Abstract
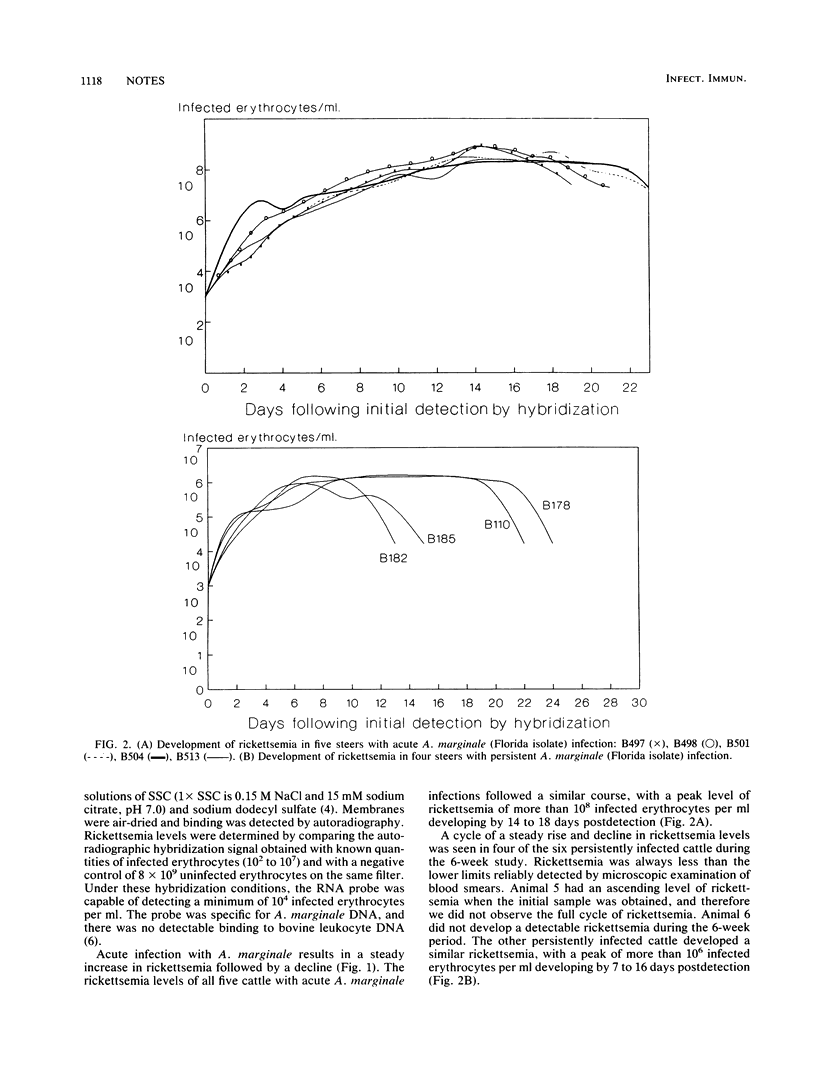
Submicroscopic levels of Anaplasma marginale rickettsemia in persistently infected cattle were determined by using nucleic acid hybridization. Within individuals, the rickettsemia levels steadily increased from less than 10(4) infected erythrocytes per ml to a peak of more than 10(6) infected erythrocytes per ml and then rapidly declined. This logarithmic variation parallels the variation of the rickettsemia level seen in acute infection and suggests that cyclic emergence of antigenic variants is a mechanism of rickettsial persistence.
Full text
PDF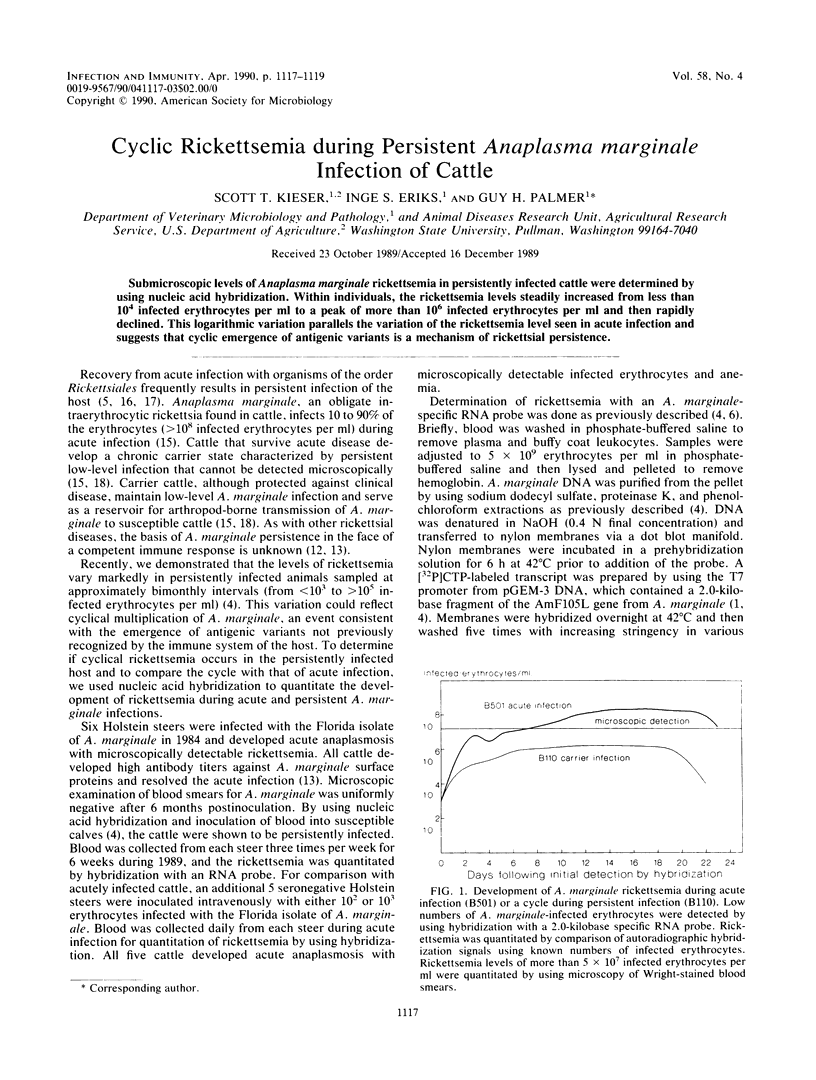

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbet A. F., Palmer G. H., Myler P. J., McGuire T. C. Characterization of an immunoprotective protein complex of Anaplasma marginale by cloning and expression of the gene coding for polypeptide Am105L. Infect Immun. 1987 Oct;55(10):2428–2435. doi: 10.1128/iai.55.10.2428-2435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buening G. M. Cell-mediated immune responses in calves with anaplasmosis. Am J Vet Res. 1973 Jun;34(6):757–763. [PubMed] [Google Scholar]
- Eckblad W. P., Magonigle R. A. Acquired cellular responsiveness in cattle cleared of Anaplasma marginale 28 months earlier. Vet Immunol Immunopathol. 1983 Jul;4(5-6):659–663. doi: 10.1016/0165-2427(83)90072-7. [DOI] [PubMed] [Google Scholar]
- Eriks I. S., Palmer G. H., McGuire T. C., Allred D. R., Barbet A. F. Detection and quantitation of Anaplasma marginale in carrier cattle by using a nucleic acid probe. J Clin Microbiol. 1989 Feb;27(2):279–284. doi: 10.1128/jcm.27.2.279-284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff W., Barbet A., Stiller D., Palmer G., Knowles D., Kocan K., Gorham J., McGuire T. Detection of Anaplasma-marginale-infected tick vectors by using a cloned DNA probe. Proc Natl Acad Sci U S A. 1988 Feb;85(3):919–923. doi: 10.1073/pnas.85.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson B. Identification and partial characterization of Rickettsia tsutsugamushi major protein immunogens. Infect Immun. 1985 Dec;50(3):603–609. doi: 10.1128/iai.50.3.603-609.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magonigle R. A., Newby T. J. Response of cattle upon reexposure to Anaplasma marginale after elimination of chronic carrier infections. Am J Vet Res. 1984 Apr;45(4):695–697. [PubMed] [Google Scholar]
- McGuire T. C., Palmer G. H., Goff W. L., Johnson M. I., Davis W. C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984 Sep;45(3):697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks E. V., Rice R. M., Kelly D. J., Stover C. K. Antigenic and genetic relatedness of eight Rickettsia tsutsugamushi antigens. Infect Immun. 1989 Oct;57(10):3116–3122. doi: 10.1128/iai.57.10.3116-3122.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberle S. M., Palmer G. H., Barbet A. F., McGuire T. C. Molecular size variations in an immunoprotective protein complex among isolates of Anaplasma marginale. Infect Immun. 1988 Jun;56(6):1567–1573. doi: 10.1128/iai.56.6.1567-1573.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Kuttler K. L., McGuire T. C. Detection of an Anaplasma marginale common surface protein present in all stages of infection. J Clin Microbiol. 1986 Jun;23(6):1078–1083. doi: 10.1128/jcm.23.6.1078-1083.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Musoke A. J., Katende J. M., Rurangirwa F., Shkap V., Pipano E., Davis W. C., McGuire T. C. Recognition of conserved surface protein epitopes on Anaplasma centrale and Anaplasma marginale isolates from Israel, Kenya and the United States. Int J Parasitol. 1988 Feb;18(1):33–38. doi: 10.1016/0020-7519(88)90033-1. [DOI] [PubMed] [Google Scholar]
- Zaugg J. L., Stiller D., Coan M. E., Lincoln S. D. Transmission of Anaplasma marginale Theiler by males of Dermacentor andersoni Stiles fed on an Idaho field-infected, chronic carrier cow. Am J Vet Res. 1986 Oct;47(10):2269–2271. [PubMed] [Google Scholar]



