Abstract
Neuroimaging techniques have lead to significant advances in our understanding of the neurobiology and treatment of drug addiction in humans. The capability to conduct parallel studies in nonhuman primates and human subjects provides a powerful translational approach to link findings in human and animal research. A significant advantage of nonhuman primate models is the ability to use drug-naïve subjects in longitudinal designs that document the neurobiological changes that are associated with chronic drug use. Moreover, experimental therapeutics can be evaluated in subjects with well-documented histories of drug exposure. The in vivo distribution and pharmacokinetics of drug binding in brain have been related to the time-course of behavioral effects associated with the addictive properties of stimulants. Importantly, the characterization of drug interactions with specific protein targets in brain has identified potential targets for medications development. Neuroimaging has proven especially useful in studying the dynamic changes in neuronal function that may be associated with environmental variables. Lastly, neuroimaging has been used effectively in nonhuman primates to characterize both transient and long-lasting changes in brain chemistry associated with chronic drug exposure. Although there is some evidence to suggest neurotoxicity in humans with long histories of stimulant use, parallel studies in nonhuman primates have not identified consistent long-term changes in such neurochemical markers. Collectively, the results of these studies of nonhuman primates have enhanced our understanding of the neurobiological basis of stimulant addiction and should have a significant impact on efforts to develop medications to treat stimulant abuse.
Keywords: Nonhuman Primates, Neuroimaging, Stimulants, PET, SPECT, fMRI, Dopamine, Serotonin
Introduction
Non-invasive neuroimaging techniques have lead to significant advances in our current understanding of the neurobiology and treatment of drug addiction in humans. Nuclear imaging with positron emission tomography (PET) and single photon emission computed tomography (SPECT) has defined the pharmacokinetics of abused stimulants in vivo in the human brain and related these findings to the time-course of behavioral effects associated with their addictive properties. With support from work that has provided new radiotracers and enhanced the resolution of imaging systems, nuclear medicine techniques have also been employed to characterize drug interactions in vivo with specific protein targets in brain, including neurotransmitter receptors and transporters. Moreover, documentation of the long-term neurobiological consequences of chronic drug use and potential neurotoxicity has lead to novel insights regarding of the pathology and treatment of stimulant addiction. Lastly, recent advances in functional magnetic resonance imaging (fMRI) have begun to localize brain circuits implicated in the acute effects of stimulants and with drug-associated environmental stimuli, providing enhanced temporal and spatial resolution. The results of theses studies have markedly enhanced our understanding of the neurobiological bases of stimulant addiction and should have a significant impact on future medications development.
The capability to conduct parallel neuroimaging studies in nonhuman primates and human subjects provides a powerful translational approach that can link findings from human and laboratory animal research. A significant advantage of nonhuman primate models is the use of initially drug naïve subjects in longitudinal designs to characterize within-subject changes in aspects of the neurobiology associated with chronic drug use. Moreover, experimental drugs under investigation can be evaluated in subjects with well-documented drug histories. As with all animal models, enhanced experimental control is a noted advantage over the necessary restrictions imposed in human clinical research. Nonhuman primates also offer several distinct advantages over other laboratory animal species. Their longevity is an important consideration. Nonhuman primates provide unique relevance to understanding the neurochemical basis of substance abuse in humans. Species differences in the complex topographical organization of the ventral striatum and its connections with surrounding areas, for example, complicate extrapolations from rodents to primates.1,2,3 Neuroimaging studies have documented that the nonhuman primate brain differs markedly from the rodent brain in the cerebral metabolic response to cocaine.4,5 Compared to rodents, nonhuman primates are more similar to humans in the pharmacokinetics and metabolism of several drug classes including 3,4-methylenedioxymethamphetamine (see6 for review).7 Lastly, nonhuman primates exhibit complex social behaviors that provide unique opportunities for examining the influence of social variables on the abuse-related effects of drugs (see 8,9 for reviews).10 Collectively, theses important species differences illustrate the importance of nonhuman primate models in neuroimaging and in substance abuse research.
With few exceptions, neuroimaging studies in nonhuman primates have employed PET, SPECT, or fMRI. Accordingly, these techniques will be the focus of the current review. In PET imaging, ligands of interest are radiolabeled with unstable atomic isotopes that emit positrons (see11,12 for basic description). When positrons collide with electrons, dual photons are emitted that can be recognized by detector arrays in the tomograph. A computer algorithm then uses this information to map the source and concentration of the radiotracer. SPECT imaging uses different radiotracers that emit a single photon. Due to methodological differences in single versus dual photon detection, SPECT imaging has lower sensitivity and resolution compared to PET imaging. Numerous radiotracers have been developed for use in PET and SPECT imaging that enable the in vivo measurement of brain neurochemistry and physiology. Development of radiotracers involves the conversion of a single atom to a heavy isotope. Accordingly, the chemical structures can be negligibly changed in ways that are unlikely to substantially alter the pharmacological properties of the labeled tracer. Since radiotracers can be used to label compounds without influencing dramatically their pharmacology, functional imaging can accurately measure drug distribution and pharmacokinetics in brain. In contrast to PET and SPECT imaging, fMRI does not require the use of radiotracers. Instead, the subject is placed in a homogeneous magnetic field where presentations of radiofrequency pulses cause transient energy changes (see11.13 for basic description). The blood oxygenation level dependent “BOLD” technique at the center of all of the fRMI studies reviewed here relies on changes in signal intensity associated with hemodynamic responses as an indirect measure of neuronal activity. Among brain imaging techniques, fMRI provides the highest spatial resolution for mapping brain activity. Each of these techniques has been used effectively in nonhuman primates to enhance our understanding of the neurobiology of stimulant addiction.
Acute Neuropharmacology of Psychostimulants
Drug Distribution and Pharmacokinetics
Some of the earliest studies to use functional neuroimaging in nonhuman primates focused on the distribution of cocaine binding in brain.14 Experiments were conducted in anesthetized baboons using PET imaging with C-11 labeled cocaine. Cocaine binding was heterogeneous with the highest concentration in the striatum. Binding in striatum was inhibited by pretreatments with pharmacological doses of cocaine and dopamine transporter (DAT) inhibitors but not by norepinephrine transporter (NET) or serotonin transporter (SERT) inhibitors. Direct comparisons in human subjects showed a similar distribution of binding with the highest concentration in the striatum followed by the thalamus, cingulate gyrus, temporal cortex, and frontal and occipital cortices. Subsequent studies documented significant overlaps in the distributions of binding of C-11 labeled cocaine and methylphenidate; both drugs were effective in inhibiting the binding of the other in the striatum.15 A remarkable direct relationship was established between self-reports of “high” induced by cocaine and the time course of striatal uptake.16 A more recent study compared the pharmacokinetics of methamphetamine to those of cocaine in brain.11 Experiments were conducted in anesthetized baboons using PET imaging and C-11 labeled d-methamphetamine, l-methamphetamine and (-) cocaine. Both d- and l-methamphetamine showed high uptake and widespread distribution in brain, with pharmacokinetics that did not differ appreciably between the enantiomers. Therefore, brain pharmacokinetics are unlikely to account for the more intense stimulant effects of d-methamphetamine reported in humans. Direct comparisons between d-methamphetamine and (-) cocaine in the same subjects showed that the slower clearance of methamphetamine is likely to contribute to its longer lasting stimulant effects relative to those of cocaine. Finally, an elegant study in pregnant nonhuman primates compared the pharmacokinetics of cocaine in maternal and fetal brains.17 Experiments were conducted in anesthetized bonnet macaques during the third-trimester of their pregnancies using PET imaging and C-11 labeled cocaine. Although the uptake of radioactivity into the fetal brain was lower and slower compared to that identified in the maternal brain, a measurable quantity of cocaine or labeled metabolites did accumulate in the fetal brain shortly after drug injection. These results add to our understanding of the risks associated with in utero exposure to cocaine, and clearly illustrate the unique advantages of nonhuman primate models in neuroimaging and substance abuse research.
Brain Activation
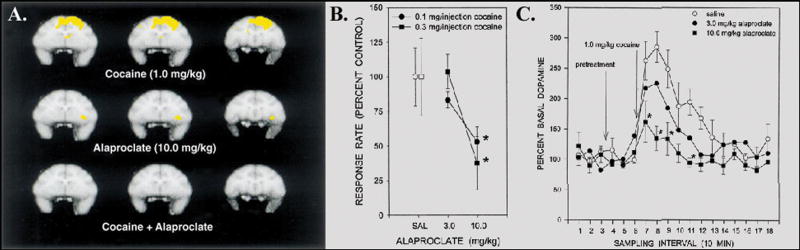
The noninvasive measurement of cerebral blood flow with PET neuroimaging and O-15 water provides a useful functional measure to characterize acute drug-induced changes in brain activity. Functional changes in cerebral blood flow using PET imaging were determined in conscious, drug-naïve rhesus monkeys following acute intravenous administration of cocaine.18,19 Use of unanesthetized subjects allowed us to eliminate potential confounding effects of anesthetics on measures of cerebral blood flow. Cocaine displayed significant dose-related effects on cerebral blood flow at 5 minutes post-injection; these effects diminished markedly by 15 minutes. Brain activation maps normalized to global flow showed prominent cocaine-induced activation of prefrontal cortex, especially dorsolaterally. These brain activation effects were blocked by pretreatment with the selective SERT inhibitor, alaproclate. Importantly, the same dose of alaproclate that blocked cocaine-induced brain activation was also effective in attenuating cocaine self-administration and cocaine-induced elevations of extracellular dopamine in squirrel monkeys.20 Hence, there was close concordance among in vivo measures of behavior, neurochemistry and functional imaging (Figure 1). More recently, fMRI has been used to characterize stimulant-induced changes in brain activity. In one study, experiments in anesthetized cynomolgus monkeys used an iron oxide nanoparticle (IRON) technique to measure changes in relative cerebral blood volume (rCBV) following acute intravenous administration of amphetamine.21 Amphetamine caused marked changes in rCBV in areas with high dopamine receptor density as well as associated circuitry. The largest increases in rCBV were observed in the parafascicular thalamus, nucleus accumbens, putamen, caudate, substantia nigra and ventral tegmental area. In a separate series of studies, experiments were conducted in conscious marmoset monkeys using BOLD techniques to characterize changes in brain activity following acute oral administration of the abused amphetamine congener MDMA.22,23 MDMA caused significant activation of the midbrain raphe nuclei and substania nigra, which represent major sources of forebrain serotonin and dopamine, respectively, as well as the hippocampus, hypothalamus and amygdala. Interestingly, there was little activation of the nucleus accumbens and prefrontal cortex, two important components of mesolimbic and mesocortical circuitry, respectively, or of other cortical areas. Collectively, the results indicate that stimulants with varied mechanisms of action may each induce a unique profile of effects on brain activity. Understanding these unique profiles can help us to better understand the neural circuits that underlie drug effects on behavior.
Figure 1.
All data were obtained from unanesthetized nonhuman primates. A. PET cerebral blood flow images in coronal orientation from a group of four rhesus monkeys showing increases in dorsolateral prefrontal areas in response to non-contingent i.v. administration of 1.0 mg/kg of cocaine (top), absence of blood flow effects in response to the serotonin reuptake inhibitor, alaproclate, administered alone (middle), and complete blockade of cocaine-induced increases in cerebral blood flow when alaproclate was administered prior to cocaine (bottom). B. Rate of responding maintained under a schedule of i.v. cocaine self-administration in a group of four squirrel monkeys. Data show responding maintained by two different unit doses of cocaine (0.1 and 0.3 mg/kg) during sessions where subjects were pretreated with saline vehicle or alaproclate (3 or 10 mg/kg). Rate of responding was dose-dependently decreased by pretreatment with alaproclate. Abscissa: Dose of alaproclate pretreatment. Ordinate: Rate of responding expressed as a percentage of control. C. Time course of cocaine-induced increases in extracellular levels of dopamine in the caudate nucleus following pretreatment with saline vehicle or alaproclate in a group of three squirrel monkeys. Alaproclate dose-dependently inhibited cocaine-induced increases in extracellular dopamine. Abscissae: Time in 10-minute sampling intervals. Ordinates: Extracellular levels of dopamine expressed as a percentage of baseline. Note the consistency of effects across three different experimental assays. The selective SERT inhibitor effectively attenuated cocaine-induced brain activation and elevations in extracellular dopamine, and cocaine self-administration.
Panel A is reproduced from Howell et al., 2002.19 Panels B and C are reproduced from Czoty et al., 2002.20
The acute effects of stimulants on cerebral blood flow and metabolism have been examined in human subjects, often those seeking to define neuronal bases for drug-induced euphoria. Acute intravenous administration of cocaine in human users resulted in significant blood flow decreases in selected frontal and basal ganglia regions, as measured by SPECT imaging.24,25,26 Cocaine-induced euphoria following acute intravenous administration was also associated with regional decreases in cerebral metabolism as measured by F-18 labeled fluorodeoxyglucose (FDG) PET imaging.27 Intravenous administration of methylphenidate in normal subjects caused variable changes in brain metabolism.28 Subjects with higher dopamine D2 receptor availability tended to show increased metabolism whereas those with lower D2 availability tended to show decreased metabolism. Similar results were observed in cocaine users, in whom methylphenidate-induced increases in metabolism in the right orbitofrontal cortex and right striatum were associated with drug craving.29 Other investigators have reported that acute cocaine administration increases cerebral blood flow mainly in the frontal and parietal regions.30 A BOLD fMRI study in cocaine-dependent subjects reported dynamic patterns of brain activation following intravenous cocaine administration.31 Some regions showed short duration actions that were correlated with ratings of “rush”. Other regions showed sustained activation associated with ratings of “craving”. Intravenous cocaine administration in cocaine users activated mesolimbic and mesocortical regions that receive dopaminergic afferents.32 This transient pattern of brain activation induced by cocaine in humans is consistent with the pattern that we have observed in conscious rhesus monkeys which are imaged 5 minutes post-injection.18,19 It is noteworthy that most studies have measured drug effects at time points up to 45 minutes post-injection; at these later times, cocaine induces decreased cerebral blood flow and metabolism in chronic cocaine users. Not surprisingly, the time at which measurements are taken provides a major determinant for results observed, especially when using cocaine, a rapid-acting, short-duration drug. Notwithstanding such differences in experimental procedures and outcomes, however, accumulated collective evidence allows us to summarize the ways in which acute drug effects on cerebral blood flow and metabolism now provide us with a tool to characterize the functional neuroanatomy that underlies the etiology of stimulant abuse (Table 1).
Table 1.
Acute effects of stimulants on cerebral metabolism, blood flow, blood volume, and blood oxygenation
| Decreases in cerebral metabolism | |||
| London et al. 1990 | Human polydrug abusers | PET, [18F]FDG | 40mg (IV) cocaine |
| Decreases in cerebral blood flow | |||
| Pearlson et al. 1993 | Human cocaine dependent | SPECT, technetium-99-m-exametazine | 48 mg (IV) cocaine |
| Wallace et al. 1996 | Human cocaine dependent | SPECT, technetium-99-m- exametazine | 40 mg (IV) cocaine |
| Johnson et al. 1998 | Human cocaine dependent | SPECT, technetium-99-m-bicisate | 0.325 and 0.650 mg/kg (IV) cocaine |
| Transient regional increases in cerebral blood flow, volume, or oxygenation | |||
| Mathew et al. 1996 | Human cocaine dependent | laser Doppler, 133 xenon | 0.3mg/kg (IV) cocaine |
| Breiter et al. 1997 | Human cocaine dependent | fMRI, BOLD | 0.6mg/kg (IV) cocaine |
| Howell et al. 2002 | Rhesus macaques | PET, (CBF), [15O]H20 | 0.3 and 1.0 mg/kg (IV) cocaine |
| Jenkins et al. 2004 | Cynomolgous macaques | fMRI, (CBV), MION | 2.5 mg/kg (IV) amphetamine |
| Kufahl et al. 2005 | Human polydrug abusers | fMRI, BOLD | 0.285 mg/kg (IV) cocaine |
| Sustained regional increases in cerebral blood oxygenation | |||
| Brevard et al. 2006 | Marmosets | fMRI, BOLD | 1.0 mg/kg (PO) MDMA |
Monoamine Transporter Occupancy
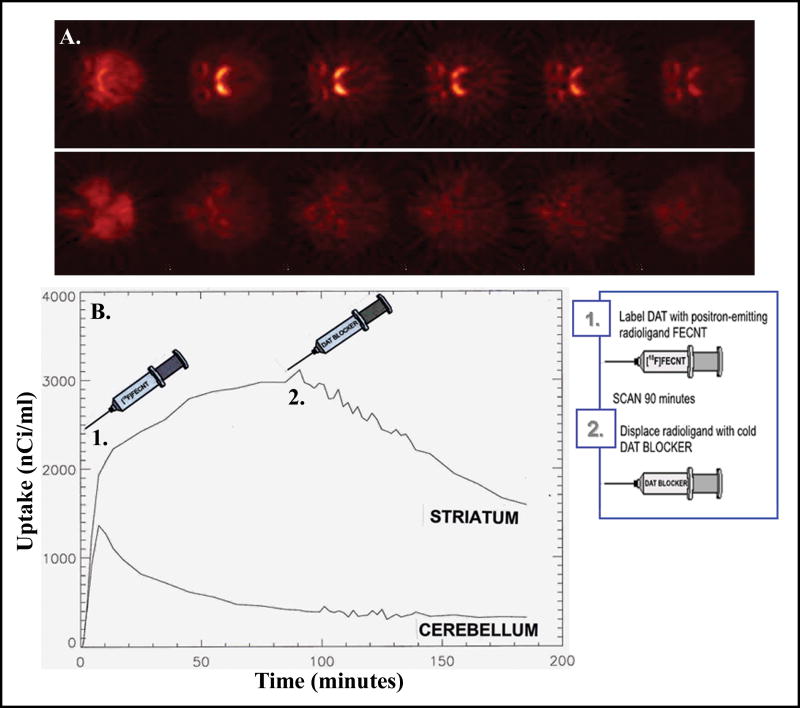
Neuroimaging has been used in nonhuman primates to study the relationship between drug occupancy at monoamine transporters on the one hand and the behavioral effects of cocaine and cocaine-like stimulants on the other (Figures 2 and 3). Studies were conducted in rhesus monkeys using PET imaging and F-18 labeled FECNT as a DAT selective radioligand. DAT occupancy by cocaine was determined by displacement of FECNT using a reference tissue method of kinetic modeling.33 The results documented that FECNT labels a cocaine-sensitive binding site, and that high levels of DAT occupancy are associated with behaviorally- active cocaine doses. In a related study, the reinforcing effects of cocaine were compared to those of its phenyltropane analog, RTI-113, in rhesus monkeys which were responding under a second-order schedule of intravenous drug self-administration.34 Both drugs reliably and equipotently maintained self-administration when DAT occupancies were 65-76% and 94-99% for optimal doses of cocaine and RTI-113, respectively. When administered as a pretreatment, RTI-113 dose-dependently reduced responding maintained by cocaine. DAT occupancies ranged between 72-84% for pretreatment doses that effectively suppressed cocaine self-administration. Similar results have been observed with the DAT-selective inhibitors RTI-177, a phenyltropane analog of cocaine, and GBR 12909, a phenylpiperazine derivative.35 Direct within-subject comparisons between drug effects on behavior and in vivo DAT occupancy measured with PET imaging were made in rhesus monkeys. At doses that decreased rates of cocaine self-administration by 50%, DAT occupancy was approximately 70% for both compounds. Doses of GBR 12909 that decreased cocaine self-administration in rhesus monkeys resulted in similar > 50% DAT occupancies in baboons, based on results from PET imaging using C-11 labeled WIN 35,428.36 Clearly, DAT inhibitors are effective in reducing cocaine self-administration. However, high levels of DAT occupancy may be required. RTI-112, a mixed-action inhibitor of DAT and SERT, did not exhibit levels of DAT occupancy above the threshold of detection at doses that significantly reduced cocaine self-administration in rhesus monkeys.35 This RTI-112 dose, however, did exhibit high levels of SERT occupancy, showing apparent in vivo selectivity for SERT over DAT at a behaviorally relevant dose. Co-administration of the selective SERT inhibitors fluoxetine or citalopram in combination with the selective DAT inhibitor RTI-336 produced more robust reductions in cocaine self-administration than RTI-336 alone, even at comparable levels of DAT occupancy.37 Hence, the effectiveness of DAT inhibitors to suppress cocaine self-administration may be enhanced by actions at other monoamine transporters. Combined inhibition of DAT and SERT warrants consideration as a viable approach in the development of cocaine medications.
Figure 2.
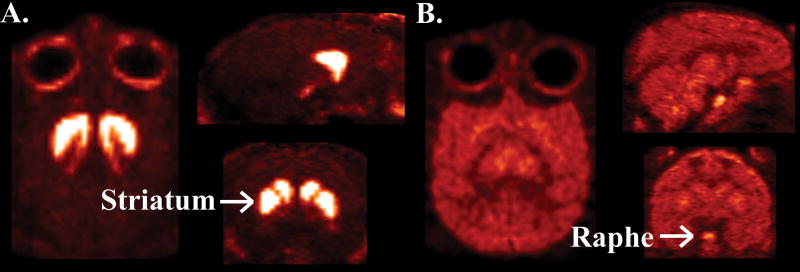
A. Representative images from a single rhesus monkey showing binding of the selective DAT radiotracer [18F]-FECNT. Images are displayed in transverse (left), sagittal (top right), and coronal (bottom right) orientations. All images are oriented in radiological convention. Binding is highly localized to striatal areas. B. Representative images from a single rhesus monkey showing binding of the selective SERT radiotracer [18F]-m-ZIENT. Images are displayed in transverse (left), sagittal (top right), and coronal (bottom right) orientations. All images are oriented in radiological convention. Binding is diffuse and extends through cortical and sub-cortical areas. However, the highest levels of binding are localized to the raphe nucleus.
Figure 3.
A. Representative images from a single rhesus monkey showing binding of the selective DAT radiotracer [18F]-FECNT at the level of the striatum (top row) or the cerebellum (bottom row). Images from left to right in each row represent increasing time over the course of the scan. Unlabelled cocaine was administered i.v. before the third image. Binding of [18F]-FECNT is rapidly and significantly reduced in the final three images. B. Representative time-activity curves from the striatum and cerebellum in the same subject depicted in panel A. Time points are identified for the administration of [18F]-FECNT (1) and cocaine (2). Abscissae: Time in minutes. Ordinates: Uptake within a given brain region in nanoCuries per milliliter.
A possible limitation to the use of selective DAT inhibitors for the treatment of cocaine addiction is their potential for abuse, given their well documented reinforcing effects.38 Phenyltropane analogs of cocaine reliably maintain self-administration in nonhuman primates,34,35,39,40,41,37 consistent with results reported for the phenylpiperazine DAT-selective blocker, GBR 12909.35,39,42 Local anesthetics that bind to the DAT and inhibit dopamine uptake are also self-administered by rhesus monkeys, with reinforcing potencies that correlate well with their in vitro affinities for DAT43 and their effectiveness in inhibiting dopamine uptake.45 More recently, the reinforcing effects of local anesthetics were compared to DAT occupancy in vivo and to drug-induced increases in dopamine in awake rhesus monkeys.45 The local anesthetics dimethocaine and procaine were selected based on their similar pharmacokinetic profiles. Substitution of dimethocaine for cocaine reliably maintained self-administration in all monkeys tested. In contrast, procaine maintained self-administration at a single dose in only one monkey. Interestingly, doses of dimethocaine that maintained maximum rates of responding produced DAT occupancy between 66-82%. These values correlate well with results of human PET imaging studies, which found that DAT occupancy by rewarding doses of cocaine was between 60-77%.16 They also correlate with PET imaging data in rhesus monkeys that reveal DAT occupancy between 65-76% following cocaine doses that maintain peak rates of responding.34 In contrast, peak doses of procaine were ineffective in maintaining self-administration and resulted in low levels of DAT occupancy, between 10-41%.45 When in vivo microdialysis was used in awake monkeys to evaluate elevations in extracellular dopamine at the maximum reinforcing doses of cocaine, dimethocaine and procaine, administration of either cocaine or dimethocaine produced robust increases in extracellular dopamine, whereas procaine was relatively ineffective. Hence, the reinforcing effects of DAT inhibitors from several distinct chemical classes were closely related to DAT occupancy and to drug-induced increases in extracellular dopamine.
It is important to note, however, that several selective DAT inhibitors from some of these same chemical classes, including phenyltropane analogs of cocaine and GBR 12909, produced lower self-administration rates than cocaine in nonhuman primates even though they produced DAT occupancies equal to or even greater than those observed for cocaine.34,35,37 In behavioral studies in rodents and nonhuman primates, these compounds displayed slower onset and longer duration of action.44,46 Hence, the reinforcing effects and pattern of self-administration of these drugs may be influenced by pharmacokinetics in addition to steady-state levels of DAT occupancy. With regard to pharmacokinetic profile, PET imaging studies with C-11 labeled WIN 35,428 have confirmed that, when compared to cocaine, GBR 12909 has a slower onset and longer duration of DAT occupancy in awake rhesus monkeys.47 The time course effects of DAT occupancy in these studies closely parallel those of drug-induced increases in extracellular dopamine measured with in vivo microdialysis in rhesus monkeys or squirrel monkeys.20 Moreover, the time course and potency differences in neurochemical effects paralleled differences in the stimulant effects of these drugs on operant behavior in squirrel monkeys.41,48 Taken together, these data offer compelling evidence that DAT occupancy measures obtained with PET imaging, including those that assess rate and those that assess “equilibrium” binding, are closely linked to functional changes in dopamine neurochemistry and in behavior.
Neuroimaging studies in human drug users have attempted to relate the acute neurochemical effects of stimulants to their reinforcing effects. Many of the results are in close agreement with preclinical studies conducted in nonhuman primates. For example, methylphenidate has affinity for the DAT comparable to cocaine. Behaviorally-relevant doses of methylphenidate can block the uptake of C-11 labeled cocaine.15 Similarly, doses of cocaine that induce euphoria can block uptake of C-11 labeled methylphenidate. In human cocaine abusers, subjective ratings of “high” correlate with percent DAT occupancy measured with PET imaging using C-11 labeled cocaine following acute administration of cocaine16 or methyphenidate.49 Approximately 50% occupancy of striatal DAT is required for subjects to identify cocaine when it is delivered as an intravenous injection.16 Therapeutic doses of methylphenidate commonly used in the treatment of attention deficit disorder also result in approximately 50% DAT occupancy. The time to reach peak uptake in brain corresponds well with the reported time course to reach peak behavioral effect.50 A subsequent study compared the levels of DAT occupancy by cocaine that was administered via different routes.51 Although similar levels of DAT occupancy were obtained across all routes of administration, smoked cocaine with the most rapid onset of action induced significantly greater self-reports of “high” than intranasal cocaine, again highlighting the importance of pharmacokinetic factors in the subjective effects of cocaine.
Dopamine Release
Competition between radiolabeled ligands and endogenous neurotransmitters for receptor binding can provide an effective means to evaluate drug-induced changes in extracellular concentrations of neurotransmitters in vivo (see52 for review). Both SPECT and PET imaging have been used in nonhuman primates to provide indirect measures of stimulant-induced changes in dopamine. For example, SPECT imaging with the dopamine D2 receptor ligand I-123 labeled iodobenzamide (IBZM) in baboons and rhesus monkeys documented amphetamine-induced displacement of binding, ostensibly due to drug-induced elevations in extracelluar dopamine.53 After methamphetamine administration, there were positive correlations between reductions in D2 receptor binding in baboons and peak dopamine release measured with microdialysis in vervet monkeys.54 Moreover, pretreatment with the dopamine synthesis inhibitor, alpha-methyl-paratyrosine, attenuated amphetamine-induced increases in extracellular dopamine and displacement of D2 receptor binding, confirming that the latter effect was mediated through dopamine release. PET imaging with F-18 labeled fluoroclebopride (FCP) as a reversible D2 receptor ligand also characterized stimulant-induced dopamine release in rhesus monkeys.55 Intravenous administration of cocaine, amphetamine, methylphenidate and methamphetamine each increased rates of FCP washout from the basal ganglia in manners consistent with the ability of each of these drugs to elevate extracellular dopamine. The effects of cocaine, amphetamine and methylphenidate have been replicated in dopamine D2 receptor PET studies. C-11 labeled raclopride studies in baboons36,56, 57 and F-18 labeled fallypride studies in rhesus monkeys58 document that these effects can be demonstrated with several radioligands and in several primate species. More recent studies in baboon and rhesus monkeys have begun to document the usefulness of drug-induced displacement of F-18 labeled fallypride to characterize dopamine release in extrastriatal brain regions. These regions include thalamus, cingulate cortex and hippocampus, which are important constituents of nigrostrial, mesolimbic and mesocortical dopamine systems, respectively.59,60 Taken together, these results validate the use of functional neuroimaging as a measure neurotransmitter release in nonhuman primates and provide a solid foundation for human studies.
Early human imaging studies extended the findings observed in animals to cocaine abusers by identifying positive correlations between stimulant-induced displacement of D2 receptor ligands and euphoric effects of acute stimulant administration. For example, PET imaging with C-11 labeled raclopride evaluated effects of cocaine on D2 receptor binding in the putamen.61 All subjects reported subjective stimulation and euphoria in response to cocaine and there were corresponding decreases in D2 receptor binding that were likely to reflect cocaine-induced elevations in dopamine. Another study that directly compared cocaine addicts to healthy control subjects demonstrated that addicts exhibited reduced dopamine release in the striatum and reduced self-reports of “high” in response to methylphenidate.62 In contrast, addicts showed increased thalamic responses to methylphenidate that were associated with cocaine craving; these were not seen in control subjects. These provocative findings challenged simple models in which cocaine addiction involved only enhanced striatal dopamine response to cocaine and corresponding induction of euphoria. Subsequent studies used PET imaging and C-11 raclopride to correlate methyphenidate-induced changes in dopamine release with self-reported measures of drug effects in healthy control subjects.63 The intensity of the “high” induced by methylphenidate was significantly correlated with levels of dopamine release, as measured by displacement of D2 receptor binding. Importantly, subjects who did not show increases in dopamine did not perceive the “high”. This was the first human study to demonstrate a quantitative relationship between levels of D2 receptor occupancy by dopamine and the intensity of stimulant-induced euphoria.
Dopamine D2 Receptor Availability as a Predictor of Stimulant Use
It has become well accepted that behavior, brain chemistry and neuronal function can be readily influenced by environmental conditions as well as by pharmacological challenges. Neuroimaging techniques are especially useful for studying the dynamic changes in neuronal function that may be associated with environmental variables. For example, differences in housing conditions and the dominance rank among socially housed nonhuman primates have been associated with differential levels of dopamine D2 receptors. An initial study using PET imaging with F-18 labeled FCP in socially-housed female cynomolgus monkeys documented reduced availability of D2 receptors in subordinate monkeys compared to dominant monkeys.64 However, it was unclear whether the observed differences in D2 binding reflected a predisposition that helped to determine dominance rank or a neurochemical response to dominance rank. In a subsequent series of experiments, male cynomolgus monkeys were first scanned using PET imaging with F-18 labeled FCP while individually housed. They were scanned again after they were placed in social groups in which they were allowed to establish stable social hierarchies.8 The levels of D2 receptor binding during individual housing did not predict eventual social rank. However, when the animals were rescanned after 3 months of social housing, there were significant differences between groups. Social housing increased the availability of D2 receptors in dominant monkeys without producing any changes in subordinate group members. Importantly, these neurochemical changes appeared to exert significant effects on cocaine use. Intravenous cocaine delivery reliably functioned as a reinforcer in subordinate subjects but failed to maintain self-administration in dominant monkeys. Subordinate monkeys reliably self-administered cocaine over a range of doses. In contrast, cocaine at several doses failed to maintain rates of responding higher than saline in dominant monkeys, indicating that cocaine did not function as a reinforcer in these subjects. During self-administration sessions, subordinate monkeys also displayed higher cocaine intakes compared to dominant monkeys. These provocative findings from repeated PET imaging in individual subjects document rapid neurochemical changes in response to environmental conditions, and subsequent alterations in subjects' propensity to use cocaine.
Analogous relationships between dopamine receptor densities and behavioral effects of stimulants also have been documented in human subjects. D2 receptor levels were determined in healthy men who had no history of drug abuse.65 Subjects who reported liking effects of methylphenidate had significantly lower D2 receptor levels in the striatum when compared to subjects who disliked the drug. In addition, there was a direct relationship between the intensity of unpleasant effects and D2 receptor levels. The results indicated that subjective responses to stimulants in humans may be correlated with D2 receptor levels, and that low levels of D2 receptors may contribute to stimulant abuse. These findings parallel those obtained in nonhuman primates, as described above.8 The ability to experimentally change D2 receptor density by altering environmental conditions in nonhuman primates provides especially strong support for orderly relationships between D2 receptor densities and reinforcing effects of cocaine.
Long-Term Consequences of Psychostimulant Administration
Dopamine D2 Receptor Availability
A major advantage of functional neuroimaging is the ability to employ longitudinal designs that involve repeated measures over extended periods of time. This approach has been used effectively in nonhuman primates to characterize both transient and long-lasting changes in brain chemistries that are associated with chronic drug exposure. For example, PET imaging studies conducted in socially housed cynomolgus monkeys were extended to characterize the effects of chronic cocaine exposure in dominant and subordinate individuals. Although dominant monkeys initially exhibited higher D2 receptor availability and were less likely to self-administer cocaine,8 chronic exposure to self-administered cocaine resulted in D2 levels that did not differ significantly from those found in subordinate monkeys66 The authors concluded that chronic exposure to cocaine attenuated the effects of environmental context on dopamine receptor availability. This conclusion was supported by in vitro receptor autoradiographic studies. Monkeys with long-term histories of cocaine self-administration reliably display lower D2 receptor densities in ways that correlate with cocaine dose and duration of exposure.67,68 A subsequent study examined D2 receptor availability during extended abstinence from cocaine.69 In three subjects exposed to cocaine for only one week, D2 receptor availability returned to baseline, pre-drug levels within three weeks. Five subjects that self-administered cocaine for twelve months were studied during cocaine abstinence. Three of the five subjects showed complete recovery of D2 receptor availability within three months of abstinence, whereas the other two subjects did not recover after one year of abstinence. Rate of recovery was not related to total drug intake over the twelve months of cocaine self-administration. It is interesting to note that individual differences in rate of recovery of D2 receptor availability have also been observed following drug-induced increases by the D2 receptor antagonist.70
Monoamine Transporter Availability
A recent study of DAT availability using PET imaging and F-18 labeled FCT examined the effects of cocaine self-administration in rhesus monkeys under conditions that resulted in low drug intake.71 Self-administration of a low cocaine dose over nine weeks did not significantly affect DAT availability in any brain region. This result contrasts with a previous study using quantitative in vitro receptor autoradiography in rhesus monkeys.72 In the initial stage of cocaine self-administration (e.g. after five days of exposure), DAT levels were moderately reduced. After more prolonged cocaine self-administration over three months, however, increased DAT binding densities were evident. Importantly, a higher cocaine dose per injection resulted in more robust increases in DAT compared to a lower unit dose of cocaine. The absence of cocaine-induced changes in DAT observed in the PET imaging study might thus reflect the relatively low dose and short duration of drug exposure.71 In rhesus monkeys with more prolonged histories of cocaine self-administration, significant increases in SERT in the caudate nucleus and putamen have been observed in PET imaging studies using C-11 labeled DASB.73
There has been significant interest in the potential neurotoxic effects of amphetamine derivatives that include methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA). There is evidence from multiple laboratories and studies of several species that MDMA can have selective and enduring effects on markers of brain serotonin systems that some investigators interpret to indicate neurotoxicity.74,75 These early studies were limited by biochemical and histological analyses that required between-subject comparisons in drug treated versus drug naïve control subjects. One of the first neuroimaging studies to characterize the effects of MDMA in vivo in a nonhuman primate used PET imaging and C-11 labeled McN5652 to evaluate SERT availability in a baboon.76 Following baseline PET scans, the subject was treated with MDMA twice daily for four consecutive days. Subsequent PET scans acquired at 13-40 days post-drug treatment showed reductions in SERT availability in all brain regions analyzed. Additional PET scans at nine and thirteen months showed regional differences in the apparent recovery of SERT availability, with increases back to normal levels observed in some brain regions including the hypothalamus, but no recovery in other brain regions that included the neocortex. Similar results have been reported for methamphetamine-induced reductions in DAT availability.77 Baboons were treated with one of three different doses of methamphetamine. PET imaging with C-11 labeled WIN-35,428 was used to quantify DAT availability. Two-three weeks after drug treatment, DAT availability was significantly reduced; decreases were larger in animals treated with higher methamphetamine doses. Moreover, reductions in striatal DAT determined by PET imaging were highly correlated with postmortem neurochemical determinations of dopamine axonal markers. Methamphetamine-induced reductions in DAT availability have also been observed in rhesus monkeys.78 Subjects received three injections at three-hour intervals PET imaging with C-11 CFT either one or seven days later revealed significant reductions in striatal DAT availability. Interestingly, treatment with the neuroprotective candidate drug minocycline significantly attenuated methamphetamine-induced reductions in DAT availability.
It is critical to note that studies reporting neurotoxic effects of amphetamine derivatives have relied on noncontingent drug administration and have typically administered large and/or repeated doses. In one of the first studies to characterize the neurochemical effects of self-administered MDMA in nonhuman primates, rhesus monkeys self-administered MDMA and its enantiomers for approximately eighteen months. PET imaging with C-11 labeled DTBZ was used to quantify vesicular monoamine transporter (VMAT) availability following at least two months of drug abstinence.79 The reinforcing effects of MDMA were selectively attenuated by chronic MDMA self-administration. However, there was no significant change in VMAT binding potential as determined by PET imaging. There were no significant changes in levels of serotonin or dopamine in postmortem brains. Hence, long-term self-administration of MDMA could lead to significant behavioral alterations in the absence of any significant neurochemical correlate. These results are supported by a recent study conducted in rhesus monkeys using C-11 labeled DASB to quantify SERT availability.73 In subjects with an extensive (6-18 month) histories of MDMA self-administration, there were no significant differences in SERT availability in any region of interest compared to control subjects. Studies that have more closely modeled the human abuse condition in nonhuman primates have thus failed to identify significant reproducible changes in neurochemical markers associated with neurotoxicity.
Clinical Relevance
Clinical studies that have used functional imaging to characterize the effects of stimulants have focused primarily on long-term changes in individuals with a complex histories of multidrug use. PET imaging has documented decreased blood flow in the prefrontal cortices of chronic cocaine users.80 Additional studies with PET and SPECT imaging have confirmed those results, demonstrating that brain perfusion deficits occur with high frequency.81,82,83,84 Local perfusion deficits have been linked closely to changes in cerebral metabolism. Measures of brain glucose metabolism with FDG in chronic users documented transient increases in metabolic activity in dopamine-associated brain regions during cocaine withdrawa.85 Decreases in frontal brain metabolism persisted after months of detoxification. The same pattern of decreased glucose metabolism86 and perfusion deficits80 was observed in the prefrontal cortices of a subset of cocaine users who were imaged on multiple occasions. More recently, mood disturbances have been linked to regional cerebral metabolic abnormalities in methamphetamine abusers87 (see also London and colleagues, this volume).
Chronic exposure to stimulant drugs in humans may also lead to significant reductions in neuronal markers of dopaminergic function. PET studies using C-11 labeled WIN-35,428 to quantify DAT availability in methamphetamine abusers showed reduced DAT availability in the nucleus accumbens, striatum and prefrontal cortex.88,89 The reduced DAT availability in these studies correlated with the duration of drug use and the severity of persistent psychiatric symptoms. PET imaging using C-11 labeled d-threo-methylphenidate to quantify DAT availability identified partial recovery of DAT binding in methamphetamine abusers during protracted abstinence.90 However, neuropsychological function did not improve to the same extent. The authors suggested that recovery of DAT availability is thus not sufficient for complete behavioral recovery. Dual-tracer PET imaging with FDG and C-11 labeled raclopride allows measurement of both brain metabolism and D2 receptor binding. These studies document both reduced frontal metabolism and decreased dopamine D2 receptor availability in cocaine or methamphetamine abusers.91,92 Moreover, D2 receptor availability was associated with metabolic rate in the orbitofrontal cortex. Based on such findings, the authors speculated that D2 receptor-mediated dysregulation of the orbitofrontal cortex could underlie compulsive drug taking. This intriguing suggestion awaits additional experimental evidence.
Some of the evidence for long-term changes in neurochemistry associated with stimulant use in humans can be interpreted as evidence for neurotoxicity. PET imaging studies using C-11 labeled McN-5652 to quantify SERT availability in human MDMA users reported enduring decrements in global brain binding that were correlated with the extent of prior MDMA use.93 These human studies are consistent with findings in nonhuman primates reported by the same research group. Likewise, humans with histories of methamphetamine use who were imaged after approximately three years of abstinence displayed reduced DAT availability in the caudate and putamen, based on C-11 WIN-35,428 PET studies.94 A preliminary study of amphetamine use by recreational users of MDMA also reported reduced striatal DAT binding, as determined by SPECT imaging using I-123 labeled B-CIT.95 However, this area of research has produced equivocal findings. As noted previously, a major advantage of neuroimaging techniques is the ability to employ longitudinal designs that minimize between-subject variability. Recent studies using longitudinal designs did not find a significant correlation between reductions in SERT availability and extent of MDMA. There were no improvements in markers for SERT during periods of drug abstinence.96,97
In addition to PET and SPECT neuroimaging, magnetic resonance spectroscopy (MRS) is an effective technique for quantifying neurotransmitters, their metabolites as well as putative biochemical markers for gliosis and cell death in discrete brain regions in vivo (see98 for basic description). Limited research that has been conducted in human MDMA users has provided mixed results. In one study, decreased ratios of N-acetyl-aspartate to creatine were associated with memory deficits in MDMA users.95 Other studies, however, have reported no differences in biochemical markers between MDMA users and control subjects99,100 It should be recognized that low magnetic field strength or a limited number of neuroanatomical regions of interest could lead to reduced sensitivity and potential false-negative results. Nonhuman primate research using more tightly controlled subject populations, high field strength magnets, and sufficient access to subjects to provide replication across many brain regions should allow us to address these issues.
Conclusions
Recent advances in nonhuman primate neuroimaging have documented drug-induced functional changes in brain activity under physiologically relevant conditions. Neuroimaging of cerebral blood flow changes coupled to cerebral metabolism measured with PET and fMRI is particularly well suited to define the neuronal circuitry that underlies drug effects on behavior. The ability to study drug interactions with specific protein targets in vivo has supported medications development efforts that have focused primarily on behavioral models of drug abuse. For example, neuroimaging has been used in nonhuman primates to study the relationship between drug occupancy at monoamine transporters and the behavioral effects of cocaine and cocaine-like agonist medications. Similar approaches have proven especially useful in studying dynamic changes in brain chemistry that may be associated with environmental variables. For example, differences in social status have been linked to dopamine receptor availability and propensity to use cocaine in nonhuman primates. The ability to conduct within-subject, longitudinal assessments of brain chemistry and neuronal function should enhance our efforts to document long-term changes due to chronic drug exposure and to elucidate recovery during prolonged abstinence or during treatment interventions. This review documents the close concordance that can be achieved among functional measures of neuroimaging, neurochemistry and behavior. Importantly, the clinical relevance of information derived from nonhuman primates has been established in several instances, when compared to the outcome of functional imaging studies in humans. Future progress in the field will rely on additional technical advances and novel research applications. MRI offers several advantages over PET and SPECT imaging, including improved temporal and spatial resolution. Appropriate contrast agents need to be developed that can adequately quantify specific protein targets in brain. Current technology with PET and SPECT radiochemistry should focus on the quantification of additional protein targets other than monoamine receptors and transporters. These include GABA, glutamate and other systems which play important roles in drug addiction. The long-term consequences of drug use on brain metabolism and cerebral blood flow have not received adequate attention in nonhuman primates with well controlled and documented histories of drug use. Finally, there is a clear need to apply neuroimaging techniques to evaluate classes of abused drugs other than psychostimulants. These complementary and integrative approaches should have important implications for medications development to treat drug abuse and addiction.
Table 2.
Long-term consequences of stimulants on monoamine transporters and dopamine D2 receptors
| No change in SERT availability | |||
| Buchert et al. 2006 | Humans | PET, [11C](+)McN5652 | MDMA abusers – longitudinal design |
| Thomasius et al. 2006 | Humans | PET, [11C](+)McN5652 | MDMA abusers – longitudinal design |
| Banks et al. 2008 | Rhesus macaques | PET, [11C]DASB | 0.03 and 0.3 mg/kg/infusion (IV) MDMA |
| Decreases in SERT availability | |||
| Scheffel et al. 1998 | Baboons | PET, [11C](+)McN5652 | 5 mg/kg bidaily X 4 days (SC) MDMA |
| Ricaurte et al. 2000 | Humans | PET, [11C](+)McN5652 | MDMA abusers |
| Increases in SERT availability | |||
| Banks et al. 2008 | Rhesus macaques | PET, [11C]DASB | 0.03 and 0.3 mg/kg/infusion (IV) cocaine |
| No change in DAT availability | |||
| Czoty et al. 2007 | Rhesus macaques | PET, [18F]FCT | 0.03 mg/kg/infusion (IV) cocaine - limited drug intake |
| Decreases in DAT availability | |||
| McCann et al. 1998 | Humans | PET, [11C]WIN 35,428 | methamphetamine abusers |
| Villemagne et al. 1998 | Baboons | PET, [11C]WIN 35,428 | 0.5, 1, or 2 mg/kg X 4 injections (IM) methamphetamine |
| Sekine et al. 2001, 2003 | Humans | PET, [11C]WIN 35,428 | methamphetamine abusers |
| Reneman et al. 2002 | Humans | SPECT, [123I]B-CIT | amphetamine/MDMA abusers |
| Hashimoto et al. 2007 | Rhesus macaques | PET, [11C]CFT | 2 mg/kg X 3 injections (IM) methamphetamine |
| Volkow et al. 2001a | Humans | PET, [11C]d-threo-methylphenidate | methamphetamine abusers |
| No change in vMAT levels | |||
| Fantegrossi et al. 2004 | Rhesus macaques | PET, [11C]DTBZ | 0.003 to 0.3 mg/kg/infusion (IV) MDMA |
| Decreases in D2 receptor availability | |||
| Volkow et al. 1993 | Humans | PET, [11C]raclopride | cocaine abusers |
| Volkow et al. 2001b | Humans | PET, [11C]raclopride | methamphetamine abusers |
| Nader et al. 2006 | Rhesus macaques | PET, [18F]FCP | 0.2 mg/kg/infusion (IV) cocaine |
Acknowledgments
The authors gratefully acknowledge the constructive comments of Dr. Matthew L. Banks. Research from the laboratory of the authors and preparation of the manuscript were supported in part by U.S. Public Health Service Grants DA10344, DA12514, DA16589, DA00517, and RR00165 (Division of Research Resources, National Institutes of Health).
Literature Cited
- 1.Haber SN, Kunishio K, Mizobuchi M, et al. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynd-Balta E, Haber SN. The organization of midbrain projections to the striatum in the primate: Sensorimotor-related striatum versus ventral striatum. Neuroscience. 1994;59(3):625–640. doi: 10.1016/0306-4522(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 3.Lynd-Balta E, Haber SN. The organization of midbrain projections to the ventral striatum in the primate. Neuroscience. 1994;59(3):609–623. doi: 10.1016/0306-4522(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 4.Lyons D, Friedman DP, Nader MA, et al. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci. 1996;16(3):1230–1238. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porrino LJ, Lyons D, Miller MD, et al. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002;22(17):7687–7694. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weerts EM, Fantegrossi WE, Goodwin AK. The value of nonhuman primates in drug abuse research. Experimental and Clinical Psychopharmacology. 2007;15(4):309–27. doi: 10.1037/1064-1297.15.4.309. [DOI] [PubMed] [Google Scholar]
- 7.Banks ML, Sprague JE, Kisor DF, et al. Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos. 2007;35(10):1840–1845. doi: 10.1124/dmd.107.016261. [DOI] [PubMed] [Google Scholar]
- 8.Nader MA, Czoty PW. PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: Genetic predisposition versus environmental modulation. Am J Psychiatry. 2005;162(8):1473–1482. doi: 10.1176/appi.ajp.162.8.1473. [DOI] [PubMed] [Google Scholar]
- 9.Nader MA, Czoty PW. Brain imaging in nonhuman primates: Insights into drug addiction. ILAR Journal. 2008;49(1):89–102. doi: 10.1093/ilar.49.1.89. [DOI] [PubMed] [Google Scholar]
- 10.Morgan D, Grant KA, Gage HD, et al. Social dominance in monkeys: Dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5(2):169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 11.Fowler JS, Kroll C, Ferrieri R, et al. PET studies of d-methamphetamine pharmacokinetics in primates: Comparison with l-methamphetamine and (--)-cocaine. J Nucl Med. 2007;48(10):1724–1732. doi: 10.2967/jnumed.107.040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senda M, Kimura Y, Herscovitch P, editors. Brain Imaging Using PET. Academic Press; New York, NY: 2002. [Google Scholar]
- 13.Huettel SA, Song AW, McCarty G, editors. Functional Magnetic Resonance Imaging. Sinauer Associates, Inc.; Sunderland, MA: 2003. [Google Scholar]
- 14.Fowler JS, Volkow ND, Wolf AP, et al. Mapping cocaine binding sites in human and baboon brain in vivo. Synapse. 1989;4(4):371–377. doi: 10.1002/syn.890040412. [DOI] [PubMed] [Google Scholar]
- 15.Volkow ND, Ding YS, Fowler JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry. 1995;52(6):456–463. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- 16.Volkow ND, Wang GJ, Fischman MW, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386(6627):827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- 17.Benveniste H, Fowler JS, Rooney W, et al. Maternal and fetal 11C-cocaine uptake and kinetics measured in vivo by combined PET and MRI in pregnant nonhuman primates. J Nucl Med. 2005;46(2):312–320. [PubMed] [Google Scholar]
- 18.Howell LL, Hoffman JM, Votaw JR, et al. An apparatus and behavioral training protocol to conduct positron emission tomography (PET) neuroimaging in conscious rhesus monkeys. J Neurosci Methods. 2001;106(2):161–169. doi: 10.1016/s0165-0270(01)00345-4. [DOI] [PubMed] [Google Scholar]
- 19.Howell LL, Hoffman JM, Votaw JR, et al. Cocaine-induced brain activation determined by positron emission tomography neuroimaging in conscious rhesus monkeys. Psychopharmacology. 2002;159(2):154–160. doi: 10.1007/s002130100911. [DOI] [PubMed] [Google Scholar]
- 20.Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2002;300(3):831–837. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins BG, Sanchez-Pernaute R, Brownell AL, et al. Mapping dopamine function in primates using pharmacologic magnetic resonance imaging. J Neurosci. 2004;24(43):9553–9560. doi: 10.1523/JNEUROSCI.1558-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brevard ME, Meyer JS, Harder JA, et al. Imaging brain activity in conscious monkeys following oral MDMA (“ecstasy”) Magn Reson Imaging. 2006;24(6):707–714. doi: 10.1016/j.mri.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Meyer JS, Brevard ME, Piper BJ, et al. Neural effects of MDMA as determined by functional magnetic resonance imaging and magnetic resonance spectroscopy in awake marmoset monkeys. Ann NY Acad Sci. 2006;1074:365–376. doi: 10.1196/annals.1369.036. [DOI] [PubMed] [Google Scholar]
- 24.Pearlson GD, Jeffery PJ, Harris GJ, et al. Correlation of acute cocaine-induced changes in local cerebral blood flow with subjective effects. Am J Psychiatry. 1993;150(3):495–497. doi: 10.1176/ajp.150.3.495. [DOI] [PubMed] [Google Scholar]
- 25.Wallace EA, Wisniewski G, Zubal G, et al. Acute cocaine effects on absolute cerebral blood flow. Psychopharmacology. 1996;128(1):17–20. doi: 10.1007/s002130050104. [DOI] [PubMed] [Google Scholar]
- 26.Johnson B, Lamki L, Fang B, et al. Demonstration of dose-dependent global and regional cocaine-induced reductions in brain blood flow using a novel approach to quantitative single photon emission computerized tomography. Neuropsychopharmacology. 1998;18(5):377–384. doi: 10.1016/S0893-133X(97)00168-1. [DOI] [PubMed] [Google Scholar]
- 27.London ED, Cascella NG, Wong DF, et al. Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose. Arch Gen Psychiatry. 1990;47(6):567–574. doi: 10.1001/archpsyc.1990.01810180067010. [DOI] [PubMed] [Google Scholar]
- 28.Volkow ND, Wang GJ, Fowler JS, et al. Effects of methylphenidate on regional brain glucose metabolism in humans: Relationship to dopamine D2 receptors. Am J Psychiatry. 1997;154(1):50–55. doi: 10.1176/ajp.154.1.50. [DOI] [PubMed] [Google Scholar]
- 29.Volkow ND, Wang GJ, Fowler JS, et al. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: Implications in addiction. Am J Psychiatry. 1999;156(1):19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- 30.Mathew RJ, Wilson WH, Lowe JV, et al. Acute changes in cranial blood flow after cocaine hydrochloride. Biol Psychiatry. 1996;40(7):609–616. doi: 10.1016/0006-3223(95)00033-x. [DOI] [PubMed] [Google Scholar]
- 31.Breiter HC, Gollub RL, Weisskoff RM, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 32.Kufahl PR, Li Z, Risinger RC, et al. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28(4):904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 33.Votaw JR, Howell LL, Martarello L, et al. Measurement of dopamine transporter occupancy for multiple injections of cocaine using a single injection of [F-18]FECNT. Synapse. 2002;44(4):203–210. doi: 10.1002/syn.10068. [DOI] [PubMed] [Google Scholar]
- 34.Wilcox KM, Lindsey KP, Votaw JR, et al. Self-administration of cocaine and the cocaine analog RTI-113: Relationship to dopamine transporter occupancy determined by PET neuroimaging in rhesus monkeys. Synapse. 2002;43(1):78–85. doi: 10.1002/syn.10018. [DOI] [PubMed] [Google Scholar]
- 35.Lindsey KP, Wilcox KM, Votaw JR, et al. Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: Relationship to transporter occupancy determined by positron emission tomography neuroimaging. J Pharmacol Exp Ther. 2004;309(3):959–969. doi: 10.1124/jpet.103.060293. [DOI] [PubMed] [Google Scholar]
- 36.Villemagne VL, Rothman RB, Yokoi F, et al. Doses of GBR12909 that suppress cocaine self-administration in non-human primates substantially occupy dopamine transporters as measured by [11C] WIN35,428 PET scans. Synapse. 1999;32(1):44–50. doi: 10.1002/(SICI)1098-2396(199904)32:1<44::AID-SYN6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Howell LL, Carroll FI, Votaw JR, et al. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320(2):757–765. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- 38.Howell LL, Wilcox KM. The dopamine transporter and cocaine medication development: Drug self-administration in nonhuman primates. J Pharmacol Exp Ther. 2001;298(1):1–6. [PubMed] [Google Scholar]
- 39.Bergman J, Madras BK, Johnson SE, et al. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251(1):150–155. [PubMed] [Google Scholar]
- 40.Weed MR, Mackevicius AS, Kebabian J, et al. Reinforcing and discriminative stimulus effects of beta-CIT in rhesus monkeys. Pharmacol Biochem Behav. 1995;51(4):953–956. doi: 10.1016/0091-3057(95)00032-r. [DOI] [PubMed] [Google Scholar]
- 41.Howell LL, Czoty PW, Kuhar MJ, et al. Comparative behavioral pharmacology of cocaine and the selective dopamine uptake inhibitor RTI-113 in the squirrel monkey. J Pharmacol Exp Ther. 2000;292(2):521–529. [PubMed] [Google Scholar]
- 42.Howell LL, Byrd LD. Characterization of the effects of cocaine and GBR 12909, a dopamine uptake inhibitor, on behavior in the squirrel monkey. J Pharmacol Exp Ther. 1991;258(1):178–185. [PubMed] [Google Scholar]
- 43.Wilcox KM, Paul IA, Woolverton WL. Comparison between dopamine transporter affinity and self-administration potency of local anesthetics in rhesus monkeys. Eur J Pharmacol. 1999;367(2-3):175–181. doi: 10.1016/s0014-2999(98)00967-4. [DOI] [PubMed] [Google Scholar]
- 44.Wilcox KM, Rowlett JK, Paul IA, et al. On the relationship between the dopamine transporter and the reinforcing effects of local anesthetics in rhesus monkeys: Practical and theoretical concerns. Psychopharmacology. 2000;153(1):139–147. doi: 10.1007/s002130000457. [DOI] [PubMed] [Google Scholar]
- 45.Wilcox KM, Kimmel HL, Lindsey KP, et al. In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse. 2005;58(4):220–228. doi: 10.1002/syn.20199. [DOI] [PubMed] [Google Scholar]
- 46.Kimmel HL, Carroll FI, Kuhar MJ. Locomotor stimulant effects of novel phenyltropanes in the mouse. Drug Alcohol Depend. 2001;65(1):25–36. doi: 10.1016/s0376-8716(01)00144-2. [DOI] [PubMed] [Google Scholar]
- 47.Tsukada H, Harada N, Nishiyama S, et al. Dose-response and duration effects of acute administrations of cocaine and GBR12909 on dopamine synthesis and transporter in the conscious monkey brain: PET studies combined with microdialysis. Brain Res. 2000;860(1-2):141–148. doi: 10.1016/s0006-8993(00)02057-6. [DOI] [PubMed] [Google Scholar]
- 48.Howell LL, Czoty PW, Byrd LD. Pharmacological interactions between serotonin and dopamine on behavior in the squirrel monkey. Psychopharmacology. 1997;131(1):40–48. doi: 10.1007/s002130050263. [DOI] [PubMed] [Google Scholar]
- 49.Volkow ND, Wang GJ, Fowler JS, et al. Blockade of striatal dopamine transporters by intravenous methylphenidate is not sufficient to induce self-reports of “high”. J Pharmacol Exp Ther. 1999;288(1):14–20. [PubMed] [Google Scholar]
- 50.Volkow ND, Wang GJ, Fowler JS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155(10):1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- 51.Volkow ND, Wang GJ, Fischman MW, et al. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci. 2000;67(12):1507–1515. doi: 10.1016/s0024-3205(00)00731-1. [DOI] [PubMed] [Google Scholar]
- 52.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: A critical review. J Cereb Blood Flow Metab. 2000;20(3):423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Innis RB, Malison RT, al-Tikriti M, et al. Amphetamine-stimulated dopamine release competes in vivo for [123I]IBZM binding to the D2 receptor in nonhuman primates. Synapse. 1992;10(3):177–184. doi: 10.1002/syn.890100302. [DOI] [PubMed] [Google Scholar]
- 54.Laruelle M, Iyer RN, Al Tikriti MS, et al. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse. 1997;25(1):1–14. doi: 10.1002/(SICI)1098-2396(199701)25:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 55.Mach RH, Nader MA, Ehrenkaufer RL, et al. Use of positron emission tomography to study the dynamics of psychostimulant-induced dopamine release. Pharmacol Biochem Behav. 1997;57(3):477–486. doi: 10.1016/s0091-3057(96)00449-2. [DOI] [PubMed] [Google Scholar]
- 56.Dewey SL, Smith GS, Logan J, et al. GABAergic inhibition of endogenous dopamine release measured in vivo with 11C-raclopride and positron emission tomography. J Neurosci. 1992;12(10):3773–3780. doi: 10.1523/JNEUROSCI.12-10-03773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volkow ND, Fowler JS, Gatley SJ, et al. Comparable changes in synaptic dopamine induced by methylphenidate and by cocaine in the baboon brain. Synapse. 1999;31(1):59–66. doi: 10.1002/(SICI)1098-2396(199901)31:1<59::AID-SYN8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 58.Mukherjee J, Yang ZY, Lew R, et al. Evaluation of d-amphetamine effects on the binding of dopamine D-2 receptor radioligand, 18F-fallypride in nonhuman primates using positron emission tomography. Synapse. 1997;27(1):1–13. doi: 10.1002/(SICI)1098-2396(199709)27:1<1::AID-SYN1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 59.Slifstein M, Narendran R, Hwang DR, et al. Effect of amphetamine on [(18)F]fallypride in vivo binding to D(2) receptors in striatal and extrastriatal regions of the primate brain: Single bolus and bolus plus constant infusion studies. Synapse. 2004;54(1):46–63. doi: 10.1002/syn.20062. [DOI] [PubMed] [Google Scholar]
- 60.Mukherjee J, Christian BT, Narayanan TK, et al. Measurement of d-amphetamine-induced effects on the binding of dopamine D-2/D-3 receptor radioligand, 18F-fallypride in extrastriatal brain regions in non-human primates using PET. Brain Res. 2005;1032(1-2):77–84. doi: 10.1016/j.brainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Schlaepfer TE, Pearlson GD, Wong DF, et al. PET study of competition between intravenous cocaine and [11C]raclopride at dopamine receptors in human subjects. Am J Psychiatry. 1997;154(9):1209–1213. doi: 10.1176/ajp.154.9.1209. [DOI] [PubMed] [Google Scholar]
- 62.Volkow ND, Wang GJ, Fowler JS, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 63.Volkow ND, Wang GJ, Fowler JS, et al. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther. 1999;291(1):409–415. [PubMed] [Google Scholar]
- 64.Grant KA, Shively CA, Nader MA, et al. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29(1):80–83. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 65.Volkow ND, Wang GJ, Fowler JS, et al. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156(9):1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- 66.Czoty PW, Morgan D, Shannon EE, et al. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology. 2004;174(3):381–388. doi: 10.1007/s00213-003-1752-z. [DOI] [PubMed] [Google Scholar]
- 67.Moore RJ, Vinsant SL, Nader MA, et al. Effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys. Synapse. 1998;30(1):88–96. doi: 10.1002/(SICI)1098-2396(199809)30:1<88::AID-SYN11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 68.Nader MA, Daunais JB, Moore T, et al. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: Initial and chronic exposure. Neuropsychopharmacology. 2002;27(1):35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- 69.Nader MA, Morgan D, Gage HD, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9(8):1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- 70.Czoty PW, Gage HD, Nader MA. PET imaging of striatal dopamine D2 receptors in nonhuman primates: Increases in availability produced by chronic raclopride treatment. Synapse. 2005;58(4):215–219. doi: 10.1002/syn.20200. [DOI] [PubMed] [Google Scholar]
- 71.Czoty PW, Gage HD, Nader SH, et al. PET imaging of dopamine D2 receptor and transporter availability during acquisition of cocaine self-administration in rhesus monkeys. J Addiction Med. 2007;1(1):33–39. doi: 10.1097/ADM.0b013e318045c038. [DOI] [PubMed] [Google Scholar]
- 72.Letchworth SR, Nader MA, Smith HR, et al. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21(8):2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Banks ML, Czoty PW, Gage HD, et al. Effects of cocaine and MDMA self-administration on serotonin transporter availability in monkeys. Neuropsychopharmacology. 2008;33(2):219–225. doi: 10.1038/sj.npp.1301420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DeSouza EB, Battaglia G, Insel TR. Neurotoxic effect of MDMA on brain serotonin neurons: Evidence from neurochemical and radioligand binding studies. Ann NY Acad Sci. 1990;600:682–697. doi: 10.1111/j.1749-6632.1990.tb16918.x. [DOI] [PubMed] [Google Scholar]
- 75.Green AR, Cross AJ, Goodwin GM. Review of the pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA or “Ecstasy”) Psychopharmacology. 1995;119(3):247–260. doi: 10.1007/BF02246288. [DOI] [PubMed] [Google Scholar]
- 76.Scheffel U, Szabo Z, Matthews WB, et al. In vivo detection of short- and long-term MDMA neurotoxicity--a positron emission tomography study in the living baboon brain. Synapse. 1998;29(2):183–192. doi: 10.1002/(SICI)1098-2396(199806)29:2<183::AID-SYN9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 77.Villemagne V, Yuan J, Wong DF, et al. Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: Evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci. 1998;18(1):419–427. doi: 10.1523/JNEUROSCI.18-01-00419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hashimoto K, Tsukada H, Nishiyama S, et al. Protective effects of minocycline on the reduction of dopamine transporters in the striatum after administration of methamphetamine: A positron emission tomography study in conscious monkeys. Biol Psychiatry. 2007;61(5):577–581. doi: 10.1016/j.biopsych.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 79.Fantegrossi WE, Woolverton WL, Kilbourn M, et al. Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology. 2004;29(7):1270–1281. doi: 10.1038/sj.npp.1300442. [DOI] [PubMed] [Google Scholar]
- 80.Volkow ND, Mullani N, Gould KL, et al. Cerebral blood flow in chronic cocaine users: A study with positron emission tomography. Br J Psychiatry. 1988;152:641–648. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- 81.Holman BL, Carvalho PA, Mendelson J, et al. Brain perfusion is abnormal in cocaine-dependent polydrug users: A study using technetium-99m-HMPAO and ASPECT. J Nucl Med. 1991;32(6):1206–1210. [PubMed] [Google Scholar]
- 82.Holman BL, Mendelson J, Garada B, et al. Regional cerebral blood flow improves with treatment in chronic cocaine polydrug users. J Nucl Med. 1993;34(5):723–727. [PubMed] [Google Scholar]
- 83.Strickland TL, Mena I, Villanueva-Meyer J, et al. Cerebral perfusion and neuropsychological consequences of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1993;5(4):419–427. doi: 10.1176/jnp.5.4.419. [DOI] [PubMed] [Google Scholar]
- 84.Levin JM, Holman BL, Mendelson JH, et al. Gender differences in cerebral perfusion in cocaine abuse: technetium-99m-HMPAO SPECT study of drug-abusing women. J Nucl Med. 1994;35(12):1902–1909. [PubMed] [Google Scholar]
- 85.Volkow ND, Fowler JS, Wolf AP, et al. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148(5):621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- 86.Reivich M, Alavi A, Wolf A, et al. Glucose metabolic rate kinetic model parameter determination in humans: The lumped constants and rate constants for [18F]fluorodeoxyglucose and [11C]deoxyglucose. J Cereb Blood Flow Metab. 1985;5(2):179–192. doi: 10.1038/jcbfm.1985.24. [DOI] [PubMed] [Google Scholar]
- 87.London ED, Simon SL, Berman SM, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61(1):73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- 88.Sekine Y, Iyo M, Ouchi Y, et al. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158(8):1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- 89.Sekine Y, Minabe Y, Ouchi Y, et al. Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. Am J Psychiatry. 2003;160(9):1699–1701. doi: 10.1176/appi.ajp.160.9.1699. [DOI] [PubMed] [Google Scholar]
- 90.Volkow ND, Chang L, Wang GJ, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21(23):9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Volkow ND, Fowler JS, Want GJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14(2):169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 92.Volkow ND, Chang L, Wang GJ, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: Association with metabolism in the orbofrontal cortex. Am J Psychiatry. 2001;158(12):2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 93.Ricaurte GA, McCann UD, Szabo Z, et al. Toxicodynamics and long-term toxicity of the recreational drug, 3, 4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’) Toxicol Lett. 2000;112-113:143–146. doi: 10.1016/s0378-4274(99)00216-7. [DOI] [PubMed] [Google Scholar]
- 94.McCann UD, Wong DF, Villemagne V, et al. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: Evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18(20):8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reneman L, Booij J, Lavalaye J, et al. Use of amphetamine by recreational users of ecstasy (MDMA) is associated with reduced striatal dopamine transporter densities: A [123I]beta-CIT SPECT study--preliminary report. Psychopharmacology (Berl) 2002;159(3):335–340. doi: 10.1007/s00213-001-0930-0. [DOI] [PubMed] [Google Scholar]
- 96.Buchert R, Thomasius R, Petersen K, et al. Reversibility of ecstasy-induced reduction in serotonin transporter availability in polydrug ecstasy users. Eur J Nucl Med Mol Imaging. 2006;33:188–199. doi: 10.1007/s00259-005-1850-8. [DOI] [PubMed] [Google Scholar]
- 97.Thomasius R, Zapletalova P, Petersen K, et al. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users: The longitudinal perspective. J Psychopharmacol. 2006;20:211–225. doi: 10.1177/0269881106059486. [DOI] [PubMed] [Google Scholar]
- 98.Minati L, Grisoli M, Bruzzone MG. MR spectroscopy, functional MRI, and diffusion-tensor imaging in the aging brain: A conceptual review. J Geriatr Psychiatry Neurol. 2007;20(1):3–21. doi: 10.1177/0891988706297089. [DOI] [PubMed] [Google Scholar]
- 99.Daumann J, Fischermann T, Pilatus U, et al. Proton magnetic resonance spectroscopy in ecstasy (MDMA) users. Neurosci Lett. 2004;362(2):113–116. doi: 10.1016/j.neulet.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 100.Cowan RL, Bolo NR, Dietrich M, et al. Occipital cortical proton MRS at 4 Tesla in human moderate MDMA polydrug users. Psychiatry Res. 2007;155(s):179–188. doi: 10.1016/j.pscychresns.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]