Abstract
High dose bee venom exposure in beekeepers by natural bee stings represents a model to understand mechanisms of T cell tolerance to allergens in healthy individuals. Continuous exposure of nonallergic beekeepers to high doses of bee venom antigens induces diminished T cell–related cutaneous late-phase swelling to bee stings in parallel with suppressed allergen-specific T cell proliferation and T helper type 1 (Th1) and Th2 cytokine secretion. After multiple bee stings, venom antigen–specific Th1 and Th2 cells show a switch toward interleukin (IL) 10–secreting type 1 T regulatory (Tr1) cells. T cell regulation continues as long as antigen exposure persists and returns to initial levels within 2 to 3 mo after bee stings. Histamine receptor 2 up-regulated on specific Th2 cells displays a dual effect by directly suppressing allergen-stimulated T cells and increasing IL-10 production. In addition, cytotoxic T lymphocyte–associated antigen 4 and programmed death 1 play roles in allergen-specific T cell suppression. In contrast to its role in mucosal allergen tolerance, transforming growth factor β does not seem to be an essential player in skin-related allergen tolerance. Thus, rapid switch and expansion of IL-10–producing Tr1 cells and the use of multiple suppressive factors represent essential mechanisms in immune tolerance to a high dose of allergens in nonallergic individuals.
To avoid chronic cell activation and inflammation against nonpathogenic antigens through skin, ingestion, and inhalation, the immune system has developed efficient peripheral tolerance mechanisms. Allergic diseases are caused by an aberrant immune response mediated through a key effector cell, the Th2 cell, and an associated cytokine pattern including IL-4, IL-5, and IL-13 (1). Consequently, the most pronounced findings with potential relevance to allergy therapy are related directly to the control of these Th2 immune effectors. There is strong evidence that peripheral T cell regulation plays a crucial role in the control of harmful T cell responses. Since the early 1970s, many types of suppressor mechanisms have been demonstrated, and the biology of T reg cells has been the subject of intensive investigation (2–6).
Subsets of T reg cells with distinct phenotypes and mechanisms of action include the naturally occurring, thymic-selected CD4+CD25+FoxP3+ T reg cells, and the inducible type 1 T regulatory (Tr1) cells (7–12). A great deal of uncertainty remains about differentiation factors, antigen specificity, and mechanisms of action of T reg cells. Several types of adaptive T reg cells have been described with a unique mechanism of action that varies depending on the experimental model. T reg cells act as suppressor T cells, which down-regulate effector cells and APCs in vitro and in inflammation models such as various chronic infections, organ transplantation, allergy, and autoimmunity (7–12). Although molecular mechanisms of T reg cell generation remain to be elucidated, some existing therapies for allergic diseases, such as treatment with glucocorticoids and β-2 agonists, might function to promote the numbers and function of IL-10–secreting Tr1-like cells (13, 14).
Studies on the immune response to allergens provide well-defined models for understanding the regulation and circumvention of antigen-specific T cell responses. The symptoms of IgE-mediated allergy—rhinitis, conjunctivitis, and asthma—can be ameliorated by the temporary suppression of mediators and immune cells (e.g., therapies such as antihistamines and corticosteroids) (15, 16). However, the only long-term solution for the treatment of allergy is allergen–specific immunotherapy (SIT) by the administration of high doses of allergen or allergen peptides that specifically target T cells over a long period of time (16). Successful venom and aeroallergen immunotherapy is associated with the induction of peripheral tolerance in T cells by the generation of T reg cells that secrete suppressive cytokines, IL-10, and TGF-β, suggesting that generation of Tr1 cells may play a role in healthy immune responses (12, 17, 18).
Histamine and four different histamine receptors (HRs) constitute a multifaceted system with distinct functions of receptor types because of their differential expression, which changes according to the stage of cell differentiation and influences of the microenvironment (19). It has been demonstrated that distinct patterns of HR expression on Th1 and Th2 cells determine reciprocal T cell responses after histamine stimulation (15). Th1 cells show predominant but not exclusive expression of HR1, whereas Th2 cells show increased expression of HR2. Histamine enhances Th1-type responses by triggering the HR1. In contrast, the HR2 seems to be a potent suppressor of T cell functions (15). The expression and role of these receptors in CD4+ T cells of healthy individuals during high dose allergen exposure was given particular focus in the present study.
Investigation of allergen-specific peripheral T cell response in nonallergic beekeepers in and out of the beekeeping season has enabled us to study essential questions in peripheral T cell tolerance. One bee sting contains ∼50 μg of protein consisting of several different antigens that are directly inoculated into the skin. The major allergen phospholipase A2 (PLA) is responsible for allergic reactions in almost all of the sensitized individuals and comprises ∼10–20% of bee venom's protein content (20). In the present study, we used several direct methods to analyze human-specific T cell response in beekeepers, who were followed for several consecutive years during and outside beekeeping season. This represents a relevant model to investigate mechanisms of immune tolerance to high dose allergen exposure in humans. We demonstrated that high dose allergen tolerance is associated with clonal expansion of specific IL-10–secreting Tr1 cells, which may switch in vivo from existing Th1- and Th2-like allergen-specific cells. Increased expression of HR2 on Th2 cells a few days after allergen exposure plays a suppressor role in these cells and increases IL-10 production.
RESULTS
High dose allergen exposure via skin decreases cutaneous late-phase responses and induces unresponsiveness in allergen-specific T cells
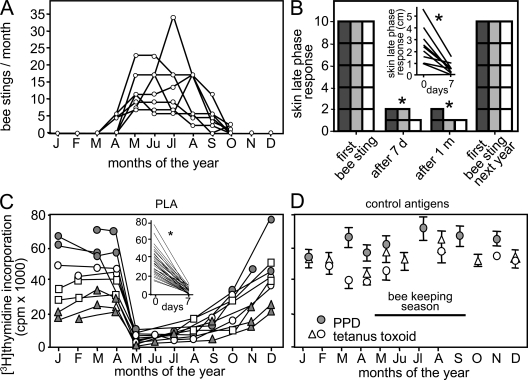
The beekeepers that were investigated in this study are not allergic to bee venom and develop cutaneous late-phase responses in response to bee stings. They do not wear protective masks or gloves and receive numerous bee stings as a result of their occupation. The beekeeping season begins in the last week of April and continues until October (Fig. 1 A). During the first week of the season, beekeepers received an average of 13 bee stings, which resulted in the development of cutaneous late-phase responses (Fig. 1 B), but did not induce fever, swelling of the draining lymph nodes, or splenomegaly. Exposure to bee venom antigens induced a significantly milder cutaneous late-phase swelling response after the first week, which continued throughout the season. At the beginning of the next beekeeping season, skin reactions to bee stings returned to the levels seen during the first week of the previous season and declined thereafter (Fig. 1 B). This pattern repeated for three consecutive years. The inset shows the initial size of the skin lesions in 9 beekeepers, which decreased significantly after 7 d (Fig. 1 B). T cells play a major role in cutaneous late-phase responses, and lesion size correlates with infiltrating T cell numbers (21). Peripheral blood T cells in the beekeepers proliferated in response to the major bee venom allergen PLA during the first 4 mo of the year. Shortly after the beginning of the beekeeping season, PLA-specific T cell proliferation declined dramatically and then returned to initial levels approximately 2 mo after the end of the beekeeping season (Fig. 1 C). The same T cell response profile was observed every year, demonstrating a short-lived peripheral T cell unresponsiveness. This unresponsiveness appeared to be antigen specific, as T cell proliferation in response to purified protein derivative (PPD) and tetanus toxoid (TT) antigens did not change in response to bee stings (Fig. 1 D).
Figure 1.
Decreased antigen-specific T cell response and cutaneous late-phase response after natural high dose antigen exposure. (A) The number of bee stings per month in nine beekeepers is. (B) Decreased cutaneous late-phase response after bee stings returns to initial levels upon no exposure. Five beekeepers who showed large cutaneous late-phase responses at the beginning of each beekeeping season were followed for 3 yr (2003–2005). The lesion size was graded as follows: 2, >3 cm; 1, 0–3 cm; and 0, no swelling (dark gray bar, 2003; gray bar, 2004; open bar, 2005). The scale of 10 demonstrates that in all of the five beekeepers, the skin lesion size was >3 cm at the beginning of each season. (inset) The direct measurement of cutaneous late-phase response lesion size in nine beekeepers in 2007 before and 7 d after bee stings. (C) PLA-induced [3H]thymidine incorporation in PBMCs after 5 d in two beekeepers followed for three years (gray circles and triangles) and two beekeepers followed for two consecutive years (open squares and circles; 67 peripheral blood samples). (inset) PLA-induced [3H]thymidine incorporation significantly decreased in all 29 experiments before and 7 d after bee stings between 2001 and 2007. (D) T cell proliferation against control antigens (PPD and TT) did not show any change in three beekeepers throughout the year. *, P < 0.001.
Increased IL-10–secreting Tr1-like T cell responses immediately after high dose venom exposure
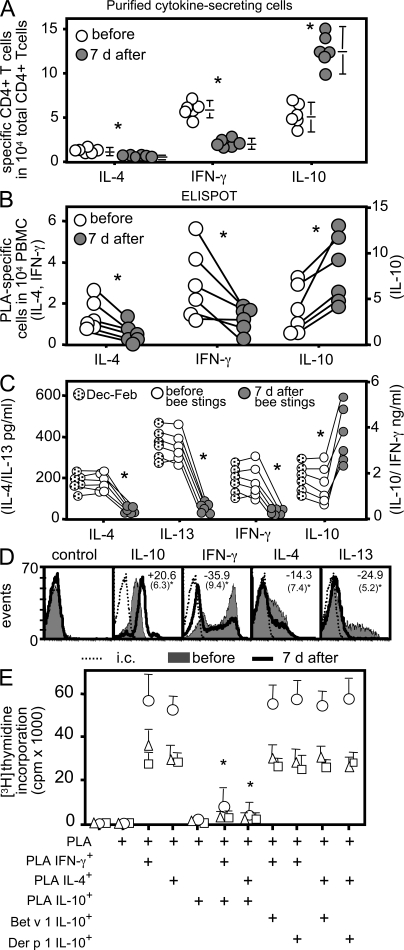
To investigate the mechanism underlying the T cell unresponsiveness, PLA-specific T cells were characterized before and after multiple bee stings. The frequency of PLA-specific T cells was determined according to their IL-4, IFN-γ, and IL-10 secretion profiles before and 7 d after the beginning of the beekeeping season. The overall number of PLA-specific T cells showed a slight increase from 12.4 to 14.8 in 10,000 CD4+ T cells. However, the number of IL-10–producing T cells increased dramatically after 7 d, from 5.52 ± 1.93 to 11.87 ± 3.12 in 10,000 CD4+ T cells (Fig. 2 A). The number of IL-4–producing CD4+ T cells, in contrast, decreased from 1.23 ± 0.32 to 0.43 ± 0.17, and IFN-γ–secreting T cells decreased from 5.66 ± 0.92 to 2.49 ± 0.76 after the first 7 d of the season. Similar changes were also seen in the frequency of IL-10, IFN-γ–, and IL-4–secreting T cells by ELISPOT (Fig. 2 B). Although the ELISPOT data were more variable, no statistically significant differences in cytokine-producing T cell numbers were noted between the two techniques. The shift in the frequency of cytokine-secreting T cells was consistent with changes in the overall cytokine profile of PLA-specific T cells after multiple bee stings (Fig. 2, C and D). Outside of beekeeping season (from December to February), PLA-specific T cells predominantly produced IL-10 and IFN-γ, as well as lower levels of IL-4 and IL-13. 7 d after the onset of the season (and after receiving multiple bee stings), PLA-stimulated T cells produced mostly IL-10 and very little IFN-γ, IL-4, or IL-13 (Fig. 2 C).
Figure 2.
Increased allergen-specific Tr1 cells and decreased Th1 and Th2 cells after bee stings. (A) PLA-specific IL-4–, IL-10–, and IFN-γ–secreting CD4+ T cells were purified 12 h after PLA stimulation, and their frequencies were calculated in six beekeepers before the beginning of beekeeping season and after 7 d. They received an average of 13 bee stings. Data are expressed as means + SEM. (B) Frequency of PLA-stimulated cytokine-secreting cells in six beekeepers by ELISPOT assay. (C) Secreted cytokines in PLA-stimulated PBMC cultures were measured by ELISA (IL-4 after 24 h, and IL-13, IFN-γ, and IL-10 after 5 d). (D) PBMCs from beekeepers were stimulated with PLA for 10 d, and intracytoplasmic cytokines were determined 12 h after anti-CD2/-CD3/-CD28 mAb stimulation. The mean percent difference (standard deviations are in parentheses) of eight experiments is shown before and 7 d after multiple bee stings inside each histogram. (E) PLA-specific IL-4–, IL-10–, and IFN-γ–secreting and Der p 1– and Bet v 1–specific IL-10–secreting T cells were purified from beekeepers after multiple bee stings. Their frequency was calculated in CD4+ T cells, and PBMCs were immediately reconstituted by increasing their frequency in cultures by 10 times. Cells were stimulated with PLA, and [3H]thymidine incorporation was determined after 5 d. Three experiments are shown. Data are expressed as means + SEM. *, P < 0.001. i.c., intracutaneous.
We next tested whether the shift in cytokine-producing cells affected T cell proliferation. PLA-specific IFN-γ–, IL-4–, and IL-10–secreting T cells were purified and added back to cultures of autologous PBMCs, such that their frequency was ∼10 times higher than their initial levels in PBMCs (Fig. 2 E). IL-10–secreting T cells specific for the unrelated allergens Bet v 1 and Der p 1 were purified from the same beekeepers. Proliferation of PBMCs in response to PLA, which was abolished after the onset of bee stings, was restored by adding PLA-specific IFN-γ– or IL-4–producing cells to the cultures (Fig. 2 E, columns 1–4). If IL-10–secreting T cells were added alone or with IFN-γ– or IL-4–producing cells, proliferation was not restored (Fig. 2 E, columns 5–7). In this case, PLA-specific IL-10–secreting T cells significantly suppressed the proliferation of PLA-specific IL-4– and IFN-γ–secreting T cells. The addition of IL-10–producing cells specific for Bet v 1– or Der p 1–specific IL-10–secreting T cells had no effect on PLA-stimulated T cell proliferation (Fig. 2 E, columns 8–11), demonstrating that the induction of T cell unresponsiveness was allergen specific.
In vivo switch of allergen-specific Th2 and Th1 cells toward IL-10–secreting Tr1 cells
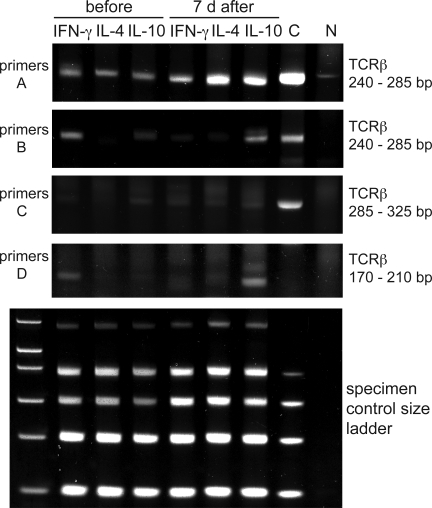
The shift in the cytokine profile to a predominantly IL-10–producing population 7 d after the onset of bee stings in vivo suggested that there was an increase in the number of allergen-specific Tr1 cells. We next asked whether this switch occurs as a result of the clonal expansion of existing antigen-specific memory Th1 and Th2 cells or whether new Tr1 cells were generated from the naive T cell pool. This was analyzed in two ways. First, we determined, the clonality of IL-4–, IL-10–, and IFN-γ–secreting T cells by combining PCR analysis to assess clonal TCR β chain gene rearrangements with heteroduplex analysis to differentiate clonal and nonclonal PCR products. Using primers spanning different parts of TCR variable β (TCRVβ) chain, junction, and diversity regions (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20080193/DC1), we found that purified PLA-specific IFN-γ–, IL-4–, and IL-10–secreting T cells consisted of clonal T cell populations (Fig. 3). The clonality of the PLA-specific T cells increased in the IL-10–secreting population 7 d after the onset of beekeeping season but decreased (primer set B) or disappeared (primer set D) in the IFN-γ–secreting T cells. Interestingly, the clone detected by primer set D disappeared from the IFN-γ–secreting population after the onset of the beekeeping season and appeared in the IL-10–secreting population.
Figure 3.
Clonality and switch to IL-10–secreting T cells after bee stings. TCRVβ gene clonality was analyzed in PLA-specific IL-4–, IL-10–, and IFN-γ–secreting T cells before and 7 d after multiple bee stings. A clonal sample (C) and a nonclonal sample (N) were included as controls. The PCR products of three different test tubes were run on three different gels, and a band that results from a clonal sample appears in a range from 240 to 285 bp (primers A, tube 1), from 240 to 285 bp (primers B, tube 2), and from 285 to 325 bp and from 170 to 210 bp (primers C and D, tube 3). The specimen control size ladder master mix generates a series of amplicons to ensure that the quality and quantity of input DNA were sufficient for the test. Heteroduplex analysis of the PCR products (except for the specimen control size ladder) on 6% TBE polyacrylamide gels stained with ethidium bromide is shown. One representative out of three experiments is shown.
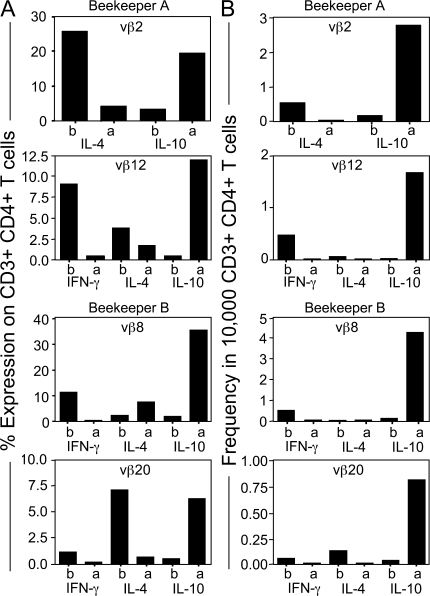
To further support an in vivo switch to Tr1 cells, we determined the frequency of TCRVβ chains among purified PLA-specific IL-4–, IL-10–, and IFN-γ–secreting T cells that were expanded in vitro with IL-2 and irradiated autologous PBMCs (Fig. 4 A). 7 d after the onset of beekeeping season, TCRVβ2-positive T cells from beekeeper A switched from a predominantly IL-4–secreting profile to a predominantly IL-10–secreting profile (Table S1, available at http://www.jem.org/cgi/content/full/jem.20080193/DC1). In addition, TCRVβ12-positive T cells switched from a predominantly IFN-γ– and IL-4–secreting profile to an IL-10–secreting profile. In beekeeper B, an increase in IL-10–producing TCRVβ8- and TCRVβ20-positive T cells occurred after the onset of bee stings, whereas cells producing IFN-γ and IL-4 decreased in these populations (Table S1). We then purified cytokine-secreting CD4+ T cell populations before and after the onset of bee stings and determined the frequency of TCRVβ-expressing CD4+ T cells among these populations (Fig. 4 B). Table S2 shows the overall numbers of cytokine-producing cells (per 10,000 CD4+ T cells) before and after the onset of beekeeping season in beekeepers A and B. When the expression of TCRVβ genes was assessed in these populations, a striking shift toward IL-10 secretion was seen among TCRVβ2- and Vβ12-expressing cells in beekeeper A (Fig. 4, A and B). In the Vβ2- and Vβ12-expressing populations, IL-10–secreting cells expanded from 0.2 to 2.9 and from 0.1 to 1.8 T cells per 10,000 CD4+ T cells, respectively. In beekeeper B, IL-10–secreting cells among the TCRVβ8- and Vβ20-positive populations expanded from 0.1 to 4.3 and 0.05 to 0.8 T cells per 10,000 CD4+ T cells, respectively. Collectively, these data suggest that continued exposure to bee venom allergen induces an in vivo expansion of IL-10–producing, allergen-specific CD4+ T cells at the expense of IFN-γ–producing (Th1) and IL-4–producing (Th2) cells.
Figure 4.
PLA-specific CD4+ IL-4– and IFN-γ–secreting CD4+ T cells switch to IL-10–secreting T cells. (A) Two beekeepers were analyzed for TCRVβ chain expression in purified PLA-specific IL-4–, IL-10–, and IFN-γ–secreting T cells by gating CD3+CD4+ T cells by flow cytometry. (B) PLA-specific IL-10–, IFN-γ–, and IL-4–secreting T cells were purified from two beekeepers before and 7 d after multiple bee stings. They were stimulated either with PLA (beekeeper A Vβ2) or anti-CD3 mAb (all of the other panels) in the presence of irradiated autologous PBMCs and were expanded with IL-2 for 12 d. The entire panel of TCR mAbs was used for staining. A significant switch in TCRVβ2 and 12% (beekeeper A) and TCRVβ8 and 20% (beekeeper B) of positive cells between T cell subsets after bee stings is shown. A change in TCRVβ-expressing CD4+ T cell frequency is accepted as significant if an increase in the frequency of IL-10–secreting T cells is observed together with a decrease in either IFN-γ– or IL-4–secreting T cells that is two times bigger than the average of four healthy controls. (B) The changes in the frequency of TCRVβ-expressing CD4+ T cells was calculated by multiplying the percentage of TCRVβ with the frequency of the same cytokine-secreting PLA-specific CD4+ T cell subset.
Role of HR2 in peripheral T cell tolerance
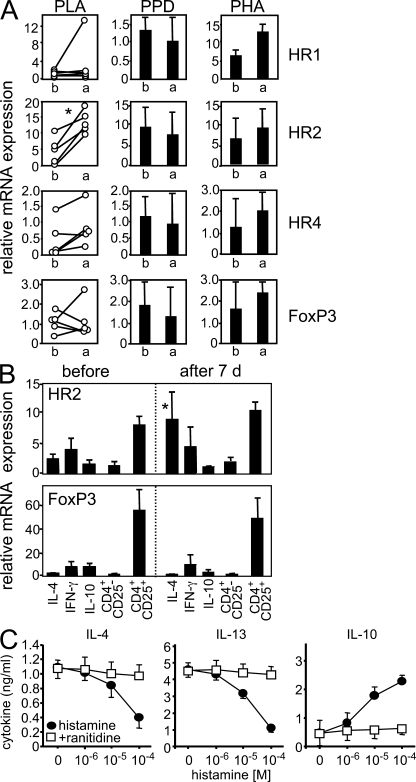
Exposure to bee venom induces the degranulation of mast cells and basophils (below the threshold of systemic anaphylaxis), and histamine is one of the major mediators of degranulation (20). Functional HRs are expressed on T cells, but so far their role in regulating immune responses after allergen exposure in humans has not been studied in detail. The expression of HR2 mRNA on allergen-specific T cells increased significantly 7 d after the onset of beekeeping season (Fig. 5 A). However, there was no change in mRNA levels of HR1, HR4, or FoxP3 in control cells stimulated with PHA or PPD. After bee stings, HR2 expression was increased exclusively on IL-4–secreting T cells and did not change on IFN-γ– or IL-10–secreting T cells (Fig. 5 B). The expression of FoxP3 was relatively low in allergen-stimulated cells compared with purified CD4+CD25+ cells and did not change in either population after the onset of beekeeping season. There was also no change in the overall percentage of CD4+CD25+ T cells in response to bee stings (unpublished data). Next, we investigated whether the increased expression of HR2 on IL-4–producing Th2 cells affects their subsequent production of IL-10 in response to histamine. PLA-specific IL-4–secreting T cells were purified and stimulated with increasing doses of histamine in the presence or absence of the HR2 antagonist ranitidine. Histamine treatment decreased the production of IL-4 and IL-13 and increased the production of IL-10 production in a dose-dependent fashion (Fig. 5 C). This effect depended on HR2 expression, as it was completely reversed by ranitidine (Fig. 5 C).
Figure 5.
HR2 is up-regulated in specific T cells after multiple bee stings and induces IL-10 production. (A) PBMCs were stimulated with PLA for 5 d. HR1, HR2, HR4, and FoxP3 mRNAs were determined 4 h after anti-CD3/-CD28 mAb stimulation before and 7 d after multiple bee stings. For comparison, PPD- and PHA-stimulated cells did not show any difference. (B) HR2 and FoxP3 were analyzed in purified allergen-specific IL-4–, IL-10–, and IFN-γ–secreting T cells immediately after purification and in CD4+CD25+ and CD4+CD25− T cells. (C) PLA-specific IL-4–secreting T cells were purified, expanded for 12 d, and stimulated with different doses of histamine in the presence or absence of 10−4 M ranitidine. Cytokines were determined by ELISA 3 d after anti-CD2/-CD3/-CD28 mAb stimulation. Data are expressed as means + SEM.
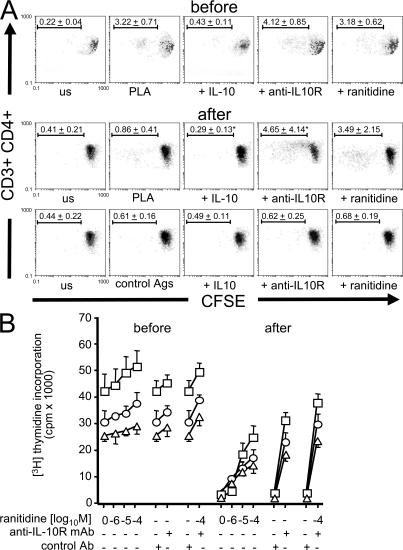
The relative roles of HR2 and IL-10 in allergen-specific T cell unresponsiveness was investigated by measuring the proliferation of PBMCs in response to PLA in the presence of ranitidine or an anti–IL-10R blocking mAb. Before the onset of beekeeping season, PLA stimulation induced proliferation in 3.22 ± 0.71% of CD3+CD4+ T cells, and IL-10 suppressed this proliferation to background levels. In PBMCs taken 7 d after the onset of beekeeping season, proliferating CD3+CD4+ T cells significantly decreased to 0.86 ± 0.41% of CD3+CD4+ cells but were rescued by blocking the IL-10R or HR2. Blocking both receptors had an additive effect (Fig. 6, A and B).
Figure 6.
HR2 and IL-10 play major roles in peripheral T cell tolerance to high dose antigen exposure. (A) PBMCs of beekeepers were labeled with CFSE, and dilutions in CFSE-expressing cells were analyzed in the presence of IL-10, anti–IL-10R mAb (blocking), and 10−4 M ranitidine before and 7 d after multiple bee stings. BSA, TT, and bee venom allergen hyaluronidase were used as control antigens in beekeepers who did not show significant proliferation to these antigens. On day 6, cells were collected, stained with anti-CD4 PE and anti-CD3 PE Texas red and analyzed by flow cytometry (means ± standard deviation of percentages of dividing cells are shown in one representative out of three beekeepers). us, unstimulated. (B) PBMCs of three different beekeepers were stimulated with PLA and different doses of ranitidine or anti–IL-10R mAb or both. [3H]Thymidine incorporation was determined on day 6. Data are expressed as means + SEM.
Predominant role of IL-10 but not TGF-β in skin-related tolerance
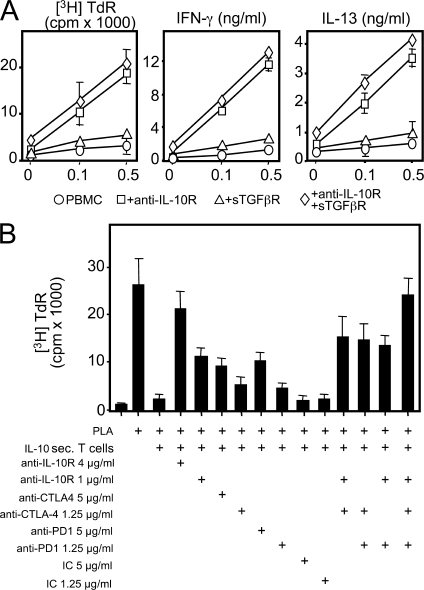
Multiple suppressive mechanisms play a role in suppression of allergen-specific Th2 cells. IL-10 and TGF-β have been suggested to cooperate in suppression of mucosal allergen-specific T cell activation (12). In our system, IL-10 appeared to play a more dominant role than TGF-β in suppression of PLA-specific T cell responses, as blocking the IL-10R, but not the TGF-β receptor, reconstituted T cell proliferation and cytokine production in PBMCs isolated after the onset of beekeeping season (Fig. 7 A). The suppressive effect of IL-10 on the expression of both IFN-γ and IL-13 was comparable. Two other mechanisms also appeared to function in immune tolerance to high dose venom allergens, as the neutralization of either CTL-associated antigen (CTLA)-4 or programmed death (PD) 1 significantly reversed suppression of PLA-specific T cell proliferation (Fig. 7 B). As noted earlier, PLA-induced T cell proliferation was significantly higher in PBMCs from beekeepers before being stung (Fig. 7 B, column 2), and suppression of proliferation could be achieved by increasing the frequency of PLA-specific IL-10–secreting T cells by 10-fold (Fig. 7 B, column 3). This suppressive effect was partially inhibited by blocking CTLA-4, PD-1, or IL-10R (Fig. 7 B, columns 4–9). Blocking two of the receptors simultaneously also partially inhibited the suppressive effect of IL-10–secreting T cells (Fig. 7 B, columns 12–14). Blocking all three receptors simultaneously had an additive effect in reconstitution of suppressed allergen-specific T cell proliferation (Fig. 7 B, column 15).
Figure 7.
Multiple suppressive mechanisms play a role in peripheral allergen tolerance. (A) Endogenous IL-10, TGF-β, or both were neutralized in PLA-stimulated PBMCs of beekeepers during beekeeping season. [3H]Thymidine incorporation (TdR), IFN-γ, and IL-13 were determined at day 5. (B) PLA-specific proliferation of PBMCs from beekeepers before multiple bee stings is suppressed by a 10 times–increased frequency of PLA-specific IL-10–secreting T cells. The activity of IL-10R, CTLA-4, and PD-1 was neutralized. [3H]Thymidine incorporation was determined at day 6. The same results were obtained in six independent experiments in A and three independent experiments in B, all performed with freshly purified cells without in vitro expansion. Data are expressed as means + SEM. IC, isotype control antibody.
DISCUSSION
The immune system must distinguish between innocuous and pathological antigens to prevent unnecessary and self-destructive immune responses (22, 23). The present study focuses on how soluble protein antigens encountered via skin exposure are tolerated. Key findings of the present study concern the time-span and allergen-dependence of allergen-specific peripheral T cell tolerance. The cytokine profile of memory T cells specific for PLA show an IL-10– and IFN-γ–predominant profile outside of beekeeping season. Upon bee venom exposure, an immediate switch of allergen-specific T cells from Th1 and Th2 cells toward IL-10–secreting Tr1 cells occurs within a few days. These sequential events are repeated every year. At the end of every season when there is no exposure, the peripheral T cell response returns to the same levels as seen before exposure within 2 to 3 mo. The changes in cytokine profile, T cell proliferation, and cutaneous late-phase response show parallel changes, demonstrating that the T cell tolerance induced by an in vivo switch to Tr1-like IL-10–secreting cells is short lived and dependent on antigen exposure. There is so far no report on in vivo turnover and life span of Tr1 cells in humans, but our data are consistent with the life span of conventional T reg cells analyzed by Vukmanovic-Stejic et al., which showed that the in vivo doubling time of FoxP3+CD25hiCD4+ T cells was relatively short (8 d) in resting conditions compared with FoxP3−CD25−CD4+ T cells (24 d) (24). Interestingly, when bee venom contact ceased, the Tr1 response substantially decreased within 2 mo after the end of the season.
Another essential finding of the present study demonstrates in vivo switch in allergen-specific IL-4– and IFN-γ–producing T cells toward IL-10–producing T cells. The consistent profile of PLA-specific T cell proliferation in beekeepers throughout consecutive years demonstrates a biphasic T cell response with substantial peripheral T cell tolerance upon high dose allergen exposure. The long-term PLA-specific T cell repertoire has been maintained in a balance of 42% IFN-γ–secreting T cells, 43% IL-10–secreting T cells, and 15% IL-4 secreting T cells. Immediately after bee stings, the repertoire changed and consisted of 80% IL-10–secreting T cells, 14% IFN-γ–secreting T cells, and 6% IL-4–secreting T cells. The stability of cytokine profiles in differentiated effector and memory T cell subsets in humans is not fully known, and it has been demonstrated that lineage-committed memory T cell subsets are responsive to cytokine signals from the opposing lineage (25, 26). The frequency of single PLA-specific CD4+ Tr1 cells ranges between 1 in 1,000 and 1 in 20,000 of the whole CD4+CD25+ T reg pool. The T cell epitope restriction pattern of PLA varies considerably from patient to patient, and at least four epitopes have been demonstrated (27, 28). In the present study, PLA-specific T cells demonstrated a clonality throughout the whole season. In both experiments, which investigated their TCR clonality by PCR and flow cytometry, an immediate switch to IL-10–producing Tr1 cells was observed in vivo after bee stings. Similarly, IL-10–secreting allergen-specific T cells represented the predominant subset and were present at a significantly higher frequency than IL-4– and IFN-γ–secreting T cells in food- and aeroallergen-sensitized individuals who did not develop an allergic response (12). Our data suggest that other mechanisms may be operative in addition to switch from one T cell subset to the other. For example, the percentage of IL-10–secreting T cells decreased in beekeeper B in Vβ2 and Vβ3-expressing T cells. The percentage of both IL-4– and IL-10–secreting cells increased in beekeeper A in TCRVβ5.1- and -12-positive T cells. One explanation for this is that the cytokine profile of these purified subsets consisted of some Th0 cells, which secrete both Th1 and Th2 cytokines as well as IL-10 and TGF-β. Further studies are needed to determine whether activation-induced cell death and deletion of T cells takes place in vivo.
Allergen-SIT in humans has been used successfully to treat allergies by inducing responsive cells to secrete IL-10, and this is analogous to the studies of low-zone tolerance (29). The IL-10–producing CD4+ Tr1-cell population is induced by T cell epitope peptide immunotherapy and whole allergen SIT (12, 17, 18, 21, 30, 31). Tr1 cells specific for a variety of antigens arise in vivo but may also differentiate from naive CD4+ T cells in the presence of IL-10 in vitro (32). The nonspecific T cell suppressor activity of IL-10 and TGF-β has been consistently reported in experiments with high amounts of exogenously added suppressor cytokines (18). However, the present study demonstrates that Tr1 cells display antigen-specific suppressor activity in very low numbers. It can be hypothesized that depending on their frequency, the first T cell that contacts the APC may be very decisive for inducing or suppressing the generation of a specific immune response. Accordingly, if the first T cell to contact the APC is a Tr1 cell, it may silence or down-regulate the maturation of the APC. IL-10 down-regulates antigen-presenting capacity such as HLA-DR expression, co-stimulatory molecules, and several cytokines in dendritic cells and monocytes/macrophages (33). Differentiation of a distinct dendritic cell subset in the presence of IL-10 has been demonstrated, which induces tolerance through the generation of Tr1 cells (34).
The present study demonstrates that HR2 represents an essential receptor that participates in peripheral tolerance to allergens by the induction of IL-10 and direct suppression of the proliferation of allergen-specific T cells. HR2 is a potent suppressor of several inflammatory and effector functions. Histamine induces the production of IL-10 by dendritic cells (35) and enhances the suppressive activity of TGF-β on T cells via HR2 (36). In the differentiation process of monocyte-derived dendritic cells, HR2 acts as a suppressive molecule for antigen-presentation capacity, suppresses IL-12 production, and enhances IL-10 production (35, 37). All of these immune tolerance–promoting effects are mediated via HR2, which is relatively highly expressed on Th2 cells and suppresses IL-4 and IL-13 production as well as T cell proliferation (15).
Allergen-specific T cell tolerance uses multiple suppressor mechanisms. The roles of CTLA-4 and PD-1 are demonstrated in the present study, in addition to IL-10R and HR2. CD4+CD25+ T cells are the only lymphocyte subpopulation in both mice and humans that expresses CTLA-4 constitutively. Its expression apparently correlates with the suppressor function of CTLA-4 (38). As demonstrated in the present study for Tr1 cells, the blocking of CTLA-4 activity has been shown to reverse the suppression in co-cultures of CD4+CD25+ and CD4+CD25− T cells (38). Similarly, the treatment of mice, which are recipients of CD4+CD45RBlow T cells with CTLA-4-blocking agents, abrogated the suppression of inflammatory bowel disease (39). These studies indicate that the engagement of CTLA-4 on the CD4+CD25+ T cells by antibody or by CD80/CD86 might lead to inhibition of the TCR-derived signals that are required for the induction of suppressor activity. PD-1 is an immunoreceptor tyrosine-based inhibitory motif–containing receptor expressed upon T cell activation. PD-1–deficient mice develop autoimmune diseases, suggesting an inhibitory role for PD-1 in immune responses (40). Members of the B7 family, PD-ligand (L) 1 and PD-L2, are ligands for PD-1. PD-1/PD-L engagement on mouse CD4 and CD8 T cells results in inhibition of proliferation and cytokine production. T cells stimulated with anti-CD3/PD-L1Fc–coated beads display dramatically decreased proliferation and IL-2 production (41).
This model represents a high dose of allergen inoculation via skin. Induction of clinical tolerance parallel to immunological tolerance should be emphasized here, because these beekeepers showed local cutaneous late-phase reactions characterized by local swelling during the first few bee stings of every new season, which significantly decreased after 1 wk. There is no detailed report on the characterization of cellular infiltration in natural bee venom–induced cutaneous late-phase response. Biopsies of skin late-phase responses were investigated in several studies with other allergens. It was demonstrated that CD3+ T cell infiltration starts at 6 h, peaks at 24 h, and declines after 48 h. CD3+CD45RO+ T cells, eosinophils, and macrophages dominate the lesions without neutrophils (42, 43). The kinetics and the type of infiltrating cells in allergen-induced skin late-phase lesions are clearly different compared with delayed-type hypersensitivity lesions, which peak at 72 h, and are characterized with infiltrating T cells and macrophages, without eosinophils (24). A significant decrease in skin late-phase lesion size and T lymphocyte and eosinophil infiltration has been demonstrated in allergen-SITs performed by the injection of allergen extracts via conventional allergen-SIT and T cell epitope peptide-SIT (21, 30, 31).
Immune response during the first confrontation of the allergens with the immune system depends on multiple factors. Biochemical properties of the allergen; other innate immune response stimulating substances in the milieu of the allergen at the time of exposure (within the same extract or coexposure with an infection or a vaccine); stability of the allergen in the tissues, digestive system, skin, or mucosa; and, finally, dose and time during the interaction with the immune system play essential roles in immune response and sensitization (23). The doses of venom allergens received during natural bee stings in the bee venom model can be estimated. The average of 13 bee stings corresponds to a high dose of allergen exposure, consisting of at least 65 μg of PLA inoculated intracutaneously. It is commonly known that a single Hymenoptera sting is sufficient to induce allergic sensitization. It is related to the number of stings and the short interval between the two stings (44). In the first month after a bee sting, >30% of adults show allergic sensitization. Skin tests become negative in 30% of patients after 2 yr and in virtually 50% after 3 yr (44). For indoor and outdoor allergens, the total amount of exposure is essentially impossible to quantify, but the risk for natural sensitization in nonatopic individuals, except for occupational exposure such as in lawn cutters, is very low (45).
Studies on T cell response to allergens in healthy individuals have demonstrated a wide range of immune response, from no detectable response to involvement of active peripheral tolerance mechanisms (17). In a high number of healthy individuals, T cells do not show any proliferative response to allergens in PBMC cultures. This can be caused by a low frequency of specific T cells caused by lack of exposure or exposure below the threshold of a sensitizing dose. Active suppression against allergens by T reg cells occurs in sensitized healthy individuals (12, 46). In individuals, who show a detectable IgG4 response, a balanced allergen-specific immune response caused by the expansion of Tr1 cells has been demonstrated (12). Although all of the beekeepers included in the present study had detectable IgE against PLA, they showed extremely high specific IgG4, which could be one of the reasons for the prevention of severe anaphylaxis. Their serum-specific IgE was in the range of allergic individuals, but specific IgG4 was a few hundred times higher than allergic individuals (47). Collectively, our results indicate that the control of Th2 and Th1 immune responses against naturally exposed harmless environmental antigens is mediated by Tr1 cells in humans. Effector (allergen-specific Th2) and suppressor (allergen-specific Tr1) T cells exist in both healthy and allergic individuals in certain amounts. Their ratio determines the development of a healthy or an allergic immune response. These data may explain the spontaneous development and spontaneous remission of allergic diseases. In addition to allergy, these mechanisms may have implications in autoimmunity, graft-versus-host disease, tumor cell growth, parasite survival and chronic infections.
MATERIALS AND METHODS
Study population and allergens.
10 beekeeper volunteers (mean age = 58 yr, range = 34–71 yr; 9 male and 1 female; average beekeeping time = 36 yr, range = 17–46 yr) who reported that they do not use protective equipment and show cutaneous late-phase swelling response to bee stings in the beginning of each season were studied. Five beekeepers were followed for 3 yr between 2003 and 2005. Physical examination was performed after 24 h, and the diameters of skin late-phase lesions were measured (21, 42, 43). Lesion size was graded as follows: 2, >3 cm; 1, 0–3 cm; and 0, no swelling. All of the beekeepers had detectable IgE against bee venom PLA (mean = 7.55 ± 1.63 RU/ml), and they showed extremely high IgG4 (2,493 ± 418 ng/ml). Four healthy individuals (mean age = 43 yr, range = 34–47 yr; two male and two female) were used as controls. The study was approved by the ethical commission of the Canton of Grissons, Switzerland. Recombinant PLA of honey bee venom (Apis mellifera) was produced in house (48). Hyaluronidase of bee venom (a gift from U. Müller, Spital Bern, Bern, Switzerland), rBet v 1 of birch pollen (Betula verrucosa), rDer p 1 of house dust mites (Dermatophagoides pteronyssinus; both from Allergopharma Joachim Ganzer KG), PPD of Mycobacterium bovis, and TT (both from Serum Institute) were used as control antigens. None of the allergens contained detectable amounts of LPS and all were >99% pure.
Purification of allergen-specific IL-4–, IFN-γ–, and IL-10–secreting cells.
PBMCs were isolated by Ficoll (Biochrom) density gradient centrifugation of peripheral venous blood, and cells were washed three times and resuspended in RPMI 1640 medium supplemented as previously described (12). 2.5 × 107 cells were stimulated with 0.3 μM of antigens in 5 ml of medium in 6-well plates in duplicates (Costar Corp.). After 12 h of stimulation in humidified 5% CO2, cells were harvested and labeled with 50 μg/ml anti–IFN-γ/CD45, anti–IL-4/CD45, or anti–IL-10/CD45 antibody-antibody conjugates (Miltenyi Biotec) for 10 min at a concentration of 108 cells/ml in ice-cold RPMI 1640 medium (49). The cells were diluted with 37°C warm medium to a final concentration of 106 cells/ml and were allowed to secrete and capture the respective cytokines for 45 min at 37°C. After capturing the secreted cytokines on their surface, cells were centrifuged at 300 g for 5 min at 4°C and resuspended at a concentration of 108 cells/ml in ice-cold buffer containing 0.5% BSA and 5 mM EDTA (both from Sigma-Aldrich) in PBS. The cells were then stained with 5 μg/ml PE-conjugated anti–IFN-γ, anti–IL-10, or anti–IL-4 for 10 min at 4°C. The cells were washed and resuspended in BSA-EDTA PBS (108 cells/ml) and magnetically labeled for 15 min at 4°C with 20 μl/107 cells of anti-PE microbeads (Miltenyi Biotec). After washing, labeled cells were purified by immunomagnetic separation (AutoMacs; Miltenyi Biotec). The cells were counterstained by FITC-labeled anti-CD4 mAb (Beckman Coulter) and were analyzed in a flow cytometer (Epics XL; Beckman Coulter), and their percentage in whole CD4+ T cells and frequency were calculated. Together with the quantitative cytokine mRNA and secreted cytokine profiles, these data demonstrate that antigen-specific Tr1, Th1, and Th2 cells can be purified from human peripheral blood (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20080193/DC1), and they are specific for the allergen that they are initially stimulated for in the purification (Fig. S3). The purity of allergen-specific CD4+ cytokine-secreting cells was between 88 and 96%. The frequency of allergen-stimulated and unstimulated cells was calculated by dividing the number of purified cytokine-secreting CD4+ T cells by the initial number of CD4+ T cells (Supplemental materials and methods).
T cell cultures.
Allergen-specific T cell proliferative response was determined by stimulation of 2 × 105 PBMCs alone or together with freshly purified allergen-specific cytokine-secreting T cells for 5 d with 0.3 μM of allergens, and 1 μg/ml TT and PPD in 200 μl of medium in 96-well flat-bottom tissue culture plates in triplicates (50). Cells were pulsed with 1 mCi/well [3H]thymidine (DuPont; New England Nuclear), and the incorporation of labeled nucleotide was determined after 8 h in an LKB β plate reader (GE Healthcare). PBMCs were stimulated for 5 d with 1 μg/ml PLA, PPD, or PHA, PHA (Sigma-Aldrich). HR1, HR2, HR4, and FoxP3 mRNAs were determined 4 h after 0.5 μg/ml anti-CD2, -CD3, and -CD28 mAb (CLB) stimulation before and 7 d after multiple bee stings. Cell proliferation was additionally measured by CFSE labeling. Cells were labeled with 5 μM CFSE (Invitrogen) and washed twice with medium before being subjected to the PLA stimulation. After 5 d, CD4+ cells were counterstained with PC5-labeled anti-CD4 and PE Texas red–labeled anti-CD3 mAbs (BD) and analyzed by flow cytometry.
Allergen-specific cytokine-secreting T cells were used immediately after purification in all experiments. IL-10 was neutralized in cultures with 4 μg/ml anti–IL-10R mAb (DNAX Research Institute) (51). TGF-β was neutralized in cultures with 100 ng/ml of recombinant human soluble TGF-β receptor II/Fc chimeric protein (R&D Systems) (18). PD-1 activity was neutralized in cultures with 5 μg/ml anti–human PD-1 (Bioscience Insight Biotechnology Limited). CTLA-4 activity was neutralized with 5 μg/ml anti–CD152 F(ab′)2 (Ancell; Enzo Biochem, Inc.). The neutralizing activity of all of these approaches was demonstrated in titrated doses. Rabbit IgG, rat IgG, mouse IgG1, or BSA (Beckman Coulter) served as controls.
Flow cytometry, ELISA, and ELISPOT.
PBMCs of beekeepers before and 7 d after they received multiple bee stings were stimulated with PLA for 10 d. Intracellular cytokines were detected after 12 h of anti-CD2, -CD3, and -CD28 mAb stimulation. 2 μM monensin (Sigma-Aldrich) was added during the last 10 h (11). The solid-phase sandwich ELISAs for IFN-γ, IL-4, IL-10, and IL-13 were performed in supernatants obtained at day 5 (11).
106 PBMCs/ml from six healthy donors were stimulated in 200 μl of medium in 96-well flat-bottom ELISPOT plates for 18 h (Euroclone Ltd.). Locally produced IL-4, IFN-γ, and IL-10 were captured by specific mAbs. After cell lysis, trapped cytokine molecules were revealed by a secondary biotinylated detection antibody, which is recognized by streptavidin conjugated to alkaline phosphatase. Colored (purple) spots developed after substrate addition were determined (ImmunoSpot; Cellular Technology Ltd.). The number of spots determined in triplicates of unstimulated wells was subtracted from 0.3 μM PLA–stimulated wells. 18 h was found to be the optimal time for the determination of frequency of cytokine-secreting cells, as it is the time point for the highest cytokine secretion before T cell proliferation starts.
Quantitative real-time PCR.
Immediately after purification, antigen-specific cytokine-secreting T cells were lysed with RNeasy lysis buffer, and the RNA was isolated using the RNeasy mini kit (QIAGEN) and eluted in 30 μl ddH2O. RT was performed with TaqMan RT reagents with random hexamers (Applied Biosystems). The PCR primers and probes were designed based on sequences available from GenBank/EMBL/DDBJ under the following accession nos.: EF1, NM_001402; IL-13, NM_002188; IFN-γ, NM_000619; IL-10, NM_000572; TGF-β1, NM_000660; HR1, NM_001098213; HR2, NM_001131055; HR4, NM_021624; and Foxp3, NM_014009. Primers were as follows: EF-1α forward, 5′-CTGAACCATCCAGGCCAAAT-3′; EF-1α reverse, 5′-GCCGTGTGGCAATCCAAT-3′; IL-13 forward A, 5′-GCCCTGGAATCCCTGATCA-3′; IL-13 reverse A, 5′-GCTCAGCATCCTCTGGGTCTT-3′; IFN-γ forward B, 5′-TCTCGGAAACGATGAAATATACAAGTTAT-3′; IFN-γ reverse B, 5′-GTAACAGCCAAGAGAACCCAAAA-3′; IL-10 forward, 5′-GGCGCTGTCATCGATTTCTT-3′; IL-10 reverse, 5′-TTGGAGCTTATTAAAGGCATTCTTC-3′; TGF-β1 forward, 5′-AAATTGAGGGCTTTCGCCTTA-3′; TGF-β1 reverse, 5′-GAACCCGTTGATGTCCACTTG-3′; HR1 forward, 5′-TCTCGAACGGACTCAGATACCA-3′; HR1 reverse, 5′-CCTGTGTTAGACCCACTCCTCAA-3′; HR1 probe, FAM-ACAGAGACAGCACCAGGCAAAGGCAA-TAMRA; HR2 forward, 5′-GCTGGGCTATGCCAACTCA-3′; HR2 reverse, 5′-GGTGCGGAAGTCTCTGTTCAG-3′; HR2 probe, FAM-CCCTGAACCCCATCCTGTATGCTGC-TAMRA; HR4 forward, 5′-GGGTCTTGAAGATTGTTACTCTGATG-3′; HR4 reverse, 5′-CTAGAATCATTGGCCCATTCACT-3′; HR4 probe, FAM-GCCAGCACCCAAACGGCCA-TAMRA; Foxp3 forward, 5′-CCCGGCCTTCCACAGAA-3′; and Foxp3 reverse, 5′-CACCCGCACAAAGCACTTG-3′ (all were from obtained from Microsynth AG). cDNAs were amplified using SYBR-PCR Master Mix (Applied Biosystems) according to the recommendations of the manufacturer in a total volume of 25 μl in an ABI PRISM 7000 Sequence Detection System (Applied Biosystems). Relative quantification was performed as previously described (52). All amplifications were performed in duplicates.
TCRVβ chain detection on CD4+ T cells.
PLA-specific IL-10–, IFN-γ–, and IL-4–secreting T cells were purified from beekeepers before and 7 d after multiple bee stings. They were stimulated either with PLA or anti-CD3 mAb (beekeeper A) in the presence of 3,000 rads of irradiated autologous PBMCs and expanded with 25 U/ml IL-2 (Proleukin; Novartis) for 12 d. Anti-CD3 stimulation was used in beekeeper A, because IFN-γ–secreting T cells did not expand with PLA stimulation. PBMCs of four healthy donors were used for controls. Cells were blocked for 10 min at room temperature with mouse serum (Dako) and were subsequently stained for 10 min with anti-CD3–FITC (clone UCHT1, IgG1 mouse; Beckman Coulter) and anti-CD4–PC5 (clone 13B8.2, IgG1 mouse; Beckman Coulter) or matching isotype controls. After washing, the cells were split into 22 samples and stained for 15 min with PE-labeled anti-TCRVβ 1, 2, 3, 5.1, 5.2, 5.3, 7, 8, 9, 11, 12, 13.1, 13.6, 14, 16, 17, 18, 20, 21.3, 22, and 23 mAbs or matching isotype controls (Beckman Coulter). The flow cytometry measurement was performed on an Epics XL MCL (Beckman Coulter). All data are shown in Table S1. The changes in TCRVβ expression were accepted as significant if the increase in IL-10–secreting T cells was two times greater than, or the decrease in either IFN-γ– or IL-4–secreting T cells was <50% of the average of four healthy controls. The frequency of CD4+ T cells with a certain TCRVβ expression was calculated by multiplying the percentage of TCRVβ with the frequency of the cytokine-secreting PLA-specific CD4+ T cell subset.
TCR clonality analysis.
PLA-specific IL-10–, IFN-γ–, and IL-4–secreting T cells were purified from beekeepers before and 7 d after multiple bee stings. They were stimulated with PLA in the presence of 3,000 rads of irradiated autologous PBMCs and expanded with 25 U/ml IL-2 (Proleukin) for 12 d. DNA was isolated with the DNA micro kit (QIAGEN), and clonality was determined using the IdentiClone TCRB Gene Clonality Assay (InVivoScribe Technologies, Inc.). This PCR-based assay identifies clonal TCR β chain gene rearrangements. Multiple consensus DNA primers that target conserved genetic regions within the TCR β chain gene were used to detect these rearrangements. Specific primers in two assay tubes (1:A and 2:B) target framework regions within the variable region and the joining region, and the third assay tube gave two PCR products and targets the diversity and joining regions (3:C/D). The specimen control size ladder master mix targets multiple genes and generates a series of amplicons to ensure that the quality and quantity of the input DNA are sufficient for the test. The DNA was added to each of the four assay tubes together with AmpliTaq Gold DNA Polymerase (Applied Biosystems), and the DNA was amplified using a standard program with a thermocycler (Mastercycler Gradient; Eppendorf). A heteroduplex analysis was done to differentiate clonal and nonclonal PCR products. PCR products from tubes 1, 2, and 3 were denatured at 94°C for 5 min and immediately chilled at 4°C in an ice water bath for 60 min. This allows reannealing of the clonal strands, which results in a single band within a polyclonal background on the gel. Normal or polyclonal DNA produces amplicons of different sizes, which results in a smear. PCR products were separated on 6% Novex TBE polyacrylamide gels (Invitrogen), stained with ethidium bromide, and scanned and analyzed using a BioImager Analyzer FLA-3000 with BASReader software (both from Fujifilm) and AIDA software (Raytest). Polyclonal and clonal control DNA were used to monitor the performance of the assay.
Statistical interpretation.
Data are expressed as means + SEM. The Wilcoxon rank sum test and the Mann-Whitney U test were used for statistical analysis.
Online supplemental material.
Table S1 demonstrates all TCRVβ measurements in three experiments with two beekeepers and four healthy individuals. Table S2 demonstrates changes in the frequency of PLA-specific cytokine-secreting T cells (in 10,000 CD4+ T cells) after bee stings are shown, which were used to calculate the frequency of the TCRVβ-expressing cells in CD4+ T cells depicted in Fig. 4 B. Fig. S1 shows the primers, target gene, and product size, used in the TCRB Gene Clonality Assay. Fig. S2 demonstrates that PLA-specific IL-4–, IFN-γ-, and IL-10–secreting T cells represent Th2-, Th1-, and Tr1-like cells, respectively. Fig. S3 depicts the antigen specificity of purified cytokine-secreting T cells as determined by stimulation with the allergen that was originally used for stimulation before purification, and several control antigens in the presence of autologous APCs. Supplemental materials and methods explains how the frequency of purified allergen-specific T cells was calculated and how the allergen-specific suppression experiment was performed. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20080193/DC1.
Supplementary Material
Acknowledgments
We thank Dr. C.H. Heusser for anti–IL-4 and anti–IFN-γ mAbs, Dr. K. Moore for anti–IL-10R blocking mAb, Dr. U. Müller for hyaluronidase, and Allergopharma Joachim Ganzer KG for Bet v 1 and Der p 1. We thank Dr. A. Faith for critical reading of the manuscript and D. Akdis for giving questionnaires to beekeepers.
This work was funded by the Swiss National Science Foundation (grants 32-112306/1 and 32-118226) and the Global Allergy and Asthma European Network.
The authors have no financial conflicts of interest.
Abbreviations used: CTLA, CTL-associated antigen; HR, histamine receptor; PD, programmed death; PLA, phospholipase A2; PPD, purified protein derivative; SIT, specific immunotherapy; TCRVβ, TCR variable β; Tr1, type 1 T regulatory; TT, tetanus toxoid.
F. Meiler and J. Zumkehr contributed equally to this paper.
References
- 1.Mosmann, T.R., H. Cherwinski, M.W. Bond, M.A. Giedlin, and R.L. Coffman. 1986. Two types of murine helper T cell clones. 1. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348–2357. [PubMed] [Google Scholar]
- 2.Sakaguchi, S., and F. Powrie. 2007. Emerging challenges in regulatory T cell function and biology. Science. 317:627–629. [DOI] [PubMed] [Google Scholar]
- 3.Roncarolo, M.G., and M. Battaglia. 2007. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat. Rev. Immunol. 7:585–598. [DOI] [PubMed] [Google Scholar]
- 4.Hill, J.A., C. Benoist, and D. Mathis. 2007. Treg cells: guardians for life. Nat. Immunol. 8:124–125. [DOI] [PubMed] [Google Scholar]
- 5.Faria, A.M., and H.L. Weiner. 2005. Oral tolerance. Immunol. Rev. 206:232–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shevach, E.M., R.A. DiPaolo, J. Andersson, D.M. Zhao, G.L. Stephens, and A.M. Thornton. 2006. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol. Rev. 212:60–73. [DOI] [PubMed] [Google Scholar]
- 7.Qin, S., S.P. Cobbold, H. Pope, J. Elliott, D. Kioussis, J. Davies, and H. Waldmann. 1993. “Infectious” transplantation tolerance. Science. 259:974–977. [DOI] [PubMed] [Google Scholar]
- 8.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J.E. De Vries, and M.G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742. [DOI] [PubMed] [Google Scholar]
- 9.Chen, Y., V.K. Kuchroo, J. Inobe, D.A. Hafler, and H.L. Weiner. 1994. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 265:1237–1240. [DOI] [PubMed] [Google Scholar]
- 10.Cong, Y., C.T. Weaver, A. Lazenby, and C.O. Elson. 2002. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J. Immunol. 169:6112–6119. [DOI] [PubMed] [Google Scholar]
- 11.Akdis, C.A., T. Blesken, M. Akdis, B. Wüthrich, and K. Blaser. 1998. Role of IL-10 in specific immunotherapy. J. Clin. Invest. 102:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akdis, M., J. Verhagen, A. Taylor, F. Karamloo, C. Karagiannidis, R. Crameri, S. Thunberg, G. Deniz, R. Valenta, H. Fiebig, et al. 2004. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 199:1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrat, F.J., D.J. Cua, A. Boonstra, D.F. Richards, C. Crain, H.F. Savelkoul, R. de Waal-Malefyt, R.L. Coffman, C.M. Hawrylowicz, and A. O'Garra. 2002. In vitro generation of interleukin 10–producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)– and Th2-inducing cytokines. J. Exp. Med. 195:603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peek, E.J., D.F. Richards, A. Faith, P. Lavender, T.H. Lee, C.J. Corrigan, and C.M. Hawrylowicz. 2005. Interleukin-10-secreting “regulatory” T cells induced by glucocorticoids and beta2-agonists. Am. J. Respir. Cell Mol. Biol. 33:105–111. [DOI] [PubMed] [Google Scholar]
- 15.Jutel, M., T. Watanabe, S. Klunker, M. Akdis, O.A.R. Thomet, J. Malolepszy, T. Zak-Nejmark, R. Koga, T. Kobayashi, K. Blaser, and A.C. Akdis. 2001. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 413:420–425. [DOI] [PubMed] [Google Scholar]
- 16.Larche, M., C.A. Akdis, and R. Valenta. 2006. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 6:761–771. [DOI] [PubMed] [Google Scholar]
- 17.Akdis, M. 2006. Healthy immune response to allergens: T regulatory cells and more. Curr. Opin. Immunol. 18:738–744. [DOI] [PubMed] [Google Scholar]
- 18.Jutel, M., M. Akdis, F. Budak, C. Aebischer-Casaulta, M. Wrzyszcz, K. Blaser, and A.C. Akdis. 2003. IL-10 and TGF-β cooperate in regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur. J. Immunol. 33:1205–1214. [DOI] [PubMed] [Google Scholar]
- 19.Thurmond, R.L., E.W. Gelfand, and P.J. Dunford. 2008. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat. Rev. Drug Discov. 7:41–53. [DOI] [PubMed] [Google Scholar]
- 20.Bilo, B.M., F. Rueff, H. Mosbech, F. Bonifazi, and J.N. Oude-Elberink. 2005. Diagnosis of Hymenoptera venom allergy. Allergy. 60:1339–1349. [DOI] [PubMed] [Google Scholar]
- 21.Tarzi, M., S. Klunker, C. Texier, A. Verhoef, S.O. Stapel, C.A. Akdis, B. Maillere, A.B. Kay, and M. Larche. 2006. Induction of interleukin-10 and suppressor of cytokine signalling-3 gene expression following peptide immunotherapy. Clin. Exp. Allergy. 36:465–474. [DOI] [PubMed] [Google Scholar]
- 22.Van Parijs, L.V., and A.K. Abbas. 1998. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 280:243–247. [DOI] [PubMed] [Google Scholar]
- 23.Akdis, C.A. 2006. Mechanisms of allergic disease. Curr. Opin. Immunol. 18:718–726. [DOI] [PubMed] [Google Scholar]
- 24.Vukmanovic-Stejic, M., Y. Zhang, J.E. Cook, J.M. Fletcher, A. McQuaid, J.E. Masters, M.H. Rustin, L.S. Taams, P.C. Beverley, D.C. Macallan, and A.N. Akbar. 2006. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J. Clin. Invest. 116:2423–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundrud, M.S., S.M. Grill, D. Ni, K. Nagata, S.S. Alkan, A. Subramaniam, and D. Unutmaz. 2003. Genetic reprogramming of primary human T cells reveals functional plasticity in Th cell differentiation. J. Immunol. 171:3542–3549. [DOI] [PubMed] [Google Scholar]
- 26.Messi, M., I. Giacchetto, K. Nagata, A. Lanzavecchia, G. Natoli, and F. Sallusto. 2003. Memory and flexibility of cytokine gene expression as separable properties of human T(H)1 and T(H)2 lymphocytes. Nat. Immunol. 4:78–86. [DOI] [PubMed] [Google Scholar]
- 27.Texier, C., S. Pouvelle, M. Busson, M. Herve, D. Charron, A. Menez, and B. Maillere. 2000. HLA-DR restricted peptide candidates for bee venom immunotherapy. J. Immunol. 164:3177–3184. [DOI] [PubMed] [Google Scholar]
- 28.Muller, U., C.A. Akdis, M. Fricker, M. Akdis, T. Blesken, F. Bettens, and K. Blaser. 1998. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J. Allergy Clin. Immunol. 101:747–754. [DOI] [PubMed] [Google Scholar]
- 29.Maurer, M., W. Seidel-Guyenot, M. Metz, J. Knop, and K. Steinbrink. 2003. Critical role of IL-10 in the induction of low zone tolerance to contact allergens. J. Clin. Invest. 112:432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexander, C., S. Ying, A.B. Kay, and M. Larche. 2005. Fel d 1-derived T cell peptide therapy induces recruitment of CD4+ CD25+; CD4+ interferon-γ+ T helper type 1 cells to sites of allergen-induced late-phase skin reactions in cat-allergic subjects. Clin. Exp. Allergy. 35:52–58. [DOI] [PubMed] [Google Scholar]
- 31.Larche, M., and D.C. Wraith. 2005. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat. Med. 11:S69–S76. [DOI] [PubMed] [Google Scholar]
- 32.Bacchetta, R., C. Sartirana, M.K. Levings, C. Bordignon, S. Narula, and M.G. Roncarolo. 2002. Growth and expansion of human T regulatory type 1 cells are independent from TCR activation but require exogenous cytokines. Eur. J. Immunol. 32:2237–2245. [DOI] [PubMed] [Google Scholar]
- 33.Moore, K.W., R. de Waal Malefyt, R.L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765. [DOI] [PubMed] [Google Scholar]
- 34.Wakkach, A., N. Fournier, V. Brun, J.-P. Breittmayer, F. Cottrez, and H. Groux. 2003. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 18:605–617. [DOI] [PubMed] [Google Scholar]
- 35.Mazzoni, A., H.A. Young, J.H. Spitzer, A. Visintin, and D.M. Segal. 2001. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J. Clin. Invest. 108:1865–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunzmann, S., P.-Y. Mantel, J.G. Wohlfahrt, M. Akdis, K. Blaser, and C.B. Schmidt-Weber. 2003. Histamine enhances TGF-beta1-mediated suppression of Th2 responses. FASEB J. 17:1089–1095. [DOI] [PubMed] [Google Scholar]
- 37.van der Pouw Kraan, T.C., A. Snijders, L.C. Boeije, E.R. de Groot, A.E. Alewijnse, R. Leurs, and L.A. Aarden. 1998. Histamine inhibits the production of interleukin-12 through interaction with H2 receptors. J. Clin. Invest. 102:1866–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi, T., T. Tagami, S. Yamazaki, T. Uede, J. Shimizu, N. Sakaguchi, T.W. Mak, and S. Sakaguchi. 2000. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte–associated antigen 4. J. Exp. Med. 192:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte–associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishimura, H., M. Nose, H. Hiai, N. Minato, and T. Honjo. 1999. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 11:141–151. [DOI] [PubMed] [Google Scholar]
- 41.Carter, L., L.A. Fouser, J. Jussif, L. Fitz, B. Deng, C.R. Wood, M. Collins, T. Honjo, G.J. Freeman, and B.M. Carreno. 2002. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur. J. Immunol. 32:634–643. [DOI] [PubMed] [Google Scholar]
- 42.Ying, S., Q. Meng, L.T. Barata, D.S. Robinson, S.R. Durham, and A.B. Kay. 1997. Associations between IL-13 and IL-4 (mRNA and protein), vascular cell adhesion molecule-1 expression, and the infiltration of eosinophils, macrophages, and T cells in allergen-induced late-phase cutaneous reactions in atopic subjects. J. Immunol. 158:5050–5057. [PubMed] [Google Scholar]
- 43.Barata, L.T., S. Ying, Q. Meng, J. Barkans, K. Rajakulasingam, S.R. Durham, and A.B. Kay. 1998. IL-4- and IL-5-positive T lymphocytes, eosinophils, and mast cells in allergen-induced late-phase cutaneous reactions in atopic subjects. J. Allergy Clin. Immunol. 101:222–230. [DOI] [PubMed] [Google Scholar]
- 44.Golden, D.B., D.G. Marsh, L.R. Freidhoff, K.A. Kwiterovich, B. Addison, A. Kagey-Sobotka, and L.M. Lichtenstein. 1997. Natural history of Hymenoptera venom sensitivity in adults. J. Allergy Clin. Immunol. 100:760–766. [DOI] [PubMed] [Google Scholar]
- 45.Gautrin, D., O. Vandenplas, J.D. DeWitte, J. L'Archeveque, C. Leblanc, C. Trudeau, C. Paulin, D. Arnoud, S. Morand, P. Comtois, et al. 1994. Allergenic exposure, IgE-mediated sensitization, and related symptoms in lawn cutters. J. Allergy Clin. Immunol. 93:437–445. [DOI] [PubMed] [Google Scholar]
- 46.Ling, E.M., T. Smith, X.D. Nguyen, C. Pridgeon, M. Dallman, J. Arbery, V.A. Carr, and D.S. Robinson. 2004. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 363:608–615. [DOI] [PubMed] [Google Scholar]
- 47.Carballido, J.M., N. Carballido-Perrig, M.K. Kägi, R.H. Meloen, B. Wüthrich, C.H. Heusser, and K. Blaser. 1993. T cell epitope specificity in human allergic and non-allergic subjects to bee venom phospholipase A2. J. Immunol. 150:3582–3591. [PubMed] [Google Scholar]
- 48.Akdis, C.A., T. Blesken, D. Wymann, M. Akdis, and K. Blaser. 1998. Differential regulation of human T cell cytokine patterns and IgE and IgG4 responses by conformational antigen variants. Eur. J. Immunol. 28:914–925. [DOI] [PubMed] [Google Scholar]
- 49.Brosterhus, H., S. Brings, H. Leyendeckers, R.A. Manz, S. Miltenyi, A. Radbruch, M. Assenmacher, and J. Schmitz. 1999. Enrichment and detection of live antigen-specific CD4+ and CD8+ T cells based on cytokine secretion. Eur. J. Immunol. 29:4053–4059. [DOI] [PubMed] [Google Scholar]
- 50.Akdis, C.A., A. Joss, M. Akdis, A. Faith, and K. Blaser. 2000. A molecular basis for T cell suppression by IL-10: CD28-associated IL-10 receptor inhibits CD28 tyrosine phosphorylation and phosphatidylinositol 3-kinase binding. FASEB J. 14:1666–1669. [DOI] [PubMed] [Google Scholar]
- 51.Liu, Y., S.H. Wei, A.S. Ho, R. de Waal Malefyt, and K.W. Moore. 1994. Expression cloning and characterization of a human IL-10 receptor. J. Immunol. 152:1821–1829. [PubMed] [Google Scholar]
- 52.Kunzmann, S., J.G. Wohlfahrt, S. Itoh, H. Asao, M. Komada, C.A. Akdis, K. Blaser, and C.B. Schmidt-Weber. 2003. SARA and Hgs attenuate susceptibility to TGF-beta1-mediated T cell suppression. FASEB J. 17:194–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.