Abstract
The role of specialized follicular helper T (TFH) cells in the germinal center has become well recognized, but it is less clear how effector T cells govern the extrafollicular response, the dominant pathway of high-affinity, isotype-switched autoantibody production in the MRL/MpJ-Faslpr (MRLlpr) mouse model of lupus. MRLlpr mice lacking the Icos gene have impaired extrafollicular differentiation of immunoglobulin (Ig) G+ plasma cells accompanied by defects in CXC chemokine receptor (CXCR) 4 expression, interleukin (IL) 21 secretion, and B cell helper function in CD4 T cells. These phenotypes reflect the selective loss of a population of T cells marked by down-regulation of P-selectin glycoprotein ligand 1 (PSGL-1; also known as CD162). PSGL-1lo T cells from MRLlpr mice express CXCR4, localize to extrafollicular sites, and uniquely mediate IgG production through IL-21 and CD40L. In other autoimmune strains, PSGL-1lo T cells are also abundant but may exhibit either a follicular or extrafollicular phenotype. Our findings define an anatomically distinct extrafollicular population of cells that regulates plasma cell differentiation in chronic autoimmunity, indicating that specialized humoral effector T cells akin to TFH cells can occur outside the follicle.
CD4 T cells control several aspects of immune responses, and there is growing recognition that individual Th functions are mediated by distinct subsets. This paradigm is particularly clear for the peripheral tissue effector lineages Th1, Th2, and Th17, which each control a distinct class of innate immune mediators (1). These inflammatory effectors can be distinguished from T cells that perform the other essential and historically emblematic Th function, the regulation of antibody responses. However, our insight into the nature of such humoral effectors is relatively limited. The classical model that Th2 cells are responsible for antibody production (2) has been criticized for failing to account for the production of the Th1-associated isotypes IgG2a and IgG2b (3). Further, although mice that lack the IL-4R signaling molecule STAT6 have severe defects in peripheral Th2 responses, they produce normal levels of the IgG isotypes upon immunization (4), indicating that Th2 development is dispensable even for IgG1 production.
More recently, consideration of the anatomy of antibody responses has provided insights into the specialized nature of B Th cells, although a comprehensive description of such CD4 Th subsets, which we refer to generally as humoral effectors, has yet to be achieved. The initial interactions between antigen-engaged CD4 T cells and B cells occur at the border of the T cell zone and follicle (5), and the early effects of Th cytokines can be observed there with the appearance of Ig heavy chain germline transcripts, the precursors to class switch recombination (CSR) (6, 7). Subsequently, subsets of B cells and Th cells migrate to the follicle and ultimately form the germinal center (GC), from which high-affinity, class-switched, and long-lived plasma cells and memory B cells emerge (5). Localization of T cells around the GC light zone as well as an ongoing CD40L requirement for affinity maturation in the GC indicate that selection of mutant B cells is a critical function of T cell help at that site (8, 9). More recent work has provided the significant insight that this T cell function is mediated by a distinct follicular helper T (TFH) cell subset (10–12).
Characterization of the follicle-resident TFH cell subset in human tonsil has been facilitated by the identification of the surface markers CXCR5 and CD57 (12). More recently, TFH cell differentiation has been achieved in vitro, allowing their further characterization in the mouse (13). TFH cells do not produce Th cytokines such as IFN-γ, IL-4, or IL-17 but likely mediate their function via CD40L and IL-21 (10–16). Although multiple functions have been ascribed to IL-21 in vitro, data from in vivo experiments indicate that it is critical for IgG production. IL-21R–deficient mice have decreased IgG1, IgG2b, and IgG3 levels, and IL-21R/IL-4 double-knockout mice have defects in the production of all switched isotypes, including IgG2a, although neither cytokine is necessary for the production of this isotype on its own (17). In vitro, IL-21 promotes B cell apoptosis in the presence of anti-IgM, though death can be rescued by anti-CD40 signaling (18, 19). Exogenous IL-21 promotes CSR and IgG secretion in vivo and in vitro and is a potent inducer of B lymphocyte–induced maturation protein 1 (16, 17, 20), and the ability of human T cells to induce Ig secretion is largely dependent on IL-21 (16, 21). These data are consistent with a role for IL-21 in TFH cell–mediated centrocyte selection and differentiation into plasma cells. Still, it is not yet clear if IL-21 functions primarily within or outside the GC, or both.
How Th cells promote antibody-forming cell (AFC) differentiation outside the follicle is poorly defined. In addition to seeding the GC, a subset of B cells at the interface of the T cell zone and follicle up-regulate CD138, migrate to the red pulp border, and form the extrafollicular focus. At this site, clumps of proliferative AFCs, or plasmablasts, undergo CSR and differentiation into long-lived plasma cells (6, 22, 23). Because T cell localization to the extrafollicular focus has not been reported, current models do not include a role for local T cell help in those responses (24). Instead, Th effects are thought to be exerted only before plasmablast migration from the T zone, though this idea has not been definitively demonstrated.
Clarifying the mechanics of the extrafollicular response is critical for understanding this important pathway for the production of autoantibodies. In normal mice, self-reactivity in B cells results in follicular exclusion and nonresponsiveness (25, 26), and provision of T cell help to such autoreactive cells leads to extrafollicular AFC differentiation rather than GC formation (27). A similar pattern of activation occurs spontaneously in some autoimmune strains (27, 28), the most well-characterized example being the MRL/MpJ-Faslpr (MRLlpr) model. In the spleens of MRLlpr mice, CXCR4+ plasmablasts form defined proliferative extrafollicular foci at the border of the T cell zone before migrating to the red pulp, in the absence of GC development (29–32). This pattern of activation is in stark contrast to autoimmune sanroque mice, in which self-reactive TFH cells promote spontaneous GC development resulting in lupus-like pathology (33). Although IgG autoantibody production in MRLlpr mice is clearly T cell dependent (34, 35), the details of this process are poorly defined. In addition to CSR, the extrafollicular focus of MRLlpr mice is also a site for somatic hypermutation (30), a process that, in mammals, is dependent on T cell help (36). Importantly, how T cells support this GC-like activity outside the follicle is unclear. Recently, IL-21 has been shown to play a role in IgG autoantibody production and pathology in MRLlpr mice (37), though the relevant source of this cytokine remains loosely defined.
To investigate the mechanisms of T cell help for the extrafollicular autoantibody response genetically, we took advantage of the well-recognized requirement for the molecule inducible T cell co-stimulator (ICOS) for Th cell differentiation and IgG antibody responses. Icos-deficient (Icos−/−) animals have marked defects in GCs (38) and CSR to both IFN-γ– and IL-4–dependent isotypes (39). A primary reason for these defects is that the development of TFH cells is ICOS dependent (13, 40, 41). Importantly, however, Icos−/− animals also have defects in IgG production early after immunization (42), suggesting that ICOS also plays a role in the extrafollicular response.
In this study, we find that Icos deficiency in MRLlpr mice causes an ontogenetic blockade of a population of effector T cells that down-regulate the surface glycoprotein P-selectin glycoprotein ligand 1 (PSGL-1). In addition to expressing CD40L, PSGL-1lo T cells are the sole producers of IL-21 and are uniquely able to induce IgG secretion in B cells in an IL-21– and CD40L–dependent manner. In their absence, plasmablast expression of activation-induced cytidine deaminase (AID) and extrafollicular development of IgG+ plasma cells are impaired. A substantial fraction of PSGL-1lo T cells express CXCR4 and localize to the extrafollicular sites in both MRLlpr and other autoimmune mice, and thus constitute a population of Th cells anatomically distinct from TFH cells.
RESULTS
ICOS co-stimulation promotes isotype switching in the extrafollicular response
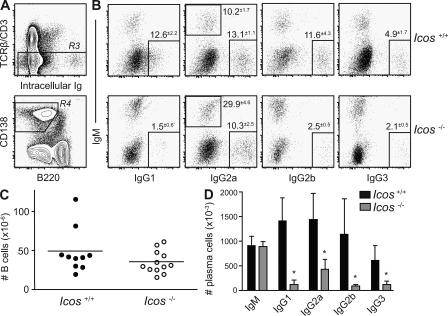
To assess the requirement for ICOS-dependent T cell help for the autoimmune extrafollicular response, we used intracellular staining to determine the number and isotype usage of plasma cells in the spleens of MRLlpr mice lacking the Icos gene. Spleen cells were stained for surface antigens and then fixed, permeabilized, and stained for both intracellular IgM and one of the four IgG isotypes. Plasma cells were identified by gating out TCRβ/CD3+ and autofluorescent cells (R3), and then gating on the B220lo CD138+ population (R4; Fig. 1 A), and region R4 was analyzed for intracellular Ig expression. The percentage of plasma cells staining positive for each isotype (Fig. 1 B) as well as their absolute number (Fig. 1 D) are shown. Both groups had similar numbers of B cells (Fig. 1 C). Although Icos-deficient animals generated equivalent numbers of IgM-secreting cells as their age-matched controls, they failed to efficiently generate plasma cells expressing IgG1, IgG2a, IgG2b, and IgG3 (Fig. 1, B and D). These data were in close agreement with serum anti–double-stranded DNA antibody levels measured by isotype-specific ELISA (unpublished data), indicating that both Th1- and Th2-associated isotypes are ICOS dependent in this model.
Figure 1.
ICOS is required for generation of IgG+ plasma cells. (A) Plasma cells were identified in the spleens of 23-wk-old Icos+/+ and Icos−/− MRLlpr animals (n = 3–5 for two experiments) by expression of CD138 and down-regulation of B220 (R4) after exclusion of T cells and autofluorescent cells (R3). (B) The percentage of plasma cells within R4 producing each antibody isotype are shown, as determined by intracellular Ig staining. Mean gate frequencies ± SEM are shown. (C) Absolute number of CD90− CD19+ B cells in the spleen (n = 10–12). Horizontal bars indicate means. (D) Absolute numbers of plasma cells were calculated by multiplying the fraction of live single cells positive for each isotype by the total cell number. Data are expressed as means ± SEM. *, P ≤ 0.05 versus Icos+/+ mice using the Mann-Whitney test.
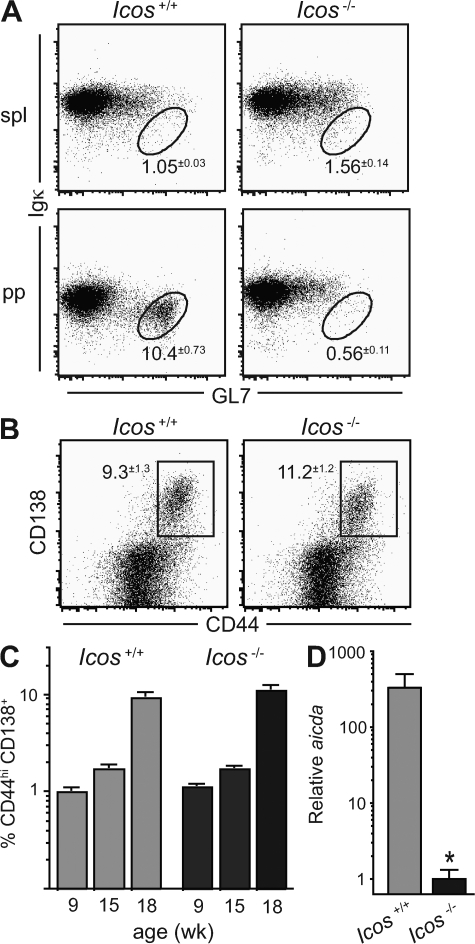
Previous work has established that GCs are ICOS dependent (38, 39). To confirm previous reports that spontaneous GC formation is minimal in the spleens of MRLlpr mice (29–32), we determined the frequency of GL7hi Igκdull B cells, a surface phenotype that corresponds to normal GC B cells in the response to nitrophenyl (NP)– chicken γ globulin (CGG; unpublished data). Although gut antigen–driven GC cells in Peyer's patches were abundant and robustly ICOS dependent (Fig. 2 A), as expected, we indeed observed very few GC B cells in the spleens of MRLlpr mice (Fig. 2 A). The GL7+ Igκhi cells we observed and the few events falling in the GC gate are not reduced by Icos deficiency, and are unlikely to be true GC cells. In contrast, the spontaneous extrafollicular response is robust in these mice (30, 31). To address whether initiation of the extrafollicular response requires ICOS-dependent T cell help, we analyzed the kinetics of early plasmablast development by gating on CD19 and determining the percentage of B cells expressing CD44 and CD138 (43). To our surprise, B cells in Icos-deficient animals up-regulated CD44 and CD138 with normal kinetics (Fig. 2, B and C), indicating that the loss of IgG+ plasma cells is not caused by reduced plasmablast formation.
Figure 2.
AID expression in extrafollicular plasmablasts, but not their formation, requires ICOS. (A) Percentage of GL7hi Igκlo GC B cells, gated on CD19, in the spleens (spl) and Peyer's patches (pp) of Icos+/+ and Icos−/− MRLlpr mice (n = 4–5 for two experiments). (B) Percentage of CD44+ cells bearing the early plasmablast marker CD138 (reference 43) in the TCRβ− CD19+ B cell compartment of 18-wk-old Icos+/+ and Icos−/− mice (n = 4–6 for three experiments). (C) Percentage of CD90− CD19+ B cells expressing CD138 and high levels of CD44 at the indicated ages (n = 4–14 per age group; three pooled experiments are shown). (D) Plasmablasts were sorted from Icos+/+ and Icos−/− mice as gated in B, and expression of aicda mRNA encoding AID was determined by quantitative PCR (two pooled mice per experiment and two experimental replicates shown). All data are displayed as means ± SEM. *, P ≤ 0.05 versus Icos+/+ mice using the Student's t test.
The normal induction of plasmablasts in knockout animals indicated that ICOS must promote IgG+ plasma cell formation farther downstream in the extrafollicular response, possibly through induction of CSR. Because a limiting step in CSR is the expression of AID, we used quantitative PCR to measure the expression of aicda, the mRNA encoding AID, in sorted TCRβ− CD19+ CD44hi CD138+ plasmablasts. We detected substantial expression of aicda in Icos+/+ plasmablasts (∼40% of the level in GC cells from NP-CGG–immunized B6 mice; not depicted), and this expression level was strikingly reduced by ∼300-fold in Icos−/− plasmablasts (Fig. 2 D), indicating that ICOS is required for the induction of CSR in the extrafollicular pathway. Because we did not observe a concomitant increase in IgM+ plasma cells with the decrease in post-switch cells in knockout animals (Fig. 1 D), it is likely that ICOS is also involved in the proliferation and/or survival of the cells that do undergo CSR.
ICOS is required for Th function and CXCR4 expression
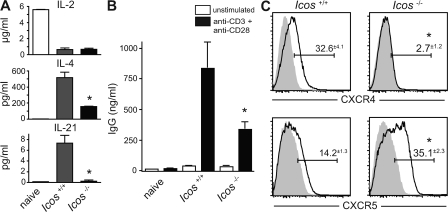
The lack of IgG+ plasma cells in mice lacking a CD4 T cell co-stimulator indicated a T cell defect ipso facto, so we proceeded to analyze the production of cytokines involved in the provision of B cell help, including the induction of CSR. To avoid the confounding relative increase in naive cells in the Icos−/− CD4 compartment (unpublished data), we sorted B220− TCRβ+ CD4+ CD44hi CD62L− effector T cells from knockout and control mice, cultured them with PMA and ionomycin for 24 h, and measured cytokine levels in the supernatants. Icos+/+ naive cells were sorted from young MRLlpr mice and stimulated as a negative control, and robust IL-2 production verified their viability and function (Fig. 3 A). Although IL-2 production by effector T cells was unaffected by Icos deletion, we observed a significant defect in the production of the B cell growth and switch factor IL-4 (Fig. 3 A). Production of other cytokines, including IFN-γ, TNF-α, IL-5, and IL-10, was also substantially reduced (not depicted), reinforcing the view suggested by the plasma cell isotype data (Fig. 1 D) that ICOS does not promote Th skewing in mouse lupus but effector cytokine secretion in general.
Figure 3.
Icos−/− effector T cells have defects in cytokine production, helper function, and CXCR4, but not CXCR5, expression. (A) Sorted TCRβ+ CD4+ B220− CD44hi CD62Llo effector T cells from Icos+/+ and Icos−/− mice were cultured with PMA and ionomycin for 24 h, and the indicated cytokines were measured in the supernatants. Icos+/+ CD44lo CD62Lhi naive T cells were stimulated as a control (representative of two experiments). (B) Effector CD4 cells were sorted as in A and cultured with sorted CD19+ B220+ CD90− CD44lo naive B cells from 8-wk-old mice in the presence or absence of anti-CD3 and anti-CD28. After 96 h, secreted IgG was measured in the culture supernatant (representative of three experiments). (C) Expression of CXCR4 and CXCR5 on CD4+ B220− T cells from 20-wk-old mice was determined by FACS (n = 3 for three experiments). The continuous line indicates specific staining, and the gray shading indicates isotype control. Values indicate the percent positive minus isotype control. All data are presented as means ± SEM. *, P ≤ 0.05 versus Icos+/+ using the Student's t test.
Because IL-4−/−IL-13−/− mice are still capable of mounting an extrafollicular IgG1 response to NP-CGG (7), however, it seemed unlikely that the modest decrease in IL-4 production was solely responsible for the robust reduction in IgG1 AFCs that we observed. Another cytokine that can promote switching to IgG1 is IL-21 (17), and strikingly, the production of IL-21 was abolished in Icos-deficient cells (Fig. 3 A). IL-21 is a Th cytokine that signals through a γc chain cytokine receptor family member, and its role in B cell plasmagenesis and class switching has recently emerged (44). To directly assess the capacity of Icos−/− CD4 T cells to induce B cell class switching in culture, we sorted CD62Llo effectors, as described in the previous paragraph, and co-cultured them with sorted CD19+ B220hi CD44lo naive B cells from young MRLlpr mice, with or without stimulation by anti-CD3 and anti-CD28 antibodies. Under these conditions, Icos−/− effectors were significantly less efficient at inducing B cell class switching as assessed by ELISA on the culture supernatants (Fig. 3 B), consistent with their impaired production of helper cytokines.
The inability of Icos−/− effectors to secrete IL-21 and efficiently promote IgG secretion was reminiscent of the finding that ICOS is required for TFH cell development in normal mice (40). Because the spleens of MRLlpr mice are nearly devoid of GCs, however, the defect we observed in extrafollicular class switching was conceptually inconsistent with a loss of TFH cells. Therefore, we hypothesized that specialized humoral effector T cells may also act in the extrafollicular focus. As TFH cells express CXCR5 in similar fashion to follicular B cells, thus colocalizing to their site of interaction, we speculated that a population of Th cells might colocalize with extrafollicular plasmablasts through expression of CXCR4. Therefore, we analyzed CXCR4 and CXCR5 expression on B220− CD4+ splenocytes and found that, indeed, a substantial percentage of CD4 T cells expressed CXCR4. Further, this CXCR4+ population was absolutely dependent on ICOS co-stimulation (Fig. 3 C). Conversely, CXCR5 expression was not only ICOS-independent but in fact elevated in its absence (Fig. 3 C). To interpret these data, it is important to consider that CXCR5 is not only expressed on TFH cells but is up-regulated on almost all CD4 T cells shortly after activation, mediating their initial localization to the T/B interface (45). Because CXCR5 expression in MRLlpr mice occurs largely on CD62L+ effector cells (see Fig. 5), the accumulation of CXCR5+ cells in Icos−/− mice likely represents effector cells in an early state of differentiation rather than TFH cells. Thus, these data suggest that this early migration program is unimpaired in the absence of ICOS but that further differentiation along an extrafollicular pathway is arrested. In combination with the defect in helper function in vitro and reduction in AID expression in vivo, the loss of CXCR4 expression suggested strongly to us that ICOS is required for the development of a TFH cell–like CD4 effector subset that acts on plasmablasts.
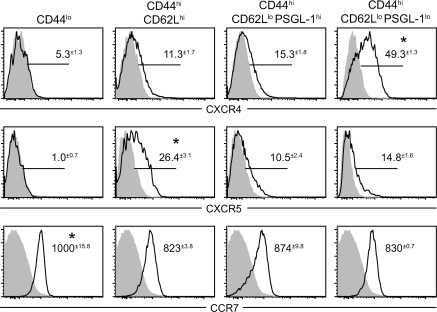
Figure 5.
PSGL-1lo T cells have an extrafollicular chemokine receptor profile. Expression levels of the chemokine receptors CXCR4, CXCR5, and CCR7 were compared between naive CD4 T cells and three subsets of CD44hi effector cells gated according to the expression of CD62L and PSGL-1, as in Fig. 4 C (n = 3 for three experiments). Specific staining is indicated by the solid line, and isotype control staining is indicated by the gray shading. For CXCR4 and CXCR5, values indicate the mean percent positive minus isotype control ± SEM. For CCR7, values are the mean fluorescence intensity ± SEM. *, P < 0.05 compared with all other groups using the Student's t test.
Extrafollicular helper cells can be identified by down-regulation of PSGL-1
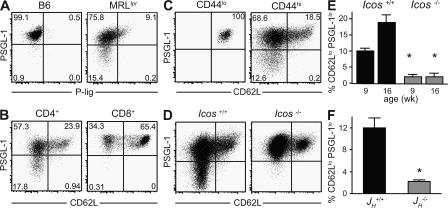
A parallel approach that we used to assess Th cell development in Icos−/− mice was to stain for other surface markers associated with Th cells in antibody responses. In the ovalbumin response, effector cells have been separated according to CD62L expression and P-selectin binding such that P-selectin ligand+ (P-lig+) cells mediate delayed-type hypersensitivity, whereas P-lig− CD62Llo cells induce IgG1 (46). P-lig, which is detected using a P-selectin–Ig fusion protein, is generated by carbohydrate modification of PSGL-1, whereas unmodified PSGL-1 expression does not confer P-selectin binding. Because PSGL-1 has been reported to be constitutively expressed by T cells, we were surprised to find that among CD4 T cells that were negative for P-lig, a minor subset also down-regulated the PSGL-1 scaffold altogether (Fig. 4 A). Down-regulation of PSGL-1 was limited to the CD44hi and CD62Llo subset (Fig. 4 C). Thus, CD44hi antigen–experienced CD4 T cells can be subdivided into three surface phenotypes according to the expression of CD62L and PSGL-1. CD8 T cells, in contrast, remain uniformly PSGL-1hi (Fig. 4 B).
Figure 4.
A novel population of CD4 T cells marked by PSGL-1 down-regulation requires both ICOS and B cells for their development. (A) B220− TCRβ+ CD4+ T cells from B6 and MRLlpr mice were stained with anti-PSGL-1 and a P-selectin–Ig fusion protein to detect P-lig. (B and C) TCRβ+ B220− T cells were gated on CD4 or CD8, and CD4+ cells were separated according to CD44 expression, as indicated, and the expression of PSGL-1 and CD62L was determined on these populations. (D) Expression of PSGL-1 and CD62L on CD4+ B220− T cells from Icos+/+ and Icos−/− mice was determined by FACS. Percentages are shown. (E) The percentages of CD4 T cells that had down-regulated PSGL-1 at 9 and 16 wk of age are shown (n = 4–7). (F) The percentage of PSGL-1lo T cells in 25-wk-old B cell–deficient JH−/− MRLlpr mice was compared with age-matched JH+/+ controls (n = 3). A–D show representative data from a minimum of five animals. Data in E and F are expressed as means ± SEM. *, P ≤ 0.05 compared with age-matched controls using the Student's t test.
PSGL-1lo CD4 cells increase in frequency in MRLlpr mice as disease progresses, reaching nearly 20% of the CD4 T cell compartment by 16 wk of age (Fig. 4 D). When we analyzed PSGL-1 expression on Icos−/− T cells, we found a marked reduction in the frequency of PSGL-1lo cells (Fig. 4, D and E) parallel to the loss of CXCR4 described in Fig. 3 C. Interestingly, the development of PSGL-1lo cells was also dependent on B cells, as MRLlpr mice bearing an Ig heavy chain deletion (JH−/−) have a significant reduction in their numbers (Fig. 4 F). Further, CXCR4 expression was almost exclusively confined to the PSGL-1lo CD62Llo population, and these cells expressed low levels of CXCR5 (Fig. 5). The expression of CXCR5 that we observed in the CD62L+ population is consistent with recent activation (45) rather than a TFH phenotype, which is CD62Llo (46). Although CXCR4, a coreceptor for HIV, is expressed broadly on human CD4 T cells, including GC T cells (47), it has not been well characterized in mouse T cells. Conditional deletion of CXCR4 in T cells does not affect GC formation, indicating that it is not critical for TFH cell function (48); the T cell–specific requirement for CXCR4 in the extrafollicular response was not addressed in that study.
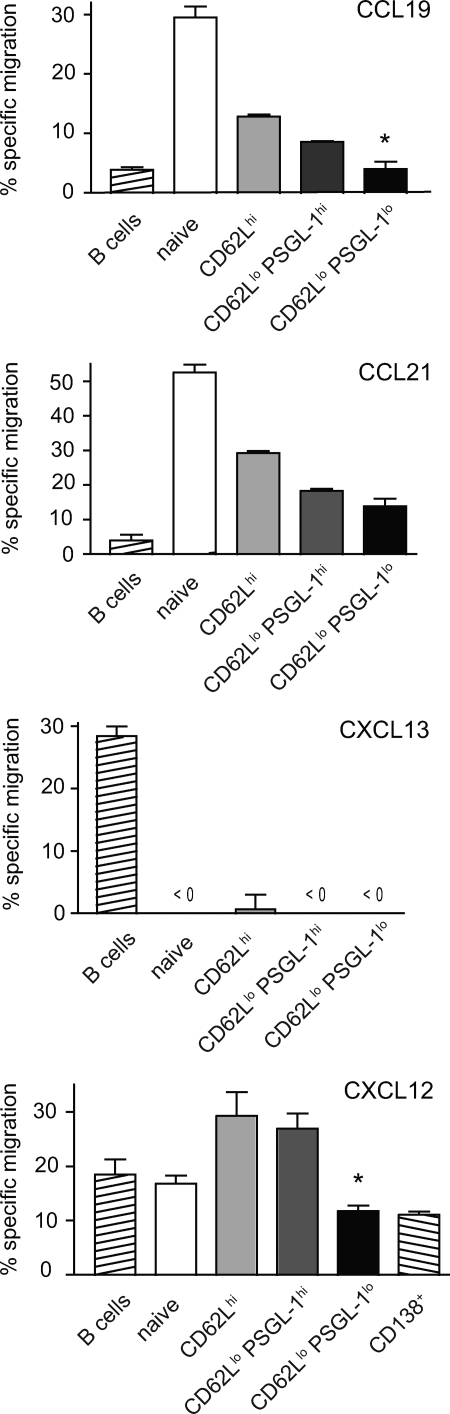
Lymphocyte homing is not mediated solely by the acquisition of new chemokine receptors but also by modulation of expression or activity of competing receptors. Although nearly all CD4 T cells up-regulate CXCR5 after activation, CCR7 activity prevents the majority of these cells from localizing to the follicle (49). CCR7 levels were reduced on all CD44hi subsets, including PSGL-1lo cells, compared with naive cells (Fig. 5), though not to a striking extent. PSGL-1 itself has recently been implicated in binding and chemotaxis to CCR7 ligands (50). To evaluate the chemotactic responses of PSGL-1lo T cells to CCR7, CXCR5, and CXCR4 ligands, we performed transmigration assays in vitro. As expected, all effector T cell subsets were less responsive to the CCR7 ligands CCL19 and CCL21 than their naive counterparts, with the PSGL-1lo subset migrating the least (Fig. 6). Loss of PSGL-1 may therefore contribute to egress from the T cell zone.
Figure 6.
PSGL-1lo T cells from MRLlpr mice have chemotactic properties consistent with extrafollicular localization. Migration of T cell subsets or control populations to the ligands for CCR7 (CCL19 and CCL21), CXCR5 (CXCL13), and CXCR4 (CXCL12) in a Transwell chemotaxis assay. Error bars indicate means ± SEM, and results are representative of three experiments. *, P < 0.05 compared with CD62Llo PSGL-1hi cells using the Student's t test.
Importantly, no responsiveness to the B cell zone chemokine CXCL13, which acts through CXCR5, was observed in the PSGL-1lo subset despite robust migration by B cells (Fig. 6), indicating that they are not TFH cells. We initially presumed that the expression of CXCR4 in PSGL-1lo T cells would confer an enhanced chemotactic response to its ligand, CXCL12, but the regulation of responses to this chemokine in vitro are more complex. Plasma cells, which migrate in a CXCR4-dependent manner to sites rich in CXCL12, rapidly lose responsiveness to this chemokine in vitro despite high expression of its receptor (51, 52). Consistent with these reports, we observed depressed in vitro migration to CXCL12 in CD138+ plasmablasts, and this reduction was mirrored in PSGL-1lo T cells (Fig. 6).
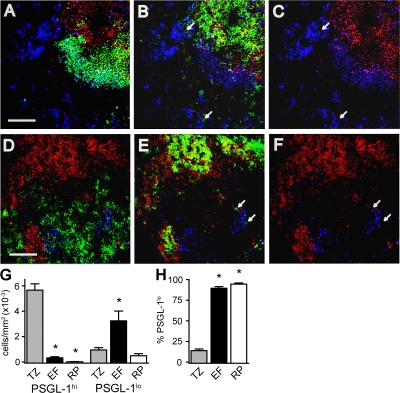
Although these data were consistent with the localization of PSGL-1lo T cells to niches high in CXCL12, such as extrafollicular foci and the red pulp, we proceeded to directly assess their splenic localization using immunofluorescence microscopy. The T cell zone is clearly identified by colocalization of PSGL-1 and CD4 (Fig. 7 B). Extrafollicular foci are identifiable as dense clusters of plasmablasts at the border of the T cell zone and red pulp, with plasmablasts being distinguished from follicular B cells both by high levels of IgM staining and loss of B220 (Fig. 7 A). Using the image processing software ImageJ, we were able to enumerate the frequency of PSGL-1hi and PSGL-1lo T cells in the T cell zone, extrafollicular foci, and red pulp. In contrast to PSGL-1hi T cells, which were abundant in the T cell zone and rare at other sites, PSGL-1lo T cells were found at the greatest density in extrafollicular foci, though they also present to a lesser extent in the T cell zone and red pulp (Fig. 7 G). The extrafollicular localization of PSGL-1lo T cells was determined not only by their occurrence outside of B220+ follicles and CD4-dense T cell zones, but by their occurrence at the edge of the T cell zone and red pulp as defined by staining of F4/80+ red pulp macrophages (Fig. 7, D–F). Extrafollicular T cells lacking PSGL-1 were also readily identified using antibodies against TCRβ (unpublished data). In contrast to the cells in the T cell zone, of which only a minority are PSGL-1lo, T cells found in extrafollicular foci and in the red pulp were almost exclusively PSGL-1lo (Fig. 7 H), confirming that extrafollicular localization is tightly associated with the loss of PSGL-1.
Figure 7.
PSGL-1lo T cells localize to the extrafollicular focus. Four-color immunofluorescence microscopy was used to determine the expression of PSGL-1 on CD4 T cells in extrafollicular sites. For clarity, each panel displays two or three of the four channels. One field is represented in A–C, and another is represented in D–F. In all panels, red and blue signals indicate PSGL-1 and IgM staining, respectively. Green indicates B220 in A, F4/80 in D, and CD4 in B and E, and is left blank in C and F. White arrows highlight the location of extrafollicular CD4+ cells. Bar, 100 μm. (G and H) Enumeration of CD4 T cells expressing or lacking PSGL-1 in splenic T cell zones, extrafollicular sites, and the red pulp. The number of cells per millimeter squared in each site (G) and the percentage of CD4+ cells lacking PSGL-1 at each site (H) were determined from immunofluorescence micrographs. 100–300 cells, from multiple sections, were scored per mouse, n = 4. *, P < 0.05 compared with the T cell zone. EF, extrafollicular focus; RP, red pulp; TZ, T cell zone.
PSGL-1lo cells are functionally specialized to drive IgG production
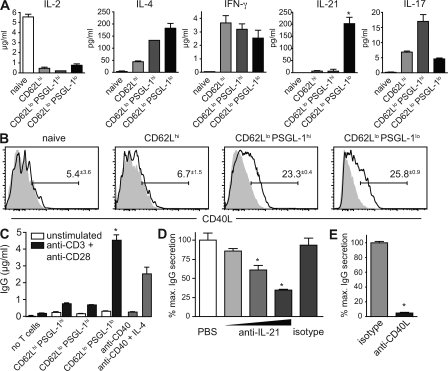
Because we had described a defect in Th function in vitro and in vivo associated with the loss of CXCR4+ PSGL-1lo T cells in the setting of Icos deficiency, we wanted to directly address whether the loss of PSGL-1 identifies effector T cells with specialized B helper functions. To this end, we analyzed their cytokine production by sorting CD44lo naive and CD44hi effector cells, the latter separated further according to their expression of CD62L and PSGL-1. Consistent with the involvement of IL-4 and IFN-γ in CSR, both of these cytokines were made by PSGL-1lo cells but were also made by other effector subsets (Fig. 8 A), as would be expected given their pleiotropic nature. Similarly, CD40L was expressed on CD62Llo cells of both PSGL-1hi and PSGL-1lo phenotypes (Fig. 8 B), consistent with the role of this effector molecule in both humoral and inflammatory responses. In striking contrast, IL-21 was secreted exclusively by PSGL-1lo cells (Fig. 8 A).
Figure 8.
PSGL-1lo T cells are unique in their ability to promote IgG secretion via IL-21. (A) TCRβ+ CD4+ B220− CD44hi effector cells were separated according to their expression of CD62L and PSGL-1 and were cultured with PMA and ionomycin for 24 h, and the indicated cytokines were measured in the supernatants. CD44lo CD62Lhi naive T cells were stimulated as a negative control. Results are representative of three experiments. (B) Percent expression of CD40L on the indicated subsets was determined by FACS (n = 3). Specific staining is indicated by a continuous line, and isotype control staining is indicated by gray shading. (C) Effector CD4 cells were sorted as in A and cultured with sorted CD19+ B220+ CD90− CD44lo naive B cells from young mice in the presence or absence of anti-CD3 and anti-CD28. After 96 h, secreted IgG was measured in the culture supernatant. B cell stimulation with an optimal dose of anti-CD40 with or without IL-4 is shown for comparison. Results are representative of three experiments. (D) IL-21 blocking antibody was added at fourfold increasing concentrations to a co-culture of naive B cells with PSGL-1lo effector cells. Isotype control antibody was added at maximum concentration. (E) CD40L blocking antibody MR1 or isotype control was added to similar co-cultures. D and E are representative of two experiments each. All data are shown as means ± SEM. *, P < 0.05 compared with other effector subsets (A and C) or isotype control (D and E) using the Student's t test.
Given the corresponding defects in the differentiation of PSGL-1lo cells and IgG+ plasma cells we observed in Icos−/− mice, we proceeded to test directly whether PSGL-1lo cells were able to induce IgG class switching and secretion by co-culturing sorted naive B cells with anti-CD3/-CD28–stimulated sorted effector T cell subsets. Although other effector subsets had little effect, activated CD62Llo PSGL-1lo cells were sufficient to robustly induce isotype switching and secretion of IgG, to an even greater extent than an optimal dose of anti-CD40 and IL-4 (Fig. 8 C). Given the unique ability of PSGL-1lo cells to both make IL-21 and drive IgG secretion, as well as the emerging role for IL-21 in class switching and plasmagenesis, we hypothesized that IL-21 was important for their helper function. Indeed, the addition of IL-21 neutralizing antibody to co-cultures of PSGL-1lo cells and B cells significantly impaired IgG production in a dose-dependent manner (Fig. 8 D), confirming the important role for this cytokine in the collaboration of extrafollicular Th cells with B cells. Further, CD40L is known to play a critical role in T/B collaboration in MRLlpr mice, and as expected, the addition of CD40L blocking antibody MR1 completely ablated the ability of PSGL-1lo helper cells to induce IgG production (Fig. 8 E).
Extrafollicular Th cells occur in other autoimmune models
Because the MRLlpr model is rather unique in the extent of the dominance of the extrafollicular pathway over the GC, it remained a possibility that the development of extrafollicular Th cells was limited to these mice. Thus, we analyzed two additional models: (NZB/BINJ × NZW/LacJ) F1 (NZB/W F1) spontaneously lupus-prone mice, as well as rat insulin promoter-driven lymphotoxin α and β (RIP-Ltαβ) transgenic mice, a genetically and mechanistically unrelated model of chronic inflammation. NZB/W F1 mice differ from MRLlpr mice in that they develop robust spontaneous GCs with age (32, 53), though both follicular and extrafollicular activation pathways are likely operative (54). RIP-LTαβ mice express both LTα and LTβ in the pancreas, kidneys, and skin, leading to massive infiltrates in these tissues (55, 56).
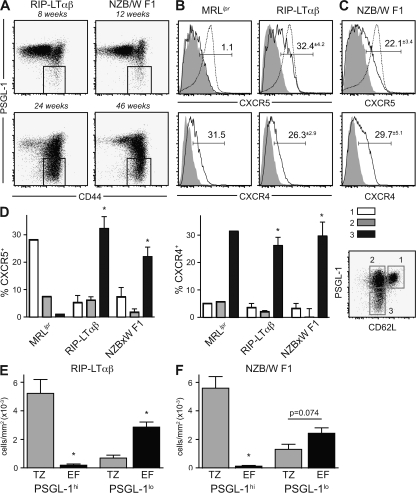
Analysis of the spleens of both NZB/W F1 and RIP-LTαβ mice revealed a striking age-dependent expansion of PSGL-1lo CD4 T cells (Fig. 9 A). Further, as in MRLlpr animals, CXCR4 was expressed specifically within the PSGL-1lo population (Fig. 9, B–D). However, PSGL-1lo cells from NZB/W F1 and RIP-LTαβ mice also contained a CXCR5+ TFH cell population (Fig. 9, B–D). These data suggested that Th cells with either a follicular or extrafollicular homing pattern could be identified within the PSGL-1lo subset of other autoimmune models. Confirming the extrafollicular homing pattern, Th cells were readily identified in the splenic extrafollicular foci of both NZB/W and RIP-LTαβ mice by immunofluorescence microscopy (Fig. 9, E and F; and not depicted). As in the MRLlpr strain, single-cell analysis of the immunofluorescence images indicated that T cells found in extrafollicular sites were almost exclusively PSGL-1lo, whereas those in the T cell zone were predominantly PSGL-1hi. Thus, Th cells with a PSGL-1lo phenotype occur at high frequency in multiple autoimmune models, a subset of which can be found in extrafollicular regions.
Figure 9.
PSGL-1lo extrafollicular T cells occur in other autoimmune models. (A) The percentage of CD44hi PSGL-1lo cells within the CD4 T cell compartment of RIP-LTαβ and NZB/W F1 mice. 6–10 mice at varying ages were analyzed, and representative plots of the indicated ages are shown. (B and C) Expression of CXCR5 and CXCR4 on PSGL-1lo cells from 24-wk-old RIP-LTαβ (B) and 46-wk-old NZB/W F1 (C) mice. Continuous lines indicate PSGL-1lo effectors, gray-shaded histograms indicate CD44lo CD62Lhi naive CD4 T cells, and dotted lines indicate B cells. An MRLlpr control was stained concomitantly with the RIP-LTαβ group; profiles may also be compared with Fig. 5. Gate frequencies above background are shown. (D) Plots of CXCR5 and CXCR4 expression on effector subsets separated by CD62L and PSGL-1 expression. *, P < 0.05 compared with PSGL-1hi effector subsets using the Student's t test. (E and F) The number of cells per millimeter squared in each site in RIP-LTαβ (E) and NZB/W F1 (F) spleens were determined from immunofluorescence micrographs as in Fig. 7. 100–300 cells across multiple sections were counted per mouse (n = 4–6 mice per group). *, P < 0.05 compared with the T cell zone using the Student's t test. EF, extrafollicular focus; TZ, T cell zone.
DISCUSSION
The extrafollicular response is the dominant pathway of autoantibody production in the MRLlpr mouse, and we find that ICOS functions in this pathway through the induction of a population of Th cells that traffics to extrafollicular focus. Although the contribution of ICOS to GC responses has received significant attention, its role in extrafollicular responses has been neglected despite an early report that IgG production the first week after immunization, largely derived from the extrafollicular response, is ICOS dependent (42). We have recapitulated these results in our own laboratory, where the percentage of CD19+ CD138+ surface IgG1+ plasmablasts 6 d after NP-CGG/alum immunization was substantially reduced in B6 Icos ligand−/− mice versus B6 controls (0.14 ± 0.11% vs. 1.87 ± 0.85%, respectively).
The ICOS-dependent Th cells that traffic to extrafollicular sites in MRLlpr mice were isolated and characterized based on their down-regulation of PSGL-1, though their essential features are expression of CXCR4, localization to the extrafollicular sites and B cell helper activity. Among the many effector cells that are generated in MRLlpr mice, the relatively small PSGL-1lo subset is remarkably unique in its ability to induce IgG secretion. One effector mechanism that is important for this function is the secretion of IL-21, an IL-2 family member previously shown to cooperate with IL-4 for IgG antibody production (17). IL-21 has been implicated in GC responses, and there is also data to suggest that it functions in the extrafollicular pathway. Although extrafollicular isotype switching to IgG1 in response to NP-CGG can occur even in the dual absence of IL-4 and IL-13 (7), this early IgG1 production is completely blocked in IL-4−/− IL-21R−/− double-knockout mice (17). Thus, CXCR4+ extrafollicular Th cells might be a source of IL-21 in the normal extrafollicular IgG1 response. Our observation that PSGL-1lo T cells from MRLlpr mice, in contrast to TFH cells (10–12), also secrete IL-4 and IFN-γ raises the possibility that isotype instruction by these switch cytokines may continue in the extrafollicular focus. Clarification of this point will require both single-cell analysis of cytokine production within the PSGL-1lo subset and histological examination of the location of IL-21 production. The former experiment has been hampered by the lack of an intracellular staining reagent for IL-21, and both experiments may be precisely addressed with an IL-21 reporter mouse.
PSGL-1 has been sensibly thought to be constitutively expressed on T cells, because P-selectin binding is regulated not by PSGL-1 expression but by inducible glycosyltransferases that modify the PSGL-1 glycoform (57). We were surprised, therefore, to identify a population of activated CD4 T cells that had down-regulated the entire, presumably inactive, glycoprotein scaffold. Recently, however, PSGL-1 was also shown to participate in chemotactic responses to CCR7 ligands (50), providing a mechanistic reason why humoral effector T cells, which must leave the T cell zone to enter follicular or extrafollicular sites, down-regulate this molecule. Supporting a connection between PSGL-1 down-regulation and humoral effector function, CD4 T cells that are actively translating IL-4 protein in the lymph nodes of helminth-infected mice, as opposed to tissue-tropic effector cells requiring peripheral restimulation, also have a PSGL-1lo phenotype (58).
In the MRLlpr autoantibody response, in which the extrafollicular pathway dominates, PSGL-1lo humoral effector T cells express CXCR4 and localize to the extrafollicular focus. In contrast, PSGL-1lo cells from NZB/W F1 and RIP-LTαβ transgenic mice, which develop both extrafollicular and GC responses, were found to have both CXCR4- and CXCR5-expressing cells. Indeed, the expression of CXCR5, like CXCR4, was largely restricted to this population, indicating that the TFH cell subset also bears a PSGL-1lo phenotype. The follicular versus extrafollicular trafficking pattern of Th cells may be instructed by their cognate B cells, the latter differentiating along the GC or plasmablast pathway, as determined by factors such as strength of the BCR signal (59).
The effector mechanisms we defined for extrafollicular PSGL-1lo cells from MRLlpr mice, IL-21 and CD40L, are also shared by TFH cells (10, 14). These common features are not surprising given that T cell–dependent CSR and plasmagenesis occur in both GC B cells and plasmablasts, and both IL-21 and CD40L regulate these processes. It is possible that the more distinct features of follicular versus extrafollicular responses, however, such as long-lived plasma cell and memory formation, may in part reflect unique products of follicular versus extrafollicular helper cells that have yet to be defined. Alternatively, the helper cells that support extrafollicular responses may not be ontogenetically or mechanistically distinct from TFH cells but simply exhibit an alternative trafficking pattern. Illuminating the relationships between these humoral effector cells will be aided by global comparison of their effector genes and lineage specification factors. In microarray experiments, TFH cells have been shown to express B cell leukemia/lymphoma 6 (14), an essential transcription factor in GC B cells (60). Whether non-TFH PSGL-1lo T cells from MRLlpr mice also express this transcription factor is currently under investigation.
Based on our data that PSGL-1lo CD4 T cells are absent in both Icos mutant and B cell–deficient mice, we propose that the ICOS– B7-related protein 1 interaction during T/B collaboration provides one of the critical signals for the development of humoral effector cells. Our observation that Icos−/− effectors are still reasonably potent at providing B cell help in culture may be attributed to the minimal requirements, such as CD40L and IL-4, for IgG induction in vitro. An alternative, though not exclusive, explanation is that humoral effector T cells fail to migrate appropriately to sites of B cell differentiation in vivo. In this view, the lack of PSGL-1lo cells could reflect a failure of humoral effector cells to down-regulate PSGL-1, rather than fail to develop per se, an interpretation that would be consistent with the reduced CXCR4 expression we observed in Icos−/− effectors. The severe defect in IL-21 secretion, however, indicates a broader block in helper cell differentiation. Still, whether ICOS provides a specific differentiation signal or simply allows humoral effector cells to expand and survive is not clear. In addition, signaling to B cells through the ICOS ligand, B7-related protein 1, itself could potentially regulate plasma cell differentiation, complementing the activity of IL-21 and CD40L. To what extent PSGL-1lo T cell effects on plasmablasts, such as AID induction, occur within the extrafollicular focus itself versus earlier at the T/B interface remains undefined.
Functional characteristics such as cytokine production are commonly used to stratify effector CD4 T cells such as Th1, Th2, or Th17. TFH cells, in contrast, are defined essentially by their trafficking pattern, though with a view to their functional specialty. We have characterized a novel population of B Th cells that occur in chronic autoimmunity that do not conform to effector cell categories as currently defined. It remains to be clarified whether these extrafollicular Th cells merit designation as a subset distinct from their follicular counterparts, or rather are TFH cells that are recruited to an alternative site of B cell activation in an autoimmune setting.
MATERIALS AND METHODS
Mice.
MRLlpr animals were obtained from the Jackson Laboratory and were maintained in specific pathogen-free conditions at the Yale School of Medicine. RIP-LTαβ transgenic mice (55) were provided by R. Mounzer and N. Ruddle (Yale School of Medicine, New Haven, CT). Spleens from NZB/W F1 mice were provided by A. Davidson (Feinstein Institute for Medical Research, Manhasset, NY). The Institutional Animal Care and Use Committee of Yale University approved all procedures. The disrupted Icos allele was generated as described previously (61), and was backcrossed to the MRLlpr background for six generations and intercrossed, and MRLlpr.Icos−/− mice were subsequently maintained as homozygotes. The MHC and four non-MHC autoimmune susceptibility loci (B6-derived Lmb1 and MRL-derived Lmb2, Lmb3, and Lmb4) are discernable in the MRLlpr and B6lpr genomes (62, 63). At the N6 generation, markers that define each of these intervals (D4Mit17, D4Mit9, D4Mit146, D4Mit12, D4Mit33, D5Mit145, D5Mit13, D5Mit356, D5Mit24, D7Mit57, D7Mit82, D7Mit211, D7Mit147, D7Mit39, D10Mit51, D10Mit15, D10Mit11, D10Mit269, D17Mit16, and Tnf) revealed that Icos−/− and control groups were fixed for the MRL genome at all intervals except the centromeric region of Lmb1 (Table S1, available at http://www.jem.org/cgi/content/full/jem.20080840/DC1). Because MRLlpr mice carrying the B6 allele of Lmb1 have slightly increased splenic lymphoproliferation, but autoantibody production is unaffected (62), Lmb1 has a minimal impact on the development of autoimmunity. Moreover, if anything, the B6 allele contributes to an increase in lymphocyte activation and so would not invalidate our conclusions; thus, the impact of Icos deficiency may actually be slightly stronger than we describe.
In all experiments, precisely age- and sex-matched controls were included with experimental animals. In experiments with mixed-sex groups, both groups contained the same ratio of males and females.
Flow cytometry.
Spleens were extracted and homogenized by pressing through a 40-μM nylon filter. Red blood cells were lysed by hypotonic shock by brief exposure to distilled water followed by immediate isotonic restoration with 10× PBS. Surface staining was performed in ice-cold PBS with 1% FCS in the presence of FcR blocking antibody 2.4G2. For experiments involving chemokine receptor staining, antibody-labeled cells were placed at 37°C for 30 min, washed, and incubated with streptavidin-conjugated fluorophore on ice. With the exception of intracellular Ig staining, samples were analyzed unfixed, and dead cells were excluded based on staining with Hoechst 33342 (Sigma-Aldrich) added immediately before analysis. For intracellular Ig staining, cells were stained for surface antigens as described, fixed with a 1:4 dilution of Cytofix/Cytoperm (BD) in PBS for 20 min on ice, washed, blocked with 5% rat serum in Perm/Wash (BD), and incubated with anti–mouse Ig antibodies in Perm/Wash overnight at 4°C. In all experiments, cells were resuspended in PBS and analyzed on a four-laser flow cytometer (LSRII; BD). Antibody clones used were anti–mouse IgG1 (15H6; SouthernBiotech), IgG2a (R19-15; BD), IgG2b (R12-3; BD), IgG3 (R40-82; BD), IgM (gift of M. Shlomchik, Yale School of Medicine, New Haven, CT), TCRβ (H57-587; BD), CD3 (145-2C11; BD), CD138 (281-2; BD), PSGL-1 (2PH-1; BD), B220 (RA3-6B2; BD), CD19 (1D3; BD), CD44 (IM7; eBioscience), CD62L (MEL-14; BD), CXCR4 (2B11; BD), CXCR5 (2G8; BD), and CCR7 (4B12; eBioscience). The fluorochromes used in each channel were FITC or AF488, PE, PE–Texas red, PE-Cy7, allophycocyanin (APC) or AF647, APC-Cy7 or APC-AF750, Hoechst 33342, and Pacific blue.
Quantitative PCR.
Total RNA was isolated from sorted TCRβ− CD19+ CD44hi CD138+ plasmablasts using the RNeasy Mini kit (QIAGEN) and treated with amplification grade DNase I (Invitrogen). 0.5 μg RNA was reversed transcribed with Superscript III (Invitrogen) using random hexamers, and the cDNA was subjected to real-time PCR using Taqman probes, HotStart Taq DNA polymerase (QIAGEN), and a thermocycler (3000XP; Stratagene). 10-fold serial dilutions of aicda (gift of V. Odegard and D. Schatz, Yale School of Medicine, New Haven, CT) and actin standard were included to generate standard curves. Starting cDNA quantity was calculated from ct values relative to the standard curve. The amount of aicda was normalized to the amount of actin in each sample. Forward primer, reverse primer, and probe sequences were as follows: aicda, 5′-CGGCTAACCAGACAACTTCG-3′, 5′-GCATCTCGCAAGTCATCGA-3′, and FAM-CGCATCCTTTTGCCCTTGTACGA-TAMRA; and actin, 5′-AGAGGGAAATCGTGCGTGAC-3′, 5′-CAATAGTGATGACCTGGCCGT-3′, and FAM-CACTGCCGCATCCTCTTCCTCCC-TAMRA.
Cytokine measurements.
Effector T cells were sorted as indicated in the figures and cultured with 50 ng/ml PMA and 1 μM ionomycin for 24 h in complete Click's media. IFN-γ, IL-4, IL-2, and IL-17 from culture supernatants were measured by a Luminex assay using anti–mouse cytokine beads (Beadlyte; Millipore). IL-21 was detected by sandwich ELISA as follows: Immulon-4 HBX (Millipore) plates were coated with anti–mouse IL-21 (R&D Systems), and bound IL-21 from culture supernatants was detected using biotinylated anti–mouse IL-21 (R&D Systems) followed by Extravidin–horseradish peroxidase (Sigma-Aldrich). Before analysis, supernatants were concentrated ninefold in 5-kD-cutoff Amicon Ultra-4 columns (Millipore).
Chemotaxis assay.
Splenocytes were isolated in prewarmed serum-free RPMI 1640 with 0.5% BSA and 2 mM HEPES, and 106 cells in 100 μl were added to the upper chamber of 5-μM Transwell plates (Millipore) containing 500 μl of media in the lower chamber. Cells were preincubated for 1 h at 37°C in 5% CO2, and chemokines were added in 100 μl to the lower chamber and cells were allowed to migrate for 2 h. The percent migration was calculated by dividing the number recovered in the lower chamber by the number of input for each population. Specific migration was determined by subtracting the percent migrated without chemokine. Samples were run in duplicate at three chemokine dilutions. Results from the optimal chemokine concentration are shown in the figures and were as follows: 25 ng/ml CCL19, 100 ng/ml CCL21, 250 ng/ml CXCL12, and 3 μg/ml CXCL13.
B cell help assay.
CD44lo B220+ CD19+ naive B cells were sorted from 8-wk-old mice. 6 × 105 sorted effector T cells were cultured with 2 × 105 B cells in 600 μl of complete Click's media in a 48-well plate. Some wells were precoated with 20 μg/ml anti-CD3, and 1 μg/ml anti-CD28 was added in suspension. As a control, cells were stimulated with 10 μg/ml anti-CD40 with or without 50 ng/ml IL-4. Secreted IgG was measured in the culture supernatant after 96 h. IL-21 blocking antibody or isotype control (R&D Systems) were added at fivefold increments up to 50 μg/ml. CD40L blocking antibody (clone MR1; BD) was added at 200 ng/ml.
Immunofluorescence microscopy.
Spleens were snap frozen in optimal cutting temperature, cut into 6-μM sections, and fixed with ice-cold acetone. Rehydrated sections were blocked with 5% rat serum, 5% rabbit serum, and 3% BSA with 0.1% Tween, and stained with antibodies, including B220 (RA3-6B2; BD), IgM (R6-602; BD), IgM-AF647 (gift of M. Shlomchik), PSGL-1 (4RA10 or KPL-1; BD), TCRβ (H57-597; eBioscience), F4/80 (BM8; eBioscience), and CD4 (RM4-5; eBioscience). Secondary detection was performed with anti-FITC–AF488 (Invitrogen), streptavidin-Cy3 (Jackson ImmunoResearch Laboratories), or streptavidin-AF647 (Invitrogen). Images were collected on a laser-scanning confocal microscope (model 510 META; Carl Zeiss, Inc.). Individual channels were uploaded into ImageJ software (available at http://rsb.info.nih.gov/ij/index.html). CD4+ single cells were scored for their expression of PSGL-1 using the “ColocalizeRGB” and “Cell counter” plugins (default settings). Extrafollicular foci were defined as dense clusters of IgMhi B220− plasmablasts near the border of T cell zones. The red pulp was defined as the remaining area outside T cell zones, B cell follicles, and extrafollicular foci, and was confirmed on separate sections to display expression for F4/80.
Statistical analysis.
All data analysis was performed using Prism 4.0a (GraphPad Software, Inc.). In most cases, two-tailed p-values were calculated by the unpaired Student's t test. For populations that were unlikely to approximate a Gaussian distribution, two-tailed p-values were calculated using the Mann-Whitney test.
Online supplemental material.
Table S1 shows the genotypes of microsatellite markers within autoimmune susceptibility loci of a sampling of mice used in the study. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20080840/DC1.
Supplementary Material
Acknowledgments
We thank A. Rudensky for supporting J.M. Odegard during the revision of this manuscript. We are grateful to M. Shlomchik for providing the anti-IgM antibody, V. Odegard and D. Schatz for the aicda probe and PCR conditions, R. Mounzer and N. Ruddle for RIP-LTαβ transgenic mice, Anne Davidson for spleens of NZB/W F1 animals, and S. Kaech and A. Rudensky for their thoughtful comments.
This work was supported by National Institutes of Health Grants AR40072 and AR44076, and by generous support from Rheuminations, Inc., the Arthritis Foundation, and the Connecticut Chapter of the Lupus Foundation of America.
The authors declare that they have no financial conflicts of interest.
Abbreviations used: AFC, antibody-forming cell; AID, activation-induced cytidine deaminase; CGG, chicken γ globulin; CSR, class switch recombination; GC, germinal center; ICOS, inducible T cell co-stimulator; MRLlpr, MRL/MpJ-Faslpr; NP, nitrophenyl; NZB/W F1, (NZB/BINJ × NZW/LacJ) F1; P-lig, P-selectin ligand; PSGL-1, P-selectin glycoprotein ligand 1; RIP-LTαβ, rat insulin promoter-driven lymphotoxin α and β; TFH cell, follicular helper T.
B.R. Marks and L.D. DiPlacido contributed equally to this paper.
J.M. Odegard's present address is Dept. of Immunology, University of Washington, Seattle, WA 98195.
C. Dong's present address is Dept. of Immunology, M.D. Anderson Cancer Center, Houston, TX 77030.
References
- 1.Reinhardt, R.L., S.J. Kang, H.E. Liang, and R.M. Locksley. 2006. T helper cell effector fates–who, how and where? Curr. Opin. Immunol. 18:271–277. [DOI] [PubMed] [Google Scholar]
- 2.Mosmann, T.R., and R.L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145–173. [DOI] [PubMed] [Google Scholar]
- 3.Abbas, A.K., K.M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature. 383:787–793. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan, M.H., U. Schindler, S.T. Smiley, and M.J. Grusby. 1996. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 4:313–319. [DOI] [PubMed] [Google Scholar]
- 5.Okada, T., and J.G. Cyster. 2006. B cell migration and interactions in the early phase of antibody responses. Curr. Opin. Immunol. 18:278–285. [DOI] [PubMed] [Google Scholar]
- 6.Toellner, K.M., S.A. Luther, D.M. Sze, R.K. Choy, D.R. Taylor, I.C. MacLennan, and H. Acha-Orbea. 1998. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J. Exp. Med. 187:1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham, A.F., P.G. Fallon, M. Khan, S. Vacheron, H. Acha-Orbea, I.C. MacLennan, A.N. McKenzie, and K.M. Toellner. 2002. Th2 activities induced during virgin T cell priming in the absence of IL-4, IL-13, and B cells. J. Immunol. 169:2900–2906. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi, Y., P.R. Dutta, D.M. Cerasoli, and G. Kelsoe. 1998. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J. Exp. Med. 187:885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacLennan, I.C. 1994. Germinal centers. Annu. Rev. Immunol. 12:117–139. [DOI] [PubMed] [Google Scholar]
- 10.Breitfeld, D., L. Ohl, E. Kremmer, J. Ellwart, F. Sallusto, M. Lipp, and R. Forster. 2000. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192:1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaerli, P., K. Willimann, A.B. Lang, M. Lipp, P. Loetscher, and B. Moser. 2000. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192:1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, C.H., L.S. Rott, I. Clark-Lewis, D.J. Campbell, L. Wu, and E.C. Butcher. 2001. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center–localized subset of CXCR5+ T cells. J. Exp. Med. 193:1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nurieva, R.I., Y. Chung, D. Hwang, X.O. Yang, H.S. Kang, L. Ma, Y.H. Wang, S.S. Watowich, A.M. Jetten, Q. Tian, and C. Dong. 2008. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 29:138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chtanova, T., S.G. Tangye, R. Newton, N. Frank, M.R. Hodge, M.S. Rolph, and C.R. Mackay. 2004. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 173:68–78. [DOI] [PubMed] [Google Scholar]
- 15.Kim, C.H., H.W. Lim, J.R. Kim, L. Rott, P. Hillsamer, and E.C. Butcher. 2004. Unique gene expression program of human germinal center T helper cells. Blood. 104:1952–1960. [DOI] [PubMed] [Google Scholar]
- 16.Bryant, V.L., C.S. Ma, D.T. Avery, Y. Li, K.L. Good, L.M. Corcoran, R. de Waal Malefyt, and S.G. Tangye. 2007. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J. Immunol. 179:8180–8190. [DOI] [PubMed] [Google Scholar]
- 17.Ozaki, K., R. Spolski, C.G. Feng, C.F. Qi, J. Cheng, A. Sher, H.C. Morse III, C. Liu, P.L. Schwartzberg, and W.J. Leonard. 2002. A critical role for IL-21 in regulating immunoglobulin production. Science. 298:1630–1634. [DOI] [PubMed] [Google Scholar]
- 18.Parrish-Novak, J., S.R. Dillon, A. Nelson, A. Hammond, C. Sprecher, J.A. Gross, J. Johnston, K. Madden, W. Xu, J. West, et al. 2000. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 408:57–63. [DOI] [PubMed] [Google Scholar]
- 19.Mehta, D.S., A.L. Wurster, M.J. Whitters, D.A. Young, M. Collins, and M.J. Grusby. 2003. IL-21 induces the apoptosis of resting and activated primary B cells. J. Immunol. 170:4111–4118. [DOI] [PubMed] [Google Scholar]
- 20.Pene, J., J.F. Gauchat, S. Lecart, E. Drouet, P. Guglielmi, V. Boulay, A. Delwail, D. Foster, J.C. Lecron, and H. Yssel. 2004. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J. Immunol. 172:5154–5157. [DOI] [PubMed] [Google Scholar]
- 21.Kuchen, S., R. Robbins, G.P. Sims, C. Sheng, T.M. Phillips, P.E. Lipsky, and R. Ettinger. 2007. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J. Immunol. 179:5886–5896. [DOI] [PubMed] [Google Scholar]
- 22.Jacob, J., R. Kassir, and G. Kelsoe. 1991. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 173:1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McHeyzer-Williams, M.G., M.J. McLean, P.A. Lalor, and G.J. Nossal. 1993. Antigen-driven B cell differentiation in vivo. J. Exp. Med. 178:295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacLennan, I.C., K.M. Toellner, A.F. Cunningham, K. Serre, D.M. Sze, E. Zuniga, M.C. Cook, and C.G. Vinuesa. 2003. Extrafollicular antibody responses. Immunol. Rev. 194:8–18. [DOI] [PubMed] [Google Scholar]
- 25.Goodnow, C.C., J. Crosbie, S. Adelstein, T.B. Lavoie, S.J. Smith-Gill, R.A. Brink, H. Pritchard-Briscoe, J.S. Wotherspoon, R.H. Loblay, K. Raphael, et al. 1988. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 334:676–682. [DOI] [PubMed] [Google Scholar]
- 26.Mandik-Nayak, L., A. Bui, H. Noorchashm, A. Eaton, and J. Erikson. 1997. Regulation of anti–double-stranded DNA B cells in nonautoimmune mice: localization to the T–B interface of the splenic follicle. J. Exp. Med. 186:1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cyster, J.G., and C.C. Goodnow. 1995. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 3:691–701. [DOI] [PubMed] [Google Scholar]
- 28.Mandik-Nayak, L., S.J. Seo, C. Sokol, K.M. Potts, A. Bui, and J. Erikson. 1999. MRL-lpr/lpr mice exhibit a defect in maintaining developmental arrest and follicular exclusion of anti–double-stranded DNA B cells. J. Exp. Med. 189:1799–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobson, B.A., D.J. Panka, K.A. Nguyen, J. Erikson, A.K. Abbas, and A. Marshak-Rothstein. 1995. Anatomy of autoantibody production: dominant localization of antibody-producing cells to T cell zones in Fas-deficient mice. Immunity. 3:509–519. [DOI] [PubMed] [Google Scholar]
- 30.William, J., C. Euler, S. Christensen, and M.J. Shlomchik. 2002. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 297:2066–2070. [DOI] [PubMed] [Google Scholar]
- 31.William, J., C. Euler, E. Leadbetter, A. Marshak-Rothstein, and M.J. Shlomchik. 2005. Visualizing the onset and evolution of an autoantibody response in systemic autoimmunity. J. Immunol. 174:6872–6878. [DOI] [PubMed] [Google Scholar]
- 32.Luzina, I.G., S.P. Atamas, C.E. Storrer, L.C. daSilva, G. Kelsoe, J.C. Papadimitriou, and B.S. Handwerger. 2001. Spontaneous formation of germinal centers in autoimmune mice. J. Leukoc. Biol. 70:578–584. [PubMed] [Google Scholar]
- 33.Vinuesa, C.G., M.C. Cook, C. Angelucci, V. Athanasopoulos, L. Rui, K.M. Hill, D. Yu, H. Domaschenz, B. Whittle, T. Lambe, et al. 2005. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 435:452–458. [DOI] [PubMed] [Google Scholar]
- 34.Merino, R., M. Iwamoto, L. Fossati, and S. Izui. 1993. Polyclonal B cell activation arises from different mechanisms in lupus-prone (NZB × NZW)F1 and MRL/MpJ-lpr/lpr mice. J. Immunol. 151:6509–6516. [PubMed] [Google Scholar]
- 35.Peng, S.L., M.P. Madaio, D.P. Hughes, I.N. Crispe, M.J. Owen, L. Wen, A.C. Hayday, and J. Craft. 1996. Murine lupus in the absence of alpha beta T cells. J. Immunol. 156:4041–4049. [PubMed] [Google Scholar]
- 36.Lentz, V.M., and T. Manser. 2001. Cutting edge: germinal centers can be induced in the absence of T cells. J. Immunol. 167:15–20. [DOI] [PubMed] [Google Scholar]
- 37.Herber, D., T.P. Brown, S. Liang, D.A. Young, M. Collins, and K. Dunussi-Joannopoulos. 2007. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J. Immunol. 178:3822–3830. [DOI] [PubMed] [Google Scholar]
- 38.Dong, C., U.A. Temann, and R.A. Flavell. 2001. Cutting edge: critical role of inducible costimulator in germinal center reactions. J. Immunol. 166:3659–3662. [DOI] [PubMed] [Google Scholar]
- 39.Tafuri, A., A. Shahinian, F. Bladt, S.K. Yoshinaga, M. Jordana, A. Wakeham, L.M. Boucher, D. Bouchard, V.S. Chan, G. Duncan, et al. 2001. ICOS is essential for effective T-helper-cell responses. Nature. 409:105–109. [DOI] [PubMed] [Google Scholar]
- 40.Akiba, H., K. Takeda, Y. Kojima, Y. Usui, N. Harada, T. Yamazaki, J. Ma, K. Tezuka, H. Yagita, and K. Okumura. 2005. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J. Immunol. 175:2340–2348. [DOI] [PubMed] [Google Scholar]
- 41.Vogelzang, A., H.M. McGuire, D. Yu, J. Sprent, C.R. Mackay, and C. King. 2008. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 29:127–137. [DOI] [PubMed] [Google Scholar]
- 42.McAdam, A.J., R.J. Greenwald, M.A. Levin, T. Chernova, N. Malenkovich, V. Ling, G.J. Freeman, and A.H. Sharpe. 2001. ICOS is critical for CD40-mediated antibody class switching. Nature. 409:102–105. [DOI] [PubMed] [Google Scholar]
- 43.William, J., C. Euler, and M.J. Shlomchik. 2005. Short-lived plasmablasts dominate the early spontaneous rheumatoid factor response: differentiation pathways, hypermutating cell types, and affinity maturation outside the germinal center. J. Immunol. 174:6879–6887. [DOI] [PubMed] [Google Scholar]
- 44.Leonard, W.J., and R. Spolski. 2005. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat. Rev. Immunol. 5:688–698. [DOI] [PubMed] [Google Scholar]
- 45.Ansel, K.M., L.J. McHeyzer-Williams, V.N. Ngo, M.G. McHeyzer-Williams, and J.G. Cyster. 1999. In vivo–activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 190:1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell, D.J., C.H. Kim, and E.C. Butcher. 2001. Separable effector T cell populations specialized for B cell help or tissue inflammation. Nat. Immunol. 2:876–881. [DOI] [PubMed] [Google Scholar]
- 47.Estes, J.D., T.C. Thacker, D.L. Hampton, S.A. Kell, B.F. Keele, E.A. Palenske, K.M. Druey, and G.F. Burton. 2004. Follicular dendritic cell regulation of CXCR4-mediated germinal center CD4 T cell migration. J. Immunol. 173:6169–6178. [DOI] [PubMed] [Google Scholar]
- 48.Allen, C.D., K.M. Ansel, C. Low, R. Lesley, H. Tamamura, N. Fujii, and J.G. Cyster. 2004. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol. 5:943–952. [DOI] [PubMed] [Google Scholar]
- 49.Hardtke, S., L. Ohl, and R. Forster. 2005. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood. 106:1924–1931. [DOI] [PubMed] [Google Scholar]
- 50.Veerman, K.M., M.J. Williams, K. Uchimura, M.S. Singer, J.S. Merzaban, S. Naus, D.A. Carlow, P. Owen, J. Rivera-Nieves, S.D. Rosen, and H.J. Ziltener. 2007. Interaction of the selectin ligand PSGL-1 with chemokines CCL21 and CCL19 facilitates efficient homing of T cells to secondary lymphoid organs. Nat. Immunol. 8:532–539. [DOI] [PubMed] [Google Scholar]
- 51.Hauser, A.E., G.F. Debes, S. Arce, G. Cassese, A. Hamann, A. Radbruch, and R.A. Manz. 2002. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J. Immunol. 169:1277–1282. [DOI] [PubMed] [Google Scholar]
- 52.Wehrli, N., D.F. Legler, D. Finke, K.M. Toellner, P. Loetscher, M. Baggiolini, I.C. MacLennan, and H. Acha-Orbea. 2001. Changing responsiveness to chemokines allows medullary plasmablasts to leave lymph nodes. Eur. J. Immunol. 31:609–616. [DOI] [PubMed] [Google Scholar]
- 53.Ermak, T.H., H.J. Steger, and D. Wofsy. 1989. Treatment of murine lupus with monoclonal antibody to L3T4. II. Effects on immunohistopathology of thymus, spleen, and lymph node. Lab. Invest. 61:447–456. [PubMed] [Google Scholar]
- 54.Hoyer, B.F., K. Moser, A.E. Hauser, A. Peddinghaus, C. Voigt, D. Eilat, A. Radbruch, F. Hiepe, and R.A. Manz. 2004. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J. Exp. Med. 199:1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drayton, D.L., X. Ying, J. Lee, W. Lesslauer, and N.H. Ruddle. 2003. Ectopic LTαβ directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J. Exp. Med. 197:1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Picarella, D.E., A. Kratz, C.B. Li, N.H. Ruddle, and R.A. Flavell. 1992. Insulitis in transgenic mice expressing tumor necrosis factor beta (lymphotoxin) in the pancreas. Proc. Natl. Acad. Sci. USA. 89:10036–10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ley, K., and G.S. Kansas. 2004. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 4:325–335. [DOI] [PubMed] [Google Scholar]
- 58.Mohrs, K., A.E. Wakil, N. Killeen, R.M. Locksley, and M. Mohrs. 2005. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 23:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paus, D., T.G. Phan, T.D. Chan, S. Gardam, A. Basten, and R. Brink. 2006. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J. Exp. Med. 203:1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dent, A.L., A.L. Shaffer, X. Yu, D. Allman, and L.M. Staudt. 1997. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 276:589–592. [DOI] [PubMed] [Google Scholar]
- 61.Dong, C., A.E. Juedes, U.A. Temann, S. Shresta, J.P. Allison, N.H. Ruddle, and R.A. Flavell. 2001. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 409:97–101. [DOI] [PubMed] [Google Scholar]
- 62.Vidal, S., D.H. Kono, and A.N. Theofilopoulos. 1998. Loci predisposing to autoimmunity in MRL-Fas lpr and C57BL/6-Faslpr mice. J. Clin. Invest. 101:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kong, P.L., T. Zhu, M.P. Madaio, and J. Craft. 2003. Role of the H-2 haplotype in Fas-intact lupus-prone MRL mice: association with autoantibodies but not renal disease. Arthritis Rheum. 48:2992–2995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.