30% of all lymph node DCs (Fig. 4 A) and fell within gates 2 and 4, as shown for skin-draining lymph nodes in Fig. 3 C (compare Fig. 3 C with Fig. 4 A, middle). TRITC+ cells were EGFP+ at the same frequency observed for dermal DCs (compare Fig. 4 A with Fig. 2 A), but TRITC− DCs remained mainly EGFP− (Fig. 4 A). Overall, CD11chi TRITC+ cells in the lymph node phenotypically resembled and showed a similar EGFP frequency as the Dermal 1 population in Fig. 2 A, and CD11cint TRITC+ cells most closely matched the Dermal 3 population in Fig. 2 A (Fig. 4 A). These data indicate that migrating DCs retain expression of EGFP during mobilization to downstream lymph nodes and illustrate that the migrating DC population does not favor EGFP− DCs in LysM-Cre × Rosa26-stopfloxEGFP mice.
Figure 4.
EGFP reporter expression in LysM-Cre × Rosa26-stopfloxEGFP mice by DCs that migrate into lymph nodes from upstream organs in response to inflammatory stimulation. (A) TRITC was applied to skin epicutaneously in a contact-sensitizing solution, and TRITC+ cells in the draining lymph nodes 18 h later were identified (left dot plot, gated area) and further analyzed. The flow cytometry plot on the right shows an overlay of TRITC+ cells (black) over all live lymph node cells (gray) with respect to the expression of CD11c and CD11b. The percentage of EGFP+ cells within TRITC+ and TRITC− lymph node DCs was normalized to the percentage of EGFP+ Ly-6Chi monocytes in the same mouse. Graph shows individual mice per data point. TRITC− DCs refers to a gate on TRITC− CD11c+ DCs that excludes the CD11bhiCD11cint gate that is considered to contain mainly monocytes (reference 37). The TRITC− CD11c+ grouping includes all DCs and the putative monocyte gate, thereby containing all TRITC− CD11c+ and CD11cint cells in the lymph node. (B) PBS or LPS was delivered intranasally (I.N.). 24 h later, live cells from the lung-draining lymph nodes were gated to identify CD11chi CD11blo cells, and gated CD11chi CD11blo cells were examined for CD103 or CD8α expression, revealing three subpopulations: CD103+ CD8α− (CD103+) lung-derived DCs, CD8α+ DCs, and double-negative DCs. Shown is the number (C) or percentage (D) of the CD103+ CD8α− (CD103+) DCs, CD8α+ DCs, and double-negative DCs within the CD11chi CD11blo gated population (from the FACS dot plot, top left) from the lung-draining lymph nodes treated with LPS (black squares) or with PBS (gray circles) to maintain near steady-state conditions. (E) Graph shows the effect of LPS treatment, used to induce DC migration from the lung, on the expression of EGFP by various populations, normalized to Ly6Chi monocytes within the same mouse. BAL and lung were gated on CD11chiCD11bloCD103+ DCs (CD103+). Lung-draining lymph node was gated on CD11chiCD11bloCD103+ DCs (CD103+), CD11chiCD11bloCD103− DCs (CD103−), and plasmacytoid DCs. Horizontal bars represent means. (F) Percentage of blood monocyte subsets and lymph node DC populations that expressed EGFP with or without intranasal LPS administration (from mice in E). In each graph, all mice studied are compiled, and each data point corresponds to one mouse. Two to three mice per group were handled per experiment. Data represent the mean ± SEM.
We next examined the effect of tissue inflammation on populations of DCs in lung-draining lymph nodes. As mentioned earlier, CD103 expression marks approximately half of the pulmonary DCs, and its expression persists after the DC migrates to the downstream, lung-draining lymph node (40, 46). We therefore monitored expression of CD103 by CD8α− DCs in the lung-draining lymph node to determine the frequency of this migrated population therein. CD103+CD8α− DCs fell within the gate of CD11chi CD11blo DCs (Fig. 4 B). These CD103+ DCs in the lymph node reflected a frequency of EGFP expression expected from analysis of upstream tissue CD103+CD11blo DCs (Fig. 2 B) but comprised a very small component within the gate (<10% gated, and 4% of lymph node DCs overall) when noninflamed lymph nodes from mice treated with PBS were examined (Fig. 4 B). However, when we treated mice intranasally with LPS to greatly enhance DC migration (5, 40, 47) and examined the lymph node 24 h later, CD103+CD8α− DCs were the dominant population in the same gate (Fig. 4 B, top left dot plots), and quantification of their absolute (Fig. 4 C) and relative (Fig. 4 D) frequency confirmed that CD103+ DCs were now the majority (60%) of the gated CD11chiCD11blo DCs (Fig. 4 D) and represented 25% of the lymph node DCs overall. LPS treatment led to an increase in the absolute number of all DC populations (Fig. 4, B and C), but migratory populations such as the CD103+ DCs were disproportionally increased (Fig. 4, C and D; and not depicted). CD103+ DCs were EGFP+ to a similar normalized frequency as their upstream tissue counterparts (Fig. 4 E). LPS increased the proportion of all lymph node DC subsets that expressed EGFP (Fig. 4 F), but because it also increased the frequency of EGFP+ monocytes and tissue DCs, it scarcely altered the normalized data. Taking these observations together, we conclude that inflammatory stimuli, such as LPS or mediators like TNF-α and IL-1 induced during contact sensitization (48, 49), are generally necessary to provoke substantial accumulation of upstream tissue-derived DCs within lymph nodes that enter by way of the afferent lymphatic vessels, and that, in contrast and against our expectations, in the steady state the lymph node contains few nonlymphoid organ–derived DCs.
DISCUSSION
Historically, several observations led to the development of the view that lymph node DCs arose substantially from lymph-borne DCs that originated in upstream tissues. First, DCs in cannulated afferent lymph are relatively abundant and are clearly involved in transporting antigen. In contrast, committed DC precursors are sparse in the circulation of humans (4). Furthermore, when afferent lymphatics are severed, the lymph node becomes nearly void of DCs (50) and antigen presentation is blocked (51). Recently, the idea that specialized DC subsets—plasmacytoid and CD8α+ DCs—enter the lymph node from the bloodstream has been introduced, but this understanding has not modified the belief that many lymph node DCs arise from interstitial nonlymphoid organ DCs that mobilize through lymphatics (16, 17) and may even mature in the steady state as they migrate (19). In this study, we present surprising evidence that most steady-state DCs in lymph nodes that drain a variety of organs by and large do not originate from upstream tissue DCs. Instead, we find that it is only during inflammation that tissue-derived DCs comprise a major fraction of lymph node DCs.
The model system used herein used LysM-Cre × Rosa26-stopfloxEGFP mice that “report” expression of LysM in neutrophils, monocytes, and macrophages by irreversibly inducing EGFP expression. A much lower frequency of expression is observed in T and B cells, stemming from promiscuous activation of the LysM promoter in some hematopoietic stem cells (31). Although macrophages continue to express lysozyme as they mature (31), this gene is not considered to be a marker for DCs and may be turned off developmentally upon induction of DC differentiation. The current study's comparison between tissue DC populations and those in the lymph node was possible because many tissue DCs, but not most lymph node or spleen DCs, expressed EGFP downstream of lysozyme promoter usage. The discrepancy in the frequency of the EGFP reporter between splenic and nonlymphoid organ DCs is consistent with the possibility that nonlymphoid organ DCs may derive from monocytes or some other lysozyme-positive precursor, but that most lymph node or spleen DCs do not (25). Both lymph node and spleen DCs more closely expressed the frequency of EGFP observed in bone marrow DC precursors called MDPs (24, 37), which do not inevitably differentiate into monocytes and may shut off EGFP as they differentiate into alternative end-stage cells, including DCs. Although complementing former adoptive transfer studies, (23–25) our approach has the advantage of permitting examination of mice with no previous experimental manipulation, thus truly maintaining the steady state. In addition, we examined whole populations, rather than a small proportion of transferred cells that require extrapolation, to make conclusions about what might have occurred in most members of a given population. Though our data are consistent with the possibility that nonlymphoid organ DCs may arise from monocytes, and indeed we used Ly-6Chi monocytes as a reference population throughout this study, our approach cannot prove an origin from monocytes for any population. However, because EGFP+ cells cannot be precursors for EGFP− cells in these mice, the strength of the model lies in its power to reveal which relationships between populations cannot be true.
Based on the fact that nonlymphoid organ DCs are frequently EGFP+ in LysM-Cre × Rosa26-stopfloxEGFP mice and lymph nodes DCs are not, we conclude that nonlymphoid organ DCs cannot account for the origin of most steady-state lymph node DCs, with the exception of discrete populations of skin DCs, such as langerin+ DCs, in skin-draining lymph nodes. Because lung and airway DCs are well characterized and possess durable markers such as CD103 that independently enable tracking in the steady state, this conclusion is particularly clear and striking when lung-draining lymph nodes are examined (5, 40, 47). In the small intestine, little information is available as to which LP population best migrates into lymphatics. We found one population of LP DCs with a very low frequency of EGFP (Fig. 2, LP1), such that if they migrated substantially to lymph nodes in the steady state they would go undetected by our method. However, the CD8α+ phenotype of these cells is more consistent with the possibility that they are contaminants from Peyer's patches rather than part of the LP proper. The major CD11c+CD11b+MHC II+ cell we identified in the LP expressed a high frequency of EGFP, but little is known about how efficiently these cells migrate into lymphatics. DCs at the epithelial interface migrate to lymph nodes after capturing luminal intestinal antigens (52). The CD11bhi population that was recovered in both the terminal villi and LP preparations is likely to correspond to this DC, given that it copurified to some extent with the epithelium. These DCs had an overall lower frequency of EGFP expression than blood monocytes, suggesting against previous conclusions (25) that they are not completely derived from monocytes. However, a low level of contamination from Peyer's patch DCs might also, as an artifact, reduce the frequency of EGFP+ cells in this population. In any case, though lower in EGFP frequency than monocytes, these villi/LP CD11bhi DCs were EGFP+ to a greater extent than any DC subset in the mesenteric lymph node, supporting the conclusion that these DCs did not comprise a major fraction of DCs in the mesenteric lymph node but are present only to a minor degree.
A caveat to our approach might be that the EGFP− DCs within a tissue preferentially have the capacity to migrate to lymph nodes. However, this is quite unlikely for several reasons. First, in all cases in which we can identify small populations of tissue-derived DCs, by gating on langerin+ DCs in skin-draining lymph nodes or CD103+CD11blo DCs in lung-draining lymph nodes, we see a close reflection of the EGFP frequency observed in the corresponding upstream tissue DC population. Second, during inflammation, upstream tissue-derived DCs are quite abundant in lymph nodes, and there is no bias toward EGFP− DCs. Thus, in all cases in which we analyze DCs confirmed to migrate from upstream organs to lymph nodes, we observe the expected frequency of EGFP. Finally, momentarily disregarding an approach based on analysis of EGFP frequency in LysM-Cre × Rosa26-stopfloxEGFP mice, an examination of total numbers of CD11bloCD103+ DCs in steady-state versus inflamed lung-draining lymph nodes (Fig. 4 B, bottom graphs) leads to a similar conclusion as that drawn from data that depend on the premises made in studies of LysM-Cre × Rosa26-stopfloxEGFP mice.
Although we conclude that the presence of nonlymphoid organ DCs is more minimal than expected in lymph nodes, this finding does not necessarily dispel the possibility that there is a relatively high frequency of DCs trafficking through lymph constitutively. For example, the lifespan of lymph-migrating DCs, especially in the steady state, may be quite brief within downstream lymph nodes. All of the available evidence indeed suggests that DC turnover in general is very rapid in lymph nodes (15, 27). Skin-resident dermal and epidermal DCs may in particular survive and persist in lymph nodes longer than other DC populations (53, 54). Our finding that these skin DC populations were the most enriched nonlymphoid organ–derived DCs in all lymph node examined corresponds with the idea that lifespan may be the major regulator of the presence of migratory tissue DCs within the lymph node.
Even as ephemeral constituents within lymph nodes, migratory DCs could still play a major role in immunity in the steady state. However, their failure to accumulate in lymph nodes for substantial durations fits best with a role in which they supply antigen, already processed or not, to other DCs within lymph nodes (55, 56). Transfer of antigen from migratory DCs to other DCs may be at least as substantial, or more so, in the steady state as it has been proposed to be during infection (56). The appearance of parietal antigens in CD8α+ DCs (10), a population of so-called lymph node–resident DCs that our data uphold do not derive from upstream tissue DCs, suggests that even large fragments of tissue antigens are not restricted within the lymph node to migratory DCs but are instead readily shared with other DCs. Alternatively, migratory DCs may often possess a phenotype distinct from that expected. CD11cintCD11bhi lymph node cells are not considered by most to be classical DCs, and they share this phenotype with Ly-6Clo monocytes (33, 57), making it tempting to consider all such cells as putative monocytes (37). Cells with this phenotype were abundant in steady-state lymph nodes, were EGFP+ like upstream tissue counterparts, and may reflect an important DC type with the capacity to directly present antigen or transfer it to other DCs. Particle-bearing, phagocytic monocyte-derived DCs that emigrate from skin to lymph nodes also express low levels of CD11c and very high levels of CD11b (20). Yet, little consideration has been given to the possibility that these cells have an important role in steady-state lymph nodes or, ultimately, in antigen presentation, in part because of the evidence that CD11chi cells are required for immune priming, even when CD11cint cells are not deleted in CD11c-DTR mice (58).
Because we cannot link lymph node DCs substantially to upstream tissue DCs, more credence may be given to the possibility that lymph node DCs mainly enter lymph nodes during the steady state through high endothelial venules. This notion still requires direct experimental support. Another interesting possibility to be investigated in future work is that the upstream tissue feeds the lymph node with DCs that enter through lymphatics, but that these cells circulate in lymph in an unexpected form, other than as descendents of tissue-resident DCs. The recent evidence that hematopoietic progenitor cells are found in lymph raises the possibility that they might seed the lymph node, although these progenitors were not retained in lymph nodes themselves and were argued to play a more important role in generating tissue DCs during inflammation rather than lymph node DCs in the steady state (59). As our findings challenge long-held views about the role of steady-state trafficking DCs in comprising lymph node populations of DCs, the present work highlights the need to investigate in more detail how lymph nodes are populated by DCs in the steady state, and the relationship between lymph-migrating and nonmigratory DCs in presenting antigen under homeostatic conditions.
MATERIALS AND METHODS
Mice.
LysM-Cre mice (29) were crossed to a reporter strain in which the Rosa26 promoter is upstream of a floxed stop cassette and the EGFP gene (30). This cross was provided to us by T. Graf (Centre for Genomic Regulation, Barcelona, Spain) (31), and we subsequently backcrossed the mice 12 generations on the C57BL/6 background before these experiments. Wild-type C57BL/6 and control mice in which EGFP is knocked into the Rosa26 locus for constitutive expression without the need for deletion of a floxed stop cassette (35) were purchased from the Jackson Laboratory. Mice were housed in a specific pathogen-free environment at Mount Sinai School of Medicine and were used in accordance with protocols approved by the Institutional Animal Care and Utilization Committee.
Cell suspension of spleen, lymph nodes, and nonlymphoid organ DCs.
Skin-draining lymph nodes (brachial, axillary, and inguinal), lung-draining lymph node (mediastinal), and mesenteric lymph node were excised, teased with needles and digested in collagenase D (Roche) for 30 min at 37°C. After digestion, 100 μl of 100 mM EDTA was added for 5 min. Cells were then lightly pressed through a 70-μm nylon filter, washed, collected, and stained for flow cytometry.
Spleen and lung cell suspensions were initially processed similarly to lymph nodes. However these tissues required an additional step of erythrocyte lysis after the first filtration. Spleen and lung cells were resuspended for 1 min in 1 ml of 1× lysing buffer (BD), followed by the addition of 10 ml of 0.3 mM EDTA/0.06% BSA/HBSS. Cells were then centrifuged at 1,200 rpm for 5 min, filtered, washed, collected, and stained for flow cytometry.
To obtain single-cell suspensions of BAL fluid, immediately after death, the trachea of a mouse was exposed and the mouse was positioned upright by inserting one side of a blunt forceps behind the trachea. An 18-gauge needle attached to a 3-ml syringe was inserted through the largest upper-cartilage ring, and the forceps was gently clamped down on the inserted needle. The airways were flushed four times with 1 ml of 0.5 mM EDTA/HBSS. Collected cells were resuspended in FACS blocking solution (HBSS containing 0.1 mM EDTA, 0.03% BSA [wt/vol], and 0.01% of each rabbit and human serum [vol/vol]) and stained for 30 min with conjugated antibodies.
To isolate LP DCs, part of the small bowel that included the jejunum and ileum was excised by separation from the mesentery. The remaining pieces of mesentery containing fat tissue were removed, and intestines were opened with scissors along the intestinal length and repeatedly washed from intestinal content. Next, intestinal tissues were incubated in the presence of 1 mM DTT (Sigma-Aldrich) at 37°C for 20 min and PBS with 1.3 mM EDTA at 37°C for 1 h on a shaker. The supernatants that contained a suspension of intestinal epithelial cells, intraepithelial lymphocytes, and a significant fraction of villi DCs were collected, washed and filtered. The remaining pieces of intestinal tissue were subjected to further processing. To enrich the LP DC fraction, the muscularis layer of the gut was separated from the rest with the use of a dissection microscope (model SZ51; Olympus) and discarded, whereas the remaining tissue that contained the villi and underlying submucosa (further referred to as the LP) was subjected to tissue digestion with 0.2 mg/ml of type IV collagenase (Sigma-Aldrich) at 37°C for 1 h. These tissues were homogenized, filtered, and washed. Obtained cells suspensions were stained and analyzed by flow cytometry.
Epidermal and dermal cell suspensions were acquired by splitting cut ears into two pieces. Both pieces were floated in RPMI 1640 medium containing 1.25 mg/ml of Dispase (BD), with the dermis side down overnight at 4°C. The next day, the epidermis and dermis were separated, placed in 1 ml of RPMI 1640 medium containing 1.75mg/ml Liberase III (Roche), cut into small pieces, and incubated at 37°C for 25 min. After 25 min of incubation, 100 μl of 100 mM EDTA was added for an additional 5 min of incubation. Cells were pressed through a 70-μm nylon filter, washed, collected, and stained for FACS.
Flow cytometry analysis.
The following mAbs from BD (except where another vendor is noted) were used for staining: biotinylated mAbs to CD3, B220, Ter119, and I-Ab; PE-conjugated mAbs to CD103 (eBioscience), CD8, B220, I-Ab, or CD115 (eBioscience); PerCP-Cy5.5–conjugated mAbs to Gr-1 (recognizes Ly-6C and Ly-6G) and CD11b; and allophycocyanin (APC)-conjugated mAbs to CD11c and pan-NK (eBioscience). Biotinylated mAbs were detected with streptavidin-conjugated APC (Invitrogen). Conjugated isotype-matched control mAbs were obtained from eBiosciences or BD.
To distinguish MDPs from monocytes in bone marrow (37), we stained for two combinations of lineage markers. Lineage stain 1 used a combination of mAbs to CD3, B220, Ter119, I-Ab, CD11c, and pan-NK (detected in the APC channel). Cells that were negative for these markers were evaluated with lineage stain 2, composed of PerCP-conjugated anti–Gr-1 and CD11b mAbs. CD115+ bone marrow cells positive for lineage stain 2 were monocytes, and those negative for both lineage stains were considered MDPs (CD115+ Lin−) (37).
Intracellular staining for langerin was performed after extracellular staining was done. Cells were fixed and permeabilized with Cytofix/Cytoperm solution (BD) for 20 min at 4°C. Then, cells were washed in 1× Perm/Wash buffer (BD). Control goat IgG (R&D Systems) or goat anti–mouse/rat/human langerin antibody (Santa Cruz Biotechnology, Inc.) was diluted in 1× Perm/Wash buffer at a 1:400 dilution and incubated with permeabilized cells for 20 min at 4°C. Cells were washed twice in 1× Perm/Wash buffer, and the secondary antibody, anti–goat PE (Invitrogen), was diluted in 1× Perm/Wash buffer at a 1:300 concentration and incubated with permeabilized cells for 20 min at 4°C. Finally, cells were washed once in 1× Perm/Wash buffer, followed by a wash in PBS. Cells were resuspended in PBS and analyzed by flow cytometry.
Fluorescent labeling of DC migration in the skin.
To analyze migrating skin DCs, TRITC (Sigma-Aldrich) was prepared as a stock solution at 100 mg/ml in DMSO and further diluted to a final concentration of 10 mg/ml in a 1:1 acetone/dibutylphthalate mixture (Sigma-Aldrich). The back skin of mice was shaved in three areas, and 25-μl aliquots of the TRITC solution were applied to each area, as described previously (60). 18 h later, brachial, axillary, and inguinal lymph nodes were excised and pooled, stained for flow cytometry, and analyzed.
Enhanced pulmonary DC migration to the lung-draining lymph nodes.
Intranasal delivery of 5 μg LPS (Sigma-Aldrich) in 30 μl PBS was used to enhance pulmonary DC migration to the lung-draining lymph nodes, as previously described (40). PBS alone, administered similarly by intranasal delivery, was used as a control. 24 h after intranasal delivery, mice were killed and tissues were processed for analysis.
Online supplemental material.
Fig. S1 analyzes EGFP intensity to determine if EGFP+ DCs might proliferate and dilute EGFP in the process. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081430/DC1.
Supplementary Material
Acknowledgments
We thank Dr. Florent Ginhoux for helpful discussion and Dr. Molly Ingersoll for critical reading of the manuscript.
This work was supported by National Institutes of Health grants AI49653 (to G.J. Randolph) and HL086899 (to M. Merad). C. Jakubzick was supported in part by a National Institutes of Health Postdoctoral Training Grant and was the recipient of a Primary Caregiver Technical Supplement from the National Institute of Allergy and Infectious Diseases. G.J. Randolph is an Established Investigator of the American Heart Association.
The authors have no conflicting financial interests.
Abbreviations used: BAL, bronchoalveolar lavage; EGFP, enhanced GFP; LP, lamina propria; LysM, lysozyme M; MDP, macrophage DC progenitor; TRITC, tetramethylrhodoamine-5-(and-6)-isothiocyanate.
References
- 1.Pugh, C.W., G.G. MacPherson, and H.W. Steer. 1983. Characterization of nonlymphoid cells derived from rat peripheral lymph. J. Exp. Med. 157:1758–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackay, C.R., W.L. Marston, and L. Dudler. 1990. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J. Exp. Med. 171:801–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Randolph, G.J., V. Angeli, and M.A. Swartz. 2005. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5:617–628. [DOI] [PubMed] [Google Scholar]
- 4.Steinman, R.M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9:271–296. [DOI] [PubMed] [Google Scholar]
- 5.MacPherson, G.G., C.D. Jenkins, M.J. Stein, and C. Edwards. 1995. Endotoxin-mediated dendritic cell release from the intestine. Characterization of released dendritic cells and TNF dependence. J. Immunol. 154:1317–1322. [PubMed] [Google Scholar]
- 6.Kupiec-Weglinski, J.W., J.M. Austyn, and P.J. Morris. 1988. Migration patterns of dendritic cells in the mouse. Traffic from the blood, and T cell–dependent and –independent entry to lymphoid tissues. J. Exp. Med. 167:632–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holt, P.G., S. Haining, D.J. Nelson, and J.D. Sedgwick. 1994. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J. Immunol. 153:256–261. [PubMed] [Google Scholar]
- 8.Huang, F.P., N. Platt, M. Wykes, J.R. Major, T.J. Powell, C.D. Jenkins, and G.G. MacPherson. 2000. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemmi, H., M. Yoshino, H. Yamazaki, M. Naito, T. Iyoda, Y. Omatsu, S. Shimoyama, J.J. Letterio, T. Nakabayashi, H. Tagaya, et al. 2001. Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-beta1-dependent cells. Int. Immunol. 13:695–704. [DOI] [PubMed] [Google Scholar]
- 10.Scheinecker, C., R. McHugh, E.M. Shevach, and R.N. Germain. 2002. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J. Exp. Med. 196:1079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurts, C., H. Kosaka, F.R. Carbone, J.F. Miller, and W.R. Heath. 1997. Class I–restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 186:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, J.W., M. Epardaud, J. Sun, J.E. Becker, A.C. Cheng, A.R. Yonekura, J.K. Heath, and S.J. Turley. 2007. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat. Immunol. 8:181–190. [DOI] [PubMed] [Google Scholar]
- 13.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919–923. [DOI] [PubMed] [Google Scholar]
- 14.Diacovo, T.G., A.L. Blasius, T.W. Mak, M. Cella, and M. Colonna. 2005. Adhesive mechanisms governing interferon-producing cell recruitment into lymph nodes. J. Exp. Med. 202:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamath, A.T., S. Henri, F. Battye, D.F. Tough, and K. Shortman. 2002. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 100:1734–1741. [PubMed] [Google Scholar]
- 16.Randolph, G.J., J. Ochando, and S. Partida-Sanchez. 2008. Migration of dendritic cells and their precursors. Annu. Rev. Immunol. 26:293–316. [DOI] [PubMed] [Google Scholar]
- 17.Villadangos, J.A., and P. Schnorrer. 2007. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat. Rev. Immunol. 7:543–555. [DOI] [PubMed] [Google Scholar]
- 18.Ohl, L., M. Mohaupt, N. Czeloth, G. Hintzen, Z. Kiafard, J. Zwirner, T. Blankenstein, G. Henning, and R. Forster. 2004. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 21:279–288. [DOI] [PubMed] [Google Scholar]
- 19.Wilson, N.S., L.J. Young, F. Kupresanin, S.H. Naik, D. Vremec, W.R. Heath, S. Akira, K. Shortman, J. Boyle, E. Maraskovsky, et al. 2008. Normal proportion and expression of maturation markers in migratory dendritic cells in the absence of germs or Toll-like receptor signaling. Immunol. Cell Biol. 86:200–205. [DOI] [PubMed] [Google Scholar]
- 20.Randolph, G.J., K. Inaba, D.F. Robbiani, R.M. Steinman, and W.A. Muller. 1999. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 11:753–761. [DOI] [PubMed] [Google Scholar]
- 21.Ginhoux, F., F. Tacke, V. Angeli, M. Bogunovic, M. Loubeau, X.M. Dai, E.R. Stanley, G.J. Randolph, and M. Merad. 2006. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 7:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakubzick, C., F. Tacke, F. Ginhoux, A.J. Wagers, N. van Rooijen, M. Mack, M. Merad, and G.J. Randolph. 2008. Blood monocyte subsets differentially give rise to CD103+ and CD103− pulmonary dendritic cell populations. J. Immunol. 180:3019–3027. [DOI] [PubMed] [Google Scholar]
- 23.Naik, S.H., D. Metcalf, A. van Nieuwenhuijze, I. Wicks, L. Wu, M. O'Keeffe, and K. Shortman. 2006. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 7:663–671. [DOI] [PubMed] [Google Scholar]
- 24.Fogg, D.K., C. Sibon, C. Miled, S. Jung, P. Aucouturier, D.R. Littman, A. Cumano, and F. Geissmann. 2006. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 311:83–87. [DOI] [PubMed] [Google Scholar]
- 25.Varol, C., L. Landsman, D.K. Fogg, L. Greenshtein, B. Gildor, R. Margalit, V. Kalchenko, F. Geissmann, and S. Jung. 2007. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J. Exp. Med. 204:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onai, N., A. Obata-Onai, M.A. Schmid, T. Ohteki, D. Jarrossay, and M.G. Manz. 2007. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat. Immunol. 8:1207–1216. [DOI] [PubMed] [Google Scholar]
- 27.Liu, K., C. Waskow, X. Liu, K. Yao, J. Hoh, and M. Nussenzweig. 2007. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 8:578–583. [DOI] [PubMed] [Google Scholar]
- 28.Landsman, L., C. Varol, and S. Jung. 2007. Distinct differentiation potential of blood monocyte subsets in the lung. J. Immunol. 178:2000–2007. [DOI] [PubMed] [Google Scholar]
- 29.Clausen, B.E., C. Burkhardt, W. Reith, R. Renkawitz, and I. Forster. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8:265–277. [DOI] [PubMed] [Google Scholar]
- 30.Mao, X., Y. Fujiwara, A. Chapdelaine, H. Yang, and S.H. Orkin. 2001. Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood. 97:324–326. [DOI] [PubMed] [Google Scholar]
- 31.Ye, M., H. Iwasaki, C.V. Laiosa, M. Stadtfeld, H. Xie, S. Heck, B. Clausen, K. Akashi, and T. Graf. 2003. Hematopoietic stem cells expressing the myeloid lysozyme gene retain long-term, multilineage repopulation potential. Immunity. 19:689–699. [DOI] [PubMed] [Google Scholar]
- 32.Geissmann, F., S. Jung, and D.R. Littman. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 19:71–82. [DOI] [PubMed] [Google Scholar]
- 33.Sunderkotter, C., T. Nikolic, M.J. Dillon, N. Van Rooijen, M. Stehling, D.A. Drevets, and P.J. Leenen. 2004. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 172:4410–4417. [DOI] [PubMed] [Google Scholar]
- 34.Qu, C., E.W. Edwards, F. Tacke, V. Angeli, J. Llodra, G. Sanchez-Schmitz, A. Garin, N.S. Haque, W. Peters, N. van Rooijen, et al. 2004. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J. Exp. Med. 200:1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zong, H., J.S. Espinosa, H.H. Su, M.D. Muzumdar, and L. Luo. 2005. Mosaic analysis with double markers in mice. Cell. 121:479–492. [DOI] [PubMed] [Google Scholar]
- 36.Tacke, F., F. Ginhoux, C. Jakubzick, N. van Rooijen, M. Merad, and G.J. Randolph. 2006. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J. Exp. Med. 203:583–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waskow, C., K. Liu, G. Darrasse-Jeze, P. Guermonprez, F. Ginhoux, M. Merad, T. Shengelia, K. Yao, and M. Nussenzweig. 2008. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat. Immunol. 9:676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bogunovic, M., F. Ginhoux, A. Wagers, M. Loubeau, L.M. Isola, L. Lubrano, V. Najfeld, R.G. Phelps, C. Grosskreutz, E. Scigliano, et al. 2006. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J. Exp. Med. 203:2627–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sung, S.S., S.M. Fu, C.E. Rose Jr., F. Gaskin, S.T. Ju, and S.R. Beaty. 2006. A major lung CD103 (alphaE)-beta 7 integrin-positive epithelial dendritic cell population expressing langerin and tight junction proteins. J. Immunol. 176:2161–2172. [DOI] [PubMed] [Google Scholar]
- 40.Jakubzick, C., J. Helft, T.J. Kaplan, and G.J. Randolph. 2008. Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J. Immunol. Methods. 337:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson, C., and B.L. Kelsall. 2005. Phenotype and function of intestinal dendritic cells. Semin. Immunol. 17:284–294. [DOI] [PubMed] [Google Scholar]
- 42.Ginhoux, F., M.P. Collin, M. Bogunovic, M. Abel, M. Leboeuf, J. Helft, J. Ochando, A. Kissenpfennig, B. Malissen, M. Grisotto, et al. 2007. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J. Exp. Med. 204:3133–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henri, S., D. Vremec, A. Kamath, J. Waithman, S. Williams, C. Benoist, K. Burnham, S. Saeland, E. Handman, and K. Shortman. 2001. The dendritic cell populations of mouse lymph nodes. J. Immunol. 167:741–748. [DOI] [PubMed] [Google Scholar]
- 44.Macatonia, S.E., A.J. Edwards, and S.C. Knight. 1986. Dendritic cells and the initiation of contact sensitivity to fluorescein isothiocyanate. Immunology. 59:509–514. [PMC free article] [PubMed] [Google Scholar]
- 45.Kissenpfennig, A., S. Henri, B. Dubois, C. Laplace-Builhe, P. Perrin, N. Romani, C.H. Tripp, P. Douillard, L. Leserman, D. Kaiserlian, et al. 2005. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 22:643–654. [DOI] [PubMed] [Google Scholar]
- 46.del Rio, M.L., J.I. Rodriguez-Barbosa, E. Kremmer, and R. Forster. 2007. CD103− and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J. Immunol. 178:6861–6866. [DOI] [PubMed] [Google Scholar]
- 47.Roake, J.A., A.S. Rao, P.J. Morris, C.P. Larsen, D.F. Hankins, and J.M. Austyn. 1995. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J. Exp. Med. 181:2237–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cumberbatch, M., R.J. Dearman, and I. Kimber. 1997. Langerhans cells require signals from both tumour necrosis factor-alpha and interleukin-1 beta for migration. Immunology. 92:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, B., H. Fujisawa, L. Zhuang, S. Kondo, G.M. Shivji, C.S. Kim, T.W. Mak, and D.N. Sauder. 1997. Depressed Langerhans cell migration and reduced contact hypersensitivity response in mice lacking TNF receptor p75. J. Immunol. 159:6148–6155. [PubMed] [Google Scholar]
- 50.Mebius, R.E., P.R. Streeter, J. Breve, A.M. Duijvestijn, and G. Kraal. 1991. The influence of afferent lymphatic vessel interruption on vascular addressin expression. J. Cell Biol. 115:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barker, C.F., and R.E. Billingham. 1968. The role of afferent lymphatics in the rejection of skin homografts. J. Exp. Med. 128:197–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niess, J.H., S. Brand, X. Gu, L. Landsman, S. Jung, B.A. McCormick, J.M. Vyas, M. Boes, H.L. Ploegh, J.G. Fox, et al. 2005. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 307:254–258. [DOI] [PubMed] [Google Scholar]
- 53.Garg, S., A. Oran, J. Wajchman, S. Sasaki, C.H. Maris, J.A. Kapp, and J. Jacob. 2003. Genetic tagging shows increased frequency and longevity of antigen-presenting, skin-derived dendritic cells in vivo. Nat. Immunol. 4:907–912. [DOI] [PubMed] [Google Scholar]
- 54.Hon, H., E.B. Rucker III, L. Hennighausen, and J. Jacob. 2004. bcl-xL is critical for dendritic cell survival in vivo. J. Immunol. 173:4425–4432. [DOI] [PubMed] [Google Scholar]
- 55.Inaba, K., S. Turley, F. Yamaide, T. Iyoda, K. Mahnke, M. Inaba, M. Pack, M. Subklewe, B. Sauter, D. Sheff, et al. 1998. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 188:2163–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allan, R.S., J. Waithman, S. Bedoui, C.M. Jones, J.A. Villadangos, Y. Zhan, A.M. Lew, K. Shortman, W.R. Heath, and F.R. Carbone. 2006. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 25:153–162. [DOI] [PubMed] [Google Scholar]
- 57.Tacke, F., D. Alvarez, T.J. Kaplan, C. Jakubzick, R. Spanbroek, J. Llodra, A. Garin, J. Liu, M. Mack, N. van Rooijen, et al. 2007. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jung, S., D. Unutmaz, P. Wong, G. Sano, K. De los Santos, T. Sparwasser, S. Wu, S. Vuthoori, K. Ko, F. Zavala, et al. 2002. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 17:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Massberg, S., P. Schaerli, I. Knezevic-Maramica, M. Kollnberger, N. Tubo, E.A. Moseman, I.V. Huff, T. Junt, A.J. Wagers, I.B. Mazo, and U.H. von Andrian. 2007. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 131:994–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robbiani, D.F., R.A. Finch, D. Jager, W.A. Muller, A.C. Sartorelli, and G.J. Randolph. 2000. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 103:757–768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.