Abstract
Cytochromes P450 (P450s) and glutathione S-transferases (GSTs) constitute two important enzyme families involved in carcinogen metabolism. Generally, P450s play activation or detoxifying roles while GSTs act primarily as detoxifying enzymes. We previously demonstrated that oral administration of the linear furanocoumarins, isopimpinellin and imperatorin, modulated P450 and GST activities in various tissues of mice. The purpose of the present study was to compare a broader range of naturally occurring coumarins (simple coumarins, and furanocoumarins of the linear and angular type) for their abilities to modulate hepatic drug metabolizing enzymes when administered orally to mice. We now report that all of the different coumarins tested (coumarin, limettin, auraptene, angelicin, bergamottin, imperatorin and isopimpinellin) induced hepatic GST activities, whereas the linear furanocoumarins possessed the greatest abilities to induce hepatic P450 activities, in particular P450 2B and 3A. In both cases, this corresponded to an increase in protein expression of the enzymes. Induction of P4502B10, 3A11, and 2C9 by xenobiotics often are a result of activation of the pregnane X receptor (PXR) and/or constitutive androstane receptor (CAR). Using a pregnane X receptor reporter system, our results demonstrated that isopimpinellin activated both PXR and its human ortholog SXR by recruiting coactivator SRC-1 in transfected cells. In CAR transfection assays, isopimpinellin counteracted the inhibitory effect of androstanol on full length mCAR, a Gal4-mCAR ligand binding domain fusion, and restored coactivator binding. Orally administered isopimpinellin induced hepatic mRNA expression of Cyp2b10,Cyp3a1, GSTa in CAR(+/+) wild-type mice. In contrast, the induction of Cyp2b10 mRNA by isopimpinellin was attenuated in the CAR(−/−) mice, suggesting that isopimpinellin induces Cyp2b10 via the CAR receptor. Overall, the current data indicate that naturally occurring coumarins have diverse activities in terms of inducing various xenobiotic metabolizing enzymes based on their chemical structure.
Keywords: coumarins, furanocoumarins, P450s, pregnane X-receptor, constitutive androstane receptor
Naturally occurring coumarins (NOCs) are dietary constituents found among the Rutaceae, Apiaceae, Umbellifereae, and Moraceae families (Murray, 1982). Coumarin (1,2-benzopyrone) is present in highest concentrations in cassia leaf oil (17000–87000 ppm), cinnamon bark oil (7000 ppm) and peppermint oil (20 ppm) (reviewed in (Lake, 1999)). Coumarin is also present in candies, alcoholic beverages, and fragrances, such that the estimated human exposure is estimated to be 0.04 mg/kg/day (Lake, 1999). However, many other sources of NOCs exist. For example, 49.6 mg of auraptene, a simple coumarin, was extracted from 120 g of grapefruit peel (Wangensteen et al., 2003). Furanocoumarins are also present in citrus oils, in which the total furanocoumarin content was recently estimated to be 2900 ppm, 2200 ppm, and 1100 ppm in lemon, grapefruit, and bitter orange oils, respectively (Frerot and Decorzant, 2004). NOCs are also present as Crude Drugs in Traditional Chinese Medicine and other phytomedicines. Human exposure to furanocoumarins following consumption of tablets prepared from Dorstenia species in Brazil was estimated to be 2–5.36 mg/d (Cardoso et al., 2006). Concentrations of imperatorin and isoimperatorin were reported to be 40–60 μg/mL and 12–44 μg/mL (respectively) in the Crude Drug Radix Angelica Dahurica (Cardoso et al., 2006). Several coumarins, including 7-hydroxycoumarin, 7-methoxycoumarin, 5,7-dimethoxycoumarin (limettin), 5-methoxypsoralen, 8-methoxypsoralen, and 5,8-dimethoxypsoralen (isopimpinellin) were detected by LC-UV in human urine following ingestion of Umbelliferae Chinese herbal medicine (Wang and Jiang, 2006). The range of pharmacologic/toxicologic properties of NOCs are diverse, and include furanocoumarin-induced photoxicity (Berenbaum, 1995), modulation of P450 3A4 (Guo and Yamazoe, 2004), anticarcinogenesis/chemoprevention of cancer (Kelly et al., 2000) (Prince et al., 2006) (Kleiner et al., 2002b) (Cai et al., 1997) (Wattenberg et al., 1979) (Tanaka et al., 1998) (Baba et al., 2002), antimicrobial properties (Ngwendson et al., 2003), and cancer cell cytoxicity (Setzer et al., 2000).
Many of the cancer chemopreventive effects of NOCs have been attributed to their abilities to modulate xenobiotic metabolizing enzymes. For example, of the NOCs investigated, bergamottin was one of the most potent at suppressing mouse hepatic ethoxyresorufin O-dealkylase activity, with an IC50 of 1.2 × 10−7 M (Cai et al., 1993). Furthermore, bergamottin (200 nmol, topical) inhibited the formation of the major benzo[a]pyrene (B[a]P)-DNA adduct, anti-benzo[a]pyrene-7,8-diol, 9,10-epoxide-dGuo in mouse epidermis in vivo (Cai et al., 1997). Bergamottin was also effective at blocking B[a]P-induced skin tumor initiation in mice (Cai et al., 1997). Thus, the ability of bergamottin to suppress mouse skin tumor initiation by B[a]P is likely mediated by its ability to suppress cytochrome P450 1A1 dependent metabolic activation of B[a]P into the anti-benzo[a]pyrene-7,8-diol, 9,10-epoxide. However, there is selectivity in the P450-inhibitory properties of NOCs. Bergamottin was actually a weak inhibitor of murine P450 1B1, and was not effective at suppressing P450 1B1-dependent metabolism of 7,12-dimethylbenz[a]anthracene (DMBA) into the syn-DMBA diol-epoxide derived DNA adducts (Kleiner et al., 2002a). In contrast, imperatorin and isopimpinellin were similarly effective at suppressing both P450 1A1 and P450 1B1 activities (Kleiner et al., 2003) and were effective at suppressing DNA adduct formation and skin tumor initiation from both B[a]P and DMBA (Cai et al., 1997; Kleiner et al., 2002b). The double bond on the furan ring is important in P450 suppression, as evidenced by studies with coriandrin. Coriandrin is a mechanism-based inactivator of P450 1A1 (Cai et al., 1996); whereas dihydrocoriandrin, which lacks the double bond on the furan ring, lacked the ability to inhibit P450 (Cai et al., 1993). Indeed, most furanocoumarins are more potent than simple coumarins at suppressing P450 1A1, 1A2, 1B1, 2B1, 3A4 activities in general (Cai et al., 1993; Kleiner et al., 2003; Prince et al., 2006). It has also been demonstrated in several rodent models that coumarins induce Phase II enzymes (reviewed in (Hayes and Pulford, 1995)). Wattenberg and colleagues (Wattenberg et al., 1979; Sparnins and Wattenberg, 1981) showed that coumarin enhanced forestomach glutathione S-transferase (GST) activity and sulfhydryl levels following two weeks of dietary administration to mice. Coumarin in the diet also induced NAD(P)H quinone oxidoreductase (NQO1) activity in small intestine of mice, and both coumarin and 3-hydroxycoumarin induced GST activity in the small intestine of mice (McMahon et al.,2001). It was also shown that coumarin increased aflatoxin B1 (AFB1) aldehyde reductase, GSTA5, GSTP1, and NQO1 expression in rat liver (Kelly et al., 2000). Coumarin in the diet also protected rats from AFB1 hepatocarcinogenesis (Kelly et al., 2000). Thus, induction of GSTs and other carcinogen-detoxifying enzymes is another mechanism by which NOCs can inhibit chemical-induced carcinogenesis.
We previously demonstrated that orally administered imperatorin and isopimpinellin blocked DNA adduct formation by DMBA and/or benzo[a]pyrene (B[a]P) in lungs, mammary gland, forestomach, and skin epidermis of mice (Kleiner et al., 2001). In lung, forestomach, and skin epidermis, P450 activities were inhibited. However, we observed an increase in P450-mediated enzyme activities [using 7-ethoxyresorufin and pentoxyresorufin as substrates] and GST activities in liver and GST activity in lungs of mice, following oral administration of isopimpinellin and/or imperatorin (Kleiner et al., 2001). We observed that the induction of P450 activities, which was apparently specific to the liver, was fairly specific to furanocoumarins, which are known to also suppress P450 activities Interestingly, long-term administration of grapefruit juice to rats resulted in an increase in liver microsomal nifedipine oxidation activity and P450 content (Mohri et al., 2000). However, it is known that grapefruit juice in humans has a predominantly inhibitory effect on drug metabolism (Lundahl et al., 1998), so these differences could be due to species-specific effects. The purpose of the current study was to examine a broader range of naturally occurring simple and furanocoumarins, and to explore potential mechanisms for their effects on hepatic enzyme activities.
Experimental Procedures
Caution
Certain naturally occurring coumarins may be phototoxic and should be handled with care.
Chemicals and Reagents
Coumarin, limettin, phenobarbital (PB), NADP+, glucose 6-phosphate, glucose 6-phosphate dehydrogenase, 1-chloro-2,4-dinitrobenzene (CDNB), 1,2-dichloro-4-nitrobenzene (DCNB), 2,6-dichloroindophenolate hydrate (DCPIP), cumene hydroperoxide, dicumarol were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO). Angelicin, bergamottin, imperatorin, and isopimpinellin were purchased from Indofine Chemical Co. (Somerville, NJ). Auraptene was a generous gift from Naoki Yoshimi (Kinki University, Japan). The chemical structures of the coumarins used are shown in Figure 1. AIN-76A semi-purified diet was obtained from Dyets (Bethlehem, PA).
Figure 1.
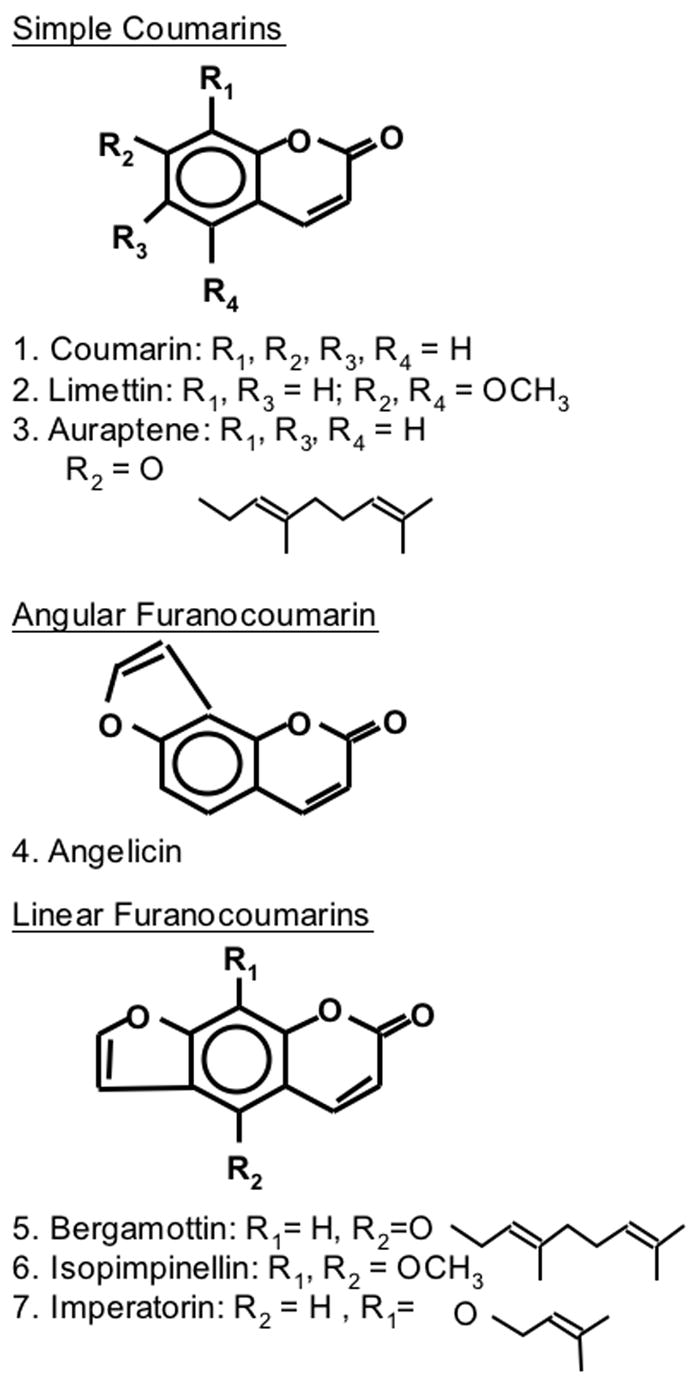
Structures of coumarins used.
Animals
C57BL/6 mice and SENCAR mice were purchased from the National Cancer Institute (Frederick, MD). All mice were housed in a temperature- and humidity-controlled AAALAC facility with a 12 h light/dark cycle. All procedures were approved by the U.T.M.D. Anderson Cancer Center Institutional Animal Care and Use Committee in accordance with NIH guidelines. Mice were maintained on AIN-76A diet (Dyets, Bethlehem, PA) and allowed access to food and water ad libitum.
Structure-Activity Relationship Study
Groups of male and female C57BL/6 mice (7–9 weeks of age) were fed AIN-76A semi-purified diet and were treated orally (100 mg/kg body weight, suspended in corn oil) by gavage once daily for 3 consecutive days. Vehicle control mice received corn oil (0.1 mL/25 g body weight) only. At 24 h after the final dose, mice were sacrificed by cervical dislocation, and hepatic tissue isolated as previously described (Kleiner et al., 2001). Livers from male and female mice were pooled together in each treatment group. Each compound was tested in 2–3 separate experiments. Liver/body weight ratios were calculated as the weight of the liver (g) divided by the body weight (g) × 100.
Dose-Response Study
Female SENCAR mice (7–9 weeks of age) were fed AIN-76A semi-purified diet and were treated orally with imperatorin or isopimpinellin (35, 70, and 150 mg/kg body weight, suspended in corn oil) by gavage once daily for 4 consecutive days. Vehicle control mice received corn oil (0.1 mL/25 g body weight) only. At 24 h after the final dose, mice were sacrificed by cervical dislocation, and livers were homogenized in 0.05 M Tris buffer, pH 7.5, containing 0.25 M sucrose (1:5, w/v). Liver cytosol and endoplasmic reticulum (microsomal)-enriched fractions were isolated by differential centrifugation as previously described (Kleiner et al., 2001). Livers from individual mice were assayed separately (3–4 mice per group).
Enzyme assays
To probe for P450s 1A1/2, 2B9/10, and 3A11, liver microsomal samples were assayed using ethoxyresorufin, pentoxyresorufin, and testosterone as substrates, respectively (Burke et al., 1985; Burke et al., 1994) (Sonderfan et al., 1987). For P450s 1A1/2 and 2B9/10, liver microsomes (0.25 mg/mL protein) were preincubated with an NADPH generating system (0.336 mM NADP+, 0.5 mM glucose 6-phosphate, 0.15 mM MgCl2 and 1 U of glucose 6-phosphate dehydrogenase) in 0.05 M Tris buffer, pH 7.5 (total volume 1.0 mL) at 37°C for 5 min. Reactions were initiated with 7-ethoxy or 7-pentoxyresorufin (5 μM) and terminated at 10 min (ethoxyresorufin) or 20 min (pentoxyresorufin) using 2.5 mL ice-cold methanol. Samples were allowed to precipitate on ice and centrifuged for 10 min at 3000 × g. Supernatants were analyzed spectrofluorometrically for product (resorufin) an excitation of 550 nm and emission of 585 nm. For the P450 3A11 assay, liver microsomal protein (0.25 mg/mL) was preincubated with the NADPH generating system in 0.1 M KH2PO4 buffer, pH 7.4, the reactions were initiated with 0.25 mM substrate (testosterone), and incubated for 10 min at 37°C. Reactions were terminated with acetonitrile, and product (6β-hydroxytestosterone) was analyzed by reverse-phase HPLC with UV254nm detection. Experimental values were extrapolated using a standard curve in the linear range. Background (boiled protein) was subtracted from experimental values.
Liver cytosolic GST activities were assayed using general and specific substrates as follows (reviewed in (Hayes and Pulford, 1995)). CDNB is a general substrate for GST, although the GST μ form appears to have relatively high activity using CDNB as a substrate. DCNB is a more specific substrate for GST μ. Conversely, GSTs π and α have relatively higher activity using ethacrynic acid (EA) as a substrate. Liver cytosolic GST activities were assessed spectrophotometrically (Shimadzu, Columbia, MD, kinetic mode) at 340 nm (CDNB), 345 nm (DCNB), and 270 nm (EA) at 25°C. Activities were calculated using extinction coefficients of 9.6 nM−1/cm−1, 8.5 nM−1/cm−1, and 5.0 nM−1/cm−1, respectively, as previously described (Habig et al., 1974). Substrate and GSH concentrations for each assay were 1 mM DCNB, 5 mM GSH; 1 mM CDNB, 1 mM GSH, and 0.2 mM EA, 0.25 mM GSH. Liver cytosolic NQO activity was assayed spectrophotometrically at 600 nm using 2,6-dichloroindophenol (1.25 mM) as a substrate and an extinction coefficient of 21 nM−1/cm−1 as previously described (Prochaska and Talalay, 1986).
Protein concentration was estimated using the Bradford method (Bradford, 1976) with BSA as a standard.
Western blots
Microsomal and cytosolic tissue samples (25 μ g) were separated electrophoretically in 10% (for P450) and 12% (for GST) SDS-PAGE gels under reducing conditions according to the method of Laemmli (Laemmli, 1970), and transferred electrophoretically to PVDF membranes (0.45 μ m) as previously described (Towbin et al., 1979). Blots were probed with appropriate dilutions of primary antibodies [anti-ratP450 1A1 (with cross-reactivity to P450 1A2, Daiichi 1:1000), anti-rat P450 2B4 (1:2000), anti-rat P450 3A2 (Daiichi, 1:7500), anti-GSTα, π, or μ(1:1000 each, Oxford Biomedical Research, Inc., Oxford, Mich.), anti-β-actin antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-albumin (Accurate Chemicals)], followed by secondary antibodies and enhanced chemiluminescent detection previously described (Prince et al., 2006). Protein standards included: 3-methylcholanthrene induced rat liver microsomes (P4501A1) and phenobarbital induced rat liver microsomes (P4502B9/10). GST standards (purified from rat liver), were purchased from (Oxford Biochemicals, Inc.): GSTα, GST Ya–Yc (p/n GS 11); GSTπ, GST Yp (p/n GS 40), and GSTμ, GSTYb1-Yb1 (p/n GS 22). The intensities of immunostained proteins were determined using Image J software (http://rsb.info.nih.gov/ij/).
P450 content
Concentrations of P450 were determined in liver microsomes by measuring the differences in the spectra (dithionite + carbon monoxide) − dithionite as described previously (Omura and Sato, 1964). The molar extinction coefficient of 91/mM/cm was used for the absorbance change between 450 and 490 nm.
PXR transfection assays
Functional cell-based assays have been developed using transient expression of the receptor with a reporter that expresses luciferase or alkaline phosphatase. The receptor can be expressed as either a full-length protein or as a chimaera that comprises the ligand-binding domain of PXR fused to the DNA-binding domain from the yeast transcription factor galactose 4 (Gal4). Expression plasmids for the full-length receptor are transfected with reporter plasmids that contain either multiple PXR response elements upstream of a heterologous promoter or, alternatively, an intact promoter of a PXR target gene. The PXR–GAL4 chimeric receptor assay uses a reporter construct that contains several copies of the GAL4 DNA-binding element. This latter assay format has the advantage of avoiding interference from endogenous receptors expressed within the host cell. For the current study, CV-1 cells were cotransfected with Gal-L-PXR or Gal-L-SXR together with MH100-tk-luc, CMX-β-gal as previously described (Blumberg et al., 1998). Additional cells were transfected with full length PXR or SXR, together with hRXRalpha, (DR3)3-tk-luc and CMX-β-gal. To determine whether isopimpinellin facilitated interaction between the SRC-1 receptor interaction domain (RID) and PXR/SCR, CV-1 cells were cotransfected with Gal-SRC-1 RID and/or VP-L-PXR or VP-L-SXR, together with MH100-tk-luc and CMX-β -gal. Transfected cells were incubated with media containing indicated amount of ligand or solvent control for 18–24 hours before subjected to luciferase and β -gal assay. Interaction between SRC-1 RID and PXR/SXR was assessed by the relative luciferase activity normalized by β-gal activity.
CAR transfection assays
Transfection assays were perfomed as previously described (Tzameli et al., 2000). Briefly, HepG2 cells, obtained from ATCC (Manassas, VA) were maintained in Dulbecco’s modified Eagle medium, supplemented with 10% fetal bovine serum (Hyclone). Cells (105) were plated onto 24-well dishes supplemented with charcoal-stripped serum and transfected as shown in Figure 8. Cells were co-transfected with pCMX-mCAR (filled bars) or pCMX vector (open bars), G5-TK-luciferase reporter (LXRE)3-firefly luc and pRL-tk-renilla luc as an internal control. Cells were grown to 70–80% confluence and then treated with 4 μM androstanol (to suppress CAR activation), 0.1% DMSO, or 4 μM androstenedione in addition to either 250 nM TC, or 0.1–100 μM isopimpinellin. At 24 h after treatment, luciferase activity was detected by dual-luciferase assy kit (Promega) on a luminometer, and the ratio of firefly luciferase to renilla luciferase was calculated. Data represent means ± SD of at least 3 separate experiments.
Figure 8.
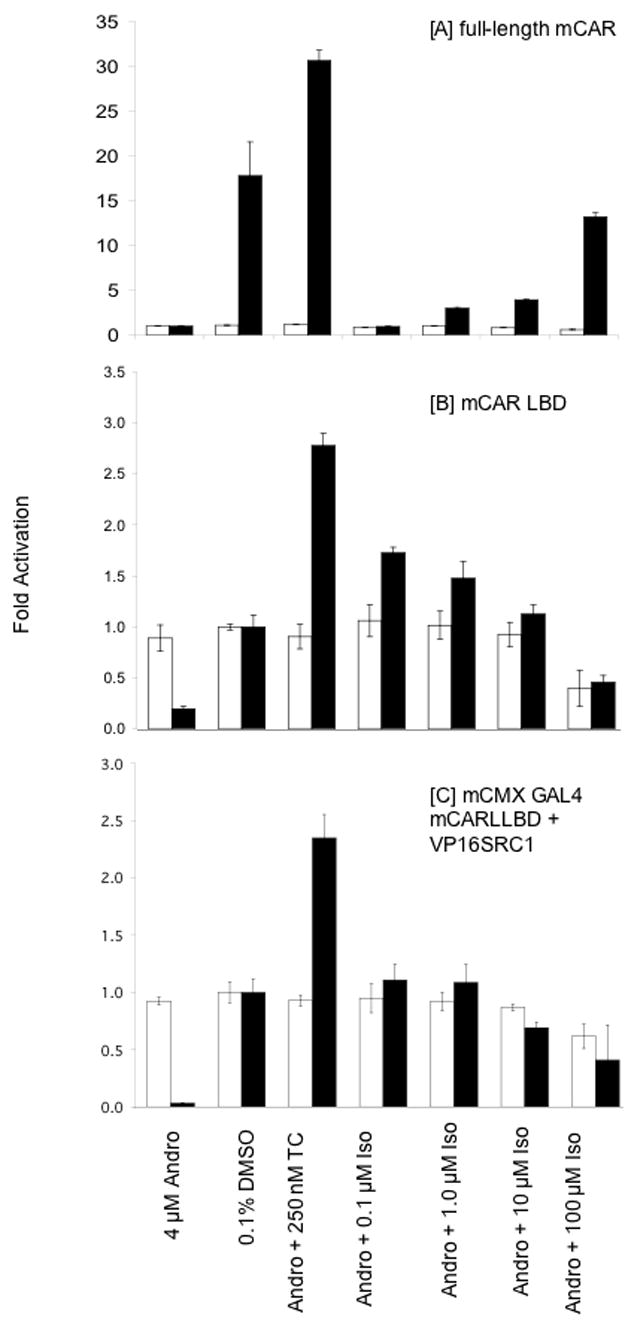
Activation of mCAR mediated reporter gene expression by isopimpinellin. HepG2 cells were co-transfected with [A] pCMX-mCAR (filled bars) or pCMX vector (open bars), [B] pCMX-Gal4 mCARLBD, and [C] pCMX-Gal4 mCARLBD + VP16SRC1. Cells were co-transfected with G5-TK-luciferase reporter (LXRE)3-luc and pRL-tk-renilla luc as an internal control. Cells were grown to 70–80% confluence and then treated with 4 μM androstenedione (to suppress CAR activation), 0.1% DMSO, or 4 μM androstenedione in addition to either 250 nM TC, or 0.1–100 μM isopimpinellin. At 24 h after treatment, luciferase activity was detected on a luminometer, and the ratio of firefly luciferase to renilla luciferase was calculated. Data represent means ± SD of at least 3 separate experiments.
CAR(−/−) knockout mouse studies
CAR(+/+) wild-type and CAR(−/−) knockout mice were bred and genotyped as previously described (Wei et al., 2000). Mice were housed in a temperature- and humidity-controlled AAALAC facility with a 12 h light/dark cycle. All procedures were approved by the Institutional Animal Care and Use Committee in accordance with NIH guidelines. Mice were allowed access to food and water ad libitum. For each genotype [CAR(+/+) and CAR(−/−)] groups of 3 male and 3 female mice (7–9 weeks old) were treated with isopimpinellin (150 mg/kg in corn oil, gavage, 24 h), vehicle (corn oil, 0.1 mL/25 g bw), or the positive control TC (3 mg per kg body weight, i.p, 6 h). At the appropriate time-points, mice were sacrificed and livers were removed and snap-frozen in liquid nitrogen. Total liver RNAs were analyzed for Cyp or GST RNA expression by Northern blots as previously described (Wei et al., 2000). Blots were scanned and the intensity of the bands were compared using Image J analysis (http://rsb.info.nih.gov/ij/).
Statistical analysis
Data represent means ± SD or range of at least 2–3 separate experiments. Statistically significant differences were assessed using ANOVA followed by Fisher’s protected least significant difference (PLSD) test. Fisher PLSD tests were performed on a Macinotosh computer with Statview 5.0 software (Altura Software, Inc.).
Results
Structure-Activity Relationship of the Effects of NOCs on murine hepatic drug-metabolizing enzymes
Initial studies were done to determine the extent to which different NOCs modulated hepatic drug-metabolizing enzyme activity. In this regard, a series of NOCs, including simple coumarins with aliphatic side chains of increasing length (coumarin, limettin, auraptene); an angular furanocoumarin (angelicin); and linear furanocoumarins (isopimpinellin, bergamottin) (structures shown in Figure 1) were administered to male and female C57BL/6 mice by gavage for 3 consecutive days. PB was used as a positive control (that induces P450s). The following parameters were measured: liver weight, liver microsomal P450 1A, 2B, and 3A activity and expression; and liver cytosolic NQO and GST enzyme activities. The results are summarized in Figure 2. As shown in Figure 2A, liver weights were significantly increased in all treatment groups. The greatest effects on liver weight were observed in mice treated with isopimpinellin and bergamottin, with 59% and 45% increases in liver/body weight ratios, respectively. There was an 18% increase in liver/body weight ratio in the PB-treated mice. Although only the PB-treated group showed a significant increase in P450 1 and P450 2 activity (Figures 2B and 2C, respectively), bergamottin and isopimpinellin treatment showed an apparent increase in P450 2B activities (Figure 2C) by ~5-fold each. Similarly, P450 3A activities were significantly increased by bergamottin, isopimpinellin, and PB treatment by 3–4-fold (Figure 2D). There was also an increase in P450 3A activity by angelicin treatment, but this effect was not statistically significant. P450 content was increased mainly in the PB and isopimpinellin-treated mice, but no changes were apparent in the other groups (Figure 2E).
Figure 2.
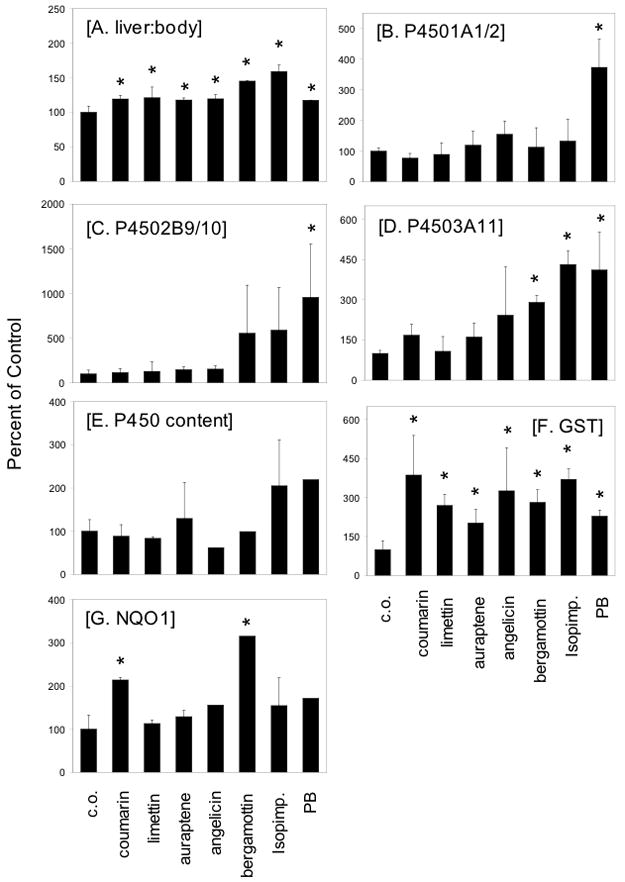
Effects of oral administration of different naturally occurring coumarins on liver:body weight (A), P450 1A activity (B), P450 2B activity (C), P450 3A activity (D), P450 content (E), GST activity (using DCNB as a substrate, panel F), and NQO activity (G). Male and female C57BL/6 mice were treated with NOCs or PB (100 mg/kg, p.o.) once a day for 3 consecutive days. At 24 h after the final dose, mice were sacrificed by cervical dislocation and livers removed. Liver weights were calculated as a percentage of liver weight divided by body weight. Data are represented as a percentage of the corn oil (vehicle) control mice (means ± SD or range). Substrates (and concentration of substrate) for P450 1A, 2B, 3A, GST, and NQO were ethoxyresorufin (5 μM), pentoxyresorufin (5 μM), testosterone (0.25 mM), DCNB (1 mM), and 2,6-dichloroindophenol (1.25 mM), respectively. Control values for each assay were: 5.37±0.48% (A), 89.4 ± 9.4 pmol/min/mg (B), 6.60 ± 2.72 pmol/min/mg (C), 1.30 ± 0.14 nmol/min/mg (D), 0.87 ± 0.23 nmol/mg (E), 31.0 ± 10.4 nmol/min/mg (F), and 242 ± 78 nmol/min/mg (G). Livers from male and female mice (3–4 per group) were pooled together in each experiment. This experiment was performed 2–3 times. * Data were significantly different from corn oil control (P 0.05, Student’s t-test)
Also in Figure 2, the effects of different NOCs on carcinogen-detoxifying enzymes are summarized. GST activities using DCNB as a substrate were significantly increased in all groups, with the highest increase in the coumarin, angelicin, and isopimpinellin treated mice (Figure 2F). There were significant increases in NQO activities in the coumarin and bergamottin treated groups by 2–3 fold; several other NOCs as well as PB increased NQO activities but these values were not significantly different from the control group (Figure 2G).
Western blot analysis was also performed to determine the effects of different naturally occurring coumarins on hepatic P450 and GST protein expression. As shown by densitometry analysis in Figure 3, angelicin, bergamottin, isopimpinellin, and PB increased P450 1A1/1A2 protein expression. The original western blots are shown in Supplemental Figure 1. In addition, bergamottin, isopimpinellin, and PB increased P450 2B10 and 3A11 protein expression. The simple coumarins (coumarin, limettin, auraptene) did not have any apparent effect on P450 protein expression. However, as shown by densitometry analysis all the coumarins tested increased liver cytosolic GSTα protein expression, had modest effects on GSTπ, and no effect on GSTμ. Interestingly, PB had a greater effect on inducing GSTμ than GSTα or GSTπ in this assay.
Figure 3.
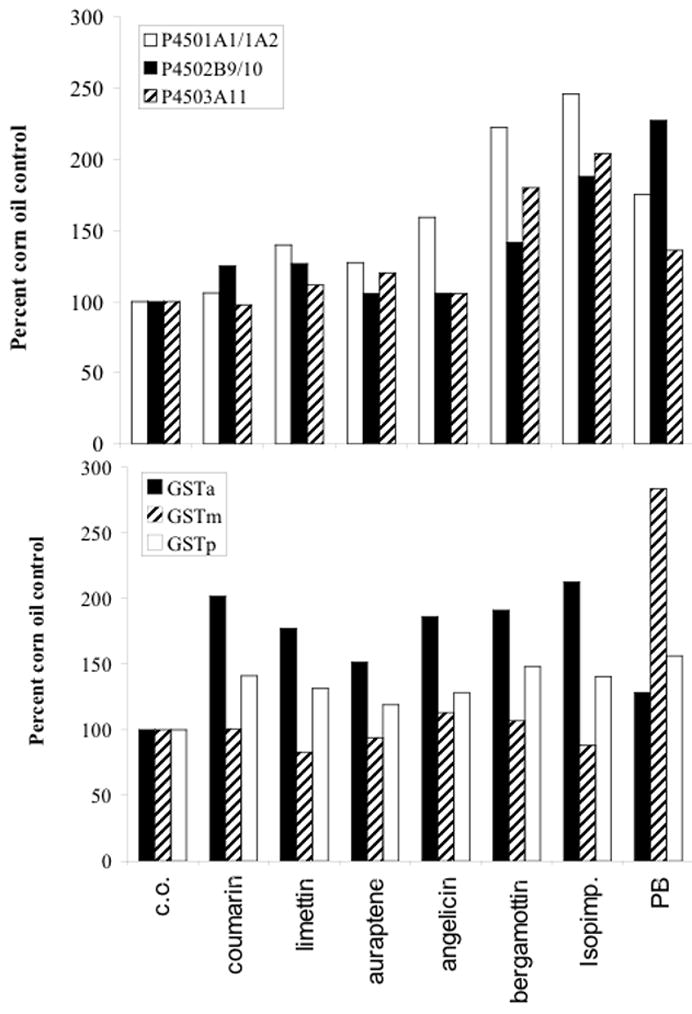
Densitometric analysis of western blots of effects of oral administration of different naturally occurring coumarins on hepatic microsomal P450 (panel A) and cytosolic GST (Panel B) protein expression. Male and female C57BL/6 mice were treated with NOCs or PB (100 mg/kg, p.o.) once a day for 3 consecutive days. At 24 h after the final dose, mice were sacrificed by cervical dislocation and livers removed. Microsomal and cytosolic liver fractions were analyzed by western blot for P450 and GST expression, respectively. β-actin and albumin were used loading controls for microsomal and cytosolic samples, respectively. Figures represent pooled samples from 3 mice each and are expressed as a percentage of the corn oil (vehicle) control group.Original western blotsare shown in Supplemental Figure 1.
Dose-response study
To further characterize the effects of the furanocoumarins, imperatorin and isopimpinellin on hepatic P450 and GST activities in mice, a dose-response study was conducted and enzyme assays performed on liver fractions. Mice were treated orally once daily for 4 consecutive days with corn oil, imperatorin, or isopimpinellin (35, 70, and 150 mg/kg), and sacrificed 24 h after the final dose. Figure 4 shows the results of these experiments. There were significant increases in P450 1 (panel A) and P450 2B (panel B) activities following oral treatment with either imperatorin or isopimpinellin, with the greatest effects being on P450 2B activities. These effects did not show a linear dose response, probably because imperatorin and isopimpinellin can also inhibit P450 1 and P450 2B activity (Cai et al., 1993). However, these results are consistent with our published results (Kleiner et al., 2001) using a 70 mg/kg oral dose. P450 3A activity (panel C) was also increased by imperatorin and isopimpinellin. Liver cytosolic NQO (panel D) was also increased at all doses in both the imperatorin and isopimpinellin groups. Increases in NQO activity were maximal at the 35 mg/kg dose. Both imperatorin and isopimpinellin increased liver cytosolic GST activities using CDNB, DCNB, and EA as substrates. The increases in these activities corresponded with increasing doses of imperatorin, reaching a maximal effect at the highest dose (150 mg/kg). For isopimpinellin, there was a maximal effect at 70 mg/kg, and no further increase was observed at 150 mg/kg. The maximum induction of GST activities (using CDNB, DCNB, and EA as substrates) by imperatorin was 260%, 300%, and 170% of the corn oil control, respectively. The maximum induction of GST activities (using CDNB, DCNB, and EA as substrates) by isopimpinellin was 250%, 350%, and 200% of the corn oil control, respectively. There were no differences in GST activities using cumene hydroperoxide as a substrate even at the highest doses of imperatorin and isopimpinellin (data not shown). Taken together, these results indicate that multiple oral doses of imperatorin and isopimpinellin elevated enzyme activities associated with murine P450s 1A1/2, 2B9/10, and 3A11. In addition, these compounds also elevated NQO and GST enzyme activities using CDNB, DCNB, and EA as substrates. It is interesting to note that this pleiotropic pattern of altered enzyme activities is similar to a number of compounds, including ethoxyquin and oltipraz (Manson et al., 1997; Kelly et al., 2000) and raises some important questions regarding the mechanism(s) that are involved.
Figure 4.
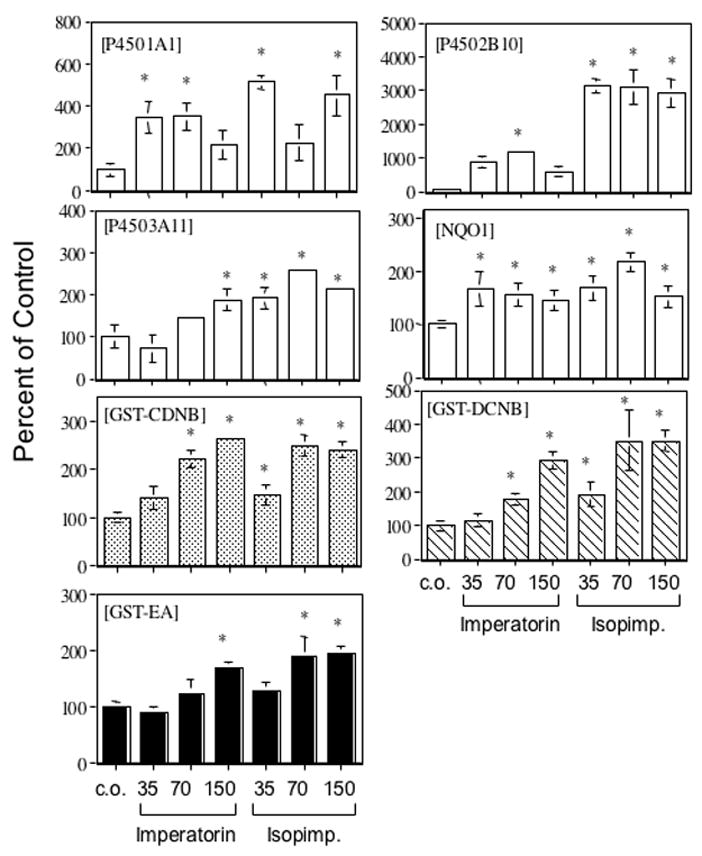
Dose-response of the effects of orally administered imperatorin and isopimpinellin on hepatic Phase I and Phase II enzyme activities. Mice were treated as described in Experimental Procedures. Liver microsomal samples were assayed for P450 1 activity (A), P450 2B activity (B), and P450 3A activity (C). Liver cytosolic samples were assayed for NQO activity (D), and GST activities using CDNB as a substrate (E), DCNB as a substrate (F), or ethacrynic acid as a substrate (G). Substrates (and concentration of substrate) for P450 1A, 2B, 3A, NQO, GST-CDNB, GST-DCNB, and GST-EA were ethoxyresorufin (5 μM), pentoxyresorufin (5 μM), testosterone (0.25 mM), 2,6-dichloroindophenol (1.25 mM), CDNB (1 mM), DCNB (1 mM), and ethacrynic acid (0.2 mM) and), respectively. Data are expressed as % of control (means ± SE, n = 3–9). Control values for each assay were as follows: 45.9 ± 14.3 pmol/min/mg (A); 6.5 ± 1.8 pmol/min/mg (B); 7.31 ± 2.19 nmol/min/mg (C); 28.9 ± 2.5 nmol/min/mg (D); 1000 ± 94 nmol/min/mg (E); 30.0 ± 0.5 nmol/min/mg protein (F); and 15.2 ± 1.6 nmol/min/mg (G).* Significantly different than corn oil control (p < 0.05).
To further evaluate the effects of imperatorin and isopimpinellin on various Phase I and Phase II enzyme activities, western blot analyses were performed to examine protein expression levels in liver. As shown by the densitometry analysis in Figure 5, P450 1A1/1A2 and P450 2B10 protein levels were elevated after four consecutive oral doses of imperatorin or isopimpinellin (35, 70, and 150 mg/kg). The original western blots are shown in Supplemental Figure 2. In particular, P450 2B10 protein levels were significantly elevated. Furthermore, we found that hepatic expression of GST α was greatly increased, particularly at the highest doses, whereas only a modest increase in GST π and no increase in GST μ protein expression was observed. However, expression of GSTμ was higher than GSTα or GST π in the control group. These results are consistent with the observations made in Figure 3 and indicate that the effects were more pronounced when a higher dose was used. These results indicate that the increased enzyme activities observed in liver following oral dosing with imperatorin and isopimpinellin corresponded to an increase in their respective protein expression.
Figure 5.
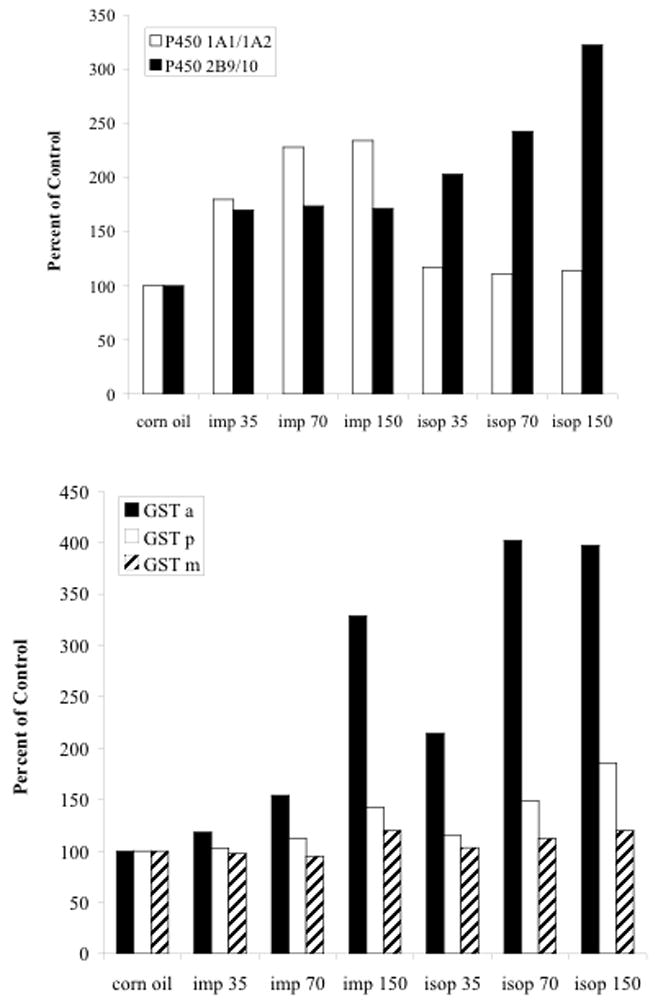
Densitometric analysis of the effects of orally administered imperatorin and isopimpinellin on hepatic microsomal P450 (upper panel) and cytosolic GST (lower panel) protein expression. Mice were treated with imperatorin or isopimpinellin (35, 70, and 150 mg/kg, p.o.) once daily for 3 consecutive days. β -actin was used as a loading control for both microsomes and cytosol. Samples from duplicate mice per group were analyzed and the data are expressed as percentage of the corn oil (vehicle) control group. The original western blots are shown in Supplemental Figure 2.
PXR Transfection Assays
The inducing effects of the furanocoumarins may be due to activation of nuclear receptor PXR (pregnane X receptor, NR112). PXR or its human ortholog SXR (steroid X receptor) is activated in response to a number of xenobiotics, including hyperforin and hypericin, two phytochemicals found in St. John’s wort that are responsible for induction of CYP3A4 (reviewed in (Mannel, 2004)). To address whether isopimpinellin activates PXR, cells transfected with either full-length PXR or luciferase reporters driven by the PXR-DR3 element were used. We also tested the effects of isopimpinellin on SXR as species differences in activation of PXR and/or SXR are well-established (LeCluyse, 2001). As shown in Figure 6 and Table 1, isopimpinellin activated both PXR and SXR in a dose-dependent manner but was far less potent that the positive controls PCN and RIF, respectively. In this regard, whereas the effective dose that activated GAL-PXR expression by 50% was ~63 nM PCN, it was 18,000 nM isopimpinellin. For GAL-SXR, RIF activated GAL-SXR by 50% at ~1200 nM, whereas a concentration of isopimpinellin required to activate GAL-SXR was 35,000 nM. The concentration of isopimpinellin to reach maximal effect was 30 times higher than PCN and 10 times higher than RIF in GAL-PXR and GAL-SXR reporter systems, respectively. Isopimpinellin was also less potent than PCN and RIF on PXR-DR3 and SXR-DR3 activation. Furthermore, isopimpinellin was less potent at activating SXR than PXR. We also tested the ability of isopimpinellin to recruit coactivator SRC-1 (steroid receptor coactivator-1) to PXR or SXR using mammalian two-hybrid assay. In this experiment isopimpinellin (50 μM) induced interaction of the PXR LBD (ligand binding domain) and -SRC-1 RID (receptor interaction domain) by over 4-fold compared to the control (Figure 7A). This effect was similar to the effects of 10 μM PCN, the positive control. Isopimpinellin (50 μM) and RIF (10 μM) induced interaction of the PXR LBD and SRC-1 by ~2–3 fold, respectively (Figure 7B). Neither isopimpinellin, PCN, nor RIF activated expression of luciferase reporter through SRC-1 alone or VPPXR/SXR alone. Taken together, these results provide a possible explanation for the hepatic induction of DME in mice. However, the dose-response suggests that isopimpinellin may be less effective in humans and less effective than traditional inducers PCN and RIF.
Figure 6.
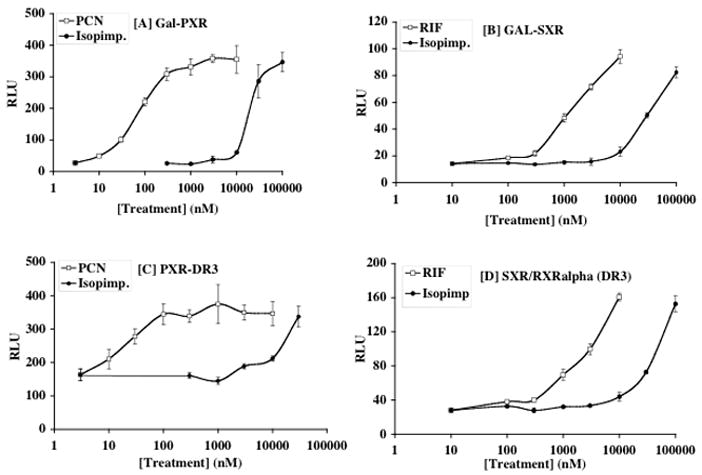
CV-1 cells were cotransfected with Gal-L-PXR (A) or Gal-L-SXR (B) together with MH100-tk-luc, CMX-β-gal. In panels (C) and (D), cells were transfected with full length PXR or SXR, together with hRXRalpha, (DR3)3-tk-luc and CMX-β-gal. Transfected cells were incubated with media containing indicated amount of ligand or solvent control for 18–24 h before subjected to luciferase and β-gal assay. Transcriptional activation was assessed by the relative luciferase activity normalized by β-gal activity. Figures represent means ± SD.
Table 1.
Comparison of the effects of isopimpinellin on PXR and SXR activation in transfected cells
| GAL-PXR | ED50 (nM) | Max (nM) | GAL-SXR | ED50 (nM) | Max (nM) |
|---|---|---|---|---|---|
| PCN (+) | 63 | 3,000 | RIF (+) | 1,259 | 10,000 |
| Isopimp. | 17,783 | 100,000 | Isopimp. | 35,481 | 100,000 |
| PXR-DR3 | ED50 (nM) | Max (nM) | SXR-DR3 | ED50 (nM) | Max (nM) |
|
| |||||
| PCN (+) | 20 | 100 | RIF (+) | 2,512 | 10,000 |
| Isopimp. | 15,849 | 30,000 | Isopimp. | 39,811 | 100,000 |
Figure 7.
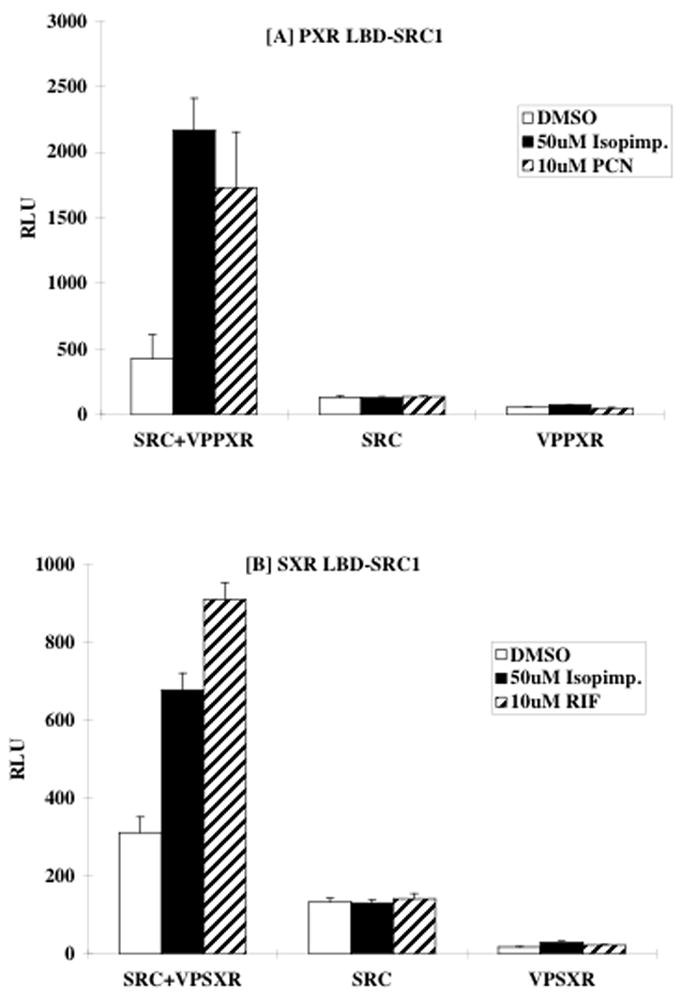
CV-1 cells were cotransfected with Gal-SRC-1 receptor interaction domain (RID) and/or VP-L-PXR (A) or VP-L-SXR (B), together with MH100-tk-luc and CMX-β-gal. Transfected cells were incubated with media containing indicated amount of ligand or solvent control for 18–24 hours before subjected to luciferase and β-gal assay. Interaction between SRC-1 RID and PXR/SXR was assessed by the relative luciferase activity normalized by β gal activity. Figures represent means ± SD.
CAR Transfection Assays
In addition to PXR, induction of P4502B10, 3A11, and 2C9 by xenobiotics may also be mediated by the constitutive androstane receptor (CAR) (Xie et al., 2000). CAR was originally characterized as a constitutive activator of retinoic acid (RA) response elements, and could activate these elements in the absence of RA (Baes et al., 1994; Zelko and Negishi, 2000). In this regard, phenobarbital (PB) is known to be a prototype inducer of Cyp2b10 and also elicits a pleiotropic response in livers of mice (Sueyoshi and Negishi, 2001). It has been demonstrated that the induction of Cyp2b10 by PB is CAR-dependent (Wei et al., 2000). Subsequently, CAR was found to be a nuclear protein that bound to the NR1 site of the phenbarbital-responsive enhancer module (PBREM) along with the retinoic X receptor (RXR) in nuclear extracts following PB treatment [reviewed in (Zelko and Negishi, 2000)]. Thus, upon PB exposure, CAR accumulates in the nucleus, forms heterodimers with RXR, binds to both NR1 and NR2 sites of PBREM and activates transcription of Cyp2b10 and Cyp3a11 [reviewed in (Zelko and Negishi, 2000)].
As an initial study to determine whether isopimpinellin could activate CAR, HepG2 cells were transiently transfected with full-length mCAR and a CAR responsive reporter. The constitutive CAR transactivation was blocked by the inverse agonist androstanol, as expected (Forman et al., 1998). This inhibitory effect of androstanol was counteracted by the CAR agonist TC, and also by increasing doses of isopimpinellin (Figure 8A). Similarly, when cells were transfected with the mCAR-LBD fused to the Gal4 DNA binding domain, both compounds counteracted the inhibitory effect of androstanol on Gal4 reporter expression (Figure 8B). Finally, in the mammalian 2-hybrid assay both compounds also counteracted the inhibitory effect of androstanol on interaction of Gal4-mCAR with the transcriptional coactivator SRC-1 (Figure 8C). In all three cases, isopimpinellin restored either CAR transactivation or coactivator binding to the constitutive level observed with CAR alone, while TC further increased both responses. However, much higher doses of isopimpinellin were required for this reversal of the androstanol effect on the intact receptor relative to the ligand binding domain. The basis for this discrepancy is unclear.
Effect of isopimpinellin on Cyp mRNA expression in CAR(−/−) mice
To further explore the role of CAR in the effects of isopimpinellin on P450 enzyme expression, we compared oral administration of isopimpinellin or corn oil (vehicle) in CAR(+/+) and CAR(−/−) mice. Figure 9 shows the Northern blot (panel A) and the densitometry analysis (panel B). The results shown in Figure 9 demonstrate that a single oral dose of isopimpinellin (150 mg/kg), as well as the positive control TC, induced both Cyp2b10 and Cyp3a11 mRNA in the CAR(+/+) wild-type mice by ~4-fold in both male and female mice. As expected, the induction of both Cyp2b10 and Cyp3a11 by TC was absent in the CAR(−/ −) mice. Similarly, induction of Cyp2b10 mRNA by isopimpinellin was attenuated in the CAR(−/ −) mice, suggesting that isopimpinellin induced Cyp2b10 via the CAR receptor. However, the increase in Cyp3a11 mRNA by isopimpinellin, unlike TC, was still observed in the CAR(−/−) mice, suggesting that isopimpinellin-mediated induction of Cyp3a11 occured via a pathway other than CAR activation.
Figure 9.
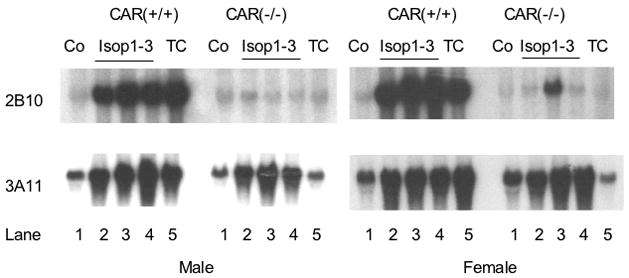
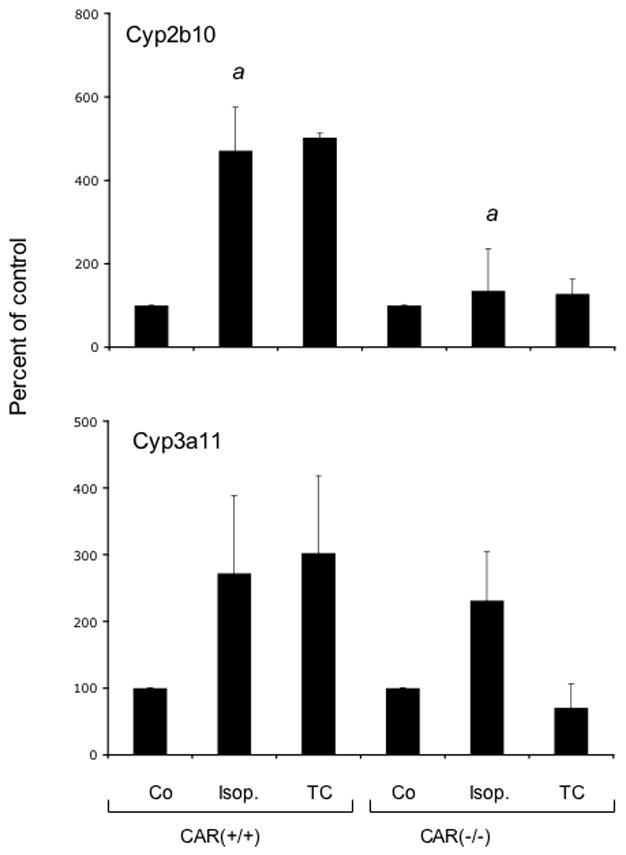
Effects of orally administered isopimpinellin on hepatic expression of Cyp2b10 and Cyp3a11 in CAR(+/+) wild type vs. CAR(−/− knockout mice. Mice were treated with a single dose of isopimpinellin (150 mg/kg, per os) and sacrificed 24 h later. Total liver RNA was analyzed by Northern blot (Panel A) for Cyp2b10 and Cyp3a11. First lane: Co, corn oil control; Lanes 2–4: isop1–3, isopimpinellin, 3 individual mice; Lane 5: TC, mice were treated with a known inducer, TC (3 mg per kg body weight, i.p., 6 h). Panel B, densitometry analysis of the effects of isopimpinellin on Cyp2b10 and Cyp3a11. Individual values (both male and female) were pooled together and the data are expressed as a percentage of the control (means ± SD or range: corn oil control, n=2; isop. n=6; TC, n=2). Bars with the superscript a were significantly different from each other (p ≤0.05).
Effect of isopimpinellin on GST mRNA expression in CAR(−/−) mice
The effects of isopimpinellin on GSTa1 and GSTp mRNA expression were also evaluated in CAR(+/+) wild-type and CAR(−/−) knockout mice. Figure 10 shows the Northern blot (panel A) and densitometry analysis (panel B) of total liver RNA from this analysis. The constitutive expression of GSTp was high in the male mice, and so did not appear much increased in CAR(+/+) mice treated with either isopimpinellin or TC. In the female mice, constitutive GSTp mRNA was lower, and was increased ~ 8-fold and ~4-fold in the CAR(+/+) mice treated with isopimpinellin and TC, respectively. In CAR(−/ −) mice treated with isopimpinellin, GSTp was still increased by at least ~5-fold. The induction of GSTp mRNA by isopimpinellin appeared to be partially attenuated in the CAR(−/−) mice. There was no increase in GSTp mRNA in CAR(−/ −) mice treated with TC. Isopimpinellin increased GSTa1 mRNA in both male and female CAR(+/+) wild type mice by ~10–11 fold, and TC increased GSTa1 mRNA in male and female CAR(+/+) mice by ~8–10 fold. In the CAR(−/ −) mice, isopimpinellin increased GSTa1 mRNA by ~8-fold in the males and by ~3-fold in the females. In contrast, TC did not increase GSTa1 mRNA at all in either male of female CAR(−/−) mice.
Figure 10.
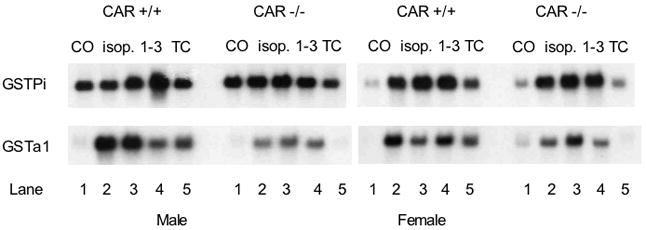
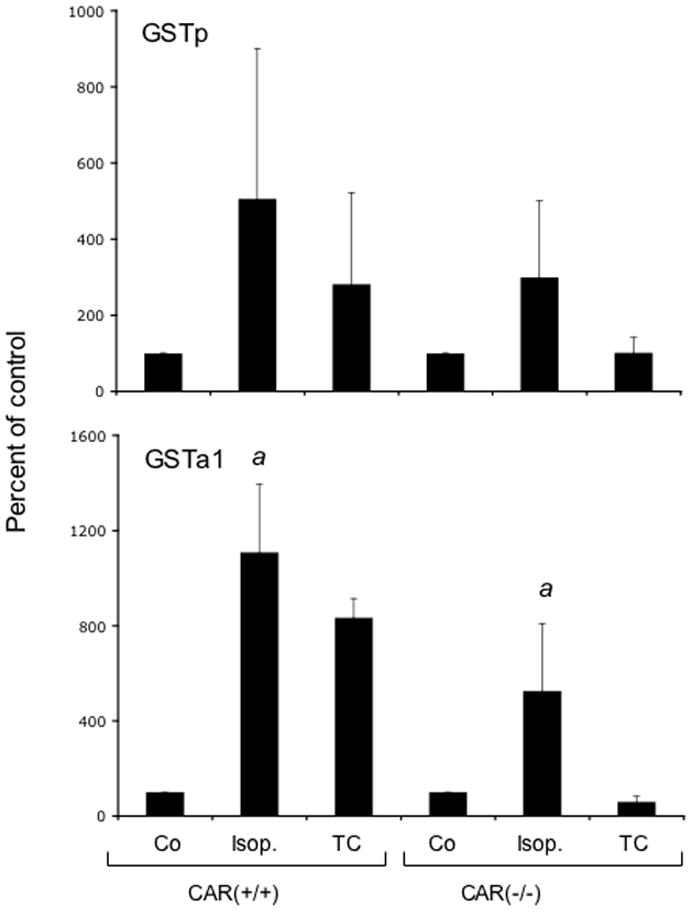
Effects of orally administered isopimpinellin on hepatic expression of GSTPi and GSTa1in CAR(+/+) wild type vs. CAR(−/−) knockout mice. Mice were treated with a single dose of isopimpinellin (150 mg/kg, per os) and sacrificed 24 h later. Total liver RNA was analyzed by Northern blot (Panel A) for GSTPiand GSTa1: First lane: Co, corn oil control; Lanes 2–4: isop1–3, isopimpinellin, 3 individual mice; Lane 5: TC, mice were treated with a known inducer, TC (3 mg per kg body weight, i.p., 6 h). Panel B, densitometry analysis of the effects of isopimpinellin on GSTPi and GSTa1. Individual values (both male and female) were pooled together and the data are expressed as a percentage of the control (means ± SD or range: corn oil control, n=2; isop. n=6; TC, n=2). Bars with the superscript a were significantly different from each other (p ≤0.05).
Discussion
The objective of the current study was to further characterize the modulation of hepatic drug metabolizing enzymes in mice by using a range of structurally diverse NOCs. The current results show that all the coumarins tested induced GST activity and protein expression, with predominant effects on GST α In addition, linear furanocoumarins induced hepatic P450s, predominantly P450 2B10 and 3A11. The angular furanocoumarin (angelicin) showed modest increases in P450 expression. In the case of isopimpinellin and PB, this increase in P450 expression also corresponded to an increase in liver weight and P450 content. The increase in liver/body weight, P450 content, and P450 protein expression is consistent with de novo protein synthesis. Another interesting feature of linear furanocoumarins is that they also inhibit the activities of P450 1, 2B, and 3A family members (Cai et al., 1993; He et al., 1998; Koenigs and Trager, 1998a; Koenigs and Trager, 1998b; Kleiner et al., 2003). Thus, the net effect on the activities of these enzymes in the liver is complicated by induction of the apoprotein and concomitant suppression of its activity. This is a likely explanation for the high variability of P4502B9/10 activity in the bergamottin and isopimpinellin groups. In fact, when examining hepatic P450 2B9/10 activities in mice treated with imperatorin and isopimpinellin for 3 consecutive days, the time of sacrifice determined the overall P450 2B9/10 activity. At 1 h after the final dose, no elevation was observed, whereas at 24 h after the final dose, P450 2B9/10 activity was increased by 100-fold (Kleiner et al., 2001). In addition to inhibition of enzyme activity, metabolism and clearance may also contribute to these differences.
Bergamottin, imperatorin, and isopimpinellin induced a rather pleiotropic enzyme induction response in the liver of mice following oral exposure. Among naturally occurring coumarins, there may be a dissociation between P450 and GST induction depending on structure. Notably, the simple coumarins also induced GST activity and protein expression but lacked the effects on P450 induction. There did not appear to be a correlation between the length of the aliphatic side chains and the effects of the NOCs on Phase I and Phase II DME. For example, bergamottin and isopimpinellin appeared to be approximately equally effective, whereas coumarin, limettin, and auraptene had little effect on increasing P450 2B9/10 and 3A11 expression. Hence, the differences were more apparent by the presence of the furan ring, which appears to confer P450 modulating activity to both suppress P450 enzyme activities and lead to their induction. It has been demonstrated that oxidation of the double bond on the furan ring of 8-methoxypsoralen leads to a reactive intermediate that covalently binds to the P450 2A6, resulting in mechanism-based inactivation (Koenigs and Trager, 1998a; Koenigs and Trager, 1998b). Furthermore, [14C]coriandrin was previously shown to covalently bind to a protein with an approximate molecular mass of 49 kDa (Cai et al., 1996). This covalent binding was NADPH-dependent, and was inhibited by electrophile trapping agents and P4501A1 inhibitors (7,8-benzoflavone, monoclonal antibody against 3-methylcholanthrene inducible P450s, polyclonal antibody against P4501A1, and ethoxyresorufin). Coriandrin, imperatorin, isopimpinellin, and bergamottin, also suppressed P450 content but had no effect on heme when incubated with liver microsomes in vitro (Cai et al., 1996). Taken together, these previous observations strongly suggest that furanocoumarins are metabolized into an electrophilic intermediate that covalently bind to P450s. However, the exact nature of the covalent adduct has not been elucidated.
Several observations were noted from the dose-response studies with imperatorin and isopimpinellin. Even the lowest doses of isopimpinellin induced P450 2B9/10 activity and protein expression to nearly the same extent as the highest dose. It is also interesting that a 2–3 fold increase in the protein expression (as measured by the optical density) corresponded to a 300-fold increase in enzyme activity. In contrast, imperatorin also increased P450 2B9/10 protein expression (by about 1.5–2 fold) but increased enzyme activity by ~100-fold. The highest dose (150 mg/kg) of imperatorin had less of an effect on P450 2B9/10 activity than the 70 mg/kg dose. Imperatorin was 10–100 times more potent than isopimpinellin at blocking P450 2B activity using pentoxyresorufin as a substrate (Cai et al., 1993; Kleiner et al., 2003). In contrast, imperatorin and isopimpinellin were ~equally effective at blocking P450 1A1 mediated ethoxyresorufin O-dealkylase activity (Cai et al., 1993; Kleiner et al., 2003). This could explain why both compounds had an irregular dose-response for P450 1A1 activity following oral administration to mice, but only imperatorin had this effect on P450 2B activity. Western blots were not done on P450 3A in the dose-response study, but the enzyme activity showed significant increases, even at the 35 mg/kg dose in the case of isopimpinellin. This was consistent with the results comparing several NOCs in Figures 2–3. GST activities (with several different substrates) were all elevated in livers of mice treated with either imperatorin or isopimpinellin. Furthermore, western blot analyses revealed that protein levels of GST α were elevated in liver of mice following oral dosing with imperatorin and isopimpinellin.
Based on the effects of isopimpinellin on P450 enzyme induction we hypothesized that it may activate PXR and perhaps its human ortholog SXR. PXR/SXR is a low affinity, broad-substrate receptor that is activated in response to steroids, phytoestrogens, and foodstuffs (Blumberg et al., 1998). This presumably provides omnivores/herbivores an adaptive response to detoxify food-borne xenobiotics. For example, the black swallowtail, Papilio polyxenes, consumes a diet that consists predominantly of furanocoumarin-containing plants in the Apiaceae and Rutaceae families, whereas the tiger swallowtail, P. glaucus, rarely ingests this diet (Hung et al., 1996). The abilities of these species to survive on furanocoumarin-containing diets (which are normally very toxic) are related to the constitutive and inducible expression of CYP6B, which metabolizes furanocoumarins (Hung et al., 1996; Li et al., 2002). Several transcriptional regulatory elements of CYP6B have been identified including XRE-xan (Xenobiotic Response Element to Xanthotoxin), XRE-Ahr (Xenobiotic Response Element to Aryl hydrocarbon Receptor), ARE (Antioxidant Response Element), and an imperfect PRE (PXR Responsive Element) (Li et al., 2002). PXR/SXR is known to regulate both CYP2B and CYP3A gene expression (Xie et al., 2000) so we investigated it as a possible mechanism for the P450 inducing effects of isopimpinellin. Indeed, in our study, isopimpinellin activated both PXR and SXR at ~17 μM (PXR) and ~35 μM (SXR) in CV-1 cells transfected with either the GAL4 chimera or the full-length receptor. The mammalian two-hybrid assay confirmed that isopimpinellin induced an interaction between the receptor interaction domain of SRC-1 and the ligand-binding domain of both PXR and SXR at 50 μM. Similarly, 8-methoxypsoralen, which differs in structure from isopimpinellin by one methoxy group, induced the expression of CYP3A4 in human hepatocytes, and activated both rat and human PXR at an EC50 of 14 μM (Yang and Yan, 2007). The possibility also exists that PXR regulates the induction of GSTs by isopimpinellin and other related coumarins (Falkner et al., 2001). In this regard, known PXR activators/ligands (e.g., pregnenolone, 17α-hydroxypregnenolone, RU486) were shown to induce a GSTA2 reporter gene expression in HepG2 cells transiently transfected with the plasmid p1.62GSTYaLUC and pPXR (Falkner et al., 2001). Furthermore, mutation of the ARE core sequence (GTGACaaaGC to GGGACaaaGC) abolishes this response by t-butylhydroquinone and 17αhydroxypregnenolone (Falkner et al., 2001). These results suggest that induction of GSTA2 by pregnanes may be mediated by the interaction of PXR with factors binding to the ARE (Falkner et al., 2001). Alternatively, linear furanocoumarins such as isopimpinellin may induce GSTs via the ARE similar to simple coumarins as noted by (McMahon et al., 2001).
Since the predominant effect of isopimpinellin was induction of P4502B10, we hypothesized that it may also act via the CAR receptor. To address this hypothesis we compared the effects of isopimpinellin to the positive control, TC, in CAR(−/−) knockout mice and transfection experiments. The transfection assay results demonstrated that isopimpinellin counteracted the inhibitory effect of androstanol on full length mCAR and also a Gal4-mCAR ligand binding domain fusion. In contrast to the superactivation observed with TC, the transactivation observed with isopimpinellin plus androstanol was comparable to that observed with full length mCAR alone, and the same result was observed with direct transactivation by a Gal4-mCAR ligand binding fusion protein and in the mammalian two-hybrid interaction of this fusion with the transcriptional coactivator SRC-1. These results strongly indicate that isopimpinellin is a direct CAR ligand able to compete effectively with the inverse agonist androstanol. However, they also suggest that isopimpinellin binding does not augment CAR transactivation beyond the apparently constitutive activity observed in the absence of exogenous ligands. In the CAR knockout mouse studies, our results showed that the positive control, TC, induced Cyp2b10, Cyp3a11, GSTp, and GSTa mRNA in CAR(+/+) wild-type but not CAR(−/−) mice as previously described (Wei et al., 2000). The induction of hepatic Cyp2b10 mRNA by isopimpinellin also appeared to be dependent on CAR because the induction of Cyp2b10 was attenuated in CAR(−/−) mice. However, Cyp3a11, GSTp, and GSTa mRNA were elevated by isopimpinellin in both CAR(+/+) wild-type and CAR(−/−) knockout mice.
The role of hepatic P450 induction by furanocoumarins in their cancer chemopreventive activities is complex. Another chemopreventive agent, oltipraz, is thought to suppress aflatoxin-B1 hepatocarcinogenesis through its induction of GSTs. However, oltipraz was also found to modulate hepatic P450 by acting both as a reversible inhibitor and an inducer of P450 1A and 2B (Langouet et al., 1997). We note that in our previous studies, the P450 inducing effects of imperatorin and isopimpinellin were apparently isolated to the liver, because skin epidermis, forestomach, and lung P450 activities remained unchanged or suppressed at 24 h after the last of 4 consecutive oral doses (Kleiner et al., 2001). B[a]P and/or DMBA DNA adduct formation was also reduced in these tissues (Kleiner et al., 2001). Perhaps the initial suppression of P450 in the liver followed by induction of hepatic P450s, GSTs, and NQO coupled with sustained suppression of P450s in the extrahepatic tissues allows carcinogens to be metabolized and cleared in the liver.
In conclusion, the current study compared a broad range of NOCs for their abilities to modulate P450s and GSTs. Overall, most/all coumarins induced GST expression and activities, with predominant effects on GSTα. However, mainly the furanocoumarins induced P450 1, 2, and 3 expression and activities. Taken together, the current evidence suggests that isopimpinellin interacts with PXR, SXR and CAR, and that the induction of Cyp2b10 mRNA may be mediated through its interactions with CAR. In the future, we will further study additional mechanism(s) whereby isopimpinellin and other NOCs induce Phase I and Phase II enzymes. The effects of NOCs on xenobiotic metabolizing enzymes suggest that further research on their biological/toxicological effects are warranted.
Supplementary Material
Acknowledgments
This research was supported by CA79442, CA16672, and ES07784. We also thank the Center for Research on Environmental Disease for the summer undergraduate research internship program of which Ms. Pontius and Ms. Abey participated.
Abbreviations
- B[a]P
Benzo[a]pyrene
- CAR
constitutive androstane receptor
- CDNB
1-chloro-2,4-dinitrobenzene
- DCNB
1,2-dichloro-4-nitrobenzene
- DCPIP
2,6-dichloroindophenolate hydrate
- DMBA
7,12-dimethylbenz[a]anthracene
- DME
drug metabolizing enzymes
- EA
ethacrynic acid
- GST
glutathione S-transferase
- LBD
ligand binding domain
- mCAR
murine CAR
- NOC
naturally occurring coumarins
- NQO
NAD(P)H quinone oxidoreductase
- PB
Phenobarbital
- PBREM
phenobarbital response element
- PXR
pregnane X receptor
- RXR
retinoid X receptor
- SXR
steroid X receptor
- TC
1,4-bis[2-(3,5-dichloropyridyloxy)]benzene
- XREM
xenobiotic response element
Footnotes
Conflict of Interest Statement
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baba M, Jin Y, Mizuno A, Suzuki H, Okada Y, Takasuka N, Tokuda H, Nishino H, Okuyama T. Studies on cancer chemoprevention by traditional folk medicines XXIV. Inhibitory effect of a coumarin derivative, 7-isopentenyloxycoumarin, against tumor-promotion. Biol Pharm Bull. 2002;25:244–246. doi: 10.1248/bpb.25.244. [DOI] [PubMed] [Google Scholar]
- Baes M, Gulick T, Choi HS, Martinoli MG, Simha D, Moore DD. A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol. 1994;14:1544–1552. doi: 10.1128/mcb.14.3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum M. Phototoxicity of plant secondary metabolites: insect and mammalian perspectives. Arch Insect Biochem Physiol. 1995;29:119–134. doi: 10.1002/arch.940290204. [DOI] [PubMed] [Google Scholar]
- Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burke MD, Thompson S, Elcombe CR, Halpert J, Haaparanta T, Mayer RT. Ethoxy-, pentoxy- and benzyloxyphenoxazones and homologues: a series of substrates to distinguish between different induced cytochromes P-450. Biochem Pharmacol. 1985;34:3337–3345. doi: 10.1016/0006-2952(85)90355-7. [DOI] [PubMed] [Google Scholar]
- Burke MD, Thompson S, Weaver RJ, Wolf CR, Mayer RT. Cytochrome P450 specificities of alkoxyresorufin O-dealkylation in human and rat liver. Biochem Pharmacol. 1994;48:923–936. doi: 10.1016/0006-2952(94)90363-8. [DOI] [PubMed] [Google Scholar]
- Cai Y, Baer-Dubowska W, Ashwood-Smith MJ, Ceska O, Tachibana S, DiGiovanni J. Mechanism-based inactivation of hepatic ethoxyresorufin O-dealkylation activity by naturally occurring coumarins. Chem Res Toxicol. 1996;9:729–736. doi: 10.1021/tx950208b. [DOI] [PubMed] [Google Scholar]
- Cai Y, Bennett D, Nair RV, Ceska O, Ashwood-Smith MJ, DiGiovanni J. Inhibition and inactivation of murine hepatic ethoxy- and pentoxyresorufin O-dealkylase by naturally occurring coumarins. Chem Res Toxicol. 1993;6:872–879. doi: 10.1021/tx00036a018. [DOI] [PubMed] [Google Scholar]
- Cai Y, Kleiner H, Johnston D, Dubowski A, Bostic S, Ivie W, DiGiovanni J. Effect of naturally occurring coumarins on the formation of epidermal DNA adducts and skin tumors induced by benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene in SENCAR mice. Carcinogenesis. 1997;18:1521–1527. doi: 10.1093/carcin/18.8.1521. [DOI] [PubMed] [Google Scholar]
- Cardoso CA, Pires AE, Honda NK. A method for quantitative determination of furanocoumarins in capsules and tablets of phytochemical preparations. Chem Pharm Bull (Tokyo) 2006;54:442–447. doi: 10.1248/cpb.54.442. [DOI] [PubMed] [Google Scholar]
- Falkner KC, Pinaire JA, Xiao GH, Geoghegan TE, Prough RA. Regulation of the rat glutathione S-transferase A2 gene by glucocorticoids: involvement of both the glucocorticoid and pregnane X receptors. Mol Pharmacol. 2001;60:611–619. [PubMed] [Google Scholar]
- Forman BM, Tzameli I, Choi HS, Chen J, Simha D, Seol W, Evans RM, Moore DD. Androstane metabolites bind to and deactivate the nuclear receptor CAR-beta. Nature. 1998;395:612–615. doi: 10.1038/26996. [DOI] [PubMed] [Google Scholar]
- Frerot E, Decorzant E. Quantification of total furocoumarins in citrus oils by HPLC coupled with UV, fluorescence, and mass detection. J Agric Food Chem. 2004;52:6879–6886. doi: 10.1021/jf040164p. [DOI] [PubMed] [Google Scholar]
- Guo LQ, Yamazoe Y. Inhibition of cytochrome P450 by furanocoumarins in grapefruit juice and herbal medicines. Acta Pharmacol Sin. 2004;25:129–136. [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- He K, Iyer KR, Hayes RN, Sinz MW, Woolf TF, Hollenberg PF. Inactivation of cytochrome P450 3A4 by bergamottin, a component of grapefruit juice. Chem Res Toxicol. 1998;11:252–259. doi: 10.1021/tx970192k. [DOI] [PubMed] [Google Scholar]
- Hung CF, Holzmacher R, Connolly E, Berenbaum MR, Schuler MA. Conserved promoter elements in the CYP6B gene family suggest common ancestry for cytochrome P450 monooxygenases mediating furanocoumarin detoxification. Proc Natl Acad Sci U S A. 1996;93:12200–12205. doi: 10.1073/pnas.93.22.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly VP, Ellis EM, Manson MM, Chanas SA, Moffat GJ, McLeod R, Judah DJ, Neal GE, Hayes JD. Chemoprevention of aflatoxin B1 hepatocarcinogenesis by coumarin, a natural benzopyrone that is a potent inducer of aflatoxin B1-aldehyde reductase, the glutathione S-transferase A5 and P1 subunits, and NAD(P)H:quinone oxidoreductase in rat liver. Cancer Res. 2000;60:957–969. [PubMed] [Google Scholar]
- Kleiner HE, Reed MJ, DiGiovanni J. Naturally occurring coumarins inhibit human cytochromes P450 and block benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene DNA adduct formation in MCF-7 cells. Chem Res Toxicol. 2003;16:415–422. doi: 10.1021/tx025636d. [DOI] [PubMed] [Google Scholar]
- Kleiner HE, Vulimiri SV, Miller L, Johnson WH, Jr, Whitman CP, DiGiovanni J. Oral administration of naturally occurring coumarins leads to altered phase I and II enzyme activities and reduced DNA adduct formation by polycyclic aromatic hydrocarbons in various tissues of SENCAR mice. Carcinogenesis. 2001;22:73–82. doi: 10.1093/carcin/22.1.73. [DOI] [PubMed] [Google Scholar]
- Kleiner HE, Vulimiri SV, Reed MJ, Uberecken A, DiGiovanni J. Role of cytochrome P450 1a1 and 1b1 in the metabolic activation of 7,12-dimethylbenz[a]anthracene and the effects of naturally occurring furanocoumarins on skin tumor initiation. Chem Res Toxicol. 2002a;15:226–235. doi: 10.1021/tx010151v. [DOI] [PubMed] [Google Scholar]
- Kleiner HE, Vulimiri SV, Starost MF, Reed MJ, DiGiovanni J. Oral administration of the citrus coumarin, isopimpinellin, blocks DNA adduct formation and skin tumor initiation by 7,12-dimethylbenz[a]anthracene in SENCAR mice. Carcinogenesis. 2002b;23:1667–1675. doi: 10.1093/carcin/23.10.1667. [DOI] [PubMed] [Google Scholar]
- Koenigs LL, Trager WF. Mechanism-based inactivation of cytochrome P450 2B1 by 8-methoxypsoralen and several other furanocoumarins. Biochemistry. 1998a;37:13184–13193. doi: 10.1021/bi981198r. [DOI] [PubMed] [Google Scholar]
- Koenigs LL, Trager WF. Mechanism-based inactivation of P450 2A6 by furanocoumarins. Biochemistry. 1998b;37:10047–10061. doi: 10.1021/bi980003c. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lake BG. Coumarin metabolism, toxicity and carcinogenicity: relevance for human risk assessment. Food Chem Toxicol. 1999;37:423–453. doi: 10.1016/s0278-6915(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Langouet S, Maheo K, Berthou F, Morel F, Lagadic-Gossman D, Glaise D, Coles B, Ketterer B, Guillouzo A. Effects of administration of the chemoprotective agent oltipraz on CYP1A and CYP2B in rat liver and rat hepatocytes in culture. Carcinogenesis. 1997;18:1343–1349. doi: 10.1093/carcin/18.7.1343. [DOI] [PubMed] [Google Scholar]
- LeCluyse EL. Pregnane X receptor: molecular basis for species differences in CYP3A induction by xenobiotics. Chem Biol Interact. 2001;134:283–289. doi: 10.1016/s0009-2797(01)00163-6. [DOI] [PubMed] [Google Scholar]
- Li W, Petersen RA, Schuler MA, Berenbaum MR. CYP6B cytochrome p450 monooxygenases from Papilio canadensis and Papilio glaucus: potential contributions of sequence divergence to host plant associations. Insect Mol Biol. 2002;11:543–551. doi: 10.1046/j.1365-2583.2002.00363.x. [DOI] [PubMed] [Google Scholar]
- Lundahl JU, Regardh CG, Edgar B, Johnsson G. The interaction effect of grapefruit juice is maximal after the first glass. Eur J Clin Pharmacol. 1998;54:75–81. doi: 10.1007/s002280050424. [DOI] [PubMed] [Google Scholar]
- Mannel M. Drug interactions with St John's wort : mechanisms and clinical implications. Drug Saf. 2004;27:773–797. doi: 10.2165/00002018-200427110-00003. [DOI] [PubMed] [Google Scholar]
- Manson MM, Ball HW, Barrett MC, Clark HL, Judah DJ, Williamson G, Neal GE. Mechanism of action of dietary chemoprotective agents in rat liver: induction of phase I and II drug metabolizing enzymes and aflatoxin B1 metabolism. Carcinogenesis. 1997;18:1729–1738. doi: 10.1093/carcin/18.9.1729. [DOI] [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, Wolf CR, Cavin C, Hayes JD. The Cap'n'Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- Mohri K, Uesawa Y, Sagawa K. Effects of long-term grapefruit juice ingestion on nifedipine pharmacokinetics: induction of rat hepatic P-450 by grapefruit juice. Drug Metab Dispos. 2000;28:482–486. [PubMed] [Google Scholar]
- Murray RDH, Mendez J, Brown SA. In: The Natural Coumarins: Occurrence, Chemistry and Biochemistry. Murray RDH, Mendez J, Brown SA, editors. John Wiley & sons, Ltd; New York: 1982. pp. 97–111. [Google Scholar]
- Ngwendson JN, Bedir E, Efange SM, Okunji CO, Iwu MM, Schuster BG, Khan IA. Constituents of Peucedanum zenkeri seeds and their antimicrobial effects. Pharmazie. 2003;58:587–589. [PubMed] [Google Scholar]
- Omura T, Sato R. The Carbon Monoxide-Binding Pigment of Liver Microsomes. I. Evidence for Its Hemoprotein Nature. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- Prince M, Campbell CT, Robertson TA, Wells AJ, Kleiner HE. Naturally occurring coumarins inhibit 7,12-dimethylbenz[a]anthracene DNA adduct formation in mouse mammary gland. Carcinogenesis. 2006;27:1204–1213. doi: 10.1093/carcin/bgi303. [DOI] [PubMed] [Google Scholar]
- Prochaska HJ, Talalay P. Purification and characterization of two isofunctional forms of NAD(P)H: quinone reductase from mouse liver. J Biol Chem. 1986;261:1372–1378. [PubMed] [Google Scholar]
- Setzer WN, Setzer MC, Schmidt JM, Moriarity DM, Vogler B, Reeb S, Holmes AM, Haber WA. Cytotoxic components from the bark of Stauranthus perforatus from Monteverde, Costa Rica. Planta Med. 2000;66:493–494. doi: 10.1055/s-2000-8595. [DOI] [PubMed] [Google Scholar]
- Sonderfan AJ, Arlotto MP, Dutton DR, McMillen SK, Parkinson A. Regulation of testosterone hydroxylation by rat liver microsomal cytochrome P-450. Arch Biochem Biophys. 1987;255:27–41. doi: 10.1016/0003-9861(87)90291-8. [DOI] [PubMed] [Google Scholar]
- Sparnins VL, Wattenberg LW. Enhancement of glutathione S-transferase activity of the mouse forestomach by inhibitors of Benzo[a]pyrene-induced neoplasia of the forestomach. J Natl Cancer Inst. 1981;66:769–771. [PubMed] [Google Scholar]
- Sueyoshi T, Negishi M. Phenobarbital response elements of cytochrome P450 genes and nuclear receptors. Annu Rev Pharmacol Toxicol. 2001;41:123–143. doi: 10.1146/annurev.pharmtox.41.1.123. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kawabata K, Kakumoto M, Hara A, Murakami A, Kuki W, Takahashi Y, Yonei H, Maeda M, Ota T, Odashima S, Yamane T, Koshimizu K, Ohigashi H. Citrus auraptene exerts dose-dependent chemopreventive activity in rat large bowel tumorigenesis: the inhibition correlates with suppression of cell proliferation and lipid peroxidation and with induction of phase II drug-metabolizing enzymes. Cancer Res. 1998;58:2550–2556. [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzameli I, Pissios P, Schuetz EG, Moore DD. The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol Cell Biol. 2000;20:2951–2958. doi: 10.1128/mcb.20.9.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Jiang SY. Simultaneous determination of urinary metabolites of methoxypsoralens in human and Umbelliferae medicines by high-performance liquid chromatography. J Chromatogr Sci. 2006;44:473–478. doi: 10.1093/chromsci/44.8.473. [DOI] [PubMed] [Google Scholar]
- Wangensteen H, Molden E, Christensen H, Malterud KE. Identification of epoxybergamottin as a CYP3A4 inhibitor in grapefruit peel. Eur J Clin Pharmacol. 2003;58:663–668. doi: 10.1007/s00228-002-0537-3. [DOI] [PubMed] [Google Scholar]
- Wattenberg LW, Lam LK, Fladmoe AV. Inhibition of chemical carcinogen-induced neoplasia by coumarins and alpha-angelicalactone. Cancer Res. 1979;39:1651–1654. [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- Xie W, Barwick JL, Simon CM, Pierce AM, Safe S, Blumberg B, Guzelian PS, Evans RM. Reciprocal activation of xenobiotic response genes by nuclear receptors SXR/PXR and CAR. Genes Dev. 2000;14:3014–3023. doi: 10.1101/gad.846800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yan B. Photochemotherapeutic agent 8-methoxypsoralen induces cytochrome P450 3A4 and carboxylesterase HCE2: evidence on an involvement of the pregnane X receptor. Toxicol Sci. 2007;95:13–22. doi: 10.1093/toxsci/kfl120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelko I, Negishi M. Phenobarbital-elicited activation of nuclear receptor CAR in induction of cytochrome P450 genes. Biochem Biophys Res Commun. 2000;277:1–6. doi: 10.1006/bbrc.2000.3557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


