Abstract
In cardiac muscle, Ca2+-induced Ca2+ release (CICR) from the sarcoplasmic reticulum (SR) defines the amplitude and time course of the Ca2+ transient. The global elevation of the intracellular Ca2+ concentration arises from the spatial and temporal summation of elementary Ca2+ release events, Ca2+ sparks. Ca2+ sparks represent the concerted opening of a group of ryanodine receptors (RYRs), which are under the control of several modulatory proteins and diffusible cytoplasmic factors (e.g., Ca2+, Mg2+, and ATP). Here, we examined by which mechanism the free intracellular Mg2+ ([Mg2+]free) affects various Ca2+ spark parameters in permeabilized mouse ventricular myocytes, such as spark frequency, duration, rise time, and full width, at half magnitude and half maximal duration. Varying the levels of free ATP and Mg2+ in specifically designed solutions allowed us to separate the inhibition of RYRs by Mg2+ from the possible activation by ATP and Mg2+-ATP via the adenine binding site of the channel. Changes in [Mg2+]free generally led to biphasic alterations of the Ca2+ spark frequency. For example, lowering [Mg2+]free resulted in an abrupt increase of spark frequency, which slowly recovered toward the initial level, presumably as a result of SR Ca2+ depletion. Fitting the Ca2+ spark inhibition by [Mg2+]free with a Hill equation revealed a Ki of 0.1 mM. In conclusion, our results support the notion that local Ca2+ release and Ca2+ sparks are modulated by Mg2+ in the intracellular environment. This seems to occur predominantly by hindering Ca2+-dependent activation of the RYRs through competitive Mg2+ occupancy of the high-affinity activation site of the channels. These findings help to characterize CICR in cardiac muscle under normal and pathological conditions, where the levels of Mg2+ and ATP can change.
INTRODUCTION
Control of intracellular Ca2+ homeostasis plays a central role in excitation-contraction coupling of cardiac myocytes. The action potential and depolarization of the cell membrane leads to activation of L-type Ca2+ channels. The resulting influx of Ca2+ initiates CICR from the SR. This Ca2+ release occurs through Ca2+ channels of the SR, called RYRs, which are of the type II isoform (RYR2) in cardiac myocytes (Otsu et al., 1990). The global cytosolic elevation of the intracellular Ca2+ concentration ([Ca2+]i) arises from the spatial and temporal summation of elementary Ca2+ release events, Ca2+ sparks (Cheng et al., 1993; Niggli and Shirokova, 2007). It appears that these local Ca2+ release events represent the concerted opening of a group of RYR2 (Niggli, 1999; Wang et al., 2001; Berridge, 2006). To reestablish the resting intracellular Ca2+ concentration, Ca2+ ions are sequestered back into SR by the Ca2+ pump (SERCA) while Ca2+ entering via L-type Ca2+ current is extruded from the cell via the Na+-Ca2+ exchanger.
The SR Ca2+ release process itself is regulated both by cytosolic Ca2+ and by Ca2+ from the luminal side of SR, which appears to modulate the cytosolic Ca2+ sensitivity (DelPrincipe et al., 1999; Meissner, 2002; Laver, 2005; Keller et al., 2007; Gyorke and Terentyev, 2008). Besides a variety of regulatory proteins located in the RYR macromolecular complex, several diffusible modulators of the RYR have been described, most notably Mg2+ and ATP (Fabiato and Fabiato, 1975; Meissner, 2002), the concentrations of which are interdependent because of Mg2+ buffering by ATP. At rest, cardiac myocytes contain around 3–5 mM of total ATP and 0.5–1.2 mM of free Mg2+ (Hess et al., 1982; Blatter and McGuigan, 1986). In ischemia the concentration of ATP declines, whereas the concentration of free Mg2+ ([Mg2+]free) is known to rise around threefold (Murphy et al., 1989; Kléber, 1990).
Mg2+ ions have been shown to suppress SR calcium release in a variety of skeletal and cardiac muscle preparations. It is suggested that Mg2+ interacts with Ca2+-activating and -inactivating sites on the RYRs, the A-site and I-site, respectively (Laver et al., 1997a). As [Mg2+]free is lowered, Mg2+ dissociates from the low-affinity I-site at which binding of either Ca2+ or Mg2+ inhibits channel opening. At even lower [Mg2+]free, it also dissociates from the high-affinity A-site at which either Ca2+ or Mg2+ can bind, but at which only Ca2+ activates the channel, whereas Mg2+ binding at this site competitively inhibits Ca2+ activation (Ashley and Williams, 1990; Laver et al., 1997b).
Very recently, Mg2+ has been suggested to also exhibit a stimulating effect on RYR2 in an intermediate concentration range of Ca2+ (10–100 μM) (Chugun et al., 2007). These authors showed that Mg2+ renders the RYR2 more sensitive to modulators of CICR, such as caffeine, β,γ-methylene ATP, procaine, and calmodulin. These new findings suggest that the role of Mg2+ may be more complex than previously thought.
Most experiments characterizing the interactions of Mg2+ with the RYRs were conducted using lipid bilayers, SR vesicles, or skinned muscle fibers (Lamb, 2000; Meissner, 2004). These studies have yielded important information about the interactions between Mg2+ and RYRs on the molecular level. However, much less data are available describing how these observations are applicable to CICR and the generation of Ca2+ sparks in cardiac myocytes, under more physiological conditions and with preserved ultrastructure. Although an inhibition of Ca2+ spark formation by elevated Mg2+ concentrations has been reported in permeabilized cardiomyocytes (Lukyanenko et al., 2001), the phenomenon was not studied in detail and virtually no information is available for low Mg2+ concentrations. Some studies have been published describing the behavior of RYRs and Ca2+ sparks at different [Mg2+] in frog skeletal muscle (Lacampagne et al., 1998; Shtifman et al., 2002; Zhou et al., 2004), but these findings may be quite different from those one would expect in cardiomyocytes. There are numerous differences between the skeletal and cardiac RYR isoforms, such as, for example, activation and inactivation by Ca2+, ATP, and caffeine (Lamb, 2000; Copello et al., 2002). Finally, the Mg2+ modulation of RYR2 is important not only for understanding its role in Ca2+ homeostasis and fluxes, but also for interpreting changes of Ca2+ signaling under ischemic conditions, where [Mg2+]i is elevated (and [ATP] is low).
Here, we analyzed spatiotemporal features of Ca2+ sparks recorded from permeabilized mouse ventricular myocytes at different intracellular concentrations of Mg2+ and ATP. The findings indirectly reflect the activity of the RYRs in their natural microenvironment as a function of [Mg2+]free and [ATP]free and allowed us to identify the predominant mechanism for Ca2+ spark modulation by Mg2+ and ATP in cardiac myocytes.
MATERIALS AND METHODS
Isolation of Cardiomyocytes
Animals were housed and handled according to the guidelines of the Swiss Animal Protection law, and the experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (1996, National Academy of Sciences, Washington, DC). Cardiac ventricular myocytes were isolated from adult mice using established enzymatic procedures as described previously (Szentesi et al., 2004). In brief, mice were killed by cervical dislocation and the excised hearts were mounted on a Langendorff perfusion system. After perfusion with nominally Ca2+-free Tyrode's solution for 3–5 min, 14 U/ml collagenase II (Worthington type 2), 0.2 U/ml protease (type XIV; Sigma-Aldrich), and 50 μM Ca2+ were added to the solution. After an additional 6–9 min of perfusion, the hearts were removed from the Langendorff apparatus and cut in small pieces, followed by gentle trituration to obtain a cell suspension. Subsequently, the Ca2+ concentration was slowly raised to the final concentration of 1.8 mM.
Cell Permeabilization and Confocal Ca2+ Imaging
Isolated cardiac myocytes were permeabilized by exposure to saponin (0.005%; 30–45 s) in recording solution that contained: 110 mM K+ aspartate, 1 mM EGTA, 10 mM phosphocreatine, 5 U/ml creatine phosphokinase, 5 mM reduced l-glutathione, 4% dextran (mol wt 40,000), 50 μM fluo-3, and 10 mM HEPES, pH 7.2. Concentration of [Ca2+]free was adjusted to 50 nM, and concentrations of Mg2+ and ATP of the various solutions are shown in Table I. [Ca2+]free, [Mg2+]free, and [ATP]free concentrations were calculated using WebMAX 10/06 software (http://www.stanford.edu/∼cpatton/maxc.html). In addition, [Ca2+]free was confirmed to be within ±4 nM of the calculated values using a fluorescence spectrometer (NanoDrop 3300; NanoDrop Products) and the ratiometric indicators Indo-1. Averaged measurements for [Mg2+]free with Mag-Indo-1 were exactly as calculated. The free Ca2+ concentration of 50 nM is very close to the recently measured 42 nM in resting mouse cardiomyocytes (Williams and Allen, 2007) and was chosen to have a nonzero resting spark frequency. During all experiments, the solutions were exchanged by complete replacement. Fluo-3 was excited with the 488-nm line of an argon-ion laser. Fluorescence was detected at >500 nm with a confocal laser-scanning microscope operating in line-scan mode (500 lines/s; μRadiance [Bio-Rad Laboratories]; 60× water-immersion objective [DIC H; Nikon]). The fluorescence was normalized and expressed as F/F0, where F0 is the baseline fluorescence at the beginning of each recording. The frequency and spatiotemporal parameters of the Ca2+ sparks were determined from the line-scan images using a computer algorithm similar to that described previously (Cheng et al., 1999; Rios et al., 2001). Ca2+ spark frequencies were expressed as the number of recorded sparks per second and per 100 μm of scanned distance. All experiments were performed at room temperature (20–22°C).
TABLE I.
Calculated Concentrations of [Mg2+]free, Free [ATP], and [MgATP] in Solutions Used in Different Sets of Experiments
| Mg2+total | Mg2+free | ATPtotal | ATPfree | Mg-ATP | |
|---|---|---|---|---|---|
| A | 0.00 | 0.00 | 3.00 | 3.00 | 0.00 |
| 1.53 | 0.10 | 3.00 | 1.58 | 1.42 | |
| 2.14 | 0.20 | 3.00 | 1.07 | 1.93 | |
| 2.97 | 0.50 | 3.00 | 0.54 | 2.46 | |
| 3.73 | 1.00 | 3.00 | 0.30 | 2.70 | |
| 5.99 | 3.00 | 3.00 | 0.11 | 2.89 | |
| B | 0.39 | 0.20 | 0.29 | 0.10 | 0.19 |
| 2.14 | 0.20 | 3.00 | 1.07 | 1.93 | |
| 5.61 | 0.20 | 8.40 | 3.00 | 5.40 | |
| C | 0.00 | 0.00 | 0.30 | 0.30 | 0.00 |
| 0.37 | 0.10 | 0.57 | 0.30 | 0.27 | |
| 0.75 | 0.20 | 0.84 | 0.30 | 0.54 | |
| 1.87 | 0.50 | 1.65 | 0.30 | 1.35 | |
| 3.73 | 1.00 | 3.00 | 0.30 | 2.70 | |
| 11.20 | 3.00 | 8.40 | 0.30 | 8.10 |
(A) In this set of solutions, total ATP remains constant, but [Mg2+]free and [ATP]free both vary. (B) [Mg2+]free is kept constant. (C) [Mg2+]free is variable but [ATP]free is constant. Values representing variable [Mg2+]free or [ATP]free are shown in bold for clarity.
Data Analysis
Data from line-scan images were averaged during the indicated intervals within each cell and are presented as mean ± SEM obtained from n different cells. Statistical differences between datasets were evaluated by Student's t test. Significance (*) was defined at P < 0.05. For multivariate analysis of Ca2+ sparks, we applied a random-effects linear regression model using the inverse of the variance of the Ca2+ spark frequency as analytical weight, mean spark frequency as dependent variable, and [Mg2+], [ATP], and [MgATP] as independent variables. Statistical analyses were performed in Stata software (version 10; Stata Corp.).
Online Supplemental Material
Video 1 contains the animation of a surface plot showing Ca2+ spark frequency as a function of both [Mg2+]free and [ATP]free. In addition, Table S1 lists detailed results of the univariable and bivariable regression analysis. The online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.200810119/DC1.
RESULTS
Free [Mg2+] Affects Local Ca2+ Sparks
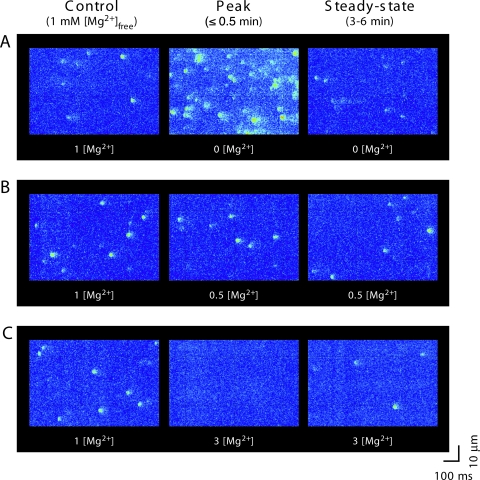
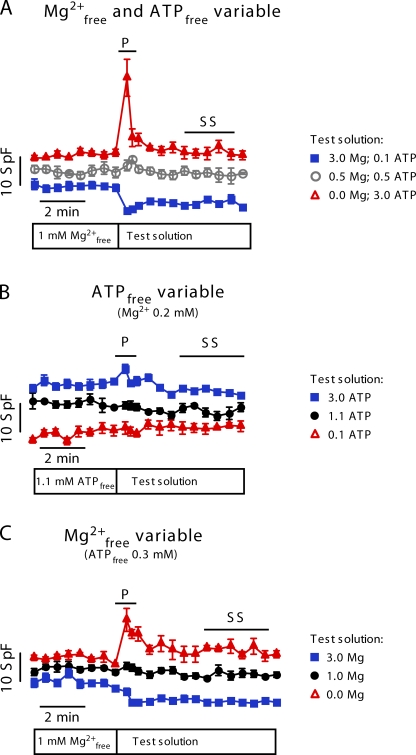
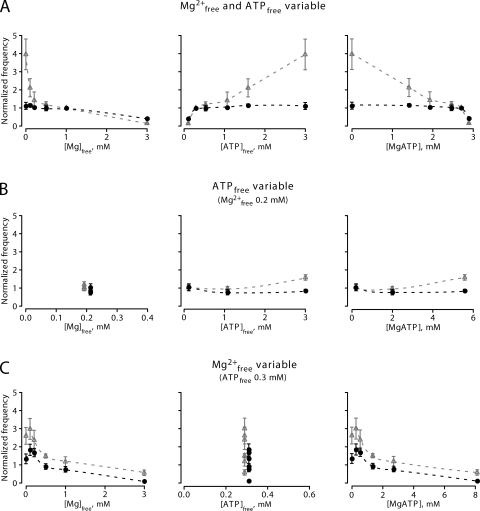
In a first series of experiments, we examined how different free Mg2+ concentrations affected the local Ca2+ release events (Ca2+ sparks) at a constant level of total ATP ([ATP]total = 3 mM; for solution composition see Table I, A). Cardiomyocytes were permeabilized and initially bathed in control solution containing 1 mM [Mg2+]free. After acquiring confocal line-scan images during ∼4 min in control, the solution was rapidly exchanged with various test concentrations of free Mg2+ (in the range of 0–3 mM). As already evident from the raw confocal line-scan images taken immediately after the solution change and 4 min later, this generally lead to biphasic changes in the Ca2+ spark frequency (Fig. 1). When analyzing the time-course of the spark frequency, we noted transient changes within the first few seconds, after which the spark frequency approached a new steady-state (Fig. 2 A). For example, lowering [Mg2+]free resulted in an abrupt and up to fourfold increase in spark frequency, which subsequently declined almost to the control level. In contrast, increasing [Mg2+]free induced a reduction of the spark frequency, which then partly recovered to an intermediate level. The maximal frequency was maintained only transiently and was limited to the first few seconds after solution exchange, as it can be clearly seen in the trace with nominally 0 mM [Mg2+]free. Therefore, for further analysis we divided the data into two groups: (1) the peak changes (1–15 s after the solution switch), and (2) the steady-state measurements (3–6 min after solution exchange). The data are summarized in Fig. 3 A, where peak values are shown in gray and steady-state frequency in black. As noticeable when comparing the gray with the black trace in Fig. 3 A (left), the spark steady-state frequency recovered more completely upon reducing [Mg2+]free than upon increasing [Mg2+]free to 3 mM.
Figure 1.
Changes in [Mg2+]free affect the frequency of spontaneous Ca2+ sparks. Representative examples of confocal line-scan images recorded from permeabilized cardiomyocytes. (A) Solution change from 1 mM [Mg2+]free (control) to nominally Mg2+ free (with 3 mM [ATP]free). (B) To 0.5 mM [Mg2+] (with 0.54 mM [ATP]free). (C) To 3 mM [Mg2+] (with 0.11 mM [ATP]free). The images were obtained before the solution change (left; 1 mM [Mg2+]), 5–30 s after (middle), and 3–6 min after (right) exchange of solution.
Figure 2.
Time-course of averaged Ca2+ spark frequencies after changes in [Mg2+]free and [ATP]free. Traces are offset slightly for clarity. SpF denotes spark frequency (per s−1 100 μm−1). [Mg2+]free was initially 1 mM in all experiments (control solution). For composition of the test solution, see symbol legend on the right. (A) At the moment indicated by the bar below the traces, test solutions were applied with different levels of [Mg2+]free and [ATP]free, but at constant ATPtotal (3 mM). (B) [ATP]free was varied at constant [Mg2+]free (0.2 mM). (C) [Mg2+]free was varied at constant [ATP]free (0.3 mM). Time intervals for data considered in further analyses are indicated by horizontal lines. P, peak values; SS, steady-state values.
Figure 3.
Summary of Ca2+ spark frequency variations and their dependence on [Mg2+]free, [ATP]free, and [MgATP]. Spark frequencies were determined from the peak (P; 0–30 s; gray triangles and lines) and from the steady-state (SS; 3–6 min; black circles and lines) after solution exchange, as shown by lines in Fig. 2. (A) Spark frequencies at constant total ATP ([Mg2+]free and [ATP]free varied simultaneously). (B) Spark frequencies at constant [Mg2+]free (0.2 mM) but variable [ATP]free. (C) Spark frequencies at constant [ATP]free (0.3 mM) but variable [Mg2+]free. The spark frequencies were plotted versus [Mg2+]free (left), [ATP]free (middle), and [MgATP] (right) to isolate the dependence on each compound from the others.
As a complication for the interpretation of these observations, the open probability of the RYRs also depends on ATP because the channel has an activating adenine binding site (Kermode et al., 1998). Taking this complexity into account, the changes in Ca2+ spark frequency as a function of [Mg2+]free observed in our experiments could also be attributed to variations in [ATP]free (or [MgATP]) because the concentrations of the three compounds are interdependent. Therefore, we re-plotted our results in dependence of [ATP]free and [MgATP] (Fig. 3; see also Fig. 4). Indeed, the high frequency of Ca2+ sparks also correlated with a high concentration of [ATP]free, which corresponds to the solution with low [Mg2+]free (Fig. 3 A, middle). Thus, our observations could, in principle, also be explained by the activation of the RYRs by [ATP]free. However, the dependence of the spark frequency on [MgATP] was the opposite of what one would expect for RYR activation by [MgATP], and therefore we could rule out [MgATP] as a main activator of Ca2+ sparks (Fig. 3 A, right).
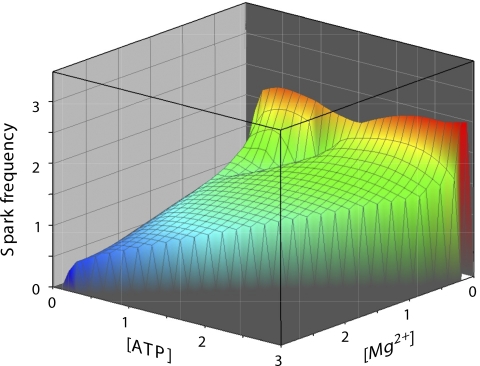
Figure 4.
Ca2+ spark frequency is represented as a surface and is shown as a function of both [Mg2+]free and [ATP]free. The bivariate correlation coefficients obtained from a multivariate regression model for the Ca2+ spark frequency dependence on [Mg2+]free was −0.425 (P = 0.005) and on [ATP]free was +0.116 (P = 0.47). For details of the multivariate analysis see Materials and methods and Table S1.
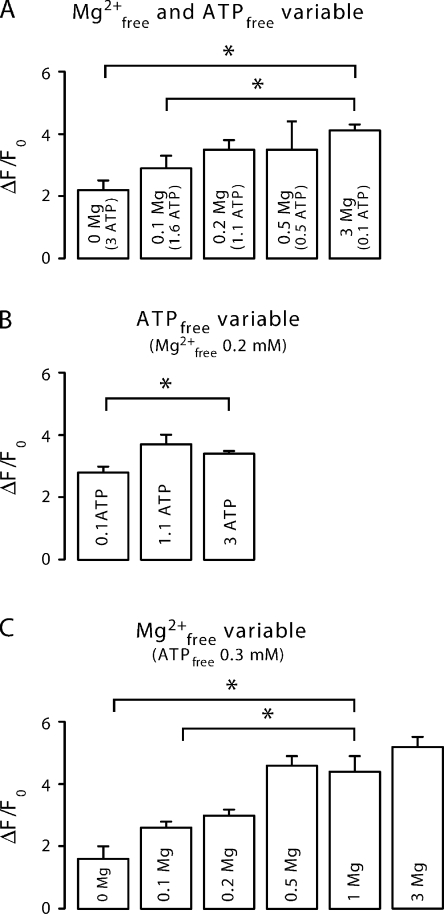
Because Ca2+ sparks represent a major pathway for the SR Ca2+ leak, some of the observations were transient because of secondary alterations of SR Ca2+ content subsequent to increases (or decreases) of the spark frequency. This is because Ca2+ spark frequency and some of the spatiotemporal spark parameters also strongly depend on the SR Ca2+ loading, as RYRs are regulated by luminal Ca2+ (Lukyanenko et al., 2001; Laver, 2005; Keller et al., 2007). To estimate these changes in SR Ca2+ content, we rapidly applied a solution containing 20 mM caffeine at the end of the experiment (Fig. 5 A). We noted that increasing [Mg2+]free to 3 mM lead to an accumulation of Ca2+ inside the SR, whereas a reduction of [Mg2+]free resulted in a lower SR Ca2+ content, as expected.
Figure 5.
Dependence of SR Ca2+ content on the concentration of [Mg2+]free and [ATP]free. SR Ca2+ loading was estimated by application of 20 mM caffeine at the end of the experiments (after 6–8 min) and recording of the resulting fluorescence transient. (A) SR Ca2+ content at constant total ATP ([Mg2+]free and [ATP]free varied simultaneously). (B) SR Ca2+ content at constant [Mg2+]free (0.2 mM) but variable [ATP]free. (D) SR Ca2+ content at constant [ATP]free (0.3 mM) but variable [Mg2+]free.
Free [ATP] Only Slightly Affects Spark Frequency
We next investigated whether binding of ATP to the adenine binding site of the RYR could be the mechanism responsible for our observations (Kermode et al., 1998; Yang and Steele, 2001; Meissner, 2004). The EC50 for channel activation by ATP has been estimated to be 0.2 mM for sheep cardiac RYR (Kermode et al., 1998). This is in the range of concentrations present in our experiments (0.11–3 mM; Table I, A). Therefore, we decided to examine the Ca2+ spark properties in the range of 0.1–3 mM [ATP]free with solutions designed to have a constant concentration of [Mg2+]free (0.2 mM; see Table I, B). As shown in Fig. 2 B, in these experiments the observed changes in Ca2+ spark frequency were quite small. Only the highest level of [ATP]free resulted in a modest transient increase, which may correlate with an activation of the RYRs by ATP (Figs. 2 B and 3 B). Collectively, varying [ATP]free at constant [Mg2+]free affected sparks properties much less than when changing [Mg2+]free (compare Fig. 3, A and B).
To reveal differences in the SR Ca2+ load as a function of [ATP]free, we applied 20 mM caffeine at the end of each experiment. Again, the findings were opposite to the results observed before; lowering [ATP]free to 0.1 mM resulted in a reduction of the SR Ca2+ load (Fig. 5 B). However, increasing [ATP]free from 1.1 to 3 mM did not change SR Ca2+ loading much.
Changing [Mg2+]free at Constant [ATP]free Affects Ca2+ Sparks
Another approach to avoid a possible interference by free [ATP] and to isolate the Ca2+ spark modulation by Mg2+ is to design solutions in which [ATP]free remains constant despite changes of [Mg2+]free. Thus, we next performed a series of experiments at constant [ATP]free (0.3 mM) while varying the [Mg2+]free from 0 to 3 mM (solutions in Table I, C). Again, we observed transient changes of the Ca2+ spark frequency immediately after the change of solution (Fig. 2 C). However, in this series of experiments, the adjustments of the steady-state levels were somewhat more pronounced than in the initial experiments (Fig. 3 C). As before, the steady-state SR Ca2+ content was dependent on [Mg2+]free (Fig. 5 C). Note that even after 4–6 min in nominally Mg2+-free solution, Ca2+ sparks were still detectable and the store not yet completely empty.
Multivariate Statistical Analysis of Ca2+ Spark Frequencies
As already mentioned, in our experiments the Ca2+ spark frequency depended on several variables that are interdependent (e.g., [Mg2+]free, [ATP]free, [MgATP], and SR Ca2+ content). This complication was the main reason to design several sets of solutions that simplify this problem by keeping at least one variable constant. An alternative approach is to analyze the dataset with multivariate statistical methods. We applied a random-effects linear regression model to determine these more complex relationships. To simplify the analysis somewhat, we made the following assumption: we assumed that immediately after the solution change the Ca2+ concentration inside the SR has not yet changed. Collectively, the bivariable analysis essentially revealed the same correlations and dependencies we had derived from the specifically designed solutions: a significant negative association between Ca2+ spark frequency and [Mg2+]free with a regression coefficient of −0.45 (P = 0.005) and a weak positive association with [ATP]free (coefficient of +0.12; P = 0.47). When comparing [Mg2+]free with [MgATP], the latter was found to have only a minimal inhibitory effect. For details of this analysis see Materials and methods and Table S1 (available at http://www.jgp.org/cgi/content/full/jgp.200810119/DC1). Because [Mg2+]free and [ATP]free turned out to be the most important variables, we visualized the result in a two-dimensional surface plot of Ca2+ spark frequency as a function of both [Mg2+]free and [ATP]free (Fig. 4). Overall, the surface plot also clearly reveals that [Mg2+]free had a much stronger effect on the spark frequency than changes of [ATP]free. For example, at the high Ca2+ spark frequency observed in low [Mg2+]free, reducing the ATP concentration did not lower the frequency by much. However, high free [Mg2+]free was able to reduce the Ca2+ spark frequency drastically, even in the presence of a stimulatory concentration of [ATP]free.
Free [Mg2+] and Spatiotemporal Parameters of the Ca2+ Sparks
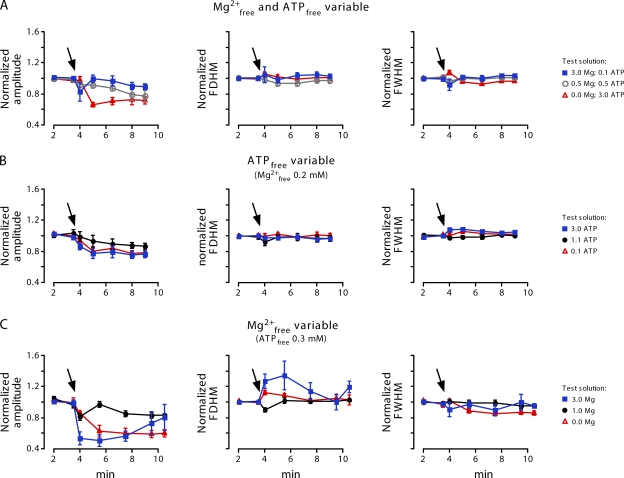
In addition to the spark frequency we analyzed the Ca2+ spark amplitude, the full-width at half magnitude and the full duration at half magnitude (Fig. 6). These spatiotemporal parameters were also slightly affected, particularly in 0 and 3 mM [Mg2+]free. Lowering the Mg2+ level led to a reduction of the spark amplitude almost to the limit of detection. In contrast, elevated Mg2+ levels initially resulted in a reduction of the amplitude, which later recovered almost to control. The reduced Ca2+ spark amplitude reflects RYR inhibition by Mg2+, but also correlates with the SR Ca2+ load (see Fig. 5 C).
Figure 6.
Summary of spatiotemporal Ca2+ spark parameters. Normalized amplitude, full duration at half magnitude (FDHM), and full-width at half magnitude (FWHM) are plotted versus time. Initially, all cells were in control solution, which was exchanged for test solution at the arrow. For composition of the test solution see symbol legend on the right. (A) [Mg2+]free was varied at constant ATPtotal (3 mM). (B) [ATP]free was varied at constant [Mg2+]free (0.2 mM). (C) [Mg2+]free was varied at constant [ATP]free (0.3 mM).
DISCUSSION
Many ion channels and transporters are modulated by or require Mg2+ and ATP. Alterations of their respective concentrations, as they are known to occur, for example, during ischemia, are expected to affect the functioning of a multitude of proteins. As far as the SR and Ca2+ cycling are concerned, the RYRs and the SERCA appear to be particularly relevant. RYRs are known to be activated by ATP and inhibited by Mg2+ (Lamb, 2000; Meissner, 2004). The SERCA ultimately requires ATP to refill the SR, and the maximal Ca2+ gradient across the SR membrane depends on the ADP/ATP ratio (Shannon et al., 2002). Furthermore, the SERCA requires the presence of Mg2+ because it has a regulatory Mg2+ binding site (MacLennan, 1970). Thus, even on the level of SR function, changes of [Mg2+] and/or [ATP] may result in complex modifications of SR Ca2+ uptake and release.
Analysis of Ca2+ sparks allows observation of the Ca2+ release mechanism in a reasonably well-preserved structural and molecular environment in situ. As in studies with skeletal muscle fibers (Lacampagne et al., 1998; Shtifman et al., 2002; Zhou et al., 2004), we performed the experiments by using saponin-permeabilized cells under close to physiological conditions. Previous studies with RYRs reconstituted into lipid bilayers or with SR vesicles have shown that the open probability of the RYRs depends on the cytosolic concentration of free Mg2+ (Meissner and Henderson, 1987; Laver and Lamb, 1998; Lamb, 2000; Meissner, 2002; Laver et al., 2004; Dias et al., 2006). Besides a direct inhibition of the RYR2, Mg2+ can also influence its sensitivity to other modulators, particularly to caffeine, ATP, or calmodulin (Chugun et al., 2007). It was also found that the activity of SERCA depends on the level of Mg2+ and, ultimately, on [MgATP] (MacLennan, 1970; Smith et al., 2001). The SERCA activity will indirectly affect the Ca2+ sparks via SR luminal Ca2+ concentration, which within a cell changes according to the SR Ca2+ leak/uptake balance (Shannon et al., 2002). Collectively, this indicates that the effects of Mg2+ in cells could be quite complicated.
It should be noted that Ca2+ spark recordings also have some limitations. Particularly, at low [Mg2+]free (and thus low Ca2+ spark amplitude), the Ca2+ spark frequency could be underestimated because of two reasons: (1) because the lowering of [Mg2+]free has a tendency to initiate “macrosparks” or miniwaves (at constant [Ca2+]), which are difficult to resolve as individual sparks; and (2) because the threshold for spark detection allows us to detect only events larger than a certain amplitude (in our case it was adjusted to 0.4 F/F0) (Cheng et al., 1999). This may also lead to a slight underestimation of the spark frequency, especially when the average amplitude of the sparks becomes small.
Free [Mg2+] and Local Ca2+ Release Signals
Here, we assessed by which mechanism(s) changes in [Mg2+]free modify Ca2+ sparks in cardiac myocytes. The experiments revealed that rapid adjustments of [Mg2+]free result in a biphasic change of the spark frequency, suggesting indeed a complex situation. The initial transient rise (or fall) in Ca2+ spark frequency occurred when the level of SR Ca2+ loading was still close to control conditions. However, the initial change in Ca2+ spark frequency disturbed the uptake/leak balance of the SR, and, after some delay, the system approached a new steady-state, in which the uptake of Ca2+ into SR again matched the leak of Ca2+ through RYRs. Indeed, application of 20 mM caffeine during the new steady-state revealed that the [Mg2+] had a marked influence on SR Ca2+ content. For example, elevated [Mg2+] induced an accumulation of Ca2+ inside the SR, presumably resulting from the inhibition of the RYRs and the reduction of the Ca2+ leak. The opposite behavior was observed at low [Mg2+]free, where the Ca2+ spark activity and thus the SR Ca2+ leak were very pronounced. These adjustments of the luminal SR Ca2+ concentration also explain the observed trend of the spark frequency toward a new steady-state level after a transient increase (or decrease). In terms of Ca2+ spark frequency, the system approached a new equilibrium quite rapidly (with a τdecay of <1 min). A similar autoregulatory tendency of SR Ca2+ release, albeit for signals triggered by L-type Ca2+ currents, has also been noted in intact cells (Eisner et al., 1998; Terentyev et al., 2002). Also, one would expect that in the steady-state Ca2+ sparks should disappear completely in zero MgATP because under these conditions the SERCA would stop working. Most likely, the duration of our experiments was sufficiently short to prevent total depletion of the SR Ca2+. Thus, Ca2+ sparks were still detectable at the end of our recordings. In addition, there might be some residual Mg2+ within the permeabilized cells that is not yet completely washed out.
Because the free concentrations of Mg2+, ATP, and MgATP are mutually dependent, all three concentrations changed during the first series of experiments, where the total [ATP]total remained constant. Therefore, we decided to perform additional experiments with solutions designed to keep one of these variables constant (see Table I). Using this approach we found that changes in [MgATP], as well as total [ATP]total, did not correlate with the Ca2+ spark frequencies in a causal manner. Previously, ATP binding has been shown to have only a mild stimulatory effect on the cardiac RYR (Lamb, 2000), consistent with our observations. Interestingly, lowering [ATP]free to 0.1 mM led to a decrease in SR loading. Because this maneuver did not acutely elevate the Ca2+ spark frequency, the reduction in SR loading most likely did not result from enhanced RYR activation, but rather from changes of SERCA activity. ATP, as well as Mg2+, also modulates SERCA2a activity. SERCA2a has a high-affinity ATP binding site (Kd ∼1 μM), which is the substrate site, and a second lower-affinity site (Kd ∼200 μM), which serves a regulatory role (Bers, 2001). Thus, lowering [ATP] could also decrease the transport of Ca2+ into the SR and induce a change in the fine balance between Ca2+ leak and uptake.
This result is somewhat different from the observations made by Yang and Steele (2001) in rat ventricular cardiomyocytes. In their study, the increase of the spark frequency when lowering [ATP]free was more pronounced and SR loading was higher at lower [ATP]free. However, they used a higher concentration of Mg2+ (1 mM instead of 0.2 mM), and in most cases lower concentrations of [ATP]free, which may explain this difference. Similarly, a much lower [ATP]free and higher Ca2+ (230 nM) may explain why ATP removal was found to inhibit the RYRs in a study on Ca2+ waves (Smith and O'Neill, 2001). From our Fig. 3 A it seems that under extreme conditions with a simultaneous decrease in [ATP]free (to 0.11 mM) and increase in [Mg2+]free (to 3 mM), the Ca2+ spark frequency cannot recover to the control level. Keeping the level of [ATP]free constant (0.3 mM) did not change the situation (Fig. 3 C). Thus, increasing the SR Ca2+ content was not sufficient to overcome the suppressed RYR activity under these conditions. The low SR Ca2+ content despite suppressed RYR activity most likely underlies the low activity state of CICR found under these conditions.
Possible Mechanisms for Ca2+ Spark Inhibition by Mg2+
The initial transient changes of the Ca2+ spark frequency most likely resulted from a direct interaction of Mg2+ with the RYRs because secondary adjustments of SR Ca2+ content had not yet developed. In previous studies on the single-channel level in lipid bilayers, it has been noted that the RYR2 has at least two binding sites for modulation by Mg2+, and both sites can also bind Ca2+ (Laver et al., 1997a; Lamb, 2000). Ca2+ activates the RYR by binding at the activating site (or A-site) with a Ki of ∼1 μM. The affinity of the A-site for Mg2+ is ∼40-fold lower, and Mg2+ binding at the A-site prevents activation of the channel by Ca2+ (Laver and Honen, 2008). The inhibitory I-site of the RYR2 has a very low Ca2+ affinity (Ki > 1 mM), but a similar affinity for Mg2+. Binding of either ion inactivates the channel. These findings suggest that, at a low Ca2+ concentration, Mg2+ acts mostly at the activation site, particularly when [Mg2+]free is <1 mM. However, increasing [Mg2+]free will inhibit RYRs further via the inhibitory site.
Fitting the Mg2+ dependence of the Ca2+ spark frequencies observed here by a Hill equation for inhibition (Laver et al., 2004):
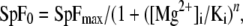 |
where SpF is spark frequency, yielded a Ki = 0.35 mM, n = 0.86, when both [Mg2+]i and [ATP] were changing. For the data where only [Mg2+]free was changing and [ATP]free remained constant at 0.3 mM, the Ki was 0.1 mM and n = 0.66. Thus, the observed Ki for Ca2+ spark generation (∼0.1 mM) is in the range of the Mg2+ affinity of the A-site on the RYR2 (see above). This suggests that the main mechanism of Ca2+ spark suppression by Mg2+ is by binding to the A-site of the RYRs, which is in contrast to skeletal muscle (Laver et al., 1997a). Mg2+ binding to the A-site, which competes with binding of activating Ca2+ to the same site, is most likely also responsible for the reduced Ca2+ spark amplitude observed in elevated [Mg2+]free.
Interestingly, when we kept the [ATP]free constant at 0.3 mM, the steady-state Ca2+ spark frequency was quite different from the situation with a constant total ATP. This observation is consistent with a regulatory effect of ATP on the modulation by [Mg2+], which has been shown for skeletal muscle (Jona et al., 2001), and which may be present in cardiac muscle too.
Implications of the Results Obtained
Under physiological conditions, cellular-free [ATP]i and [Mg2+]i levels are tightly regulated and very stable. However, both [Mg2+]i and [ATP]i are altered during ischemia (Murphy et al., 1989; Kléber, 1990). While the concentration of free (and total) [ATP]i declines, the concentration of [Mg2+]i becomes elevated. Besides the disruption of other ATP-dependent processes, these pathological disturbances of the intracellular milieu and the release of free Mg2+ will affect the Ca2+ homeostasis and several ion channels (Bers, 2001). In particular, the elevation of [Mg2+]i will inhibit the RYRs, subsequently limiting the Ca2+ release from the SR. This suppression of the CICR is thought to be beneficial for the survival of the ischemic cardiomyocytes because it will dramatically reduce the energy demand of the affected cells. During ischemia, other factors, such as the acidic pH, will contribute to RYR2 inhibition and CICR shutdown, leading to a highly Ca2+-loaded SR (Overend et al., 2001). Therefore, for a complete appreciation of disease-related alterations of Ca2+ spark and Ca2+ signaling, a better understanding of isolated processes is required, such as the mechanism of RYR inhibition by Mg2+ as characterized here. To this end, several computer models incorporating the interactions of Mg2+ with Ca2+ signaling in cardiac cells have been developed (Laver et al., 1997a; Zahradnikova et al., 2003; Michailova et al., 2004; Valent et al., 2007) and our present data should enable further progress in these efforts.
Acknowledgments
The authors thank Dr. Peter Jueni for advice on the biostatistics and Daniel Luethi for technical assistance.
This work was supported by the Swiss National Science Foundation (grant 31-109693 to E. Niggli), the Swiss Cardiovascular Research and Training Network (SCRTN), and the Swiss Foundation for Research on Muscle Diseases.
Lawrence G. Palmer served as editor.
References
- Ashley, R.H., and A.J. Williams. 1990. Divalent cation activation and inhibition of single calcium release channels from sheep cardiac sarcoplasmic reticulum. J. Gen. Physiol. 95:981–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge, M.J. 2006. Calcium microdomains: organization and function. Cell Calcium. 40:405–412. [DOI] [PubMed] [Google Scholar]
- Bers, D.M. 2001. Excitation-Contraction Coupling and Cardiac Contractile Force. Kluwer, Dordrecht, Netherlands. 427 pp.
- Blatter, L.A., and J.A.S. McGuigan. 1986. Free intracellular magnesium concentration in ferret ventricular muscle measured with ion selective micro-electrodes. Q. J. Exp. Physiol. 71:467–473. [DOI] [PubMed] [Google Scholar]
- Cheng, H., W.J. Lederer, and M.B. Cannell. 1993. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 262:740–744. [DOI] [PubMed] [Google Scholar]
- Cheng, H., L.S. Song, N. Shirokova, A. Gonzalez, E.G. Lakatta, E. Rios, and M.D. Stern. 1999. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys. J. 76:606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugun, A., O. Sato, H. Takeshima, and Y. Ogawa. 2007. Mg2+ activates the ryanodine receptor type 2 (RyR2) at intermediate Ca2+ concentrations. Am. J. Physiol. Cell Physiol. 292:C535–C544. [DOI] [PubMed] [Google Scholar]
- Copello, J.A., S. Barg, A. Sonnleitner, M. Porta, P. Diaz-Sylvester, M. Fill, H. Schindler, and S. Fleischer. 2002. Differential activation by Ca2+, ATP and caffeine of cardiac and skeletal muscle ryanodine receptors after block by Mg2+. J. Membr. Biol. 187:51–64. [DOI] [PubMed] [Google Scholar]
- DelPrincipe, F., M. Egger, and E. Niggli. 1999. Calcium signalling in cardiac muscle: refractoriness revealed by coherent activation. Nat. Cell Biol. 1:323–329. [DOI] [PubMed] [Google Scholar]
- Dias, J.M., C. Szegedi, I. Jona, and P.D. Vogel. 2006. Insights into the regulation of the ryanodine receptor: differential effects of Mg2+ and Ca2+ on ATP binding. Biochemistry. 45:9408–9415. [DOI] [PubMed] [Google Scholar]
- Eisner, D.A., A.W. Trafford, M.E. Diaz, C.L. Overend, and S.C. O'Neill. 1998. The control of Ca release from the cardiac sarcoplasmic reticulum: regulation versus autoregulation. Cardiovasc. Res. 38:589–604. [DOI] [PubMed] [Google Scholar]
- Fabiato, A., and F. Fabiato. 1975. Effects of magnesium on contractile activation of skinned cardiac cells. J. Physiol. 249:497–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorke, S., and D. Terentyev. 2008. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc. Res. 77:245–255. [DOI] [PubMed] [Google Scholar]
- Hess, P., P. Metzger, and R. Weingart. 1982. Free magnesium in sheep, ferret and frog striated muscle at rest measured with ion-selective micro-electrodes. J. Physiol. 333:173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jona, I., C. Szegedi, S. Sarkozi, P. Szentesi, L. Csernoch, and L. Kovacs. 2001. Altered inhibition of the rat skeletal ryanodine receptor/calcium release channel by magnesium in the presence of ATP. Pflugers Arch. 441:729–738. [DOI] [PubMed] [Google Scholar]
- Keller, M., J.P. Kao, M. Egger, and E. Niggli. 2007. Calcium waves driven by “sensitization” wave-fronts. Cardiovasc. Res. 74:39–45. [DOI] [PubMed] [Google Scholar]
- Kermode, H., A.J. Williams, and R. Sitsapesan. 1998. The interactions of ATP, ADP, and inorganic phosphate with the sheep cardiac ryanodine receptor. Biophys. J. 74:1296–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kléber, A.G. 1990. Consequences of acute ischemia for the electrical and mechanical function of the ventricular myocardium. A brief review. Experientia. 46:1162–1167. [DOI] [PubMed] [Google Scholar]
- Lacampagne, A., M.G. Klein, and M.F. Schneider. 1998. Modulation of the frequency of spontaneous sarcoplasmic reticulum Ca2+ release events (Ca2+ sparks) by myoplasmic [Mg2+] in frog skeletal muscle. J. Gen. Physiol. 111:207–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, G.D. 2000. Excitation-contraction coupling in skeletal muscle: comparisons with cardiac muscle. Clin. Exp. Pharmacol. Physiol. 27:216–224. [DOI] [PubMed] [Google Scholar]
- Laver, D.R. 2005. Coupled calcium release channels and their regulation by luminal and cytosolic ions. Eur. Biophys. J. 34:359–368. [DOI] [PubMed] [Google Scholar]
- Laver, D.R., and B.N. Honen. 2008. Luminal Mg2+, a key factor controlling RYR2-mediated Ca2+ release: cytoplasmic and luminal regulation modeled in a tetrameric channel. J. Gen. Physiol. 132:429–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver, D.R., and G.D. Lamb. 1998. Inactivation of Ca2+ release channels (ryanodine receptors RyR1 and RyR2) with rapid steps in [Ca2+] and voltage. Biophys. J. 74:2352–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver, D.R., T.M. Baynes, and A.F. Dulhunty. 1997a. Magnesium inhibition of ryanodine-receptor calcium channels: evidence for two independent mechanisms. J. Membr. Biol. 156:213–229. [DOI] [PubMed] [Google Scholar]
- Laver, D.R., V.J. Owen, P.R. Junankar, N.L. Taske, A.F. Dulhunty, and G.D. Lamb. 1997b. Reduced inhibitory effect of Mg2+ on ryanodine receptor-Ca2+ release channels in malignant hyperthermia. Biophys. J. 73:1913–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver, D.R., E.R. O'Neill, and G.D. Lamb. 2004. Luminal Ca2+-regulated Mg2+ inhibition of skeletal RyRs reconstituted as isolated channels or coupled clusters. J. Gen. Physiol. 124:741–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko, V., S. Viatchenko-Karpinski, A. Smirnov, T.F. Wiesner, and S. Gyorke. 2001. Dynamic regulation of sarcoplasmic reticulum Ca2+ content and release by luminal Ca2+-sensitive leak in rat ventricular myocytes. Biophys. J. 81:785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan, D.H. 1970. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J. Biol. Chem. 245:4508–4518. [PubMed] [Google Scholar]
- Meissner, G. 2002. Regulation of mammalian ryanodine receptors. Front. Biosci. 7:2072–2080. [DOI] [PubMed] [Google Scholar]
- Meissner, G. 2004. Molecular regulation of cardiac ryanodine receptor ion channel. Cell Calcium. 35:621–628. [DOI] [PubMed] [Google Scholar]
- Meissner, G., and J.S. Henderson. 1987. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J. Biol. Chem. 262:3065–3073. [PubMed] [Google Scholar]
- Michailova, A.P., M.E. Belik, and A.D. McCulloch. 2004. Effects of magnesium on cardiac excitation-contraction coupling. J. Am. Coll. Nutr. 23:514S–517S. [DOI] [PubMed] [Google Scholar]
- Murphy, E., C. Steenbergen, L.A. Levy, B. Raju, and R.E. London. 1989. Cytosolic free magnesium levels in ischemic rat heart. J. Biol. Chem. 264:5622–5627. [PubMed] [Google Scholar]
- Niggli, E. 1999. Localized intracellular calcium signaling in muscle: calcium sparks and calcium quarks. Annu. Rev. Physiol. 61:311–335. [DOI] [PubMed] [Google Scholar]
- Niggli, E., and N. Shirokova. 2007. A guide to sparkology: the taxonomy of elementary cellular Ca2+ signaling events. Cell Calcium. 42:379–387. [DOI] [PubMed] [Google Scholar]
- Otsu, K., H.F. Willard, V.K. Khanna, F. Zorzato, N.M. Green, and D.H. MacLennan. 1990. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J. Biol. Chem. 265:13472–13483. [PubMed] [Google Scholar]
- Overend, C.L., D.A. Eisner, and S.C. O'Neill. 2001. Altered cardiac sarcoplasmic reticulum function of intact myocytes of rat ventricle during metabolic inhibition. Circ. Res. 88:181–187. [DOI] [PubMed] [Google Scholar]
- Rios, E., N. Shirokova, W.G. Kirsch, G. Pizarro, M.D. Stern, H. Cheng, and A. Gonzalez. 2001. A preferred amplitude of calcium sparks in skeletal muscle. Biophys. J. 80:169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon, T.R., K.S. Ginsburg, and D.M. Bers. 2002. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ. Res. 91:594–600. [DOI] [PubMed] [Google Scholar]
- Shtifman, A., C.W. Ward, T. Yamamoto, J. Wang, B. Olbinski, H.H. Valdivia, N. Ikemoto, and M.F. Schneider. 2002. Interdomain interactions within ryanodine receptors regulate Ca2+ spark frequency in skeletal muscle. J. Gen. Physiol. 119:15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G.A., J.I. Vandenberg, N.S. Freestone, and H.B. Dixon. 2001. The effect of Mg2+ on cardiac muscle function: is CaATP the substrate for priming myofibril cross-bridge formation and Ca2+ reuptake by the sarcoplasmic reticulum? Biochem. J. 354:539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G.L., and S.C. O'Neill. 2001. A comparison of the effects of ATP and tetracaine on spontaneous Ca2+ release from rat permeabilised cardiac myocytes. J. Physiol. 534:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentesi, P., C. Pignier, M. Egger, E.G. Kranias, and E. Niggli. 2004. Sarcoplasmic reticulum Ca2+ refilling controls recovery from Ca2+-induced Ca2+ release refractoriness in heart muscle. Circ. Res. 95:807–813. [DOI] [PubMed] [Google Scholar]
- Terentyev, D., S. Viatchenko-Karpinski, H.H. Valdivia, A.L. Escobar, and S. Gyorke. 2002. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ. Res. 91:414–420. [DOI] [PubMed] [Google Scholar]
- Valent, I., A. Zahradnikova, J. Pavelkova, and I. Zahradnik. 2007. Spatial and temporal Ca2+, Mg2+, and ATP2- dynamics in cardiac dyads during calcium release. Biochim. Biophys. Acta. 1768:155–166. [DOI] [PubMed] [Google Scholar]
- Wang, S.Q., L.S. Song, E.G. Lakatta, and H. Cheng. 2001. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 410:592–596. [DOI] [PubMed] [Google Scholar]
- Williams, I.A., and D.G. Allen. 2007. Intracellular calcium handling in ventricular myocytes from mdx mice. Am. J. Physiol. Heart Circ. Physiol. 292:H846–H855. [DOI] [PubMed] [Google Scholar]
- Yang, Z., and D.S. Steele. 2001. Effects of cytosolic ATP on Ca2+ sparks and SR Ca2+ content in permeabilized cardiac myocytes. Circ. Res. 89:526–533. [DOI] [PubMed] [Google Scholar]
- Zahradnikova, A., M. Dura, I. Gyorke, A.L. Escobar, I. Zahradnik, and S. Gyorke. 2003. Regulation of dynamic behavior of cardiac ryanodine receptor by Mg2+ under simulated physiological conditions. Am. J. Physiol. Cell Physiol. 285:C1059–C1070. [DOI] [PubMed] [Google Scholar]
- Zhou, J., B.S. Launikonis, E. Rios, and G. Brum. 2004. Regulation of Ca2+ sparks by Ca2+ and Mg2+ in mammalian and amphibian muscle. An RyR isoform-specific role in excitation-contraction coupling? J. Gen. Physiol. 124:409–428. [DOI] [PMC free article] [PubMed] [Google Scholar]