Abstract
A series of 2β-alkynyl and 2β-(1,2,3-triazol)substituted 3β-(substituted phenyl)tropanes were synthesized and evaluated for affinities at dopamine, serotonin and norepinephrine membrane transporters using competitive radioligand binding assays. All tested compounds were found to exhibit nanomolar or subnanomolar affinity for the dopamine transporter (DAT). One of the most potent and selective compounds in the series was 3β-(4-chlorophenyl)-2β-(4-nitrophenylethynyl)tropane (10c) that possessed an IC50 value of 0.9 nM at the DAT and Ki values of 230 nM and 620 nM at the norepinephrine transporter (NET) and serotonin transporter (5-HTT), respectively.
Keywords: Monoamine transporters; 3-phenyltropanes; alkynes; 1,2,3-triazoles; cocaine; addiction
1. Introduction
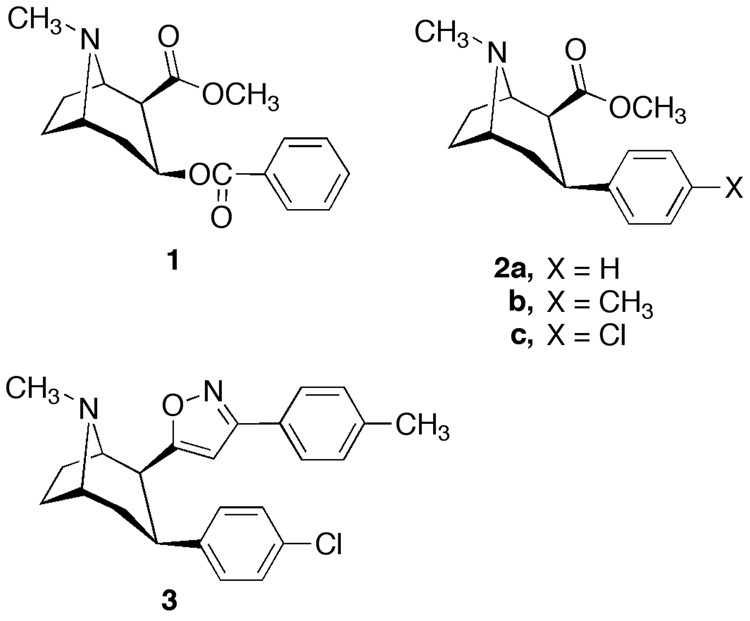
Cocaine (1, Figure 1) is one of the most powerful central nervous system (CNS) stimulants with reinforcing properties. While cocaine blocks the presynaptic reuptake of dopamine (DA), serotonin (5-HT) and norepinephrine (NE), numerous studies have strongly supported the hypothesis that the dopamine transporter (DAT) is highly significant in cocaine abuse regarding its reinforcing effects.1–5 Although other targets may be important to addiction (metabotrophic and iontropic glutamate receptors, GABA)6–8 animal behavioral and clinical finding suggest that the DAT is still an important target.9–14 Structure activity relationship (SAR) studies of a number of different classes of DAT inhibitors with the goal of the development of pharmacotherapies to treat cocaine addiction have been reported.12, 15–18 One of the most studied classes of DAT inhibitors is the 3-phenyltropanes.12, 15, 19, 20 The lead compound was 3β-phenyltropane-2β-carboxylic acid methyl ester (2a, WIN 35,065-2).21, 22 A large part of our SAR studies have been directed towards modification of the 4’-methyl and 4’-chloro analogs 2b,c.12
Figure 1.
Most of the studies were directed toward changes in the C(2) position of 2a–c. SAR studies have revealed that a variety of functional groups and substituents are well tolerated at this position without loss of high-affinity for monoamine transporters. In early studies, we showed that a variety of 2β esters and amides had high affinity for the DAT, in some cases, with considerably reduced affinity at the 5-HTT and NET.12 SAR studies from other groups and us also revealed that large lipophilic groups on the 2β-position, including alkyl, alkenyl and aryl substituents retained high DAT binding affinity.12, 23–25 In addition, exchange of the 2β carbomethoxy group with bioisosteric heterocyclic groups led to analogs with high-affinity and selectivity for the DAT. One of the most studied compounds in this series is the DAT selective 3 phenyltropane analog, RTI 336 (3). RTI 336 is currently in advanced preclinical development.10–12
Despite extensive efforts directed toward the development of a pharmacotherapy for cocaine abuse, at present no clinically approved drugs are available. In order to gain a better understanding of the molecular mechanisms of cocaine actions in the brain and find highly potent and selective monoamine uptake inhibitors, we have continued the investigation on modification of 2a. The present study was undertaken to further explore the SAR of 2-substituents of the 3β-phenyltropanes. In this paper, we describe the synthesis and monoamine transporter binding properties of several 2β-alkynyl and 2β-(1,2,3-triazol)substituted 3β-(substituted phenyl)tropane derivatives, and report that 3β-(4-chlorophenyl)-2β-(4- nitrophenylethynyl)tropane (10c) has high potency and good selectivity for the DAT relative to the 5-HTT and NET.
2. Chemistry
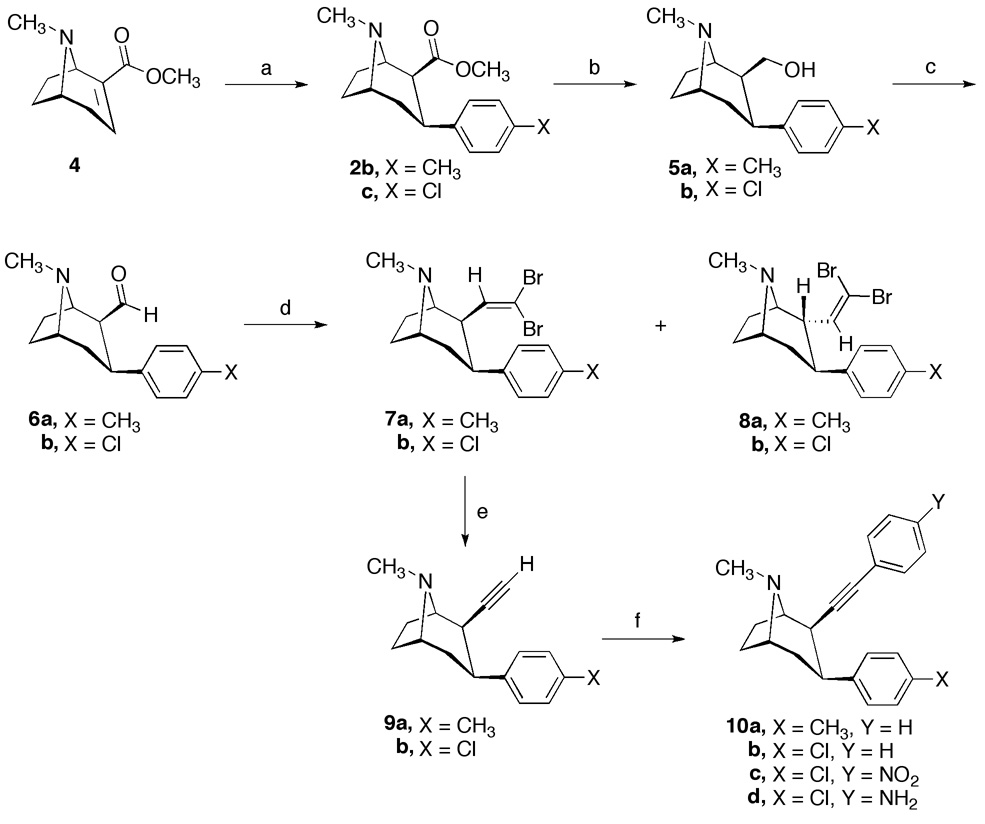
The synthesis of 2β-alkynyl-3β-(substituted phenyl)tropane analogs 9a,b and 10a–d starting with anhydroecgonine methyl ester (4), is outlined in Scheme 1. The 1,4-addition of 4 with the appropriate Grignard reagent at −45 °C in ethyl ether followed by trifluroacetic acid (TFA) led to the corresponding 3β-aryl substituted compounds 2b,c.26 Lithium Aluminum hydride reduction of the 2β-ester group of 2b,c afforded alcohols 5a,b. Swern oxidation of 5a,b provided aldehydes 6a,b. The aldehydes 6a,b were not stable and underwent epimerization at the C(2)-position during silica gel chromatography. Therefore, crude 6a,b, used without purification, were treated with carbon tetrabromide, triphenylphosphine, and zinc to give the 2β-isomers 7a,b in the order of 54–56% yield as well as the minor 2α-isomers 8a,b in 1.7% and 1.4% yield, respectively, after column chromatography. With the C(2)-position no longer susceptible to epimerization, 7a,b were treated with 2 equivalents of butyl lithium to afford 2β- ethynyltropanes 9a,b exclusively. Sonogashira coupling of ethyne 9a or 9b with iodobenzene using tetrakis(triphenylphosphine)palladium(0), and copper(I) iodide in 1:1 mixture of benzene-triethylamine gave 2β-phenylethynyltropanes 10a and 10b, respectively. Finally, coupling of 9b with 1-iodo-4-nitrobenzene or 4-iodoaniline furnished 10c and 10d. The relative stereochemistry of each compound was determined by 1H NMR spectral analysis, particularly with the aid of coupling constants of C(2)-H and C(3)-H. The vicinal couplings of J2eq, 3ax = 5.4–5.7 Hz and J2ax, 3ax = 11.5–11.9 Hz for the 2β- and 2α-substituents, respectively, are in good agreement with stereochemical assignments.
Scheme 1.
Reagents and conditions: (a) Grignard reagent, −45 °C, 2 h, then −78 °C, TFA; (b) LiAIH4, THF, room temperature; (c) Swern oxidation; (d) CBr4, PPh3, Zn, CH2Cl2, room temperature; (e) 2 BuLi, THF, −78 °C to room temperature; (f) Arl, Pd(PPh3)4, Cul, benzene-Et3N, 40 °C.
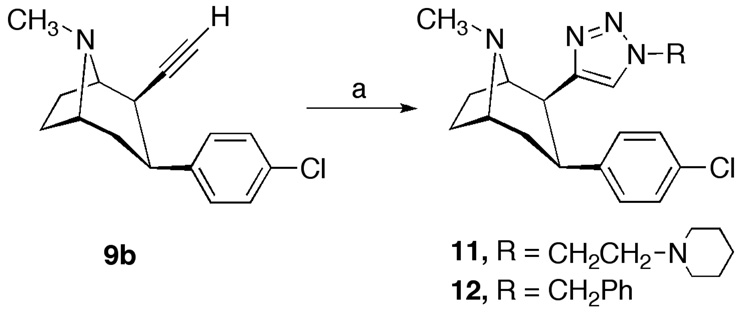
Recently, the copper(I)-catalyzed 1,2,3-triazole formation from terminal alkynes and azides, also known as the “click reaction”, has found growing applications in drug discovery due to the favourable physicochemical properties of triazole, which can readily associate with biological targets through hydrogen bonding and dipole interactions.27, 28 Accordingly, 2β-(1,2,3-triazol)substituted tropanes 11 and 12 were synthesized by treatment of 9b with 1-(2-azidoethyl)piperidine or benzylazide in the presence of copper sulfate and sodium ascorbate (Scheme 2).
Scheme 2.
Reagents and conditions: (a) azide, CuSO4, sodium ascorbate, tBuOH-H2O, room temperature.
3. Biology
The binding affinities for the target compounds at the DAT, NET, and 5-HTT were determined via competitive binding assays using the previously reported procedures.29, 30 The final concentration of radioligands in the assays were 0.5 nM [3H]WIN35,428 for the DAT, 0.5 nM [3H]nisoxetine for the NET and 0.2 nM [3H]paroxetine for the 5-HTT. The results of the binding studies, along with binding data of cocaine and 2a10 for comparison are listed in Table 1. Since the NET and 5-HTT have only one binding site, Ki values were calculated for inhibition of binding at these two transporters.
Table 1.
Monoamine transporter binding potencies for 2β-substituted 3β-(substituted phenyl)tropane derivatives.
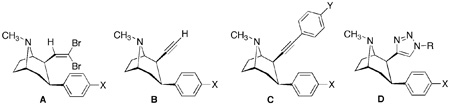 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compda | clogP | Structure | X | Y | R | DAT, IC50 (nM)d [3H]WIN35,428 |
NET, Ki (nM)d [3H]nisoxetine |
5-HTT, Ki (nM)d [3H]paroxetine |
| cocaineb | 89.1 | 1990 | 95 | |||||
| 2a | -- | -- | -- | -- | -- | 23 | 556 | 182 |
| 7a | 5.21 | A | CH3 | 0.23 ± 0.05 | 3.4 ± 0.6 | 39 ± 10 | ||
| 7b | 5.35 | A | Cl | 0.32 ± 0.05 | 3.5 ± 0.3 | 7 ± 2 | ||
| 8a | 5.21 | Ac | CH3 | 3.6 ± 1.1 | 55 ± 2 | 252 ± 13 | ||
| 8b | 5.35 | Ac | Cl | 1.4 ± 0.2 | 49 ± 2 | 76 ± 9 | ||
| 9a | 3.40 | B | CH3 | 11 ± 3 | 150 ± 20 | 54 ± 15 | ||
| 9b | 3.53 | B | Cl | 3.6 ± 0.4 | 75 ± 13 | 18 ± 2 | ||
| 10a | 5.87 | C | CH3 | H | 0.8 ± 0.3 | 31 ± 1 | 730 ± 190 | |
| 10b | 6.01 | C | Cl | H | 0.8 ± 0.2 | 14 ± 2 | 81 ± 4 | |
| 10c | 5.74 | C | Cl | NO2 | 0.9 ± 0.2 | 230 ± 20 | 620 ± 150 | |
| 10d | 4.73 | C | Cl | NH2 | 2.2 ± 0.5 | 50 ± 3 | 19 ± 3 | |
| 11 | 3.89 | D | Cl | 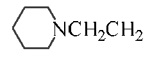 |
8 ± 1 | 430 ± 50 | 138 ± 11 | |
| 12 | 4.25 | D | Cl | C6H5CH2 | 47 ± 12 | 2560 ± 130 | 161 ± 27 | |
All compounds were tested as the HCl salt.
Data taken from reference 9.
2α-stereoisomer.
All values are mean ± standard error of three or four experiments performed in triplicate.
4. Results and Discussion
One of the most interesting features of the SAR of 3-phenyltropane analogs for the DAT is that exchanging the 2β-ester group in WIN 35,065-2, with amide, ether, heterocyclic, keto, alkyl, alkenyl or aryl substitutents at the C(2)-position provides compounds with equal or greater affinity than that of WIN 35,065-2 at the DAT.12 Although the DAT tolerates ligands having a broad variety of 2β-substituents with little change in the affinity of the ligand, the nature of the substituents has a profound effect on the monoamine selectivity. In order to gain additional information on the structural requirements for high-affinity and good selectivity at the C(2)-position, we designed and synthesized a new series of 2-substituted 3β-(substituted phenyl)tropane derivatives.
All the compounds possessed high affinities at the DAT with nanomolar or subnanomolar IC50 values ranging from 0.23 nM to 47 nM. No correlation was observed between the DAT binding affinities with the calculated partition coefficients (clogP) of the compounds. Generally, the 3β-(4-chlorophenyl) substituted tropanes except 7b were equal or more potent than the compounds with a 3β-(4-methylphenyl) substitution. The 2α-isomers 8a and 8b were approximately 16- and 4-fold less potent than the corresponding 2β-isomers 7a and 7b, respectively. This was consistent with the previous findings that exchange of the 2β-subsituent in the tropane derivatives with the 2α moiety decreased the relative binding affinity for the DAT.31
Among the tested 2β-substituted tropanes, the 2β-dibromovinyltropanes 7a and 7b possessed the highest potency at the DAT with IC50 values of 0.23 nM and 0.32 nM, respectively. Replacement of the vinyl group with the ethynyl substitution afforded 9a and 9b with decreased affinities at the DAT (11 nM vs. 0.23 nM and 3.6 nM vs. 0.32 nM IC50). It is interesting to note that attachment of a phenyl group to the end of the ethyne group gave 10a and 10b, which regained the binding affinity by 4 to 14-fold, respectively. In addition, replacing the phenylethynyl group at the C(2)-position of the 3β-(4-chlorophenyl) analogue 10b (IC50 = 0.8 nM) with the 4-nitrophenylethynyl substitution to give 10c had no effect on binding affinity (IC50 = 0.9 nM). However, the addition of a 4-amino group to 10b resulted in approximately 3-fold loss of binding affinity at the DAT. These findings support previous reports that a large pocket is present in the DAT binding site occupied by the 2β-substituent.12 The lipophilic interactions as well as the possible π-interactions between the 2β-substituents and the binding site may play an important role for the high affinity of these ligands. Finally, as analogs of the reported 2β- heterocyclic tropanes, the 2β-(1,2,3-triazol)substituted tropane derivatives 11 and 12 also possessed high-affinity at the DAT with IC50 values of 8 nM and 47 nM, respectively.
In terms of monoamine selectivity, all the compounds exhibited lower binding affinities at the NET and 5-HTT relative to the DAT. However, no correlation was observed between the 2β-substituents and the monoamine selectivity. 3β-(4-Chlorophenyl)-2β-(4-nitrophenylethynyl)tropane (10c) with an IC50 value of 0.9 nM at the DAT and Ki values of 230 nM at the NET and 620 nM at the 5-HTT, respectively, was one of the most potent and selective compounds for the DAT relative to the NET and 5-HTT in the series.
In summary, several novel 3β-(substituted phenyl)tropanes with various 2β-alkynyl and 2β-(1,2,3-triazol) substituents were synthesized and evaluated for their monoamine transporter binding affinities. All the tested compounds demonstrated higher potency at the DAT than cocaine and were more selective relative to binding at the NET and 5-HTT. One of the most potent and selective compounds in the series, 3β-(4-chlorophenyl)-2β-(4-nitrophenylethynyl)tropane (10c) had an IC50 value of 0.9 nM at the DAT and was 256- and 689-fold selective for the DAT over the NET and 5-HTT, respectively. These 2β-substituted tropanes are promising leads for further investigation of highly potent and selective monoamine inhibitors.
5. Experimental
Melting points were determined using a MEL-TEMP II capillary melting point apparatus and are uncorrected. Nuclear magnetic resonance (1H NMR and 13C NMR) spectra were obtained on a Bruker Avance DPX-300 MHz NMR spectrometer. Chemical shifts are reported in parts per million (ppm) with reference to internal solvent. Mass spectra (MS) were run on a Perkin-Elmer SCIEX API 150 EX mass spectrometer outfitted with ESI (turbospray) source or on a Hewlett Packard 5989A instrument by electron impact. Elemental analysis was performed by Atlantic Microlab Inc., Atlanta, GA. Optical rotations were measured on an AutoPol III polarimeter, purchased from Rudolf Research. Analytical thin-layer chromatography (TLC) was carried out using EMD silica gel 60 F254 TLC plates. TLC visualization was achieved with a UV lamp or in an iodine chamber. Flash column chromatography was done on a CombiFlash Companion system using Isco prepacked silica gel columns or using EM Science silica gel 60Å (230–400 mesh). Unless otherwise stated, reagent-grade chemicals were obtained from commercial sources and were used without further purification. All moisture- and air-sensitive reactions and reagent transfers were carried out under dry nitrogen.
5.1. 3β-(4-Methylphenyl)-2β-hydroxymethyltropane (5a)
To a stirred solution of 2b26 (11.4 g, 0.04 mol) in anhydrous THF (100 mL) at 0 °C under nitrogen was added LiAlH4 (3.04 g, 0.080 mol). After stirring at room temperature for 2 h, the reaction was quenched by slow addition of H2O (20 mL). The organic layer was separated and the aqueous layer was extracted with EtOAc (3 × 30 mL). The combined organic phases were washed with brine (3 × 50 mL) and dried (Na2SO4). Removal of the solvent under reduced pressure afforded crude 5a (9.60 g, 98%) as a white solid: mp 78–80 °C; 1H NMR (CDCl3) δ 7.25 (d, J = 8.1 Hz, 2H), 7.12 (d, J = 8.1 Hz, 2H), 3.75 (dd, J = 10.8, 2.1 Hz, 1H), 3.51-3.45 (m, 1H), 3.39 (dd, J = 10.8, 2.1 Hz, 1H), 3.37-3.29 (m, 1H). 3.05 (ddd, J = 12.9, 5.7, 5.7 Hz, 1H), 2.51 (ddd, J = 12.9, 12.9, 3.0 Hz, 1H), 2.32 (s, 3H), 2.27 (s, 3H), 2.26-2.04 (m, 2H), 1.82-1.57 (m, 3H), 1.52-1.43 (m, 1H); MS (ESI) m/z 246.4 (M + 1). The desired compound was used in the next step without further purification.
5.2. 3β-(4-Chlorophenyl)-2β-hydroxymethyltropane (5b)
The procedure for 5a was followed using 10.0 g (0.034 mol) of 2c26 to give 8.90 g (99%) of 5b as a white solid: mp 82–84 °C; 1H NMR (CDCl3) δ 7.38-7.25 (m, 4H), 3.75 (dd, J = 11.1, 2.1 Hz, 1H), 3.50-3.42 (m, 1H), 3.38-3.28 (m, 2H), 3.12-3.00 (m, 1H), 2.49 (ddd, J = 12.9, 12.9, 3.2 Hz, 1H), 2.27 (s, 3H), 2.26-2.04 (m, 2H), 1.80-1.57 (m, 3H), 1.50-1.44 (m, 1H); MS (ESI) m/z 266.3 (M + 1). The desired compound was used in the next step without further purification.
5.3. 3β-(4-Methylphenyl)-2β-(2,2-dibromovinyl)tropane (7a) and 3β-(4-methylphenyl)-2α-(2,2-dibromovinyl)tropane (8a)
To a stirred solution of oxalyl chloride (2 M solution, 16.5 mL, 33.0 mmol) in anhydrous CH2Cl2 (100 mL) at −78 °C under nitrogen was added anhydrous DMSO (4.68 mL, 66.0 mmol). After stirring for 15 min, a solution of 5a (5.40 g, 22.0 mmol) in anhydrous CH2Cl2 (100 mL) was added and the reaction mixture was stirred at −78 °C for another 30 min. TEA (18.4 mL, 132 mmol) was then added and the reaction mixture was warmed to room temperature and stirred for 2 h. The reaction was quenched by addition of H2O (10 mL). The organic layer was separated and washed with NH4Cl (3 × 50 mL), brine (50 mL) and dried (Na2SO4). Removal of the solvent under reduced pressure afforded aldehyde 6a (5.42 g) as an oil, which was used in the next step without further purification.
To a stirred solution of CBr4 (14.6 g, 0.044 mol) in anhydrous CH2Cl2 (165 mL) at 0 °C under nitrogen was added PPh3 (11.5 g, 0.044 mol) followed by zinc dust (2.88 g, 0.044 mol). After stirring at room temperature for 16 h, the reaction mixture was cooled to 0 °C and a solution of aldehyde 6a (5.42 g) was added. The reaction mixture was stirred at room temperature for 1 h and filtered through a short pad of Celite. The filtrate was washed with brine (3 × 50 mL), dried (Na2SO4) and concentrated under reduced pressure. Flash column chromatography on silica gel (300 g) using 0→30% ether in hexanes with 5% TEA afforded 2β-isomer 7a (4.94 g, 56%) and 2α-isomer 8a (0.15 g, 1.7%). 7a: white solid; mp 48–50 °C; 1H NMR (CDCl3) δ 7.08 (d, J = 8.1 Hz, 2H), 7.01 (d, J = 8.1 Hz, 2H), 6.70 (d, J = 9.4 Hz, 1H), 3.35-3.26 (m, 1H), 2.19-2.13 (m, 1H), 3.07 (ddd, J = 12.6, 5.7, 5.7 Hz, 1H), 2.64 (ddd, J = 5.7, 3.5, 9.4 Hz, 1H), 2.31 (s, 3H), 2.22 (s, 3H), 2.21-2.01 (m, 3H), 1.80-1.58 (m, 3H); 13C NMR (75 MHz; CDCl3) δ 139.8, 138.9, 135.8, 129.0, 127.8, 87.7, 66.1, 62.4, 51.5, 42.2, 35.9, 35.1, 26.6, 25.2, 21.2; MS (ESI) m/z 400.2 (M + 1). The free base was converted to the hydrochloride salt: mp 241 °C (dec.); [α]20D−41.67° (c 0.24, CH3OH); Anal. Calcd for C17H21Br2N·HCl·0.75H2O: C, 45.46; H, 5.27; N, 3.12. Found: C, 45.32; H, 5.35; N, 3.01.
8a
white solid; mp 115–117 °C; 1H NMR (CDCl3) δ 7.07 (s, 4H), 6.09 (d, J = 9.1 Hz, 1H), 3.30-3.16 (m, 2H), 3.03 (ddd, J = 11.5, 2.6, 9.1 Hz, 1H), 2.60 (ddd, J = 11.5, 11.7, 5.4 Hz, 1H), 2.39 (s, 3H), 2.30 (s, 3H), 2.20-1.94 (m, 2H), 1.87 (ddd, J = 11.7, 11.7, 2.4 Hz, 1H), 1.77-1.54 (m, 3H); 13C NMR (75 MHz; CDCl3) δ 140.1, 140.0, 136.2, 129.4, 127.7, 89.9, 64.4, 61.4, 49.7, 40.4, 39.9, 39.7, 26.4, 23.7, 21.2; MS (ESI) m/z 400.3 (M + 1). The free base was converted to the hydrochloride salt: mp 210 °C (dec.); [α]20D+23.53° (c 0.26, CH3OH); Anal. Calcd for C17H21Br2N·HCl·0.25H2O: C, 46.39; H, 5.15; N, 3.18. Found: C, 46.21; H, 5.36; N, 3.11.
5.4. 3β-(4-Chlorophenyl)-2β-(2,2-dibromovinyl)tropane (7b) and 3β-(4-Chlorophenyl)-2α-(2,2-dibromovinyl)tropane (8b)
The procedure for 7a and 8a was followed using 5.30 g (0.02 mol) of 5b to give 4.53 g (54%) of 2β-isomer 7b and 0.12 g (1.4%) of 2α-isomer 8b. 7b: white solid; mp 88–90 °C; 1H NMR (CDCl3) δ 7.30-7.20 (m, 2H), 7.10-7.03 (m, 2H), 6.70 (d, J = 9.5 Hz, 1H), 3.32-3.26 (m, 1H), 3.18-3.10 (m, 1H), 3.07 (ddd, J = 12.9, 5.6, 5.4 Hz, 1H), 2.62 (ddd, J = 5.6, 3.3, 9.5 Hz, 1H), 2.22 (s, 3H), 2.20-2.02 (m, 3H), 1.80-1.57 (m, 3H); 13C NMR (75 MHz; CDCl3). δ 140.5, 139.2, 132.0, 129.3, 128.2, 88.2, 65.9, 62.1, 51.3, 42.1, 36.0, 35.0, 26.6, 25.2; MS (ESI) m/z 420.3 (M + 1). The free base was converted to the hydrochloride salt: mp 235 °C (dec.); [α]20D−34.9° (c 0.22, CH3OH); Anal. Calcd for C16H18Br2ClN·HCl: C, 42.14; H, 4.20; N, 3.07. Found: C, 42.27; H, 4.21; N, 3.14.
8b
oil; 1H NMR (CDCl3) δ 7.25 (d, J = 8.4 Hz, 2H), 7.13 (d, J = 8.4 Hz, 2H), 6.07 (d, J = 9.4 Hz, 1H), 3.30-3.16 (m, 2H), 3.00 (ddd, J = 11.9, 2.4, 9.4 Hz, 1H), 2.61 (ddd, J = 11.9, 12.2, 5.4 Hz, 1H), 2.39 (s, 3H), 2.20-1.94 (m, 2H), 1.85 (ddd, J = 12.2, 12.6, 2.4 Hz, 1H), 1.77-1.53 (m, 3H); 13C NMR (75 MHz; CDCl3) δ 141.7, 139.4, 132.4, 129.3, 128.8, 90.4, 64.5, 61.4, 50.1, 40.3, 40.1, 39.6, 26.3, 23.7; MS (ESI) m/z 420.5 (M + 1). The free base was converted to the hydrochloride salt: mp 140 °C (fusion); [α]20D+26.1° (c 0.23, CH3OH); Anal. Calcd for C16H18Br2ClN·HCl: C, 42.14; H, 4.20; N, 3.07. Found: C, 42.27; H, 3.97; N, 3.02.
5.5. 3β-(4-Methylphenyl)-2β-ethynyltropane (9a)
To a stirred solution of 7a (400 mg, 1.00 mmol) in anhydrous THF (10 mL) at −78 °C under nitrogen was added BuLi (1.6 M solution, 1.31 mL, 2.10 mmol). After stirring at −78 °C for 1 h, the reaction mixture was warmed to room temperature and the stirring was continued for another 1 h. The reaction was quenched by addition of saturated NH4Cl. The organic layer was separated and the aqueous layer was extracted with EtOAc (3 × 30 mL). The combined organic phases were washed with brine (3 × 30 mL), dried (Na2SO4) and concentrated under reduced pressure. Flash column chromatography on silica gel (12 g Isco prepacked column) using 0→5% ether in hexanes with 5% TEA afforded 9a (130 mg, 54%) as an oil: 1H NMR (CDCl3) δ 7.22-7.08 (m, 4H), 3.38-3.28 (m, 2H), 2.97 (ddd, J = 12.9, 5.4, 5.4 Hz, 1H), 2.73-2.67 (m, 1H), 2.40-2.00 (m, 10H), 1.76-1.51 (m, 3H); 13C NMR (75 MHz; CDCl3) δ 139.4, 136.0, 128.8, 128.1, 84.8, 71.6, 66.7, 61.9, 42.3, 41.6, 35.9, 35.1, 26.1, 25.2, 21.2; MS (ESI) m/z 240.4 (M + 1). The free base was converted to the hydrochloride salt: mp 210 °C (dec.); [α]20D−114.2° (c 0.28, CH3OH); Anal. Calcd for C17H21N·HCl·0.25H2O: C, 72.84; H, 8.09; N, 5.00. Found: C, 72.66; H, 8.08; N, 4.99.
5.6. 3β-(4-Chlorophenyl)-2β-ethynyltropane (9b)
The procedure for 9a was followed using 650 mg (1.55 mmol) of 7b to give 260 mg (65%) of 9b as a white solid: mp 123–125 °C; 1H NMR (CDCl3) δ 7.32-7.18 (m, 4H), 3.40-3.30 (m, 2H), 2.98 (ddd, J = 12.9, 5.4, 5.4 Hz, 1H), 2.72-2.65 (m, 1H), 2.33 (s, 3H), 2.30-2.00 (m, 4H), 1.78-1.53 (m, 3H); 13C NMR (75 MHz; CDCl3) δ 141.0, 132.3, 129.7, 128.2, 84.4, 71.9, 66.6, 61.8, 42.3, 41.5, 35.9, 35.1, 26.1, 25.2; MS (ESI) m/z 260.4 (M + 1). The free base was converted to the hydrochloride salt: mp 145 °C (fusion); [α]20D−106.3° (c 0.26, CH3OH); Anal. Calcd for C16H18ClN·HCl·1.25H2O: C, 60.29; H, 6.80; N, 4.39. Found: C, 60.37; H, 6.89; N, 4.27.
5.7. 3β-(4-Methylphenyl)-2β-(phenylethynyl)tropane (10a)
To a stirred mixture of 9a (40.0 mg, 0.17 mmol), CuI (3.18 mg, 0.017 mmol) and Pd(PPh3)4 (6.00 mg, 0.005 mmol) in 1:1 mixture of benzene-TEA (5 mL) at room temperature under nitrogen was added iodobenzene (0.075 mL, 0.67 mmol). The reaction mixture was stirred at 40 °C for 1 h. After cooling to room temperature, the reaction mixture was diluted with EtOAc (50 mL), washed with NH4Cl (10 mL), brine (3 × 30 mL) and concentrated under reduced pressure. The resultant residue was partitioned between ether (10 mL) and 6 N HCl (10 mL). The aqueous layer was separated, basified to pH 11 with NH4OH and extracted with EtOAc (3 × 30 mL). The combined EtOAc extracts were washed with brine (3 × 30 mL), dried (Na2SO4) and concentrated under reduced pressure. Flash column chromatography on silica gel (12 g Isco prepacked column) using 2% TEA in hexanes afforded 10a (45.0 mg, 86%) as an oil: 1H NMR (CDCl3) δ 7.34-7.08 (m, 9H), 3.47-3.40 (m, 1H), 3.39-3.30 (m, 1H), 3.06 (ddd, J = 12.9, 5.4, 5.4 Hz, 1H), 2.93-2.87 (m, 1H), 2.42-2.03 (m, 9H), 1.80-1.54 (m, 3H); 13C NMR (75 MHz; CDCl3) δ 139.8, 136.1, 131.8, 128.8, 128.5, 128.0, 127.3, 124.6, 91.2, 83.9, 66.6, 61.9, 42.5, 42.1, 36.8, 35.3, 26.5, 25.4, 21.2; MS (ESI) m/z 316.5 (M + 1). The free base was converted to the hydrochloride salt: mp 228 °C (dec.); [α]20 D −229.0° (c 0.25, CH3OH); Anal. Calcd for C23H25N·HCl·0.25H2O: C, 77.51; H, 7.49; N, 3.93. Found: C, 77.30; H, 7.45; N, 3.92.
5.8. 3β-(4-Chlorophenyl)-2β-(phenylethynyl)tropane (10b)
The procedure for 10a was followed using 78.0 mg (0.30 mmol) of 9b and 0.13 mL (1.20 mmol) of iodobenzene to give 70.0 mg (70%) of 10b as an oil: 1H NMR (CDCl3) δ 7.29 (s, 5H), 7.20 (m, 4H), 3.50-3.40 (m, 1H), 3.39-3.30 (m, 1H), 3.07 (ddd, J = 12.9, 5.4, 5.4 Hz, 1H), 2.92-2.84 (m, 1H), 2.37 (s, 3H), 2.35-2.05 (m, 3H), 1.78-1.53 (m, 3H); 13C NMR (75 MHz; CDCl3) δ 141.5, 132.4, 131.8, 130.0, 128.2, 127.5, 124.2, 90.6, 84.2, 66.5, 61.8, 42.3, 42.0, 36.6, 35.1, 26.5, 25.4; MS (ESI) m/z 336.5 (M + 1). The free base was converted to the hydrochloride salt: mp 234 °C (dec.); [α]20D−228.1° (c 0.21, CH3OH); Anal. Calcd for C22H22ClN·HCl·0.5H2O: C, 69.29; H, 6.34; N, 3.67. Found: C, 69.65; H, 6.12; N, 3.73.
5.9. 3β-(4-Chlorophenyl)-2β-(4-nitrophenylethynyl)tropane (10c)
The procedure for 10a was followed using 78.0 mg (0.30 mmol) of 9b and 299 mg (1.20 mmol) of 1-iodo-4-nitrobenzene to give 110 mg (96%) of 10c as a white solid: mp 62–64 °C; 1H NMR (CDCl3) δ 8.12-8.02 (m, 2H), 7.40-7.23 (m, 6H), 3.50-3.41 (m, 1H), 3.40-3.30 (m, 1H), 3.12 (ddd, J = 12.6, 5.4, 5.4 Hz, 1H), 2.98-2.90 (m, 1H), 2.36 (s, 3H), 2.32-2.05 (m, 3H), 1.80-1.54 (m, 3H); 13C NMR (75 MHz; CDCl3) δ 146.6, 141.1, 132.5, 132.4, 131.3, 129.7, 128.3, 123.4, 97.0, 82.7, 66.3, 61.8, 42.7, 42.1, 36.3, 35.1, 26.3, 25.3; MS (ESI) m/z 381.6 (M + 1). The free base was converted to the hydrochloride salt: mp 115–117 °C; [α]20D−265.3° (c 0.23, CH3OH); Anal. Calcd for C22H21ClN2O2·HCl·0.25H2O: C, 62.64; H, 5.38; N, 6.64. Found: C, 62.44; H, 5.31; N, 6.49.
5.10. 3β-(4-Chlorophenyl)-2β-(4-aminophenylethynyl)tropane (10d)
The procedure for 10a was followed using 78.0 mg (0.30 mmol) of 9b and 263 mg (1.20 mmol) of 4-iodoaniline to give 65.0 mg (62%) of 10d as a white solid: mp 188–190 °C; 1H NMR (CDCl3) δ 7.27 (s, 4H), 7.01 (d, J = 6.0 Hz, 2H), 6.50 (d, J = 6.0 Hz, 2H), 3.64 (br s, 2H), 3.45-3.38 (m, 1H), 3.38-3.30 (m, 1H), 3.02 (ddd, J = 12.6, 5.4, 5.4 Hz, 1H), 2.88-2.81 (m, 1H), 2.37 (s, 3H), 2.32-2.03 (m, 3H), 1.80-1.53 (m, 3H); 13C NMR (75 MHz; CDCl3) δ 146.0, 141.6, 132.9, 132.2, 130.0, 128.2, 114.7, 113.9, 87.9, 84.5, 66.6, 61.8, 42.2, 42.0, 36.7, 35.1, 26.5, 25.4; MS (ESI) m/z 351.3 (M + 1). The free base was converted to the hydrochloride salt: mp 240 °C (dec.); [α]20D−199.2° (c 0.26, CH3OH); Anal. Calcd for C22H23ClN2·2HCl·1.25 H2O: C, 59.20; H, 6.21; N, 6.28. Found: C, 59.57; H, 6.11; N, 6.05.
5.11. 3β-(4-Chlorophenyl)-2β-[1-(2-piperidin-1-yl)ethyl-1H-[1,2,3]triazol-4-yl]tropane (11)
To a stirred suspension of 9b (130 mg, 0.50 mmol) and 1-(2-azidoethyl)-piperidine (77.1 mg, 0.50 mmol) in 1:1 mixture of tBuOH-H2O (4 mL) at room temperature under nitrogen was added a freshly prepared 1 M sodium ascorbate solution (0.50 mL, 0.50 mmol) followed by 0.3 M CuSO4 solution (0.167 mL, 0.05 mmol). After stirring for 10 h, the reaction mixture was diluted with EtOAc (50 mL), washed with brine (3 × 30 mL) and dried (Na2SO4). The solvent was concentrated under reduced pressure. Flash column chromatography on silica gel (12 g Isco prepacked column) using 0→10% ether in hexanes with 5% TEA afforded 11 (135 mg, 65%) as a white solid: mp 250 °C (dec.); 1H NMR (CDCl3) δ 7.77 (s, 1H), 7.02-6.92 (m, 2H), 6.83-6.73 (m, 2H), 4.37-4.16 (m, 2H), 3.34-3.09 (m, 4H), 2.60 (t, J = 6.0 Hz, 2H), 2.40-2.21 (m, 4H), 2.20-1.92 (m, 6H), 1.80-1.30 (m, 9H); 13C NMR (75 MHz; CDCl3) δ 147.4, 141.2, 131.6, 129.1, 128.0, 124.1, 66.8, 61.8, 58.6, 54.5, 47.7, 45.7, 42.2, 35.6, 35.0, 26.3, 26.2, 25.2, 24.3; MS (EI) m/z 414.2 (M+). The free base was converted to the hydrochloride salt: mp 145 °C (fusion); [α]20D−66.0° (c 0.24, CH3OH); Anal. Calcd for C23H32ClN5·2HCl·1.5H2O: C, 53.75; H, 7.26; N, 13.63. Found: C, 54.13; H, 7.21; N, 13.27.
5.12. 3β-(4-Chlorophenyl)-2β-(1-benzyl-1H-[1,2,3]triazol-4-yl)tropane (12)
The procedure for 11 was followed using 130 mg (0.50 mmol) of 9b and 0.066 mL (0.50 mmol) of benzylazide to give 155 mg (79%) of 12 as a white solid: 118–119 °C; 1H NMR (CDCl3) δ 7.63 (s, 1H), 7.40-7.27 (m, 3H), 7.10-6.95 (m, 4H), 6.77 (d, J = 8.4 Hz, 2H), 5.55 (d, J = 15.0 Hz, 1H), 5.31 (d, J = 15.0 Hz, 1H), 3.46-3.40 (m, 1H), 3.32-3.18 (m, 3H), 2.30-1.93 (m, 6H), 1.88-1.53 (m, 3H); 13C NMR (75 MHz; CDCl3)δ 148.3, 141.1, 135.9, 131.7, 129.0, 128.9, 128.4, 128.1, 127.3, 123.6, 66.7, 61.8, 53.7, 46.0, 42.2, 35.4, 34.9, 26.3, 25.2; MS (EI) m/z 393.2 (M+). The free base was converted to the hydrochloride salt: mp 118 °C (fusion); [α]20D −110.9° (c 0.23, CH3OH); Anal. Calcd for C23H25ClN4·HCl·0.75H2O: C, 62.37; H, 6.26; N, 12.65. Found: C, 62.44; H, 6.29; N, 12.53.
Acknowledgements
This research was supported by the National Institute on Drug Abuse, Grant No. DA05477.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J. Pharmacol. Exp. Ther. 1989;251:150–155. [PubMed] [Google Scholar]
- 2.Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14(7):299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 3.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 4.Wilcox KM, Rowlett JK, Paul IA, Ordway GA, Woolverton WL. On the relationship between the dopamine transporter and the reinforcing effects of local anesthetics in rhesus monkeys: practical and theoretical concerns. Psychopharmacology (Berl) 2000;153(1):139–147. doi: 10.1007/s002130000457. [DOI] [PubMed] [Google Scholar]
- 5.Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120(1):10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- 6.Herman BH, Elkashef A, Vocci F. Medications for the treatment of cocaine addiction: Emerging candidates. Drug Discovery Today: Therapeutic Strategies. 2005;2(1):87–92. [Google Scholar]
- 7.Kalivas PW. Neurobiology of cocaine addiction: implications for new pharmacotherapy. Am. J. Addict. 2007;16(2):71–78. doi: 10.1080/10550490601184142. [DOI] [PubMed] [Google Scholar]
- 8.Knackstedt LA, Kalivas PW. Pharmacotherapy targets for regulating cocaine-induced plasticity. Drugs of the Future. 2006;31(10):893–900. [Google Scholar]
- 9.Newman AH. Novel pharmacotherapies for cocaine abuse 1997–2000. Exp. Opin. Ther. Patents. 2000;10(7):1095–1122. [Google Scholar]
- 10.Carroll FI, Howard JL, Howell LL, Fox BS, Kuhar MJ. Development of the dopamine transporter selective RTI-336 as a pharmacotherapy for cocaine abuse. AAPS J. 2006;8(1):E196–E203. doi: 10.1208/aapsj080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll FI, Fox BS, Kuhar MJ, Howard JL, Pollard GT, Schenk S. Effects of dopamine transporter selective 3-phenyltropane analogs on locomotor activity, drug discrimination, and cocaine self-administration after oral administration. Eur. J. Pharmacol. 2006;553(1–3):149–156. doi: 10.1016/j.ejphar.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Runyon SP, Carroll FI. Dopamine transporter ligands: recent developments and therapeutic potential. Curr Top Med Chem. 2006;6(17):1825–1843. doi: 10.2174/156802606778249775. [DOI] [PubMed] [Google Scholar]
- 13.Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem. Pharmacol. 2008;75(1):196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Rothman RB, Baumann MH, Prisinzano TE, Newman AH. Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem. Pharmacol. 2008;75(1):2–16. doi: 10.1016/j.bcp.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll FI, Lewin AH, Mascarella SW. Dopamine Transporter Uptake Blockers: Structure-Activity Relationships. In: Reith MEA, editor. Neurotransmitter Transporters: Structure, Function, and Regulation. 2nd Edition. Totowa, NJ: Humana Press; 2001. pp. 381–432. [Google Scholar]
- 16.Newman AH, Kulkarni S. Probes for the dopamine transporter: new leads toward a cocaine-abuse therapeutic--A focus on analogues of benztropine and rimcazole. Med. Res. Rev. 2002;22(5):429–464. doi: 10.1002/med.10014. [DOI] [PubMed] [Google Scholar]
- 17.Prisinzano T, Rice KC, Baumann MH, Rothman RB. Development of Neurochemical Normalization ("Agonist Substitution") Therapeutics for Stimulant Abuse: Focus on the Dopamine Uptake Inhibitor, GBR12909. Curr. Med. Chem.-Central Nervous System Agents. 2004;4:47–59. [Google Scholar]
- 18.Carrera MR, Meijler MM, Janda KD. Cocaine pharmacology and current pharmacotherapies for its abuse. Bioorg. Med. Chem. 2004;12(19):5019–5030. doi: 10.1016/j.bmc.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Carroll FI. 2002 Medicinal Chemistry Division Award address: monoamine transporters and opioid receptors. Targets for addiction therapy. J. Med. Chem. 2003;46(10):1775–1794. doi: 10.1021/jm030092d. [DOI] [PubMed] [Google Scholar]
- 20.Dutta AK, Zhang S, Kolhatkar R, Reith ME. Dopamine transporter as target for drug development of cocaine dependence medications. Eur. J. Pharmacol. 2003;479(1–3):93–106. doi: 10.1016/j.ejphar.2003.08.060. [DOI] [PubMed] [Google Scholar]
- 21.Clarke RL. The Tropane Alkaloids, Chapter 2. In: Manske RHF, editor. The Alkaloids. Vol. 16. New York: Academic Press; 1977. [Google Scholar]
- 22.Clarke RL, Daum SJ, Gambino AJ, Aceto MD, Pearl J, Levitt M, Cumiskey WR, Bogado EF. Compounds affecting the central nervous system. 3β-Phenyltropane- 2-carboxylic esters and analogs. J. Med. Chem. 1973;16:1260–1267. doi: 10.1021/jm00269a600. [DOI] [PubMed] [Google Scholar]
- 23.Davies HML, Saikali E, Huby NJS, Gilliat VJ, Matasi JJ, Sexton T, Childers SR. Synthesis of 2β-Acyl-3β-aryl-8-azabicyclo[3.2.1]octanes and their binding affinities at dopamine and serotonin transport sites in rat striatum and frontal cortex. J. Med. Chem. 1994;37:1262–1268. doi: 10.1021/jm00035a005. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Kelkar SV, Lomenzo SA, Izenwasser S, Katz JL, Kline RH, Trudell ML. Synthesis, dopamine transporter affinity, dopamine uptake inhibition, and locomotor stimulant activity of 2-substituted 3β-phenyltropane derivatives. J. Med. Chem. 1997;40:858–863. doi: 10.1021/jm960739c. [DOI] [PubMed] [Google Scholar]
- 25.Kozikowski AP, Araldi GL, Prakash KR, Zhang M, Johnson KM. Synthesis and biological properties of new 2beta-alkyl- and 2beta-aryl-3-(substituted phenyl)tropane derivatives: stereochemical effect of C-3 on affinity and selectivity for neuronal dopamine and serotonin transporters. J. Med. Chem. 1998;41(25):4973–4982. doi: 10.1021/jm9802564. [DOI] [PubMed] [Google Scholar]
- 26.Carroll FI, Gao Y, Rahman MA, Abraham P, Parham K, Lewin AH, Boja JW, Kuhar MJ. Synthesis, ligand binding, QSAR, and CoMFA study of 3β-(p-substituted phenyl)tropane-2β-carboxylic acid methyl esters. J. Med. Chem. 1991;34:2719–2925. doi: 10.1021/jm00113a008. [DOI] [PubMed] [Google Scholar]
- 27.Moses JE, Moorhouse AD. The growing applications of click chemistry. Chem. Soc. Rev. 2007;36(8):1249–1262. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]
- 28.Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discov. Today. 2003;8(24):1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 29.Boja JW, Rahman MA, Philip A, Lewin AH, Carroll FI, Kuhar MJ. Isothiocyanate derivatives of cocaine: Irreversible inhibition of ligand binding at the dopamine transporter. Mol. Pharmacol. 1991;39:339–345. [PubMed] [Google Scholar]
- 30.Carroll FI, Gray JL, Abraham P, Kuzemko MA, Lewin AH, Boja JW, Kuhar MJ. 3-Aryl-2-(3'-substituted-1',2',4'-oxadiazol-5'-yl)tropane analogues of cocaine: Affinities at the cocaine binding site at the dopamine, serotonin, and norepinephrine transporters. J. Med. Chem. 1993;36(20):2886–2890. doi: 10.1021/jm00072a007. [DOI] [PubMed] [Google Scholar]
- 31.Carroll FI, Runyon SP, Abraham P, Navarro H, Kuhar MJ, Pollard GT, Howard JL. Monoamine Transporter Binding, Locomotor Activity, and Drug Discrimination Properties of 3-(4-Substituted-phenyl)tropane-2-carboxylic Acid Methyl Ester Isomers. J. Med. Chem. 2004;47(25):6401–6409. doi: 10.1021/jm0401311. [DOI] [PubMed] [Google Scholar]