Abstract
Tpl2 (Tumor progression locus 2), also known as Cot/MAP3K8, is a hematopoietically expressed serine-threonine kinase. Tpl2 is known to have critical functions in innate immunity in regulating tumor necrosis factor–α, Toll-like receptor, and G protein–coupled receptor signaling; however, our understanding of its physiological role in T cells is limited. We investigated the potential roles of Tpl2 in T cells and found that it was induced by interleukin-12 in human and mouse T cells in a Stat4-dependent manner. Deficiency of Tpl2 was associated with impaired interferon (IFN)-γ production. Accordingly, Tpl2−/− mice had impaired host defense against Toxoplasma gondii with reduced parasite clearance and decreased IFN-γ production. Furthermore, reconstitution of Rag2−/− mice with Tpl2-deficient T cells followed by T. gondii infection recapitulated the IFN-γ defect seen in the Tpl2-deficient mice, confirming a T cell–intrinsic defect. CD4+ T cells isolated from Tpl2−/− mice showed poor induction of T-bet and failure to up-regulate Stat4 protein, which is associated with impaired TCR-dependent extracellular signal-regulated kinase activation. These data underscore the role of Tpl2 as a regulator of T helper cell lineage decisions and demonstrate that Tpl2 has an important functional role in the regulation of Th1 responses.
Mature CD4+ T cells can be divided into distinct T helper cell lineages characterized by the effector cytokines produced upon activation. IFN-γ production defines the Th1 lineage that protects against intracellular organisms (1), IL-4 production is a hallmark of the Th2 lineage that defends against helminths and boosts humoral immunity (2), and IL-17 production distinguishes the Th17 lineage that defends against extracellular bacteria and yeast (3). The differentiation of naive CD4+ T cells into the appropriate lineage is critical for tailoring the immune response to invading pathogens and is determined in part by the cytokine milieu provided by DC.
One such cytokine, IL-12, is especially important because its expression during infection determines the type and duration of adaptive immune response (4). Specifically, IL-12 is required for Th1 effector cell differentiation from naive CD4+ T cells and for the secretion of the potent inflammatory cytokine, IFN-γ (5–7). IFN-γ, in turn, plays a major role in cell-mediated immunity by enhancing the bactericidal responses of macrophages, stimulating antigen presentation to T cells, inducing B cell antibody class switching, enhancing cytotoxic responses of NK cells, and promoting the differentiation of Th1 cells. The importance of both IL-12 and IFN-γ in host defense has been clearly demonstrated by cytokine and receptor KO mice, which have increased susceptibility to infection (8–11).
Despite the obvious importance of IL-12 in both innate and adaptive immunity, our understanding of the molecular basis of this cytokine's action is far from complete. The first step is that IL-12 activates the receptor-associated kinases Jak2 and Tyk2, which subsequently activate the transcription factor Stat4 (12). The importance of Tyk2, Jak2, and Stat4 in IL-12 signaling is substantiated by strong genetic evidence (8–11). Deficiency of Tyk2 greatly diminishes IL-12 signaling (13), but deficiency of Jak2 has even more profound consequences, including embryonic lethality caused by its role in erythropoiesis (14). Other signaling molecules, such as the p38 mitogen-activated protein kinse (MAPK), have also been implicated in IL-12 signaling, but their actions have not yet been fully defined (15, 16).
Further delineation of genes regulated by IL-12 and Stat4 and elucidation of how they contribute to the biology of developing CD4+ T cells will be important in understanding the actions of this cytokine and transcription factor and aid in the development of therapeutic interventions for inflammatory and autoimmune diseases exacerbated by IL-12 and IFN-γ. To this end, we and others have performed microarray analysis to identify IL-12–regulated genes (17). One gene that was prominently induced by IL-12 was Tpl2/Cot (tumor progression locus 2/Cancer Osaka thyroid; also known as MAP3K8). Originally identified as a protooncogene (18), Tpl2 is a serine-threonine kinase belonging to the MAPK family that has essential functions in innate immune cells where it transmits signals via Toll-like receptors, the TNF family of receptors (19), and G protein–coupled receptors (20). When overexpressed in a variety of cell types, it activates all of the MAPK pathways, NFAT, and NF-κB (21–24). Its signaling output, however, appears to be cell type dependent and signal dependent (20, 25). In APCs, it is reported to be an obligatory upstream activator of the extracellular signal-regulated kinase (ERK) pathway and to function as a critical regulator of TNF-α production in response to TLR signals (19). However, surprisingly little is known about its functions in normal T cells.
Herein, we demonstrate that Tpl2 is induced by IL-12 and is required by T cells for optimal IL-12–induced IFN-γ production. In vitro stimulation of Tpl2−/− CD4+ T cells revealed impaired IL-12– and TCR-induced IFN-γ production and inefficient polarization toward the Th1 phenotype. Furthermore, in response to infection with the Th1-inducing parasite Toxoplasma gondii, Tpl2-deficient mice developed a higher pathogen load associated with decreased systemic IFN-γ production, defining Tpl2 as a positive regulator of Th1 responses both in vitro and in vivo. Interestingly, Tpl2 appears to contribute to Th1 differentiation by ensuring proper induction of two key transcription factors, T-bet and Stat4. Thus, in addition to its key functions in the innate immune response, Tpl2 also has critical functions in T cells in promoting expression of transcription factors that drive optimal Th1 differentiation.
RESULTS
Tpl2 is induced by IL-12 stimulation
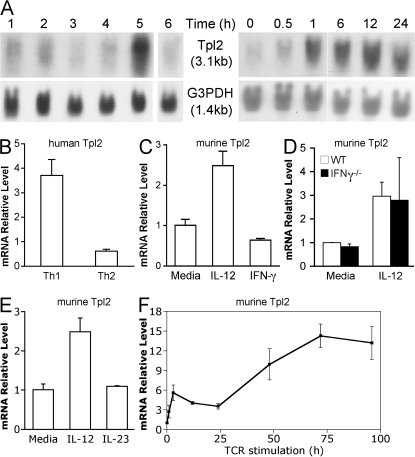
To identify genes rapidly induced by IL-12, microarray analysis was conducted using human PBMC enriched for CD3+ T cells after stimulation for 6 h. Approximately 90 genes were differentially induced by IL-12 stimulation. The top 30 most highly IL-12–inducible genes are depicted in Fig. S1 (available at http://www.jem.org/cgi/content/full/jem.20081461/DC1). These include known IL-12–inducible genes such as IFNG, IL12RB2, IL18R1, TBX21 (T-bet), and GZMB, lending validity to this study (17). In addition, the MAPK family member Tpl2 (also known as MAP3K8) was noted to be IL-12 inducible. A previous microarray study of polarized Th1 cells stimulated with IL-12 also included Tpl2 as a Th1-specific gene, but these results were not verified (17). To confirm these microarray results, we first performed Northern blot analysis on PBMC stimulated with various cytokines. Consistent with the microarray data, IL-12 alone specifically induced Tpl2 mRNA (Fig. 1 A, lane 5). Furthermore, Tpl2 was preferentially induced in human naive CD4+ cord blood T cells that had been cultured under Th1, compared with Th2, differentiating conditions for 7 d (Fig. 1 B). IFN-γ and IL-4 mRNA levels were measured by real-time PCR to confirm the proper polarization of the Th1 and Th2 cells, respectively (unpublished data).
Figure 1.
Tpl2 is an IL-12–inducible gene in human and mouse cells. (A, left) Human PBMC were cultured for 3 d in RPMI containing 1 μg/ml PHA followed by an additional day of culture in 40 U/ml IL-2 to maximize expression of IL-12R (43). The cells were then rested for 12 h in serum-free medium, followed by stimulation with medium alone (lane 1), IL-2 (lane 2), IL-4 (lane 3), IL-7 (lane 4), IL-12 (lane 5), or IL-15 (lane 6) at 10 ng/ml for 6 h, and Tpl2 mRNA expression was determined by Northern blot analysis using a Tpl2-specific probe. (A, right) A kinetic analysis of Tpl2 mRNA induction was performed by Northern blot analysis of 10 ng/ml IL-12–stimulated human PHA blasts. Data are representative of two independent experiments. White lines indicate that intervening lanes have been spliced out. (B) Human cord blood CD4+ T cells were cultured under Th1 or Th2 inducing conditions, as described in Materials and methods, and analyzed for Tpl2 message level by real-time PCR. Data are representative of two separate experiments. (C–E) For analysis of Tpl2 expression in mouse cells, CD4+ T cells were purified from mouse spleens. Cells were then grown for 3 d on plates coated with anti-CD3 and -CD28, rested for 5 h in cytokine-free medium, restimulated with IL-12 or IFN-γ (C), IL-12 (D), or IL-23 (E) for 4 h, and subsequently analyzed for Tpl2 expression by real-time PCR. Mouse cells analyzed were from WT (C and E; n = 3 replicate experiments) or IFN-γ–deficient mice (D; representative of two biological replicates from one experiment). (F) For transcriptional analysis of Tpl2 in response to TCR signals, 2 × 106 CD4+ T cells were stimulated in 2 ml of media on a 12-well plate coated with anti-CD3 and -CD28 for the indicated times and analyzed for Tpl2 expression by real-time PCR. Data are representative of two independent experiments. Error bars represent SD from replicates within a representative experiment.
Because IL-12 is a potent inducer of IFN-γ and Th1 cells produce IFN-γ, these studies did not discriminate between induction of Tpl2 directly by IL-12 or indirectly through IFN-γ. The time course of Tpl2 mRNA induction in response to IL-12 was very rapid, within 1 h of stimulation (Fig. 1 A, right), suggesting a direct mode of regulation. To formally test this hypothesis, we stimulated cells directly with IL-12 or IFN-γ. IL-12 stimulation of human PBMC induced Tpl2 expression, whereas direct stimulation with IFN-γ did not (unpublished data). The same was true in purified mouse CD4+ T cells, demonstrating that Tpl2 was regulated by IL-12 directly in both human and mouse cells (Fig. 1 C). Furthermore, IL-12 stimulation induced Tpl2 expression even in IFN-γ–deficient mouse T cells, excluding an indirect mode of regulation through IFN-γ (Fig. 1 D).
To determine if Tpl2 induction is specific to IL-12 stimulation, we investigated whether IL-23, which shares the p40 ligand subunit and the IL-12Rβ1 receptor chain with IL-12 (26), could induce Tpl2 expression. Although IL-23 induced production of IL-17 mRNA as expected (not depicted), IL-23 failed to induce Tpl2 expression (Fig. 1 E). These data provide further evidence of direct and specific induction of Tpl2 by IL-12. However, previous studies on cell lines have suggested that Tpl2 is also induced by TCR signaling (18, 24, 27). Indeed, activation of CD4+ T cells with plate-bound anti-CD3 and -CD28 led to rapid and sustained induction of Tpl2 (Fig. 1 F). We therefore conclude that Tpl2 is dynamically regulated in T lymphocytes by both T cell receptor engagement and IL-12.
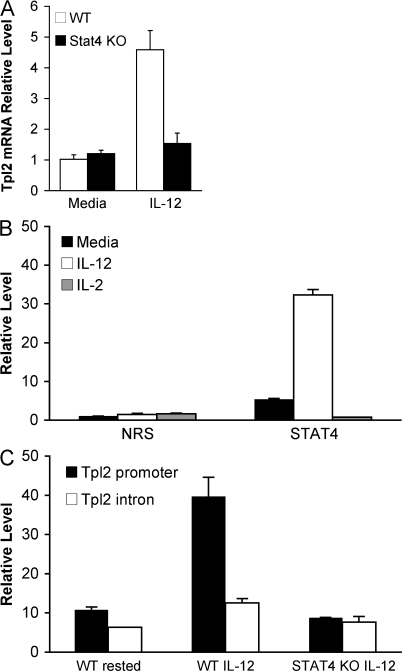
Because the major IL-12 signaling intermediate is Stat4, we investigated whether Tpl2 up-regulation is dependent on this transcription factor. Notably, IL-12 stimulation failed to induce Tpl2 mRNA expression in Stat4-deficient CD4+ T cells, suggesting that Tpl2 gene expression is regulated by Stat4 (Fig. 2 A). Examination of the mouse Tpl2 locus revealed several putative Stat binding motifs within 1 kb upstream of the Tpl2 transcriptional start site. Therefore, we performed chromatin immunoprecipitation (ChIP) experiments to test for direct binding of Stat4 to the Tpl2 promoter. ChIP with an anti-Stat4 antibody revealed binding to the Tpl2 promoter using two different primer sets designed to interrogate different regions of the promoter (unpublished data). IL-12–inducible association was quantitated by ChIP using real-time PCR (Fig. 2 B). To further confirm the specificity of this association, an intronic sequence within the Tpl2 gene was amplified and failed to show any significant Stat4 binding, which is similar to what was seen using Stat4-deficient T cells (Fig. 2 C). Thus, we conclude that Stat4 binds to the Tpl2 promoter in an IL-12–inducible manner and is required for IL-12–dependent Tpl2 gene expression.
Figure 2.
Tpl2 is a direct Stat4 target gene. (A) The Stat4 dependency of Tpl2 induction by IL-12 was examined by stimulating WT or Stat4-deficient mouse CD4+ T cell blasts with IL-12 for 4 h and analyzing Tpl2 mRNA induction. n = 3 replicate experiments. (B) ChIP using an anti-Stat4 antibody or nonimmune rabbit serum (NRS) was analyzed quantitatively by real-time PCR using primers designed around putative Stat sites within 1 kb upstream of the Tpl2 transcriptional start site. n = 4 independent experiments. (C) To further test the specificity of the binding, we compared the binding to the promoter region to that seen within an intronic region of the gene (intron 5). n = 1 experiment. Error bars represent SD from replicates within a representative experiment.
Tpl2 regulates T cell IFN-γ production
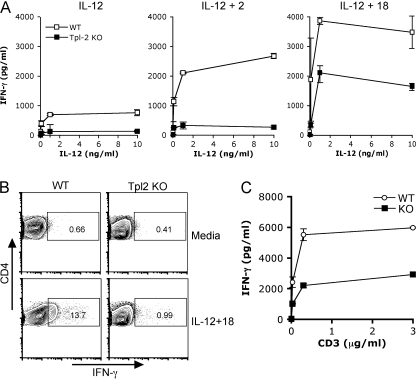
Because Tpl2 is induced by IL-12, we reasoned that Tpl2 might also be involved in IFN-γ expression and Th1 differentiation. As has previously been reported, Tpl2-deficient mice displayed normal lymphoid development, with normal numbers and proportions of thymocytes and splenocytes (19). To test the ability of T cells from Tpl2-deficient mice to respond to IL-12 stimulation, WT or Tpl2-deficient CD4+ T cells were first activated with anti-CD3 and -CD28 for 3 d to induce IL-12R expression, rested in cytokine-free medium for 5 h, and stimulated with IL-12. Tpl2-deficient T cells were significantly diminished in their capacity to produce IFN-γ (Fig. 3 A, left). Furthermore, this defect could not be overcome by stimulation with IL-12 in combination with IL-2 and was only partially rescued by IL-12 plus IL-18 (Fig. 3 A, middle and right), both of which are known to synergize with IL-12 for IFN-γ production. Although IL-12 stimulation alone was insufficient to visualize intracellular IFN-γ staining (not depicted), the combination of IL-12 and IL-18 yielded 14% IFN-γ+ cells in WT compared with only 1% in Tpl2-deficient T cells (Fig. 3 B). These data clearly indicate that there is a decrease in the proportion of Tpl2−/− CD4+ T cells capable of making IFN-γ under these conditions. The IFN-γ defect was also confirmed in human cells using a small interfering RNA (siRNA) knockdown approach (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20081461/DC1). The siRNA approach additionally demonstrates that altered IFN-γ production by Tpl2-deficient T cells is not caused by a developmental abnormality because these cells developed in the presence of Tpl2.
Figure 3.
Tpl2 is required for optimal IL-12–induced IFN-γ production. (A and B) IL-12–induced IFN-γ production was analyzed in WT and Tpl2-deficient CD4+ T cells. CD4+ T cells isolated from WT or Tpl2-deficient mouse LN and spleens were activated and expanded on anti-CD3– and -CD28–coated flasks for 3 d. Cells were then rested for 5 h in cytokine-free medium before stimulation with 0–10 ng/ml IL-12 alone or in combination with 50 U/ml IL-2 or 10 ng/ml IL-18 for 24 h. Cytokine production was measured in cell culture supernatants using cytometric bead array (A) or intracellular cytokine staining (B). (C) For intracellular cytokine staining, rested cells were restimulated with IL-12 and IL-18 for 24 h with the addition of Golgi transport inhibitor for the last 5 h of culture. Whole splenocytes were stimulated with soluble anti-CD3 for 48 h, and the production of IFN-γ was measured by cytometric bead array. A, n = 3 experiments; B, n = 2 experiments; C, n = 3 experiments. Error bars represent SD from replicates within a representative experiment.
Given that Tpl2 is also induced by TCR signals, we considered that it might also affect IFN-γ production in response to TCR-mediated signals. To this end, splenocytes were stimulated with soluble anti-CD3, and cytokine secretion was measured 24 h later. As shown in Fig. 3 C, Tpl2-deficient splenocytes produced significantly less IFN-γ than WT cells in response to TCR stimulation.
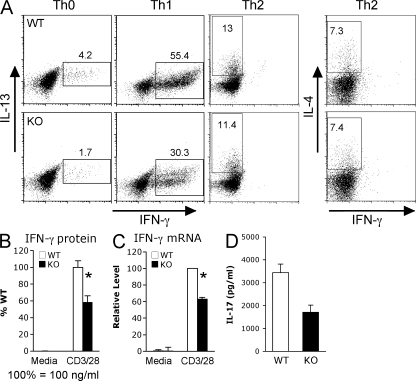
To investigate the role of Tpl2 in T helper polarization, we isolated naive CD62LhiCD44lo CD4+ T cells from WT or Tpl2−/− mice by FACS sorting and cultured the cells with appropriate cytokines and anticytokine antibodies to generate Th0, Th1, Th2, or Th17 cells. Under neutral conditions (Th0), fewer IFN-γ+ cells were generated by Tpl2-deficient cells (Fig. 4 A, left). Even when Tpl2-deficient cells were cultured under optimal Th1-polarizing conditions, differentiation into Th1 cells was still attenuated as measured by intracellular cytokine staining, ELISA, and real-time PCR (Fig. 4, A–C). However, Th2 differentiation was not further augmented by Tpl2 deficiency under optimal Th2 conditions (Fig. 4 A, right). Interestingly, IL-17 production was also impaired in Tpl2-deficient cells cultured under Th17 conditions (Fig. 4 D). These data are consistent with the idea that Tpl2 deficiency is associated with an intrinsic impairment of TCR- and IL-12–dependent Th1 responses.
Figure 4.
Tpl2 deficiency leads to impaired Th1 differentiation in vitro. Naive CD44loCD62Lhi CD4+ T cells were purified by FACS sorting and cultured with immobilized anti-CD3 and -CD28 plus the appropriate cytokines and anti-cytokine antibodies for 7 d to generate Th0, Th1, Th2, or Th17 T helper lineages. Efficiency of polarization was determined based on IFN-γ, IL-4/IL-13, or IL-17 production, as measured by intracellular cytokine staining for IFN-γ and IL-13 or IL-4 (A), cytometric bead array for IFN-γ (B), real-time PCR for IFN-γ mRNA (C), and ELISA for IL-17 protein (D). Data shown for A–C are representative of at least three independent experiments; data in D are representative of two experiments. Error bars indicate SEM; *, P < 0.05.
Tpl2-deficient T cells have reduced levels of Stat4 and T-bet
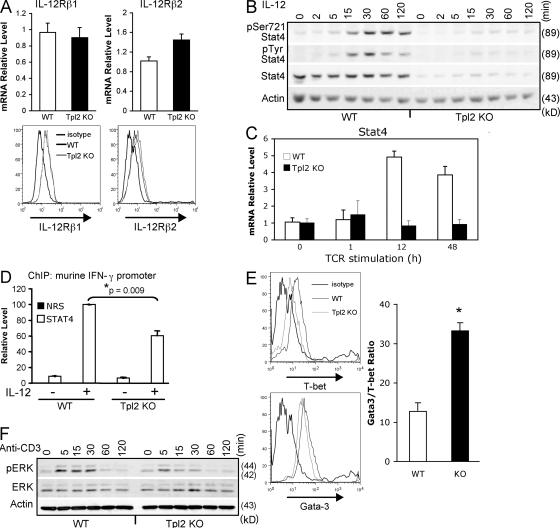
We next examined potential mechanisms underlying the IL-12 hyporesponsiveness in Tpl2-deficient T cells. Assessment of IL-12Rβ1 and IL-12Rβ2 showed similar expression levels of both subunits in activated CD4+ T cells from WT or Tpl2-deficient mice as measured by real-time PCR (Fig. 5 A, top) and surface staining (Fig. 5 A, bottom). Despite normal receptor expression, both IL-12–dependent Stat4 serine and tyrosine phosphorylation were dramatically reduced in Tpl2−/− mice, owing to reduced levels of total Stat4 protein and mRNA (Fig. 5, B and C). Specifically, freshly isolated Tpl2−/− CD4+ T cells did not have altered levels of Stat4 expression (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20081461/DC1). However, Stat4 is inducible upon T cell activation (12), and this Stat4 induction was diminished in TCR-activated Tpl2−/− T cells (Fig. 5, B and C). The reduced levels of Stat4 protein in cultured CD4+ T cells were associated with reduced recruitment of Stat4 to the mouse Ifng promoter in response to IL-12 signaling, demonstrating that this difference is functionally relevant (Fig. 5 D). Additionally, purified CD4+ T cells from Tpl2−/− mice failed to properly induce T-bet protein when stimulated with anti-CD3 and -CD28 (Fig. 5 E, left), whereas Gata3 protein was regulated normally. Accordingly, at the transcriptional level a greater than twofold increase in the ratio of Gata3/T-bet mRNAs was seen by real-time PCR (Fig. 5 E, right). Collectively, these data demonstrate that Tpl2 contributes to Th1 differentiation in activated T cells by ensuring proper induction of the key transcription factors T-bet and Stat4. These defects appeared to arise during the TCR activation phase. Therefore, we examined Tpl2-dependent events in response to TCR activation. We analyzed ERK activation in response to CD3 cross-linking and, consistent with recently published data (28), we found that ERK activation is impaired in the Tpl2-deficient T cells (Fig. 5 F). Based on these data, we propose a model for the role of Tpl2 in Th1 differentiation as depicted in Fig. S4.
Figure 5.
Tpl2 regulates Stat4 and T-bet levels. (A) CD4+ T cell blasts were generated over 3 d of culture on immobilized anti-CD3 and -CD28. Expression of IL-12 receptor β1 and β2 were determined by real-time PCR or surface staining. n = 3 separate experiments each. (B) Cells were then stimulated with IL-12 for the indicated times. Whole cell lysates were analyzed for ERK (not depicted) or Stat4 activation by Western blotting. n = 3 independent experiments. (C) TCR-dependent regulation of Stat4 was determined by culturing 2 × 106 cells in 2 ml of media on a 12-well plate coated with anti-CD3 and -CD28 for the indicated times. Stat4 expression was determined by real-time PCR analysis. Error bars represent SD from replicates within a representative experiment. n = 2 independent experiments. (D) ChIP was performed to determine whether Tpl2 deficiency affected IL-12–induced Stat4 binding to the Ifng promoter. Cells were activated and rested as in A, plated at 106/ml in 10-cm petri dishes, and stimulated for 1 h with 10 ng/ml IL-12. Immunoprecipitation was performed using anti-Stat4 antibody or nonimmune rabbit serum, and binding to the Ifng promoter was analyzed by real-time PCR. Data represent means and SE of the mean of three independent experiments. (E, left) Purified CD4+ T cells were activated with anti-CD3 and -CD28 for 3 d and analyzed by intracellular staining for T-bet and Gata3 expression. n = 2 independent experiments with a total of three individual mice. (E, right) Additionally, purified CD4+ T cells were analyzed for Gata3 and T-bet mRNA expression by real-time PCR relative to an 18S control, and the ratio of Gata3/T-bet message is shown. The ratio of Gata3/T-bet was calculated by ΔCt method. Data represent means and SEM for a total of four mice per group in two separate experiments. *, P < 0.05. (F) Tpl2-dependent TCR signaling events were analyzed in WT and Tpl2-deficient mice. Five million cells/point were stimulated with biotinylated anti-CD3 on ice for 15 min before cross-linking with streptavidin at 37°C for the indicated times. Western blots were probed with antibodies recognizing phospho-ERK, total ERK, and actin. Data are representative of two independent experiments.
Tpl2 is required for the normal host defense against the Th1-inducing parasite Toxoplasma gondii
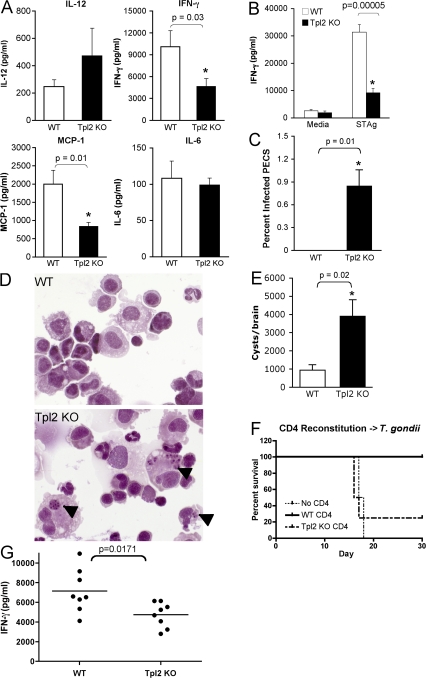
To determine whether Tpl2 plays a critical role in IFN-γ production in vivo, WT or Tpl2-deficient mice were infected i.p. with T. gondii, an obligate intracellular parasite, resistance to which critically depends upon IFN-γ production (29). 7 d after infection, mice were killed and examined for markers of acute infection. Despite the fact that Tpl2−/− APCs overproduce IL-12 both in vitro in response to TLR ligands (19, 30) and in vivo in response to T. gondii infection (Fig. 6 A), T. gondii–infected Tpl2−/− mice produced significantly less IFN-γ systemically than WT mice. Additionally, reduced amounts of MCP-1 (CCL2), an IFN-γ–inducible chemokine, were present in sera of infected mice, whereas IL-6 levels were unaffected by Tpl2 deficiency (Fig. 6 A). There were similar numbers and proportions of T cells and NK cells in spleens and peritoneal exudate cells (PECs) isolated from infected and uninfected WT or Tpl2-deficient mice (unpublished data), suggesting that altered cellular responsiveness and not development/recruitment was responsible for the defective IFN-γ production. Recall responses of lymphocytes to soluble T. gondii antigen (STAg) taken from day-7 infected mice also showed impaired antigen-specific T cell responses in the Tpl2−/− cells as measured by IFN-γ secretion (Fig. 6 B). The susceptibility of Tpl2−/− mice to T. gondii was further assessed by quantifying the number of infected PECs persisting at day 7 after infection. Tpl2−/− mice retained detectable parasites within the PECs, whereas WT mice had cleared the local infection by this time point (Fig. 6, C and D). Encysted bradyzoites are found in the muscle and nervous tissue of chronically infected animals, and their abundance can be related to parasite control during the acute phase. Consistent with the defect in IFN-γ production and increased pathogen load, Tpl2−/− mice developed fourfold greater numbers of brain cysts after 5 wk of infection (Fig. 6 E). Collectively, these data indicate that Tpl2 is essential for proper host resistance against a parasite that requires an appropriate Th1 response and is a critical mediator of IFN-γ production in vivo.
Figure 6.
Tpl2 is required for normal host responses to T. gondii. WT or Tpl2-deficient mice were infected with 20 cysts of the avirulent T. gondii strain ME49 and analyzed for host responses during acute and chronic stages of infection. Both WT and Tpl2−/− mice survived the infection for >8 wk. To explore their immune responses further, mice were killed on day 7 after infection and several parameters were analyzed, including systemic cytokine levels in serum (A), LN recall responses to STAg (B), and proportion of infected PECs (C and D). Arrowheads in D indicate intracellular T. gondii parasites. Host responses of chronically infected mice were assessed at week 5 by quantifying the numbers of encysted bradyzoites in the brain (E). Rag2-deficient mice were reconstituted with 10 million purified CD4+ T cells and infected with T. gondii >1 wk later. Survival of reconstituted infected mice was monitored (F). Serum levels of IFN-γ were also determined 7 d after infection (G). Data indicate means from at least seven mice per group from two separate infections for A, B, and G and are from four mice per group in C, E, and F, respectively. Error bars indicate standard errors of the mean.
The overproduction of IL-12 by Tpl2-deficient APCs would be expected to promote Th1 differentiation and might complicate the interpretation of the preceding experiment. To more carefully assess the in vivo function of Tpl2-deficient T cells, we reconstituted Rag2-deficient mice with WT or Tpl2−/− CD4+ T cells and then infected the mice with T. gondii. As expected, Rag2-deficient mice that did not receive any T cells rapidly succumbed to infection, whereas mice receiving WT T cells were protected (Fig. 6 F). Similar to what was observed in Tpl2−/− mice, mice adoptively transferred with Tpl2-deficient T cells produced significantly less IFN-γ than mice receiving WT T cells (Fig. 6 G). Furthermore, mice receiving Tpl2 KO T cells died shortly after infection with kinetics similar to Rag-deficient mice that had not received any T cells (Fig. 6 F), although no differences were found in reconstitution potentials between WT and Tpl2−/− CD4+ T cells (not depicted). We interpret these data to suggest that hyperproduction of IL-12 by Tpl2-deficient APCs in the complete Tpl2-deficient mice likely partially masks a more dramatic T cell phenotype in this model. Regardless though, the adoptive transfer experiment argues strongly for an intrinsic role for Tpl2 in promoting Th1 differentiation and IFN-γ production.
DISCUSSION
Although Tpl2 has been reported to be critical in innate immunity and oncogenesis, remarkably little attention has been given to its role in normal T cells. In this study, we show that Tpl2, which is inducible by TCR occupancy and IL-12, is important for T cell differentiation and effector functions. Activation of Tpl2-deficient CD4+ T cells via cross-linking of the TCR led to impaired development of Th1 responses. Furthermore, IL-12 stimulation of activated Tpl2-deficient T cells also showed major defects in IFN-γ production. Accordingly, Tpl2-deficient T cells had reduced levels of Stat4 and T-bet, two factors which are critical for Th1 differentiation. This abnormal response was physiologically relevant in host defense against the model pathogen T. gondii, in which IFN-γ induction was impaired and the pathogen burden was increased. Using adoptive transfer experiments, we established that this increased susceptibility was caused by an intrinsic T cell defect. These findings are interesting from several perspectives and have multiple implications.
First, the study defines Tpl2 as a novel IL-12–inducible gene that is a direct target of Stat4. Stat4 is clearly critical in host defense and immunoregulation, and several Stat4-dependent genes have been identified (31). However, a paucity of direct Stat4 targets have been defined. These include IFN-γ (32), Cd25 (33), the proprotein convertase Furin (34), and now Tpl2 (Map3k8). These genes are inducible in response to TCR cross-linking as well as by cytokines. Potential transcription factors required for TCR-induced Tpl2 regulation have not been examined, but like the Ifng locus, the Tpl2 locus is likely to be of interest to examine as a model gene regulated jointly by TCR and cytokine signals. In preliminary experiments, we have begun to examine epigenetic modifications in the Tpl2 gene in differentiating T cells. It will be of interest to determine whether Stat4 is essential for epigenetic regulation of this locus as it is for the Ifng locus.
Second, although several studies have examined the potential function of Tpl2 by overexpression of WT or mutant alleles in cell lines (18, 35), very little is known about the physiological role for Tpl2 in T cells. In this paper, we demonstrate that this kinase has an important nonredundant function in T cells in vivo as an inducible positive regulator of IFN-γ. It is clear that Tpl2 has a T cell–intrinsic role in IL-12 signaling and IFN-γ production because in vitro polarization of naive CD4+ T cells toward the Th1 phenotype is impaired in Tpl2−/− cells, even under conditions antagonistic to Th2 polarization (i.e., in the presence of neutralizing IL-4 antibody). In this setting, continued neutralization of Th2 responses did not reverse the Th1/IFN-γ defect in vitro, indicating that impaired IFN-γ production by Tpl2−/− CD4+ T cells cannot be explained simply by enhanced Th2 responses.
Mechanistically, it appears that Tpl2 exerts its effect by influencing the levels of T-bet and Stat4 protein in activated T cells. Although this results in impaired IL-12 signaling as determined by Stat4 recruitment to the Ifng promoter, the impaired expression of Stat4 apparently arises during initial TCR-dependent actions. A recent study has shown that Tpl2 is required for the optimal activation of ERK and MEK in response to TCR stimulation (28). Our data have confirmed that ERK phosphorylation induced by TCR occupancy is partially Tpl2-dependent in T cells (Fig. 5 F). Whether Stat4 induction by TCR signals is ERK dependent remains to be determined. However, T cells deficient in the ERK-activated transcription factor Ets-1 have been shown to have impaired Th1 responses and reduced levels of Stat4 (36). Precisely how Tpl2 contributes to the regulation of Stat4 and T-bet protein levels is unclear but is worthy of future investigation.
In addition to its role in TCR signaling, Tpl2 might also be involved in T cell costimulatory pathways. Kane et al. (37) have reported that Tpl2 associates with and is phosphorylated by protein kinase B (Akt) (38). Furthermore, they demonstrate that overexpression of an activated form of Akt mimics CD28 costimulation of IFN-γ induction in T cells. If Tpl2 is indeed involved in CD28 costimulatory signals, Tpl2 deficiency may impair CD28 costimulation and, consequently, IFN-γ production.
The in vivo relevance of Tpl2 functions in normal T cell responses was most dramatically indicated by the challenge of Tpl2−/− mice with T. gondii, a pathogen which is known to induce a vigorous Th1 response. Although Tpl2−/− mice survived the infection, the parasite burden was increased relative to WT mice. Furthermore, specific impairment of T cells was evident as assessed by recall responses to STAg. The present data stand in apparent contrast to a study indicating that enhanced TLR-induced IL-12 production by Tpl2-deficient APCs is associated with increased Th1 responses (30). We too have found enhanced IL-12 production in Tpl2-deficient DC in vivo and in vitro (Fig. 6 A and Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20081461/DC1); however, our data also indicate that Tpl2-deficient T cells are nonetheless impaired in IFN-γ production and Th1 polarization. We believe that some important factors distinguish these studies. First, the previous study used adjuvants in in vitro and in vivo experiments that may have inadvertently magnified the IL-12 overproduction defect by the APC population and led to an apparent Th1 bias in the Tpl2−/−. Additionally, although the earlier study focused on Tpl2's functional role in APCs, we have tailored our study to specifically address its role in T cells, particularly with the Rag−/− reconstitution experiment (Fig. 6, F and G). These data clearly support our contention that Tpl2 is required for optimal IFN-γ production by CD4 T cells.
Further delineation of Tpl2 functions downstream of the TCR will provide valuable information about the balance of T helper lineages and may prove useful in the treatment of inflammatory diseases. Tpl2 deficiency has already been reported to attenuate a TNF-dependent model of inflammatory bowel disease (39). Because TNF blockade has been shown to be efficacious in the treatment of several inflammatory diseases (40), Tpl2 kinase has been suggested as a potential therapeutic target. A Tpl2 inhibitor has been generated and shown to inhibit LPS-induced TNF-α production by human monocytes and in LPS-treated mice (41, 42). Despite the fact that Tpl2 inhibition might also be beneficial in treating autoimmunity driven by IFN-γ and IL-17, our data suggest that targeting Tpl2 might attenuate Th1 differentiation and impair T cell responses to infection. Clearly, further studies are needed to give a more complete understanding of the risks versus benefits of therapeutic Tpl2 blockade and the consequences in terms of T cell function.
MATERIALS AND METHODS
Cytokines, antibodies, and reagents
Human IL-2 was provided by C. Reynolds (National Cancer Institute, Frederick, MD). All other cytokines were purchased from R&D Systems. The following antibodies were purchased: anti–IFN-γ, IL-4 (BD), anti-actin, T-bet, and Gata3 (Santa Cruz Biotechnology, Inc.). PHA was purchased from Sigma-Aldrich. Anti-CD3 and -CD28 antibodies used to activate T cells were purchased from BD.
Cell culture and cytokine stimulation
Human cells.
Human cells were cultured in complete RPMI (RPMI 1640 supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg/ml streptomycin, and 2.5 μg/ml fungizone). Because resting T cells do not express IL-12R, PBMC from healthy adult donors (Department of Transfusion Medicine, Clinical Center, National Institutes of Health) were activated by culturing for 72 h in complete RPMI containing 1 μg/ml PHA, followed by an additional 24 h in complete medium containing 40 U/ml IL-2 to maximize IL-12R expression (43). Before cytokine stimulation, cells (>95% CD3+) were washed with CO2-acidified RPMI and rested overnight in serum-free RPMI supplemented with 1% BSA. The cells were then stimulated with cytokines for the indicated times. For T cell differentiation experiments, naive cord blood CD4+ cells (Cambrex) were cultured in complete RPMI containing 50 U/ml IL-2 with either 20 ng/ml IL-12 and 20 μg/ml anti–IL-4 for Th1 differentiation, or 25 ng/ml IL-4 and 20 μg/ml anti–IFN-γ for Th2 differentiation.
Mice and mouse cells.
All animal experiments were performed according to the National Institutes of Health guidelines for laboratory animals and were approved by the National Institute of Arthritis and Musculoskeletal and Skin Diseases Animal Care and Use Committee. Mice were housed in a specific pathogen-free facility. Mouse CD4+ T cells were purified from single-cell suspensions of mouse splenocytes and LN using CD4 microbeads (positive selection) or CD4+ T cell isolation kit (negative selection) and the AutoMACS cell separation system (Miltenyi Biotec). All mouse cells were cultured in complete mouse medium (complete RPMI supplemented with 10 mM Hepes, 1 mM sodium pyruvate, and 50 μM β-mercaptoethanol). For IL-12 stimulations, cells were first activated by culturing on flasks that had been precoated with anti-CD3 and -CD28 (5 μg/ml PBS each) for 3 d in the presence of 40 U/ml IL-2 to induce IL-12 receptor expression. Cells were then washed and rested in complete mouse medium for 5 h before restimulation with IL-12 and various other cytokines. Secreted cytokines were measured from cell culture supernatants by cytokine bead array (BD) or ELISA (R&D Systems).
For T helper cell polarization experiments, naive CD44loCD62Lhi CD4+ T cells were purified by FACS sorting, and 4 × 105 cells/500 μl of media were plated on 24-well plates that had been precoated with 5 μg/ml of anti-CD3 and 5 μg/ml of anti-CD28 (Th0 condition). For Th1 conditions, 10 μg/ml anti–IL-4 and 20 ng/ml of exogenous IL-12 were also added. For Th2 conditions, 10 μg/ml of anti–IL-12, 10 mg/ml of anti–IFN-γ, and 40 ng/ml of exogenous IL-4 were added. For Th17 conditions, 10 μg/ml of anti–IL-4 and 10 μg/ml of anti-IFN-γ, along with 10 ng/ml IL-6 and 5 ng/ml TGF-β, were added. Cells were cultured for 3 d and transferred into T75 flasks containing 15 ml of medium with 40 U/ml IL-2 alone (Th0) or in combination with IL-12 (Th1) or IL-4 (Th2) and grown an additional 4 d. Fully polarized Th0, Th1, or Th2 cells were harvested after 7 d culture, counted, and restimulated for IFN-γ production. Th17 cells were analyzed after the initial 3-d culture. For cytokine secretion, 2 × 105 cells/200 μl were stimulated with 5 μg/ml of anti-CD3 and 5 μg/ml of anti-CD28 for 24 h, and IFN-γ in cell culture supernatants was quantified by cytometric bead array. For mRNA analysis, 2 × 106 cells/2 ml were stimulated under the same conditions for 6 h, and IFN-γ mRNA was measured by real-time PCR. For intracellular cytokine staining, 2 × 106 cells/2 ml were stimulated with anti-CD3, anti-CD28, PMA, and ionomycin for 6–8 h and with Golgi transport inhibitor, which was present for the final 4 h of culture. Cells were fixed, permeabilized, and stained using Cytofix/Cytoperm Fixation/Permeabilization kit with GolgiPlug (BD) according to the manufacturer's protocol.
Microarray analysis of IL-12–inducible genes
PBMC were cultured as described in Human cells and left unstimulated or were stimulated with 10 ng/ml IL-12 for 6 h. Total RNA was isolated with the RNeasy Midi kit (QIAGEN). For microarray analysis, samples were further purified with Trizol (Invitrogen), followed by use of the RNeasy Mini kit (QIAGEN) according to the manufacturer's protocols. Biotin-labeled cRNA was synthesized using the High Yield RNA transcript labeling kit (BioArray; Enzo Biochem, Inc.) and hybridized to human genome U133A and U133B microarray chips according to the manufacturer's instructions (Affymetrix). Data from unstimulated or IL-12–stimulated PBMC from seven human donors were obtained. MAS5 software (Affymetrix) was used to analyze the images from each human genome U133A and U133B GeneChip Array and to obtain expression values for each probe set along with corresponding calls of present, marginal, or absent. Genes were judged to be significantly up-regulated if the expression levels for a least one corresponding probe set (as determined by Entrez Gene identifiers) satisfied the following three criteria: (1) a p-value of ≤0.05 from the right-tailed paired Student's t test on the log of the stimulated and control expression levels; (2) a geometric mean fold change of ≥1.7; and (3) at most, one of the calls for the stimulated expression levels failed to be present. Custom software written in the Interactive Data Language (IDL; ITT Visual Information Solutions, Boulder, CO) was used to conduct this analysis. The data discussed in this publication have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus and are accessible through the GEO Series accession no. GSE12839 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE12839).
Analysis of Tpl2 expression
Tpl2 mRNA expression was quantified by Northern blotting and/or real-time PCR. RNA was isolated with the RNeasy Mini kit and reverse transcribed using a first-strand cDNA synthesis kit (Roche). Real-time PCR was performed using the ABI PRISM7700 Sequence Detection System (Applied Biosystems). Analysis of Tpl2, IFN-γ, IL-17, Stat4, and GAPDH mRNA levels was performed using commercially available primer/probe sets (Applied Biosystems). Relative levels of Tpl2 and IFN-γ were determined by normalization to GAPDH levels and are presented as relative to the unstimulated control for each individual experiment, which was arbitrarily designated a value of 1. For Northern blot analysis, 10 μg of total RNA was separated on 1% agarose-glyoxal gel, transferred to nylon membrane, and hybridized to a radiolabeled full-length Tpl2 probe (provided by S. Gutkind, National Institutes of Health/National Institute of Dental and Craniofacial Research, Bethesda, MD). Blots were washed and exposed to film for autoradiography and then stripped and hybridized to a radiolabeled G3DPH probe (Clontech Laboratories, Inc.) as a control for equivalent loading.
ChIP
ChIP was performed as previously described using anti-Stat4 antibody (Santa Cruz Biotechnology, Inc.) for immunoprecipitation (44). DNA binding was quantified by real-time PCR. For amplification of the promoter region of Tpl2, the primers used were 5′-CCTGGTGAGGATGATGAGCA-3′ and 5′-TGAAGCCAGTGCCCCTGTA-3′ and the probe used was 5′-6FAM-TGAGCAGCAACTTAG-3′. For amplification of an intronic region of Tpl2 (intron 5), the primers used were 5′-CACCCCAGGACAGCATAAGC-3′ and 5′-TCCAAATTCCAACAGGGTGC-3′ and the probe used was 5′-6FAM-TGGAGTCCTGAGCACAG-3. For quantitative analysis of Stat4 binding to the IFNG promoter, the primers used for real-time PCR were 5′-TGCTTTCAGAGAATCCCACAAG-3′ and 5′-CGATGAGACAGCCCCGC-3′ and the probe used was 5′-TGGCACAGGTGGGCA-3′.
Tpl2 silencing with siRNA
The target sequence for the Tpl2-specific siRNA was 5′-AAGUCUCUGCUGCUUAGUGGC-3′, which is 106 bp downstream of the Tpl2 open reading frame ATG. This sequence had no sequence similarity to any other gene as determined by a BLAST search. Freshly isolated PBL were cultured overnight in complete RPMI 1640 without stimulation. The cultured cells were washed three times in PBS and then resuspended in T cell Nucleofector solution (Amaxa Biosystems) containing 10 nmol of either a Tpl2-specific siRNA or a scrambled siRNA (Dharmacon Research Inc., Lafayette, CO). The cells were placed in a nucleofection cuvette and electroporated in a Nucleofector instrument (Amaxa Biosystems) using program U-14. Immediately after transfection, the cells were placed in RPMI 1640 containing 1 μg/ml PHA and cultured for 24 h. The cells were then washed and cultured in RPMI with or without 10 ng/ml IL-12 for 24 h.
In vivo challenge with T. gondii
Age-matched female WT and Tpl2 KO mice were challenged i.p. with 20 cysts of the avirulent ME49 strain of T. gondii. In vivo persistence of the local infection was assessed 7 d after infection by microscopic examination of hematoxylin and eosin–stained cytospins of PECs. Serum was collected, and inflammatory cytokines were measured to monitor the acute immune response in vivo. For antigen-specific recall responses, LN were isolated and stimulated at 2 × 106/ml in a volume of 200 μl with 5 μg/ml STAg for 48 h. IFN-γ production was measured by cytometric bead array. The number of cysts within the brains of 5-wk-infected mice was also quantified. In brief, brains were isolated and homogenized by sequential passage through 19- and 21-gauge needles, and cysts were counted microscopically.
To assess the T cell–intrinsic requirement for Tpl2 in resistance to T. gondii, we generated chimeric mice by reconstituting Rag2-deficient mice with ∼10 million purified WT or Tpl2-deficient CD4+ T cells. Transferred cells were allowed to homeostatically expand for >1 wk before i.p. challenge of mice with 20 cysts of T. gondii strain ME49. Serum cytokines were assessed at day 7, and mice were subsequently observed for survival for 30 d.
Statistical analysis
Data represent mean ± SD, except where indicated. P-values were determined by one-tailed Student's t test with the null hypothesis that Tpl2 KO have decreased IL-12–induced responses or IFN-γ production where appropriate.
Online supplemental material
Fig. S1 shows the 30 genes most highly induced in human T cells by IL-12. Fig. S2 demonstrates that siRNA knockdown of Tpl2 in human PBMC impairs IL-12–dependent IFN-γ production. Fig. S3 shows that Stat4 dysregulation in Tpl2-deficient T cells is a consequence of defective TCR signaling. Fig. S4 depicts a model for the proposed role of Tpl2 in the regulation of Th1 differentiation. Fig. S5 shows that Tpl2-deficient BM-derived DCs overproduce IL-12p70 and are impaired in IL-10 production. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081461/DC1.
Supplementary Material
Acknowledgments
The authors would like to thank Jim Simone and the flow cytometry core of the National Institute of Arthritis and Musculoskeletal and Skin Diseases Office of Science and Technology for excellent advice and technical service. Additionally, we are grateful to Sandy White (Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases) for animal technical support.
This research was supported, in part, by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. W.T. Watford is supported by National Institutes of Health grant #1 K22 AR53953-01. P. Tsichlis is supported by National Institutes of Health grant #R01 CA095431.
The authors have no conflicting financial interests.
Abbreviations used: ChIP, chromatin immunoprecipitation; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; PEC, peritoneal exudate cell; siRNA, small interfering RNA; STAg, soluble T. gondii antigen.
References
- 1.Agnello, D., C.S. Lankford, J. Bream, A. Morinobu, M. Gadina, J.J. O'Shea, and D.M. Frucht. 2003. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J. Clin. Immunol. 23:147–161. [DOI] [PubMed] [Google Scholar]
- 2.Mowen, K.A., and L.H. Glimcher. 2004. Signaling pathways in Th2 development. Immunol. Rev. 202:203–222. [DOI] [PubMed] [Google Scholar]
- 3.Steinman, L. 2007. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat. Med. 13:139–145. [DOI] [PubMed] [Google Scholar]
- 4.Watford, W.T., M. Moriguchi, A. Morinobu, and J.J. O'Shea. 2003. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 14:361–368. [DOI] [PubMed] [Google Scholar]
- 5.Glimcher, L.H. 2001. Lineage commitment in lymphocytes: controlling the immune response. J. Clin. Invest. 108:s25–s30. [PubMed] [Google Scholar]
- 6.O'Shea, J.J., M. Gadina, and R.D. Schreiber. 2002. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 109:S121–S131. [DOI] [PubMed] [Google Scholar]
- 7.O'Shea, J.J., and W.E. Paul. 2002. Regulation of T(H)1 differentiation–controlling the controllers. Nat. Immunol. 3:506–508. [DOI] [PubMed] [Google Scholar]
- 8.Mattner, F., J. Magram, J. Ferrante, P. Launois, K. Di Padova, R. Behin, M.K. Gately, J.A. Louis, and G. Alber. 1996. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur. J. Immunol. 26:1553–1559. [DOI] [PubMed] [Google Scholar]
- 9.Decken, K., G. Kohler, K. Palmer-Lehmann, A. Wunderlin, F. Mattner, J. Magram, M.K. Gately, and G. Alber. 1998. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 66:4994–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper, A.M., D.K. Dalton, T.A. Stewart, J.P. Griffin, D.G. Russell, and I.M. Orme. 1993. Disseminated tuberculosis in interferon γ gene-disrupted mice. J. Exp. Med. 178:2243–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newport, M.J., C.M. Huxley, S. Huston, C.M. Hawrylowicz, B.A. Oostra, R. Williamson, and M. Levin. 1996. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941–1949. [DOI] [PubMed] [Google Scholar]
- 12.Bacon, C.M., E.F. Petricoin III, J.R. Ortaldo, R.C. Rees, A.C. Larner, J.A. Johnston, and J.J. O'Shea. 1995. Interleukin 12 induces tyrosine phosphorylation and activation of STAT4 in human lymphocytes. Proc. Natl. Acad. Sci. USA. 92:7307–7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karaghiosoff, M., H. Neubauer, C. Lassnig, P. Kovarik, H. Schindler, H. Pircher, B. McCoy, C. Bogdan, T. Decker, G. Brem, et al. 2000. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 13:549–560. [DOI] [PubMed] [Google Scholar]
- 14.Neubauer, H., A. Cumano, M. Muller, H. Wu, U. Huffstadt, and K. Pfeffer. 1998. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 93:397–409. [DOI] [PubMed] [Google Scholar]
- 15.Visconti, R., M. Gadina, M. Chiariello, E.H. Chen, L.F. Stancato, J.S. Gutkind, and J.J. O'Shea. 2000. Importance of the MKK6/p38 pathway for interleukin-12-induced STAT4 serine phosphorylation and transcriptional activity. Blood. 96:1844–1852. [PubMed] [Google Scholar]
- 16.Zhang, S., and M.H. Kaplan. 2000. The p38 mitogen-activated protein kinase is required for IL-12-induced IFN-gamma expression. J. Immunol. 165:1374–1380. [DOI] [PubMed] [Google Scholar]
- 17.Rogge, L., E. Bianchi, M. Biffi, E. Bono, S.Y. Chang, H. Alexander, C. Santini, G. Ferrari, L. Sinigaglia, M. Seiler, et al. 2000. Transcript imaging of the development of human T helper cells using oligonucleotide arrays. Nat. Genet. 25:96–101. [DOI] [PubMed] [Google Scholar]
- 18.Patriotis, C., A. Makris, S.E. Bear, and P.N. Tsichlis. 1993. Tumor progression locus 2 (Tpl-2) encodes a protein kinase involved in the progression of rodent T-cell lymphomas and in T-cell activation. Proc. Natl. Acad. Sci. USA. 90:2251–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumitru, C.D., J.D. Ceci, C. Tsatsanis, D. Kontoyiannis, K. Stamatakis, J.H. Lin, C. Patriotis, N.A. Jenkins, N.G. Copeland, G. Kollias, and P.N. Tsichlis. 2000. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 103:1071–1083. [DOI] [PubMed] [Google Scholar]
- 20.Hatziapostolou, M., C. Polytarchou, D. Panutsopulos, L. Covic, and P.N. Tsichlis. 2008. Proteinase-activated receptor-1-triggered activation of tumor progression locus-2 promotes actin cytoskeleton reorganization and cell migration. Cancer Res. 68:1851–1861. [DOI] [PubMed] [Google Scholar]
- 21.Salmeron, A., T.B. Ahmad, G.W. Carlile, D. Pappin, R.P. Narsimhan, and S.C. Ley. 1996. Activation of MEK-1 and SEK-1 by Tpl-2 proto-oncoprotein, a novel MAP kinase kinase kinase. EMBO J. 15:817–826. [PMC free article] [PubMed] [Google Scholar]
- 22.Chiariello, M., M.J. Marinissen, and J.S. Gutkind. 2000. Multiple mitogen-activated protein kinase signaling pathways connect the cot oncoprotein to the c-jun promoter and to cellular transformation. Mol. Cell. Biol. 20:1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patriotis, C., A. Makris, J. Chernoff, and P.N. Tsichlis. 1994. Tpl-2 acts in concert with Ras and Raf-1 to activate mitogen-activated protein kinase. Proc. Natl. Acad. Sci. USA. 91:9755–9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsatsanis, C., C. Patriotis, and P.N. Tsichlis. 1998. Tpl-2 induces IL-2 expression in T-cell lines by triggering multiple signaling pathways that activate NFAT and NF-kappaB. Oncogene. 17:2609–2618. [DOI] [PubMed] [Google Scholar]
- 25.Das, S., J. Cho, I. Lambertz, M.A. Kelliher, A.G. Eliopoulos, K. Du, and P.N. Tsichlis. 2005. Tpl2/cot signals activate ERK, JNK, and NF-kappaB in a cell-type and stimulus-specific manner. J. Biol. Chem. 280:23748–23757. [DOI] [PubMed] [Google Scholar]
- 26.Oppmann, B., R. Lesley, B. Blom, J.C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 13:715–725. [DOI] [PubMed] [Google Scholar]
- 27.Tsatsanis, C., C. Patriotis, S.E. Bear, and P.N. Tsichlis. 1998. The Tpl-2 protooncoprotein activates the nuclear factor of activated T cells and induces interleukin 2 expression in T cell lines. Proc. Natl. Acad. Sci. USA. 95:3827–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsatsanis, C., K. Vaporidi, V. Zacharioudaki, A. Androulidaki, Y. Sykulev, A.N. Margioris, and P.N. Tsichlis. 2008. Tpl2 and ERK transduce antiproliferative T cell receptor signals and inhibit transformation of chronically stimulated T cells. Proc. Natl. Acad. Sci. USA. 105:2987–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yap, G.S., and A. Sher. 1999. Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiology. 201:240–247. [DOI] [PubMed] [Google Scholar]
- 30.Sugimoto, K., M. Ohata, J. Miyoshi, H. Ishizaki, N. Tsuboi, A. Masuda, Y. Yoshikai, M. Takamoto, K. Sugane, S. Matsuo, et al. 2004. A serine/threonine kinase, Cot/Tpl2, modulates bacterial DNA-induced IL-12 production and Th cell differentiation. J. Clin. Invest. 114:857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lund, R.J., Z. Chen, J. Scheinin, and R. Lahesmaa. 2004. Early target genes of IL-12 and STAT4 signaling in th cells. J. Immunol. 172:6775–6782. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen, K.B., W.T. Watford, R. Salomon, S.R. Hofmann, G.C. Pien, A. Morinobu, M. Gadina, J.J. O'Shea, and C.A. Biron. 2002. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 297:2063–2066. [DOI] [PubMed] [Google Scholar]
- 33.O'Sullivan, A., H.C. Chang, Q. Yu, and M.H. Kaplan. 2004. STAT4 is required for interleukin-12-induced chromatin remodeling of the CD25 locus. J. Biol. Chem. 279:7339–7345. [DOI] [PubMed] [Google Scholar]
- 34.Pesu, M., L. Muul, Y. Kanno, and J.J. O'Shea. 2006. Proprotein convertase furin is preferentially expressed in T helper 1 cells and regulates interferon gamma. Blood. 108:983–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ballester, A., R. Tobena, C. Lisbona, V. Calvo, and S. Alemany. 1997. Cot kinase regulation of IL-2 production in Jurkat T cells. J. Immunol. 159:1613–1618. [PubMed] [Google Scholar]
- 36.Grenningloh, R., B.Y. Kang, and I.C. Ho. 2005. Ets-1, a functional cofactor of T-bet, is essential for Th1 inflammatory responses. J. Exp. Med. 201:615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kane, L.P., P.G. Andres, K.C. Howland, A.K. Abbas, and A. Weiss. 2001. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat. Immunol. 2:37–44. [DOI] [PubMed] [Google Scholar]
- 38.Kane, L.P., M.N. Mollenauer, Z. Xu, C.W. Turck, and A. Weiss. 2002. Akt-dependent phosphorylation specifically regulates Cot induction of NF-kappa B-dependent transcription. Mol. Cell. Biol. 22:5962–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kontoyiannis, D., G. Boulougouris, M. Manoloukos, M. Armaka, M. Apostolaki, T. Pizarro, A. Kotlyarov, I. Forster, R. Flavell, M. Gaestel, et al. 2002. Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn's-like inflammatory bowel disease. J. Exp. Med. 196:1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moller, B., and P.M. Villiger. 2006. Inhibition of IL-1, IL-6, and TNF-alpha in immune-mediated inflammatory diseases. Springer Semin. Immunopathol. 27:391–408. [DOI] [PubMed] [Google Scholar]
- 41.Gavrin, L.K., N. Green, Y. Hu, K. Janz, N. Kaila, H.Q. Li, S.Y. Tam, J.R. Thomason, A. Gopalsamy, G. Ciszewski, et al. 2005. Inhibition of Tpl2 kinase and TNF-alpha production with 1,7-naphthyridine-3-carbonitriles: synthesis and structure-activity relationships. Bioorg. Med. Chem. Lett. 15:5288–5292. [DOI] [PubMed] [Google Scholar]
- 42.Hu, Y., N. Green, L.K. Gavrin, K. Janz, N. Kaila, H.Q. Li, J.R. Thomason, J.W. Cuozzo, J.P. Hall, S. Hsu, et al. 2006. Inhibition of Tpl2 kinase and TNFalpha production with quinoline-3-carbonitriles for the treatment of rheumatoid arthritis. Bioorg. Med. Chem. Lett. 16:6067–6072. [DOI] [PubMed] [Google Scholar]
- 43.Wu, C.Y., M. Gadina, K. Wang, J. O'Shea, and R.A. Seder. 2000. Cytokine regulation of IL-12 receptor beta2 expression: differential effects on human T and NK cells. Eur. J. Immunol. 30:1364–1374. [DOI] [PubMed] [Google Scholar]
- 44.Morinobu, A., Y. Kanno, and J.J. O'Shea. 2004. Discrete roles for histone acetylation in human T helper 1 cell-specific gene expression. J. Biol. Chem. 279:40640–40646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.