Abstract
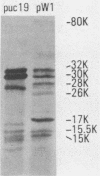
AF/R1 pili on the surface of Escherichia coli RDEC-1 promote attachment of the bacteria to rabbit intestinal brush borders. In order to characterize AF/R1 pili and manipulate their expression, we cloned the genes necessary for AF/R1 expression; determined the size of proteins produced in minicells; located the gene encoding the major structural subunit, named AfrA; and determined the DNA sequence of afrA as well as the sequence of 700 additional nucleotides upstream of afrA. Two contiguous EcoRI fragments spanning 7.9 kilobases were cloned from the 86-megadalton plasmid of RDEC-1 into vector pUC19 to make plasmid pW1. Bacteria carrying pW1 produced AF/R1 pili that were recognized by AF/R1-specific antiserum and promoted adherence of bacteria to brush borders prepared from rabbit intestine. Proteins with a molecular weight of 17,000 (17K proteins), which was the size of AfrA, as well as 15K, 15.5K, 26K, 28K, and 80K proteins were detected in minicells carrying pW1. The gene afrA was located by using an oligonucleotide probe, and its DNA sequence was determined. The DNA sequence of 700 additional nucleotides upstream was determined because this sequence may be important in the regulation of AF/R1 expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Goguen J. D., Beachey E. H. Hyperadhesive mutant of type 1-fimbriated Escherichia coli associated with formation of FimH organelles (fimbriosomes). Infect Immun. 1988 May;56(5):1023–1029. doi: 10.1128/iai.56.5.1023-1029.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendson R., Cheney C. P., Schad P. A., Boedeker E. C. Species-specific binding of purified pili (AF/R1) from the Escherichia coli RDEC-1 to rabbit intestinal mucosa. Gastroenterology. 1983 Oct;85(4):837–845. [PubMed] [Google Scholar]
- Båga M., Göransson M., Normark S., Uhlin B. E. Transcriptional activation of a pap pilus virulence operon from uropathogenic Escherichia coli. EMBO J. 1985 Dec 30;4(13B):3887–3893. doi: 10.1002/j.1460-2075.1985.tb04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Båga M., Norgren M., Normark S. Biogenesis of E. coli Pap pili: papH, a minor pilin subunit involved in cell anchoring and length modulation. Cell. 1987 Apr 24;49(2):241–251. doi: 10.1016/0092-8674(87)90565-4. [DOI] [PubMed] [Google Scholar]
- Båga M., Normark S., Hardy J., O'Hanley P., Lark D., Olsson O., Schoolnik G., Falkow S. Nucleotide sequence of the papA gene encoding the Pap pilus subunit of human uropathogenic Escherichia coli. J Bacteriol. 1984 Jan;157(1):330–333. doi: 10.1128/jb.157.1.330-333.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantey J. R., Inman L. R., Blake R. K. Production of diarrhea in the rabbit by a mutant of Escherichia coli (RDEC-1) that does not express adherence (AF/R1) pili. J Infect Dis. 1989 Jul;160(1):136–141. doi: 10.1093/infdis/160.1.136. [DOI] [PubMed] [Google Scholar]
- Cheney C. P., Boedeker E. C., Formal S. B. Quantitation of the adherence of an enteropathogenic Escherichia coli to isolated rabbit intestinal brush borders. Infect Immun. 1979 Nov;26(2):736–743. doi: 10.1128/iai.26.2.736-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney C. P., Formal S. B., Schad P. A., Boedeker E. C. Genetic transfer of a mucosal adherence factor (R1) from an enteropathogenic Escherichia coli strain into a Shigella flexneri strain and the phenotypic suppression of this adherence factor. J Infect Dis. 1983 Apr;147(4):711–723. doi: 10.1093/infdis/147.4.711. [DOI] [PubMed] [Google Scholar]
- Cheney C. P., Schad P. A., Formal S. B., Boedeker E. C. Species specificity of in vitro Escherichia coli adherence to host intestinal cell membranes and its correlation with in vivo colonization and infectivity. Infect Immun. 1980 Jun;28(3):1019–1027. doi: 10.1128/iai.28.3.1019-1027.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebright R. H., Cossart P., Gicquel-Sanzey B., Beckwith J. Mutations that alter the DNA sequence specificity of the catabolite gene activator protein of E. coli. Nature. 1984 Sep 20;311(5983):232–235. doi: 10.1038/311232a0. [DOI] [PubMed] [Google Scholar]
- Göransson M., Uhlin B. E. Environmental temperature regulates transcription of a virulence pili operon in E. coli. EMBO J. 1984 Dec 1;3(12):2885–2888. doi: 10.1002/j.1460-2075.1984.tb02225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. S., Brinton C. C., Jr Identification and characterization of E. coli type-1 pilus tip adhesion protein. Nature. 1988 Mar 17;332(6161):265–268. doi: 10.1038/332265a0. [DOI] [PubMed] [Google Scholar]
- Hultgren S. J., Lindberg F., Magnusson G., Kihlberg J., Tennent J. M., Normark S. The PapG adhesin of uropathogenic Escherichia coli contains separate regions for receptor binding and for the incorporation into the pilus. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4357–4361. doi: 10.1073/pnas.86.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Klemm P. The fimA gene encoding the type-1 fimbrial subunit of Escherichia coli. Nucleotide sequence and primary structure of the protein. Eur J Biochem. 1984 Sep 3;143(2):395–399. doi: 10.1111/j.1432-1033.1984.tb08386.x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer L., Orndorff P. E. Identification and characterization of genes determining receptor binding and pilus length of Escherichia coli type 1 pili. J Bacteriol. 1987 Feb;169(2):640–645. doi: 10.1128/jb.169.2.640-645.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland J. W., Green B. A., Foulds J., Holmes R. K. Cloning of extracellular DNase and construction of a DNase-negative strain of Vibrio cholerae. Infect Immun. 1985 Mar;47(3):691–696. doi: 10.1128/iai.47.3.691-696.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibuchi M., Hill W. E., Zon G., Payne W. L., Kaper J. B. Synthetic oligodeoxyribonucleotide probes to detect Kanagawa phenomenon-positive Vibrio parahaemolyticus. J Clin Microbiol. 1986 Jun;23(6):1091–1095. doi: 10.1128/jcm.23.6.1091-1095.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H., Hinson G. Temperature-dependent transcriptional regulation of expression of fimbriae in an Escherichia coli strain isolated from a child with severe enteritis. Infect Immun. 1987 Jul;55(7):1734–1736. doi: 10.1128/iai.55.7.1734-1736.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. K., Andrews G. P., Fritz D. L., Sjogren R. W., Jr, Boedeker E. C. Characterization of the plasmid from Escherichia coli RDEC-1 that mediates expression of adhesin AF/R1 and evidence that AF/R1 pili promote but are not essential for enteropathogenic disease. Infect Immun. 1988 Aug;56(8):1846–1857. doi: 10.1128/iai.56.8.1846-1857.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. K., Andrews G. P., Tall B. D., McConnell M. M., Levine M. M., Boedeker E. C. Characterization of CS4 and CS6 antigenic components of PCF8775, a putative colonization factor complex from enterotoxigenic Escherichia coli E8775. Infect Immun. 1989 Jan;57(1):164–173. doi: 10.1128/iai.57.1.164-173.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Krenn B. E., Klaasen P. Organization and expression of genes involved in the biosynthesis of K99 fimbriae. Infect Immun. 1984 Feb;43(2):508–514. doi: 10.1128/iai.43.2.508-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]