INTRODUCTION
This year marks the 50th anniversary of the publication of one of the most important papers in the field of epithelial transport. In 1958 Valborg Koefoed-Johnsen and Hans Ussing presented the two-membrane model (KJU model) of Na transport by the frog skin (Koefoed-Johnsen and Ussing, 1958). This idea has guided thinking throughout epithelial biology for the past half century. The life and remarkable career of Ussing has been reviewed (Larsen, 2002), and we will not do that in detail here. Rather, we will mark the milestone of this seminal work with a short description of its historical background and a brief discussion of its wide-ranging impact and implications.
BACKGROUND
Ussing joined August Krogh's Laboratory of Zoophysiology at the University of Copenhagen in 1934, shortly after Harold C. Urey had given Krogh a small sample of heavy water (Ussing, 1980). Ussing was recruited to assist Krogh in exploring the uses of heavy water in biology.
George de Hevesy, who had invented the use of tracers, had for some time tried to stimulate Krogh's interest in the use of tracers in biological research. Before the discovery of deuterium, however, the available tracers were of little biological interest. Ussing therefore joined Krogh's laboratory at a propitious moment, and he soon began to study protein turnover using deuterium-labeled amino acids. He turned from protein metabolism to membrane permeability and ion transport some 10 years later during World War II, when August Krogh in 1944 fled the Nazi-occupied Denmark to Sweden.
When Krogh left Copenhagen, he requested that Ussing oversee the ion transport studies in the Laboratory of Zoophysiology at the University of Copenhagen. Ussing accepted the responsibility and, when Krogh retired in 1945, he agreed to continue to supervise the research program that Krogh had laid out in his Croonian lecture (Krogh, 1946); the anticipated temporary detour into membrane transport became a lifelong endeavor. Not only was the problem important, but the Laboratory of Zoophysiology was also in a strong position to pursue studies of transmembrane ion movement due to the ready availability of suitable tracers, which since 1938 had been produced by the cyclotron at the Niels Bohr Institute in Copenhagen (Hahn et al., 1939; Ussing, 1980).
Although, or maybe because, Krogh's focus was on K+, Ussing decided to examine the transmembrane movement of Na+ in the search for evidence for the “sodium pump” that had been proposed, in various formulations, by earlier workers (cf. Glynn, 2002). Being fully aware of the possibilities provided by the use of tracers, Ussing and colleagues began to use 24Na+ to explore how muscle cells handle Na+. They showed that all cellular Na+ could exchange with the extracellular Na+, which implied that Na+ could leave the cell against both a concentration and an electrical gradient—i.e., that the Na+ efflux involved active transport. The surprise came when the magnitude of the Na+ efflux was evaluated and found to be larger than the available energy as deduced from the oxygen consumption. This led Ussing to propose that some of the measured Na+ efflux occurred as an exchange diffusion, where the cell membrane was proposed to be endowed with some “permutite” molecules that could move a Na+ from the intracellular across the membrane in exchange for a Na+ from the extracellular compartment, a process that would not require the direct expenditure of metabolic energy (Ussing, 1947).
The existence of exchange diffusion meant that, in the absence of additional information, one could not use steady-state isotope fluxes to measure either the active ion transport or the electrodiffusive ion leak. Not only was it necessary to abandon the muscle preparation, but to fully characterize the fluxes it was also necessary to know both the transmembrane concentration and potential differences. This led Ussing to the isolated frog skin—in part because Krogh earlier had demonstrated that frogs can take up both Na+ and Cl− from very dilute solutions (Ussing, 1980).
Ussing and colleagues showed that Na+ indeed can move across the isolated frog skin against both concentration and potential differences (Ussing, 1949a), and the flux-ratio equation was introduced as a tool to distinguish between active transport and electrodiffusion (Ussing, 1949b). This study also introduced Cu2+ as a tool to increase the transepithelial potential difference across the skin (by reducing the anion conductance). This ability of Cu2+ to reduce the skin's Cl− conductance became an important tool in the KJ&U study.
The key advance, however, was the implementation of the short-circuit technique (Ussing and Zerahn, 1951), in which one could eliminate both the concentration and the potential differences across the skin and thereby avoid many of the problems that had afflicted previous studies. (The short-circuit technique had been used by earlier workers [W.L. Francis, and E.J. Lund and P. Stapp]; Ussing learned about it while visiting the Donner Laboratory in 1948 [Ussing, 1980]. The short-circuit technique can be considered a special case of the voltage-clamp method, although since the transepithelial voltage is kept constant [at 0 mV in the short-circuit situation] the potentials across the individual membranes can vary. The voltage clamp had previously been applied to axons [Hodgkin et al., 1949; Marmont, 1949], although Ussing and Zerahn make no reference to this work.) Under short-circuit conditions the measured transepithelial current reflects the net fluxes of all actively transported ions. Ussing and Zerahn found that there was near equality between the measured current and the current predicted from the transepithelial Na+ flux, with the Na+ influx (from the outer solution to the blood side) equivalent to ∼105% of the measured current and the Na+ efflux (from the blood side to the outer solution) equivalent to ∼5% of the current but in the opposite direction. Thus, within the accuracy of the measurements, the short-circuit current represents the active Na+ transport across the epithelium. When the transepithelial potential difference (measured relative to the outer solution) was increased, the Na+ influx decreased and the Na+ efflux increased; the potential difference required to equalize the influxes and efflux was estimated to be ∼110 mV, which would be the electromotive force of the Na+ transport mechanism under the given conditions.
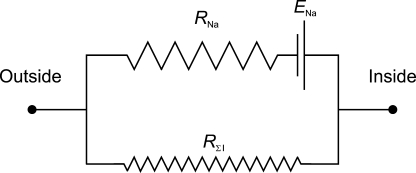
Ussing and Zerahn thus could reduce the electrical and transport characteristics of the frog skin to the equivalent circuit shown in Fig. 1, in which ENa denotes the electromotive force of the Na+ transport mechanism, RNa the resistance of the active Na+ pump-related pathway, and RΣI the resistance to other ions—including Cl− and, again, highlighting the importance of minimizing the contributions from ions other than Na+ when studying the active Na+ transport.
Figure 1.
Equivalent circuit representing the frog skin. ENa represents the electromotive force of the Na+-transporting mechanism (sodium pump). RNa represents the resistance to Na+ movement across the frog skin. RΣI represents the combined resistance to all other ions. Modified after Ussing and Zerahn (1951).
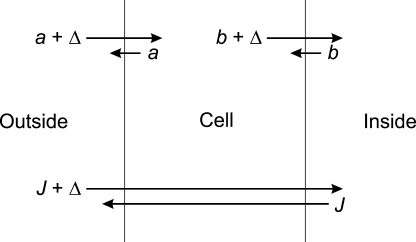
Ussing and Zerahn went on to consider how the results should be interpreted in the context of a more detailed, two-membrane model of the frog skin (Fig. 2), where the net transepithelial transport (Δ) would be equal to the net Na+ flux across both the outer (apical) and the inner (basolateral) cell membranes. The sodium pump was proposed to reside in the latter. The detailed characteristics of the two membranes were not considered further. By 1958, however, the time clearly had come to move beyond the black box approach embodied in Fig. 1 and explore the implications of adopting a more cellular approach (cf. Ussing, 1980).
Figure 2.
Two-membrane representation of the frog skin. The net Na+ flux across the skin (Δ) is the difference between two unidirectional fluxes (the influx, J + Δ, and the efflux, J), which again is equal to the difference between the influx (a) and efflux (a + Δ) across the outer membrane and the efflux (b + Δ) and influx (b) across the inner membrane. Modified after Ussing and Zerahn (1951).
THE PAPER
The experiments themselves were simple. First, it was shown using isotopic fluxes of 36Cl− that 10 μM CuSO4, added to the outer solution, reduced the Cl− permeability of the isolated frog skin (see above). Then, in the presence of Cu2+, the transepithelial potential of the skin was measured while the K+ concentration of the inner solution was increased from 2 to 80 mM, with K+ replacing Na+. The potential fell in proportion to log[K+], as if the skin were a perfect K+ electrode. This experiment was repeated with two other preparations in which the shunt pathway was minimized, either by replacing Cl− with the less permeant SO42− ion, or by reducing NaCl in the outer solution. Finally, the transepithelial potential was measured while the Na+ in the outer solution was lowered from 100 mM by substituting K+. In this case, the voltage increased as Na+ was increased (and K+ decreased). In a single Cu2+-treated skin, the slope was rather less than that predicted for a perfect Na+ electrode; another skin mounted in SO42− Ringer behaved as an almost perfect Na+ electrode down to a concentration of 1 mM. (The remarkably small dataset, especially with respect to changes in the outer solution, is perhaps explained by the statement that the ideal Na+ behavior “is seen only in skins from frogs in good condition.”)
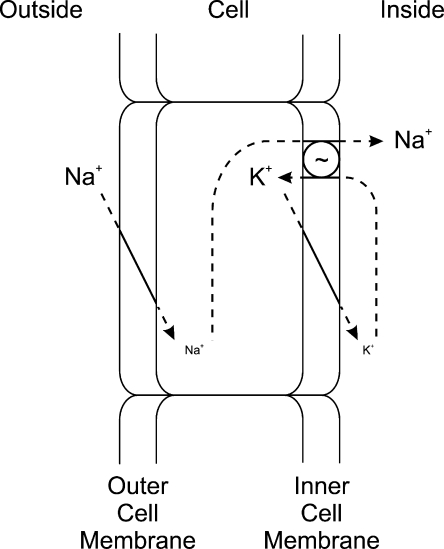
The key to the paper's impact is in the interpretation of the results. Koefoed-Johnsen and Ussing realized that, in the absence of a shunt, the electrical properties of the two membranes become independent, and the two-membrane voltages would add as if they were in series to generate the transepithelial voltage (Fig. 3).
Figure 3.
The KJU two-membrane model. Na+ enters the cell by an electrodiffusive mechanism through the outer (apical) cell membrane and pumped out the cell by a sodium pump (Na+/K+ pump) in the inner (basolateral) cell membrane. The K+ that is pumped into the cell by Na+/K+ pump leaves the cell through an electrodiffusive mechanism through the inner membrane. The potential difference across the skin (V), measured relative to the outer solution, is the difference between the potential differences across the outer membrane (Vo) and the inner membrane (Vi), where Vi is measured relative to the inner solution (opposite the convention used by KJ&U), V = Vo + Vi. Modified after Koefoed-Johnsen and Ussing (1958).
The opposite direction of the potential changes in response to the ion concentration changes in the two solutions could not be interpreted within the conceptual framework provided by the equivalent circuit in Fig. 1. It required a new way of thinking (H.H. Ussing to Larsen [2002], which led to the two-membrane model—a notion that already had been approached by Ussing and Zerahn in 1951 (Fig. 2). To account for the opposite potential changes, KJ&U proposed that the apical and basolateral cell membranes had different ion selectivities—the outer (or apical) membrane being Na+ selective; the inner (or basolateral) membrane being K+ selective. The selective Na+ permeability of the outer membrane represents the most unusual aspect of the model because most cell membranes were known to be selectively K+ permeable. Although the notion of a Na+-selective membrane is implicit in earlier studies from the Ussing laboratory (Ussing, 1949a; Ussing and Zerahn, 1951), this idea may also have been influenced by the earlier description of the Na+-selective nature of the squid axon membrane at the peak of the action potential (Hodgkin and Katz, 1949), a finding that was mentioned but not specifically cited.
It follows, then, that the active transport step must be at the inner membrane to avoid being shunted by the high Na+ permeability of the outer membrane. This scheme was attractive because it demanded that cytoplasmic Na+ concentration be maintained low by the pump, similar to the situation in other cells. The inner membrane thus looks like that of a “typical” cell; only the outer membrane has special properties. It was further postulated that the pump exchanges Na+ for K+. This was not a requirement of the two-membrane model, but turned out to be correct, and may have been influenced by findings in other cell types. The sodium pump had been shown to be a Na+/K+ pump in red blood cells (Glynn, 1956) and in the squid giant axon (Hodgkin and Keynes, 1955). Koefoed-Johnsen and Ussing allude to these results but, again, they are not specifically cited; the identification of the Na+,K+-ATPase the year before (Skou, 1957) is not mentioned at all.
IMPLICATIONS AND EXTENSIONS
Although the 1958 paper focused entirely on Na+ transport by a particular tissue, the frog skin, the power of the model became apparent as it was applied to an ever widening range of transporting epithelia. Indeed, the frog skin itself became an important model system for studying Na+-reabsorbing, high-resistance epithelia. Furthermore, with some modifications, the two-membrane hypothesis explained the functions of many different ion-transporting tissues.
In the 1950s and 1960s, the amphibian urinary bladder, particularly that of the giant toad Bufo marinus, became a second popular epithelium for studying Na+ transport. Interest in this tissue expanded with the finding of Crabbé (1961) and others that Na+ transport by the bladder could be stimulated in vitro by the adrenocorticoid aldosterone, a steroid known to be important in salt balance. The two-membrane model permitted investigators to ask which membrane step was rate-limiting for transport and controlled by the action of the hormone (Sharp and Leaf, 1966).
Like the frog skin, the small intestine has a mucosa-negative transepithelial voltage and a short-circuit current that is accounted for by active Na+ transport. Both parameters were increased by glucose as well as by non-metabolizable analogues (Schultz and Zalusky, 1964). This was consistent with earlier findings that the uptake of glucose was dependent on the presence of Na+ (Riklis and Quastel, 1958; Csáky and Thale, 1960), and the proposal of a stoichiometrical coupling between glucose and Na+ uptake (Crane, 1962). The KJU model explained this behavior neatly, with the replacement of a simple Na+ permeability in the outer membrane with a Na+ glucose cotransport system (Schultz and Zalusky, 1964).
While the frog skin and toad bladder actively transport Na+, with Cl− movement following through the shunt, some closely related mammalian epithelia transport Na+, in part, in exchange for K+. Though this represents an important difference, it can arise from a simple modification of the KJU model by including a K+ conductance in the apical membrane (O'Neil and Boulpaep, 1982; Koeppen et al., 1983). In tissues such as the renal cortical collecting duct and the distal colon, this permits part of the K+ that is transported into the cell by the pump to leave the cell across the luminal membrane and thus to be actively secreted into the urine and feces.
Other epithelia, notably the renal proximal tubule and gall bladder, transport Na+ vigorously but unlike the frog skin do not generate large transepithelial voltages. This might appear to involve a very different mechanism, but it can be accounted for by two modifications of the KJU model, one quantitative and one qualitative. The qualitative difference is in the nature of the Na+-permeability step. In the brush-border membrane of the proximal tubule, the major pathway for Na+ entry into the cell is an electroneutral exchange with one Na+ ion being exchanged for one H+ ion (Murer et al., 1976). The quantitative difference is in the leak pathway, which has a much larger conductance in the so-called leaky epithelia of the proximal tubule, small intestine, and gall bladder (Frömter and Diamond, 1972). Otherwise, the basic concepts are the same; Na+ entry is driven by passive forces (now just the Na+ concentration and pH differences across the apical cell membrane) while the Na+/K+ pump mediates exit of Na+ across the basolateral membrane, which establishes the low Na+ concentration in the cell. Several Na+ entry mechanisms involving cotransport and countertransport have been identified in different absorbing epithelia (Reuss, 2008); the generic two-membrane model describes, with minor modifications, the overall Na+ transport mechanism in each of these cases.
Secretory epithelia move salt and water in the opposite direction, from the interstitial compartment to the lumen of the organ or gland (Welsh, 1987). This involves a different set of events, which again can be thought of as modifications of the basic two-membrane KJU model, with one active and one passive transport step (in this case with Cl− being the key ion). Again the apical, or luminal, membrane has a high passive permeability (also conferred by ion channels) and the basolateral membrane an active transport mechanism. The overall transport process is still driven by the Na+/K+-ATPase, which maintains the intracellular Na+ concentration low, thereby providing the driving force for a 1Na+,1K+,2Cl− cotransport of ions into the cell from the interstitial solution. The transporters are quite different, but the conceptual framework is maintained.
The two-membrane model implicitly raises the issue of how the two cell membranes, facing the inside and outside of the frog, respectively, can have such a different complement of membrane transporters. In 1958 the tools required to address this question were lacking. Advances in our understanding of the cell biology of protein synthesis and trafficking through the endoplasmic reticulum and Golgi apparatus, coupled with the identification of the membrane transport proteins involved (see below), have led to important insights into how proteins are targeted to one membrane or the other in an epithelium (Rodriguez-Boulan et al., 2005). These sorting mechanisms are still not fully understood and remain a major area of investigation. In a sense the field of epithelial polarity began in 1958 with the Koefoed-Johnsen and Ussing paper.
A MODERN VERSION OF THE MODEL
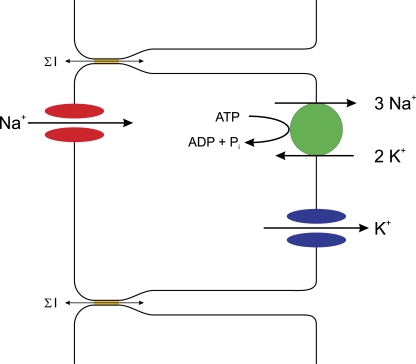
The two-membrane concept of the KJU model has remained intact since it was proposed. We have, however, gleaned a huge amount of new information concerning the molecular details of the various aspects of the scheme (Fig. 4).
Figure 4.
A modern version of the KJU two-membrane model. The apical Na+ influx occurs through ENaC channels (red). The Na+ is pumped out of the cell by a Na+,K+-ATPase (green) that in each catalytic cycle pumps three Na+ out of the cell and two K+ into the cell. The basolateral K+ influx occurs through potassium channels (blue), either inward-rectifier potassium channels or 2P potassium channels. The shunt pathway includes tight junctions, which are formed by claudins (ochre) that allow the relatively nonselective movement of many different ions (denoted by ΣI). In the frog skin, this pathway also includes mitochondria-rich cells that selectively conduct Cl− (not depicted).
Apical Na+ Permeability
The Na+ entry step in the frog skin and related high-resistance epithelia is facilitated by ion channels, consistent with the simple electrodiffusion process envisioned by Koefoed-Johnsen and Ussing. The channels were identified by fluctuation analysis and patch clamp. Molecular cloning showed that they consisted of three subunits, termed α, β, and γENaC (Garty and Palmer, 1997; Kellenberger and Schild, 2002) Last year the three-dimensional structure of a homologue, the acid-sensing channel found in sensory nerves, was solved (Jasti et al., 2007). This channel is a trimer, with each subunit having to transmembrane α-helices, which presumably form the conductive pathway.
Na+/K+ Pump
As mentioned above, the Na+/K+ pump had been identified as an ATPase in 1957 (Skou, 1957). Subsequently, it was found to consist of two major subunits (Jorgensen et al., 2003). The transporter is the founding member of the P-type family of pumps, named for the role of the phosphorylated intermediate in driving the cycle of conformational changes required for ion movement. The kinetics of the transport process have been worked out in exquisite detail. The three-dimensional structure of this protein was also published last year (Morth et al., 2007).
Basolateral K+ Permeability
The K+ permeability of the basolateral membrane of the frog skin and related epithelia is presumably also conferred by ion channels. Several K+-selective channels have been identified and/or described, but there is no consensus on which, if any, is the dominant form (Hebert et al., 2005). This may reflect the diversity of K+ channels in any one epithelium or differences among tissues. The most likely candidates include inward-rectifier K+ channels and 2-P K+ channels. A three-dimensional structure has been solved for a bacterial homologue of the former class (Kuo et al., 2003).
The Shunt Pathway
In the Koefoed-Johnsen and Ussing paper efforts were made to minimize the contribution of the “shunt” pathways that obscure the major membrane transport pathways for Na+ and K+. Under physiological conditions, however, much of the transport of Na+ will be accompanied by the concomitant uptake of anions, particularly Cl−, through the shunt. In the frog skin, the major shunt pathway for Cl− ions turned out to be through a minority cell type—the mitochondria-rich cells—arranged in parallel with the Na+-transporting cells (Larsen, 1991). This involves conductive movement through voltage-dependent anion channels that have yet to be identified at the molecular level. Another part of the shunt pathway involves a paracellular movement through tight junctions, as recognized by Ussing and his colleagues early on (Ussing and Windhager, 1964). Later, a wide range of paracellular conductances, which led to the distinction between “tight” and “leaky” epithelia, became apparent (Frömter and Diamond, 1972), and several proteins associated with the tight junctions have been identified. The most important in determining the conductive properties are the claudins, transmembrane proteins that appear to interact with each other between cells (Van Itallie and Anderson, 2006). The precise structure of the junction has not yet been determined.
SUMMARY
The field of epithelial transport has come a long way since 1958. Much of this progress has been guided by the basic principles enunciated in KJ&U. The solutions of the three-dimensional structures of both the Na+/K+ pump and the Na+ channel homologue ASIC1 last year constitute a fitting golden anniversary present for the two-membrane model. They also remind those of us in the field, as well as the interested onlookers, that for much of the past 50 years we have largely been filling in the details of the KJU model.
References
- Crabbé, J. 1961. Stimulation of active sodium transport by the isolated toad bladder with aldosterone in vitro. J. Clin. Invest. 40:2103–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane, R.K. 1962. Hypothesis for mechanism of intestinal transport of sugars. Fed. Proc. 21:891–895. [PubMed] [Google Scholar]
- Csáky, T.Z., and M. Thale. 1960. Effect of ionic environment on intestinal sugar transport. J. Physiol. 151:59–65. [PMC free article] [PubMed] [Google Scholar]
- Frömter, E., and J.M. Diamond. 1972. Route of passive ion permeation in epithelia. Nat. New Biol. 235:9–13. [DOI] [PubMed] [Google Scholar]
- Garty, H., and L.G. Palmer. 1997. Epithelial Na+ channels: function, structure, and regulation. Physiol. Rev. 77:359–396. [DOI] [PubMed] [Google Scholar]
- Glynn, I.M. 1956. Sodium and potassium movements in human red cells. J. Physiol. 134:278–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn, I.M. 2002. A hundred years of sodium pumping. Annu. Rev. Physiol. 64:1–18. [DOI] [PubMed] [Google Scholar]
- Hahn, L.A., G.C. Hevesty, and O.H. Rebbe. 1939. Do the potassium ions inside the muscle cells and blood corpuscles exchange with those present in the plasma? Biochem. J. 33:1549–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert, S.C., G. Desir, G. Giebisch, and W. Wang. 2005. Molecular diversity and regulation of renal potassium channels. Physiol. Rev. 85:319–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin, A.L., and B. Katz. 1949. The effect of sodium ions on the electrical activity of the giant axon of the squid. J. Physiol. 108:37–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin, A.L., and R.D. Keynes. 1955. Active transport of cations in giant axons from Sepia and Loligo. J. Physiol. 128:28–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin, A.L., A.F. Huxley, and B. Katz. 1949. Ionic currents underlying activity in the giant axon of the squid. Arch. Sci. Physiol. (Paris). 3:129–150. [Google Scholar]
- Jasti, J., H. Furukawa, E.B. Gonzales, and E. Gouaux. 2007. Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature. 449:316–323. [DOI] [PubMed] [Google Scholar]
- Jorgensen, P.L., K.O. Hakannson, and S.J. Karlish. 2003. Structure and mechanism of Na,K-ATPase: functional sites and their interactions. Annu. Rev. Physiol. 65:817–849. [DOI] [PubMed] [Google Scholar]
- Kellenberger, S., and L. Schild. 2002. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 82:735–767. [DOI] [PubMed] [Google Scholar]
- Koefoed-Johnsen, V., and H.H. Ussing. 1958. On the nature of the frog skin potential. Acta Physiol. Scand. 42:298–308. [DOI] [PubMed] [Google Scholar]
- Koeppen, B.M., B.A. Biagi, and G. Giebisch. 1983. Intracellular microelectrode characterization of the rabbit cortical collecting duct. Am. J. Physiol. 244:F35–F47. [DOI] [PubMed] [Google Scholar]
- Krogh, A. 1946. The active and passive exchanges of inorganic ions through the surfaces of living cells and through living membranes generally. Proc. R. Soc. Lond. B. Biol. Sci. 133:140–200. [DOI] [PubMed] [Google Scholar]
- Kuo, A., J.M. Gulbis, J.F. Antcliff, T. Rahman, E.D. Lowe, J. Zimmer, J. Cuthbertson, F.M. Ashcroft, T. Ezaki, and D.A. Doyle. 2003. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 300:1922–1926. [DOI] [PubMed] [Google Scholar]
- Larsen, E.H. 1991. Chloride transport by high resistance heterocellular epithelia. Physiol. Rev. 71:235–283. [DOI] [PubMed] [Google Scholar]
- Larsen, E.H. 2002. Hans H. Ussing—scientific work: contemporary significance and perspectives. Biochim. Biophys. Acta. 1566:2–15. [DOI] [PubMed] [Google Scholar]
- Marmont, G. 1949. Studies on the axon membrane; a new method. J. Cell. Physiol. 34:351–382. [DOI] [PubMed] [Google Scholar]
- Morth, J.P., B.P. Pedersen, M.S. Toustrup-Jensen, T.L. Sorensen, J. Petersen, J.P. Andersen, B. Vilsen, and P. Nissen. 2007. Crystal structure of the sodium-potassium pump. Nature. 450:1043–1049. [DOI] [PubMed] [Google Scholar]
- Murer, H., U. Hopfer, and R. Kinne. 1976. Sodium/proton antiport in brush-border-membrane vesicles from rat small intestine and kidney. Biochem. J. 154:597–604. [PMC free article] [PubMed] [Google Scholar]
- O'Neil, R.G., and E.L. Boulpaep. 1982. Ionic conductive properties and electrophysiology of the rabbit cortical collecting tubule. Am. J. Physiol. 243:F81–F95. [DOI] [PubMed] [Google Scholar]
- Reuss, L. 2008. Mechanisms of ion transport across cell membranes and epithelia. In The Kindey: Physiology and Pathophysiology, 4th Edition. R.J. Alpern and S.C. Hebert, editors. Academic Press, Burlington, MA. 35–56.
- Riklis, E., and J.H. Quastel. 1958. Effects of cations on sugar absorption by isolated surviving guinea pig intestine. Can. J. Biochem. Physiol. 36:347–362. [PubMed] [Google Scholar]
- Rodriguez-Boulan, E., G. Kreitzer, and A. Müsch. 2005. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 6:233–247. [DOI] [PubMed] [Google Scholar]
- Schultz, S.G., and R. Zalusky. 1964. Ion transport in isolated rabbit ileum II. Interaction between active sodium and active sugar transport. J. Gen. Physiol. 47:1043–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp, G.W.G., and A. Leaf. 1966. Mechanism of action of aldosterone. Physiol. Rev. 46:593–633. [DOI] [PubMed] [Google Scholar]
- Skou, J.C. 1957. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim. Biophys. Acta. 23:394–401. [DOI] [PubMed] [Google Scholar]
- Ussing, H.H. 1947. Interpretation of the exchange of radio-sodium in skeletal muscle. Nature. 160:262–263. [DOI] [PubMed] [Google Scholar]
- Ussing, H.H. 1949a. The active ion transport through the isolated frog skin in the light of tracer studies. Acta Physiol. Scand. 17:1–37. [DOI] [PubMed] [Google Scholar]
- Ussing, H.H. 1949b. The distinction by means of tracers between active transport and diffusion. Acta Physiol. Scand. 19:43–56. [Google Scholar]
- Ussing, H.H. 1980. Life with tracers. Annu. Rev. Physiol. 42:1–16. [DOI] [PubMed] [Google Scholar]
- Ussing, H.H., and E.E. Windhager. 1964. Nature of shunt path and active sodium transport path through frog skin epithelium. Acta Physiol. Scand. 61:484–504. [PubMed] [Google Scholar]
- Ussing, H.H., and K. Zerahn. 1951. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol. Scand. 23:110–127. [DOI] [PubMed] [Google Scholar]
- Van Itallie, C.M., and J.M. Anderson. 2006. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 68:403–429. [DOI] [PubMed] [Google Scholar]
- Welsh, M.J. 1987. Electrolyte transport by airway epithelia. Physiol. Rev. 67:1143–1184. [DOI] [PubMed] [Google Scholar]