Abstract
The peroxisome proliferator activated receptor gamma (PPARγ) is essential for the formation and function of adipocytes. PPARγ is also involved in regulating insulin sensitivity and is the functional target of the thiazolidinedione (TZDs) class of insulin sensitizing drugs. While TZDs activate PPARγ and decrease PPARγ protein levels, genetic models indicate decreased expression of PPARγ is also associated with increased insulin sensitivity. In this study, we show that resveratrol modulates PPARγ protein levels in 3T3-L1 adipocytes via inhibition of PPARγ gene expression coupled with increased ubiquitin-proteasome dependent degradation of PPARγ proteins. Resveratrol-mediated decreases in PPARγ expression are associated with repression of PPARγ transcriptional activity when assayed using a panel of PPARγ target genes in adipocytes. Finally, we demonstrate that resveratrol inhibits insulin-dependent changes in glucose uptake and glycogen levels and decreases IRS-1 and GLUT4 protein levels, indicating resveratrol represses insulin sensitivity in adipocytes. These results indicate resveratrol-mediated effects in adipocytes involve regulation of PPARγ expression and transcriptional activity along with decreased responsiveness to insulin.
Keywords: resveratrol, PPARγ, adipocyte, ubiquitin, proteasome
Introduction
A remarkable range of health benefits are ascribed to resveratrol, a bioactive plant polyphenol found in grapes, peanuts, and berries. Recent studies show that resveratrol treatment protects against diet-induced insulin resistance in rodents (1; 2) and leads to decreased lipid accumulation in murine adipocytes (3). Resveratrol is a potent activator of SIRT1 (silencing information regulator-1) (4), a histone deacetylase that mediates the effects of resveratrol in mice (1). SIRT1 is also reported to inhibit the formation of adipocytes via repression of the peroxisome proliferator activated receptor gamma (PPARγ) transcriptional activity (3).
Regulation of PPARγ activity in adipocytes provides a direct link between nutritional status, lipid metabolism, and adipocyte gene expression (5). Adipose PPARγ is also required for the maintenance of insulin sensitivity (6) yet mice heterozygous for PPARγ deficiency remain more insulin sensitive than wild-type mice when fed a high fat diet (7–9). The reduced PPARγ gene expression in the PPARγ −/+ mice correlates with decreased PPARγ protein (10), suggesting modulation of PPARγ activity and protein levels can play a role in regulating insulin sensitivity.
Activation of SIRT1 by resveratrol suggests a potential link between regulation of PPARγ activity, decreased PPARγ protein levels, and insulin sensitivity via resveratrol-mediated effects and points to a role for resveratrol in modulating insulin action associated with obesity and type 2 diabetic states. In the current study, we show that resveratrol treatment in 3T3-L1 adipocytes represses the endogenous gene expression of transcriptional targets of PPARγ such as aP2, Lpl and Pepck. In addition, resveratrol decreases PPARg gene expression while increasing targeting of PPARγ protein to the ubiquitin-proteasome system for degradation, a novel mechanism of resveratrol-mediated effects in adipocytes. We also show that resveratrol treatment in 3T3-L1 adipocytes reduces insulin sensitivity as measured by decreased insulin-dependent glucose uptake and glycogen content along with decreased protein content of IRS-1 and GLUT4. Thus, our data indicates resveratrol may function as a nutritional regulator of PPARγ activity, expression and stability while also decreasing insulin sensitivity in adipocytes.
Materials and Methods
Cell Culture
Murine 3T3-L1 preadipocytes were plated and grown to 2 days post confluence in DMEM high glucose with 10% bovine serum and penicillin/streptomycin. The cells were induced to differentiate using a standard induction cocktail of 3-isobutyl-1-methylxanthine, dexamethasone, insulin (MDI) as previously described (11). The 3T3-L1 preadipocytes and fully differentiated adipocytes were maintained in a humidified chamber at 37°C.
Preparation of Whole Cell Extracts
Cell monolayers were rinsed with phosphate-buffered saline (PBS) and harvested in a non-denaturing buffer as previously described (12). Samples were extracted for 30 minutes on ice and centrifuged at 15,521 × g at 4°C for 15 minutes. Supernatants containing whole cell extracts were analyzed for protein concentrations using a BCA kit (Pierce) according to the manufacturer’s instructions.
TZD and Resveratrol Treatment of 3T3-L1 Adipocytes
5.0 μM rosiglitazone (TZD) and 50 μM resveratrol (Sigma-Aldrich) were added to fully differentiated 3T3-L1 adipocytes at the indicated times. Resveratrol was added in the indicated concentrations when glucose uptake was assayed in the presence of increasing concentrations of resveratrol. DMSO was used as a solvent for both rosiglitazone and resveratrol.
Gel Electrophoresis and Immunoblotting
Proteins were separated in polyacrylamide (National Diagnostics) gels containing sodium dodecyl sulfate (SDS) according to Laemmli (13) and transferred to nitrocellulose. Following transfer, the membrane was blocked in 4% non-fat dry milk suspended in phosphate buffered saline, pH 7.4 with 0.1% Tween 20 (PBS-T) for 1 hour at room temperature. The membranes were incubated with mouse monoclonal anti-PPARγ or antibodies against IRS-1, IRS-2, PI3K, AKT, phospho-AKT, PTP-1B, AMPKα1, AMPKα2, β-actin, anti-insulin receptor β subunit, GLUT1, and GLUT4 as indicated for 1–2 hours. Following extensive washes with PBS-T, the results were visualized with horseradish peroxidase (HRP)-conjugated secondary antibodies and enhanced chemiluminescence (Pierce).
Real-time RT-PCR
Total RNA was purified from cultured cells using TriReagent (Molecular Research Center) according to the manufacturer’s instructions. Real-time RT-PCR was performed via two-step RT-PCR (High Capacity cDNA Archive Kit, Applied Biosystems) followed by PCR using TaqMan primer/probe pairs consisting of two sequence-specific PCR primers and a TaqMan assay-FAM™ dye-labeled MGB probe (Applied Biosystems, Taqman Gene Expression Assay) for each gene of interest. The genes of interest were fatty acid binding protein 4 (aP2), lipoprotein lipase (Lpl), and cytosolic phosphoenolpyruvate kinase (Pepck). PCR was performed using the 7900 Real-Time PCR system (Applied Biosystems) under universal cycling conditions. All results were normalized to a cyclophilin B expression control and reported as the mean or the fold change relative to baseline −/+ standard deviation.
Ubiquitin Conjugation Assay
3T3-L1 adipocytes were preincubated with 10 μM MG132 and 1 μM epoxomicin for 1 hour prior to adding 50 μM resveratrol, 5.0 μM rosiglitazone or an equal volume of DMSO as a vehicle control. The cells were harvested after 30 minutes and lysed on ice in PBS containing 1% Triton X-100, 10 mM N-ethylmaleimide, 1 mM EDTA, 1 mM PMSF, 1 μM pepstatin and 10 μM leupeptin. Whole cell extracts were incubated with protein A-sepharose (RepliGen) and the unbound supernatant was collected for immunoprecipitation using a polyclonal anti-PPARγ followed by incubation with protein A-sepharose. PPARγ-ubiquitin conjugates were detected by western blotting using monoclonal anti-PPARγ and polyclonal anti-ubiquitin antibodies.
Determination of 2-[3H] deoxyglucose Uptake
Fully differentiated 3T3-L1 adipocytes at day 6–7 post induction were incubated in the presence of resveratrol, rosiglitazone or DMSO for 6 hours. Four hours prior to measuring glucose uptake, the cells were serum-deprived in DMEM containing 6.25 mM glucose and 0.3% bovine serum albumin. At the end of each treatment, 2-[3H] deoxyglucose uptake measurements were performed in triplicate and the results were corrected for nonspecific uptake, which was measured in the presence of 5 μM cytochalasin B (14). The protein concentration of each lysate was determined using a BCA kit according to the manufacturer’s instructions.
Glycogen Content
The adipocytes were treated with rosiglitazone (5 μM) or resveratrol (50 μM) for 15 hours with serum derivation during the final 2 hours of treatment. The cells were then incubated with 30 mM glucose in the absence or presence of 100 nM insulin. At the end of 2 hours, the cells were washed three times with cold phosphate buffered saline (PBS), pH 7.4 and whole cell extracts were harvested in 200 μl of 0.2 M sodium acetate, pH 4.8, followed by sonication. After removal of 50 μl aliquots for protein concentration determination, glycogen content was measured according to the method of Gomez-lechon (15).
RESULTS
Resveratrol treatment increases proteasome-dependent PPARγ degradation and decreases PPARγ gene expression
Over-expression of SIRT1 is associated with decreased PPARγ protein levels in 3T3-L1 adipocytes (3), suggesting a link between activation of SIRT1 via resveratrol and regulation of PPARγ protein levels. To determine the effect of resveratrol on PPARγ protein levels, we assayed the steady-state levels of PPARγ proteins in fully differentiated 3T3-L1 adipocytes. As shown in Figure 1A, a six hour treatment with either rosiglitazone or resveratrol decreases the steady-state levels of PPARγ proteins in adipocytes. We and others previously demonstrated that activation of PPARγ is linked to the ubiquitin-proteasome dependent degradation of PPARγ (12; 16). Therefore, we asked if resveratrol-mediated decreases in PPARγ protein levels were also associated with direct targeting of PPARγ for ubiquitin conjugation and degradation by the proteasome. As shown in Figure 1A, a one hour pretreatment of the adipocytes with the 20S proteasome inhibitors MG132 and epoxomicin partially reverses the effect of either rosiglitazone or resveratrol, but does not return PPARγ protein levels to those observed under control conditions in the presence of proteasome inhibition. The observed decreases in PPARγ protein levels are associated with increased targeting of PPARγ for ubiquitin-dependent degradation as shown by the increase in PPARγ-ubiquitin conjugate formation in the presence of resveratrol (Figure 1B). The incomplete restoration of PPARγ protein levels in the presence of proteasome inhibition indicates that increased targeting for ubiquitin-proteasome dependent degradation is insufficient to account for the resveratrol-mediated changes in PPARγ protein levels. As shown in Figure 1C, both resveratrol and rosiglitazone also decrease Pparg gene expression.
Figure 1. Resveratrol decreases PPARγ gene and protein expression in 3T3-L1 adipocytes.
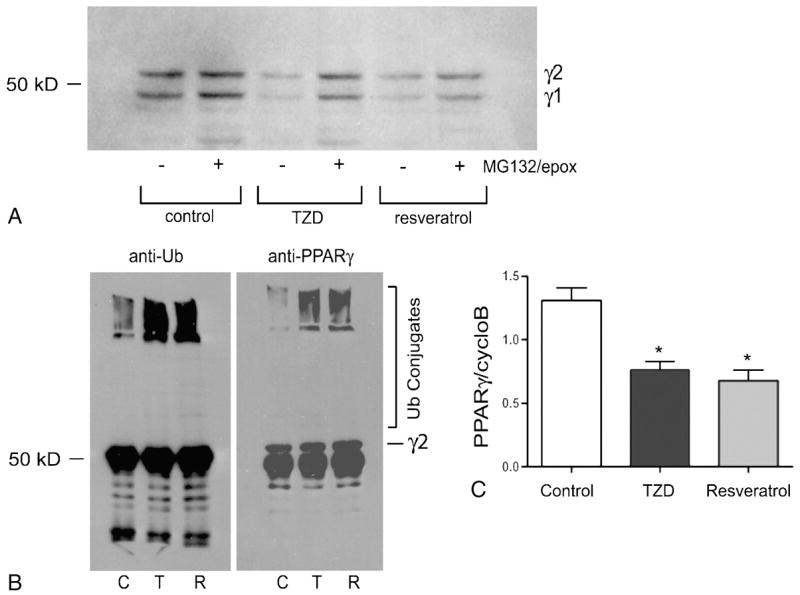
Fully differentiated 3T3-L1 adipocytes were incubated for 6 hours under control (DMSO only) conditions or in the presence of rosiglitazone (5 μM, TZD) or resveratrol (50 μM). (A) The adipocytes were pretreated for 1 hour in the presence (+) or absence (−) of MG132 (10 μM) and epoxomicin (1 μM) as indicated. Whole cell extracts were harvested and separated by SDS-PAGE followed by western blot analysis. (B) The adipocytes were preincubated with 10 μM MG132 and 1 μM epoxomicin for 1 hour prior to adding 50 μM resveratrol (R), 5.0 μM rosiglitazone (T) or an equal volume of DMSO as a vehicle control (C). Whole cell extracts were harvested after 30 minutes and subjected to immunoprecipitation using a polyclonal anti-PPARγ antibody. The immunoprecipitated proteins were analyzed by western blotting using anti-ubiquitin and anti-PPARγ antibodies as indicated. (C) Total RNA was purified and real-time RT-PCR was carried using TaqMan chemistry (Applied Biosystems). PPARγ expression is reported as the ratio of PPARγ expression to cyclophilin B gene expression. Each experiment was carried out in triplicate and reported as the average −/+ standard deviation. Statistical significance was determined using an unpaired Student’s t test. *P<0.05 compared to control.
Resveratrol treatment represses expression of endogenous genes regulated by PPARγ activity
The parallel effects of resveratrol and rosiglitazone on PPARγ protein degradation and gene expression prompted us to ask if resveratrol affects PPARγ transcriptional activity. To determine if resveratrol affects PPARγ activity, we used real-time RT-PCR to assay expression of PPARγ target genes in fully differentiated adipocytes rather than a luciferase-based assay of PPARγ transactivation. Previous studies have shown that resveratrol activates PPARγ in a variety of tissues (17–19), including macrophages (17) when measured using transactivation reporter assays. However, studies of other transcription factors such as Elk-1 and c-Fos (20) or control of T cell receptor β expression (21) have demonstrated discrepancies between luciferase-based transactivation assays and regulation of endogenous targets of transcription factor activity. These studies point to the pitfalls of measuring gene expression outside the usual chromatin structure as is the case with luciferase reporter assays. This consideration is particularly relevant since resveratrol action involves activation of SIRT1, a histone deacetylase. To circumvent this problem, we chose a small set of genes in adipocytes that are involved in lipid metabolism and insulin sensitivity and whose expression is well-described as PPARγ dependent: lipoprotein lipase (Lpl) (22; 23), the fatty acid binding protein (aP2) (24), and the cytosolic phosphoenolpyruvate carboxykinase (Pepck) (25–27). We assayed gene expression under control conditions or in the presence of resveratrol or rosiglitazone (TZD) for six or fifteen hours. As shown in Figure 2A, a six hour treatment with resveratrol represses aP2 and Lpl expression without affecting Pepck expression. Rosiglitazone (TZD) activation of PPARγ corresponds to increased expression of aP2 and Pepck in both cases with no increase in Lpl expression. However, a fifteen hour treatment (Figure 2B) with resveratrol shows repression of all three PPARγ target genes while rosiglitazone treatment continues to be associated with increased expression of aP2 and Pepck.
Figure 2. Resveratrol regulates PPARγ transcriptional activity.
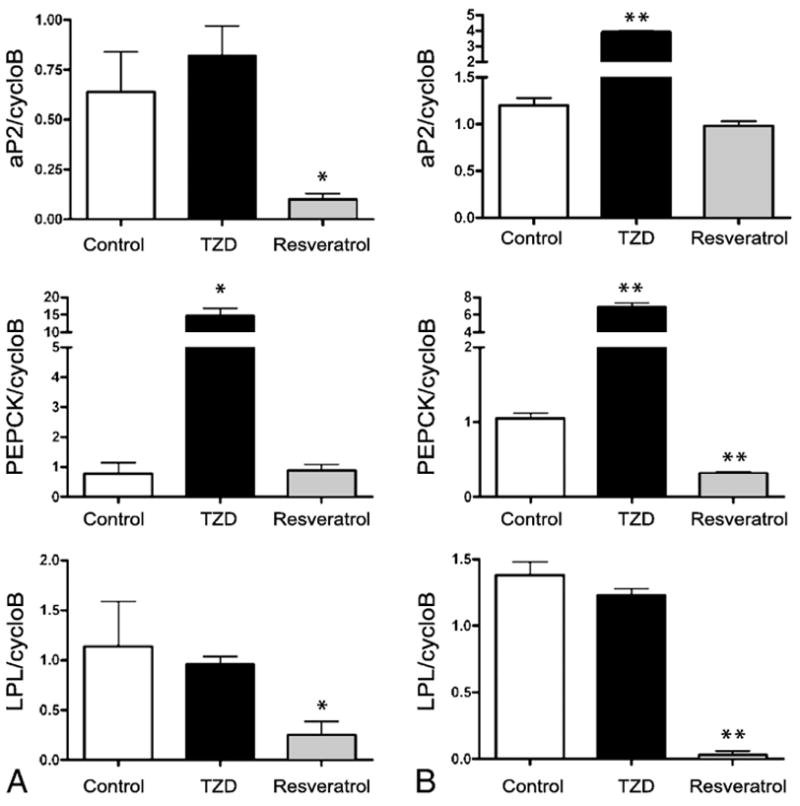
Fully differentiated 3T3-L1 adipocytes were incubated for (A) 6 hours or (B) 15 hours under control (DMSO) conditions or in the presence of rosiglitazone (5 μM) or resveratrol (50 μM). Total RNA was purified and real-time-PCR was carried using TaqMan chemistry. The levels of aP2, Pepck, and Lpl were calculated as the ratio of the gene to cyclophilin B expression. Each experiment was carried out in triplicate and reported as the average −/+ standard deviation. Statistical significance was determined using an unpaired Student’s t test. *P<0.05, ** P<0.005 (compared to control)
Resveratrol decreases insulin-dependent glucose uptake in adipocytes
Modulation of PPARγ activity and gene expression is associated with improved insulin sensitivity when assayed as plasma glucose and insulin levels (7–9). In addition, resveratrol treatment is associated with improved insulin sensitivity in murine models (1; 2). To determine if resveratrol affects insulin sensitivity in adipocytes, we measured the effect of resveratrol on glucose transport. The results in Figure 3A demonstrate that resveratrol decreases insulin-stimulated glucose uptake in adipocytes when compared to control or TZD-treated conditions. In addition to triglyceride synthesis, insulin-stimulated glucose uptake in adipocytes leads to an increase in glycogen content via stimulation of glycogen synthase activity (28; 29). Therefore, we assayed the glycogen content of the 3T3-L1 adipocytes under control, rosiglitazone, or resveratrol-treated conditions in the absence or presence of insulin. As shown in Figure 3B, resveratrol treatment is associated with decreased glycogen content under basal and insulin stimulated conditions. This is consistent with the observed resveratrol-mediated decreases in glucose uptake, indicating resveratrol reduces insulin sensitivity in adipocytes.
Figure 3. Resveratrol decreases glucose uptake and glycogen content in adipocytes.
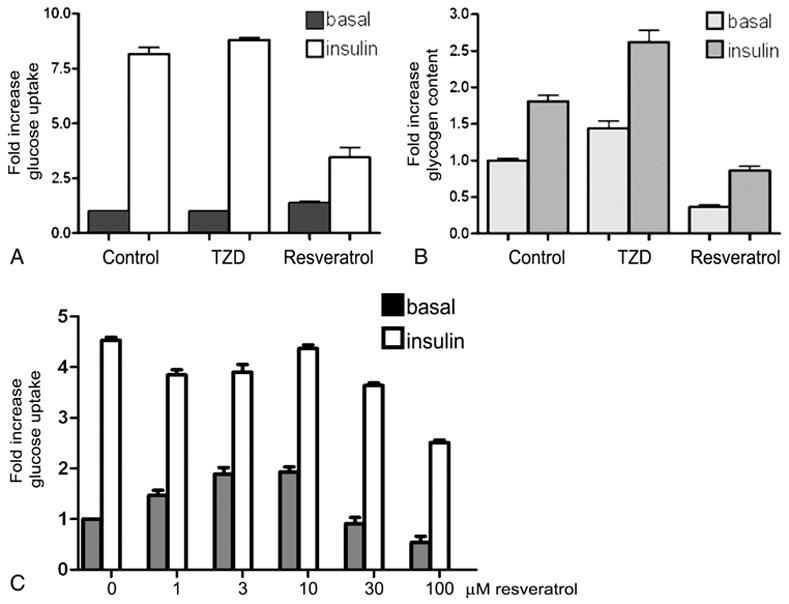
Fully differentiated adipocytes were treated with DMSO (control), rosiglitazone (5 μM) or resveratrol (50 μM) for 15 hours and serum-deprived for 2 hours prior to adding vehicle control (10 mM HCl) (■, basal) or 100 nM insulin (□, insulin). (A) Glucose uptake and (B) Glycogen content were measured as described in Materials and Methods. (C) Glucose uptake was measured in the 3T3-L1 adipocytes after treatment with the indicated concentration of resveratrol for 15 hours. Glucose uptake and glycogen content are reported as fold increase over basal control levels.
In our experiments, we used resveratrol at a concentration previously shown to affect lipid accumulation in 3T3-L1 adipocytes (3). In addition, resveratrol at 50 μM has been shown to increase SIRT1 activity 3–4 fold (4). To determine if the observed resveratrol-mediated decreases in insulin sensitivity were dose-related, we carried out glucose uptake assays in the presence of increasing concentrations of resveratrol (Figure 3C). Insulin-stimulated glucose uptake is unaffected by treatment with resveratrol at 1–10 μM although basal levels of glucose uptake trend upward. Insulin-stimulated glucose uptake is inhibited in the presence of higher concentrations (30–100 μM) of resveratrol.
Changes in insulin signaling pathway components in the presence of resveratrol
The resveratrol-mediated decreased glucose uptake suggests resveratrol affects components of the insulin signaling pathway in adipocytes. As shown in Figure 4, we assessed the content of proteins involved in the insulin signaling pathway. Resveratrol treatment decreased the basal levels of IRS-1 and the phosphorylated form of protein kinase B (AKT-P), and resulted in a small increase in insulin-stimulated AKT-P (Figure 4A). In addition, total GLUT4 levels were decreased with resveratrol treatment (Figure 4B). The observed changes in insulin sensitivity in the presence of resveratrol are consistent with decreased IRS-1 and GLUT4 protein levels, although contrary to the slight increase in insulin-stimulated AKT phosphorylation.
Figure 4. Resveratrol-mediated changes in insulin signaling components in 3T3-L1 adipocytes.
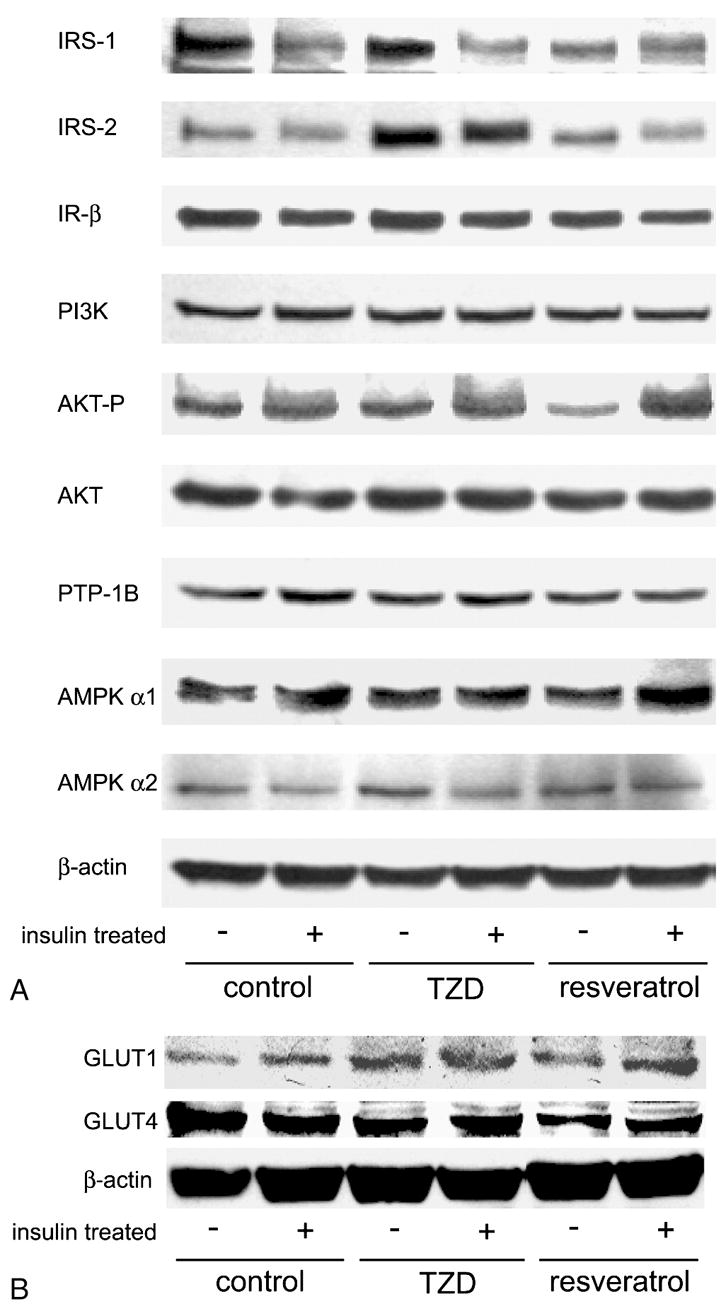
Fully differentiated adipocytes were incubated in the presence of 5 μM rosiglitazone (TZD), 50 μM resveratrol or DMSO (control) for 14 hours in the absence or presence of 400 nM insulin. Whole cell lysates were harvested and analyzed by SDS-PAGE followed by detection using chemiluminence (Pierce). β-actin is included as a loading control.
DISCUSSION
Since PPARγ was identified as the functional receptor for the thiazolidinedione (TZD) class of insulin sensitizing drugs (30), efforts to improve treatment of type 2 diabetes have included understanding the regulation of PPARγ in adipocytes. Although activation of PPARγ by the TZDs increases insulin sensitivity, studies of mice heterozygous for PPARγ show that reduced gene expression of wild-type PPARγ also improves insulin sensitivity (7; 9). The improvement in insulin sensitivity was observed with aging (8) and may include resistance to changes in insulin sensitivity that accompany a high fat diet (7; 8). This is in contrast to the effect of a dominant negative PPARγ mutation (P465L) in leptin deficient mice (P465L/ob) that results in insulin resistance in a setting of positive energy balance (31). The wild-type PPARγ −/+ genetic model indicates modulation of wild-type PPARγ expression could offer an alternative approach in the treatment of type 2 diabetes (32).
The present studies demonstrate that PPARγ transcriptional activity and protein levels are modulated in adipocytes by resveratrol, a bioactive plant polyphenol. The decreased levels of PPARγ proteins in 3T3-L1 adipocytes in response to resveratrol are mediated by decreased Pparg gene expression coupled with increased ubiquitin-proteasome-dependent degradation of PPARγ proteins, paralleling the effect of TZDs. Earlier evidence indicated PPARγ is targeted for destruction via the ubiquitin-proteasome system under basal or activated conditions, (14; 16; 33), supporting a model in which PPARγ degradation serves to limit PPARγ transcriptional activity. Therefore, downregulation of PPARγ in the presence of resveratrol describes a novel mechanism of action for resveratrol that consistent with the overall scheme of limiting PPARγ activity via ubiquitin-proteasome dependent degradation.
Resveratrol-mediated reductions in PPARγ gene expression and PPARγ proteins correlate with decreased cellular effects of insulin and insulin signaling proteins in adipocytes as assayed by decreases in protein content for IRS-1 and GLUT4 in addition to glucose uptake and glycogen content. Assessment of functional aspects of GLUT4 properties such as translocation to the plasma membrane was outside the scope of the current study. Although resveratrol treatment (2) and reduction in PPARγ expression (7–9) are associated with generally improved insulin sensitivity in murine models, the observed effect of resveratrol in adipocytes is consistent with studies on longevity demonstrating calorie restriction is associated with inhibition of insulin signaling (34). In particular, selective loss of the insulin receptor in murine adipocytes (FIRCO mice) protects against developing age and obesity-related insulin resistance (35). The reduction in insulin sensitivity in adipocytes in response to resveratrol may mimic the overall insulin sensitizing effects of calorie restriction, where the predominant feature is a loss of fat mass as a result of decreased lipid storage and increased lipolysis in adipocytes (36). Clearly, any generalized improvement in insulin sensitivity coincident with decreased glucose uptake in adipocytes will involve the interaction of adipocytes with other tissues that are glucose responsive, such as skeletal muscle and the liver as well as independent effects of resveratrol on those tissues (1; 2). While the current studies are not designed to determine if the resveratrol-mediated changes in insulin sensitivity are due to down-regulation of PPARγ activity and expression in adipocytes, our results are consistent with a recent finding from Liao et al (37) showing direct attenuation of PPARγ expression decreases glucose uptake in 3T3-L1 adipocytes. Thus, resveratrol may serve as a pharmacological tool to explore the effects of reducing PPARγ protein and gene expression in adipocytes.
Acknowledgments
This work in funded in part by NIH P50AT002776-01 from the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements and by the National Institute on Aging R03 AG025751 (to Z.E.F.). We thank Dr. Jeffrey Gimble for helpful discussions and critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1[alpha] Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006 doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 5.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medina-Gomez G, Virtue S, Lelliott C, Boiani R, Campbell M, Christodoulides C, Perrin C, Jimenez-Linan M, Blount M, Dixon J, Zahn D, Thresher RR, Aparicio S, Carlton M, Colledge WH, Kettunen MI, Seppanen-Laakso T, Sethi JK, O’Rahilly S, Brindle K, Cinti S, Oresic M, Burcelin R, Vidal-Puig A. The link between nutritional status and insulin sensitivity is dependent on the adipocyte-specific peroxisome proliferator-activated receptor-gamma2 isoform. Diabetes. 2005;54:1706–1716. doi: 10.2337/diabetes.54.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Kadowaki T, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 8.Miles PD, Barak Y, Evans RM, Olefsky JM. Effect of heterozygous PPARgamma deficiency and TZD treatment on insulin resistance associated with age and high-fat feeding. Am J Physiol Endocrinol Metab. 2003;284:E618–626. doi: 10.1152/ajpendo.00312.2002. [DOI] [PubMed] [Google Scholar]
- 9.Miles PD, Barak Y, He W, Evans RM, Olefsky JM. Improved insulin-sensitivity in mice heterozygous for PPAR-gamma deficiency. J Clin Invest. 2000;105:287–292. doi: 10.1172/JCI8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rieusset J, Seydoux J, Anghel SI, Escher P, Michalik L, Soon Tan N, Metzger D, Chambon P, Wahli W, Desvergne B. Altered growth in male peroxisome proliferator-activated receptor gamma (PPARgamma) heterozygous mice: involvement of PPARgamma in a negative feedback regulation of growth hormone action. Mol Endocrinol. 2004;18:2363–2377. doi: 10.1210/me.2003-0325. [DOI] [PubMed] [Google Scholar]
- 11.Stewart WC, Baugh JE, Jr, Floyd ZE, Stephens JM. STAT 5 activators can replace the requirement of FBS in the adipogenesis of 3T3-L1 cells. Biochem Biophys Res Commun. 2004;324:355–359. doi: 10.1016/j.bbrc.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 12.Floyd ZE, Stephens JM. Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem. 2002;277:4062–4068. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Waite KJ, Floyd ZE, Arbour-Reily P, Stephens JM. Interferon-gamma-induced regulation of peroxisome proliferator-activated receptor gamma and STATs in adipocytes. J Biol Chem. 2001;276:7062–7068. doi: 10.1074/jbc.M007894200. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Lechon MJ, Ponsoda X, Castell JV. A microassay for measuring glycogen in 96-well-cultured cells. Anal Biochem. 1996;236:296–301. doi: 10.1006/abio.1996.0170. [DOI] [PubMed] [Google Scholar]
- 16.Hauser S, Adelmant G, Sarraf P, Wright HM, Mueller E, Spiegelman BM. Degradation of the peroxisome proliferator-activated receptor gamma is linked to ligand-dependent activation. J Biol Chem. 2000;275:18527–18533. doi: 10.1074/jbc.M001297200. [DOI] [PubMed] [Google Scholar]
- 17.Ge H, Zhang JF, Guo BS, He Q, Wang BY, He B, Wang CQ. Resveratrol inhibits macrophage expression of EMMPRIN by activating PPARgamma. Vascul Pharmacol. 2007;46:114–121. doi: 10.1016/j.vph.2006.08.412. [DOI] [PubMed] [Google Scholar]
- 18.Inoue H, Jiang XF, Katayama T, Osada S, Umesono K, Namura S. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor alpha in mice. Neurosci Lett. 2003;352:203–206. doi: 10.1016/j.neulet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Ulrich S, Loitsch SM, Rau O, von Knethen A, Brune B, Schubert-Zsilavecz M, Stein JM. Peroxisome proliferator-activated receptor gamma as a molecular target of resveratrol-induced modulation of polyamine metabolism. Cancer Res. 2006;66:7348–7354. doi: 10.1158/0008-5472.CAN-05-2777. [DOI] [PubMed] [Google Scholar]
- 20.Yan B, Wang H, Kon T, Li CY. Pim-1 kinase inhibits the activation of reporter gene expression in Elk-1 and c-Fos reporting systems but not the endogenous gene expression: an artifact of the reporter gene assay by transient co-transfection. Braz J Med Biol Res. 2006;39:169–176. doi: 10.1590/s0100-879x2006000200002. [DOI] [PubMed] [Google Scholar]
- 21.Chattopadhyay S, Whitehurst CE, Chen J. A nuclear matrix attachment region upstream of the T cell receptor beta gene enhancer binds Cux/CDP and SATB1 and modulates enhancer-dependent reporter gene expression but not endogenous gene expression. J Biol Chem. 1998;273:29838–29846. doi: 10.1074/jbc.273.45.29838. [DOI] [PubMed] [Google Scholar]
- 22.Schoonjans K, Staels B, Auwerx J. The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta. 1996;1302:93–109. doi: 10.1016/0005-2760(96)00066-5. [DOI] [PubMed] [Google Scholar]
- 23.Hua XX, Enerback S, Hudson J, Youkhana K, Gimble JM. Cloning and characterization of the promoter of the murine lipoprotein lipase-encoding gene: structural and functional analysis. Gene. 1991;107:247–258. doi: 10.1016/0378-1119(91)90325-6. [DOI] [PubMed] [Google Scholar]
- 24.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 25.Devine JH, Eubank DW, Clouthier DE, Tontonoz P, Spiegelman BM, Hammer RE, Beale EG. Adipose Expression of the Phosphoenolpyruvate Carboxykinase Promoter Requires Peroxisome Proliferator-activated Receptor gamma and 9-cis-Retinoic Acid Receptor Binding to an Adipocyte-specific Enhancer in Vivo. J Biol Chem. 1999;274:13604–13612. doi: 10.1074/jbc.274.19.13604. [DOI] [PubMed] [Google Scholar]
- 26.Tontonoz P, Hu E, Devine J, Beale EG, Spiegelman BM. PPAR gamma 2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1995;15:351–357. doi: 10.1128/mcb.15.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tordjman J, Chauvet G, Quette J, Beale EG, Forest C, Antoine B. Thiazolidinediones Block Fatty Acid Release by Inducing Glyceroneogenesis in Fat Cells. J Biol Chem. 2003;278:18785–18790. doi: 10.1074/jbc.M206999200. [DOI] [PubMed] [Google Scholar]
- 28.Brady MJ, Bourbonais FJ, Saltiel AR. The activation of glycogen synthase by insulin switches from kinase inhibition to phosphatase activation during adipogenesis in 3T3-L1 cells. J Biol Chem. 1998;273:14063–14066. doi: 10.1074/jbc.273.23.14063. [DOI] [PubMed] [Google Scholar]
- 29.Jensen TC, Crosson SM, Kartha PM, Brady MJ. Specific desensitization of glycogen synthase activation by insulin in 3T3-L1 adipocytes. Connection between enzymatic activation and subcellular localization. J Biol Chem. 2000;275:40148–40154. doi: 10.1074/jbc.M004902200. [DOI] [PubMed] [Google Scholar]
- 30.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 31.Gray SL, Nora ED, Grosse J, Manieri M, Stoeger T, Medina-Gomez G, Burling K, Wattler S, Russ A, Yeo GS, Chatterjee VK, O’Rahilly S, Voshol PJ, Cinti S, Vidal-Puig A. Leptin deficiency unmasks the deleterious effects of impaired peroxisome proliferator-activated receptor gamma function (P465L PPARgamma) in mice. Diabetes. 2006;55:2669–2677. doi: 10.2337/db06-0389. [DOI] [PubMed] [Google Scholar]
- 32.Larsen TM, Toubro S, Astrup A. PPARgamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? Int J Obes Relat Metab Disord. 2003;27:147–161. doi: 10.1038/sj.ijo.802223. [DOI] [PubMed] [Google Scholar]
- 33.Floyd ZE, Stephens JM. Control of peroxisome proliferator-activated receptor gamma2 stability and activity by SUMOylation. Obes Res. 2004;12:921–928. doi: 10.1038/oby.2004.112. [DOI] [PubMed] [Google Scholar]
- 34.Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313–321. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- 35.Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 36.Barzilai N, Gabriely I. The role of fat depletion in the biological benefits of caloric restriction. J Nutr. 2001;131:903S–906S. doi: 10.1093/jn/131.3.903S. [DOI] [PubMed] [Google Scholar]
- 37.Liao W, Nguyen MT, Yoshizaki T, Favelyukis S, Patsouris D, Imamura T, Verma IM, Olefsky JM. Suppression of PPAR{gamma} Attenuates Insulin-Stimulated Glucose Uptake by Affecting Both GLUT1 and GLUT4 in 3T3-L1 Adipocytes. Am J Physiol Endocrinol Metab. 2007 doi: 10.1152/ajpendo.00695.2006. [DOI] [PubMed] [Google Scholar]


