Abstract
Macrophages play an important role in the biological response to wear particles, which can result in periprosthetic osteolysis and implant loosening. In this study, we demonstrate that polymer particles induce systemic trafficking of macrophages by non-invasive in vivo imaging and immunohistochemistry. The distal femora of nude mice were injected with 10% (weight/volume) Simplex bone cement (BC) suspensions or saline (PBS). Reporter RAW264.7 macrophages which stably expressed the bioluminescent reporter gene fluc, and the fluorescence reporter gene gfp, were injected intravenously. Bioluminescence imaging was performed immediately and periodically at 2-day intervals until day 14. Compared to the non-operated contralateral femora, the bioluminescent signal of femora injected with BC suspension increased 4.7±1.6 and 7.8 ± 2.9 fold at day 6 and 8, respectively. The same values for PBS group were 1.2±0.2 and 1.4±0.5, respectively. The increase of bioluminescence of the BC group was significantly greater than the PBS group at day 8 (p < 0.05) and day 6 (p<0.1). Histological study confirmed the presence of reporter macrophages within the medullary canal of mice that received cement particles. Modulation of the signaling mechanisms that regulate systemic macrophage trafficking may provide a new strategy for mitigating the chronic inflammatory response and osteolysis associated with wear debris.
1. Introduction
Excessive production of wear particles from joint replacements is associated with periprosthetic osteolysis, which can lead to implant loosening [1-7]. Phagocytic cells engulf particulate debris and become activated; releasing proinflammatory cytokines, chemokines, degradative enzymes, reactive oxygen radicals and other substances which stimulate osteoclasts to undermine the prosthetic bed [8-14]. The key cell in the foreign body and chronic inflammatory response to wear particles is the macrophage [15-17]. Cells of the monocyte/macrophage lineage differentiate and maturate into phagocytic macrophages, foreign body giant cells and osteoclast precursors. These cells (in communication with stromal cells and other cell types) are primarily responsible for the cascade of events culminating in periprosthetic osteolysis. Despite ongoing research into the cellular and molecular processes associated with periprosthetic osteolysis, no in vivo studies have elucidated whether remote macrophages are stimulated to migrate to wear particles, or whether these events are a local phenomenon only. If macrophage recruitment to particles is a systemic phenomenon then novel strategies to mitigate these events may be potential targets for treatment.
We hypothesized that exogenous reporter macrophages introduced from a distant site would migrate and concentrate to an area in which phagocytosable polymer particles have been implanted. To examine this hypothesis, we use a model of femoral intramedullary polymer particle placement [18] in nude mice, a murine macrophage cell line transfected with a bioluminescent reporter gene, and sequential non-invasive imaging in-vivo using bioluminescence.
2. Materials and Methods
2.1 Animals and Cells
Eight to eleven week old adult male nude mice (Charles River Laboratories, Inc., MA) were housed and fed in our institution's animal facility. The murine macrophage cell line RAW264.7 was transfected with the lentiviral vector to express the bioluminescent optical reporter gene, firefly luciferase (fluc), and a fluorescence reporter gene, green fluorescent protein (gfp) [19].
2.2 Bone Cement Particles
Simplex® P bone cement (BC) powder (Howmedica Osteonics, Allendale, NJ) was used in the study. The BC powder is composed of 15% polymethyl methacrylate (PMMA), 10% barium sulphate, and 75% methylmethacrylate styrene copolymer. The particles vary from less than 1 μm in diameter to approximately 100 μm according to the manufacturer. The particles tested negative for endotoxin using a Limulus Amebocyte Lysate kit (BioWhittaker, Walkersville, MD). 10% (weight/volume) BC suspension was prepared in phosphate buffered saline (PBS, pH 7.4).
2.3 Surgical procedure
Institutional guidelines for the care and use of laboratory animals were strictly followed. All the operations were done using general anesthesia using a mask (3% isoflurane in 100% oxygen). We used the transpatellar tendon approach for distal femoral medullary cavity injection [20]. Briefly, the patellar tendon was exposed through a 5 mm lateral skin incision, and then the lateral aspect of the femoral shaft was exposed by another 5 mm incision over the distal quadriceps. The intramedullary injection (10 μl) was performed through the patellar tendon into the inter-condylar region of the femur with a 5 mm insertion of the needle guided by palpation of the lateral femoral shaft. The quadriceps-patellar complex was repaired with suture after injection. The incisions on the skin were closed by surgical adhesive glue and suture. Buprenorphine (Ben Venue Laboratories, Bedford, OH) at 0.1 mg/kg was given subcutaneously immediately and 4 hours later post-operatively for pain control.
In addition to the BC suspension treatments, additional mouse limbs were used as negative controls (no injection, or injection of PBS only) and positive controls (injection of lipopolysaccharide from Escherichia coli O127:B8 at a concentration of 1 μg/gram bodyweight in PBS, purchased from Sigma, Saint Louis, MO,).
2.4 Bioluminescence imaging
For in vivo surveillance of the trafficking of macrophages, seven days post operation, macrophages (5 × 105 cell) suspended in 0.1 ml Hanks' balanced salt solution (HBSS, Invitrogen, Carlsbad, CA) were injected intravenously via a syringe and needle (25 gage) into the lateral tail vein of mice. Fifteen minutes after intraperitoneal administration of D-luciferin (3 mg/mouse, Biosynth International), 5-minute images were taken with an in vivo imaging system (IVIS) employing a cooled charge-coupled device camera (Caliper LifeSciences, Hopkinton, MA). Prone and lateral images were obtained from each animal at each time point to better determine the origin of photon emission. Animals were imaged at 2-day intervals post-macrophage injection. Bioluminescence images were quantified by drawing uniformly sized regions of interest (ROIs) throughout the whole experiment, over the thigh on the lateral images of the mice, and the data were collected as to photon/cm2/sec/steradian.
2.5 Histology and immunohistology
Femora were collected at day 0 immediately after particle injection (6 femora) and 3 weeks after completion of the imaging experiment (38 femora). Frozen sections were cut using a cryostat (Cambridge Instruments, Buffalo, NY). Polarized light microscope (Nikon E1000M, Japan) was used to confirm the existence of BC particles in the femoral medullary canal.
Mouse anti-GFP monoclonal antibody (Chemicon International, Temecula, CA) was used to detect exogenous macrophages tagged with GFP. Rat anti mouse macrophage/monocyte monoclonal antibody (MOMA-2, Chemicon International, Temecula, CA) was used to detect macrophages. The secondary antibody used was Alexa Fluor 488 (or 594) conjugated goat anti-mouse (or rat) IgG (Invitrogen, Carlsbad, CA). Briefly, neutral buffered formaldehyde (10%, pH7.4) fixed frozen sections were blocked by Image-iT FX signal Enhancer (Molecular Probes, Eugene, OR). Mouse anti-GFP monoclonal antibody and rat anti-MOMA2 monoclonal antibody were incubated at room temperature for 3 hours, respectively. Then the sections were incubated with Alexa Fluor 488/594 conjugated goat anti-mouse/rat IgG (Invitrogen, Carlsbad, CA) for 1 hour at room temperature in the dark. DAPI containing ProLong Gold antifade reagent (Molecular Probes, Eugene, OR) was used for nuclear staining and slide mounting.
2.6 Statistical Methods
The non-parametric Mann-Whitney U test was used for statistical analyses between groups and the signed rank test was used to compare right and left limbs in the same animals.
3. Results
3.1 Polarized light microscopy of frozen section of femora
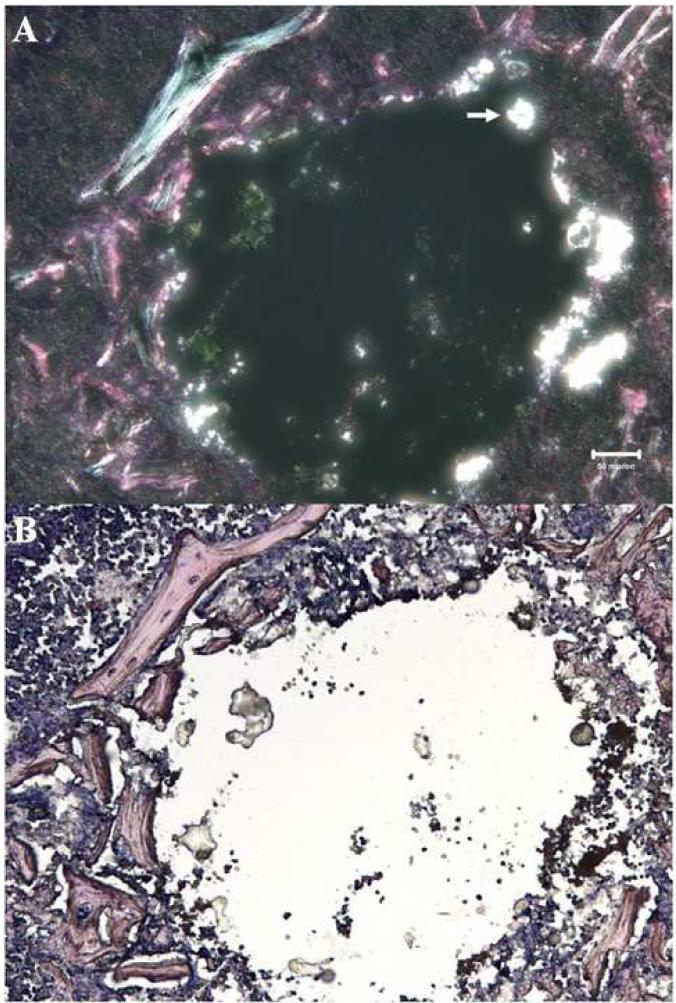
To demonstrate the presence of the BC particles in the femoral medullary canal of experimental animals, femora of selected mice were harvested immediately after injection. Frozen sections were stained with hematoxylin and eosin (H&E). The adopted histological protocol utilized reagents that precluded particle disruption and dissolution during preparation of the slides. Polarized light microscopy was used to observe the birefringence of the BC particles. As shown in Figure 1, bright white spots indicated the presence of cement particles within the medullary space of the femur, indicating successful particle injection.
Figure 1.
Polarized light microscopy of an H&E stained section of a mouse femur injected with 10% BC particles at day 0, and sacrificed immediately post-operatively. The irregular bright white spots (arrows) are areas of birefringent BC particles, which are in the medullary injection area. The multi-colored birefringent strands are cortical and trabecular fragments containing collagen fibers. The scale bar equals to 50 μm.
3.2 Imaging and bioluminescent signals of nude mice in BC and control group
We injected lipopolysaccharide (LPS) into the femoral medullary cavities of nude mice (n=10) to ensure that there was a positive response of the tagged macrophages to endotoxin. Compared to PBS injected femora, LPS injected femora had a stronger bioluminescent signal on day 6 onwards, as shown in Figure 2. This demonstrated the tagged macrophages could be induced to migrate and proliferate to an area containing endotoxin in vivo in this animal model.
Figure 2.
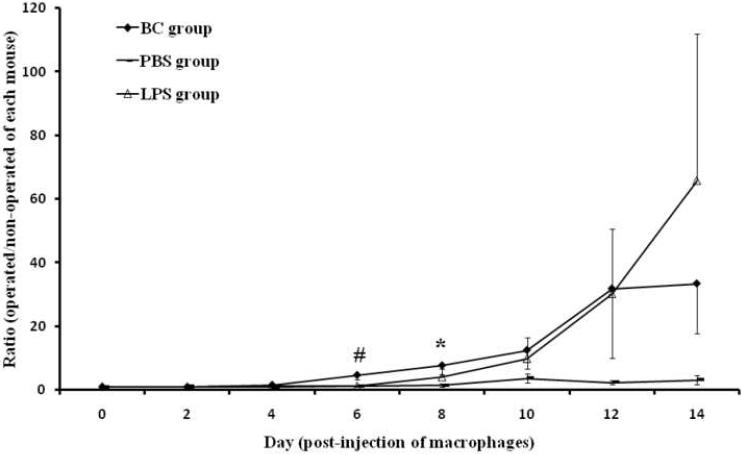
The ratio of bioluminescence of 10% (w/v) BC suspension femora, LPS, and PBS injected femora versus bioluminescence of the corresponding non-operated contralateral femur from day 0 to 14 post-injection of macrophages. The Y-axis is a normalized ratio of bioluminescence (unit: p/s/cm2/sr) from the operated femur versus non-operated femur in each animal. The values are mean ± SE. Number of animals in BC and saline injected groups are 11 and 8, respectively. # and *: indicates BC treatment group compared with PBS injected group at the same time point is statistically significant, p < 0.1 and p < 0.05, respectively;
Femora of twelve nude mice were injected with a 10% (w/v) BC suspension unilaterally and eight nude mice were injected with the carrier PBS alone. The pulmonary bioluminescent signal immediately after injection indicated a successful intravenous injection of tagged macrophages (Figure 3, day 0). One of the twelve mice in the BC group was excluded because of the lack of a pulmonary signal. As shown in Figure 3 in a typical experimental animal, a strong signal was seen in the lungs on day 0. From day 6 onwards, a strong bioluminescent signal was detected in the operated left femur receiving BC particles. Weaker signals could be seen in the vicinity of the kidney, spine and skull, as the tagged macrophages distributed throughout the body. In Figure 2, the bioluminescent signals for the ratios of operated divided by non-operated femora for the BC particle and PBS treatments are shown. From day 0 to day 4 post-injection of macrophages, there were few differences between the operated and non-operated femora in both groups (the ratios of day 0 to day 4 were 1.0, 1.0, and 1.6, respectively). However from day 6 onwards, there were higher bioluminescent signals detected from femora receiving cement particles compared to those receiving the carrier PBS alone. The ratios of the bioluminescence of BC injected femora versus non-operated femora in the experimental group were 4.7 ± 1.6 and 7.8 ± 2.9 at day 6 and day 8, whereas the values for the control group were 1.2 ± 0.2 and 1.4 ± 0.5, respectively. The increased bioluminescent signals of the BC group were significantly higher than those from the PBS group at day 8 (p < 0.05) and a trend was seen at day 6 (p < 0.1). The average bioluminescent signal at day 8 from the BC particle group was higher (3.7 ± 2.1 × 106 p/s/cm2/sr) than the PBS group (3.00 ± 0.8 × 103 p/sec/cm2/sr). The signals from the contralateral non-operated limbs in the BC particle and PBS groups were 6.0 ± 3.6 × 105 p/s/cm2/sr and 3.1 ± 0.9 × 103 p/s/cm2/sr, respectively.
Figure 3.
In vivo imaging of 10% BC particles injected in the left femur of nude mice from day 0 to day 10. Images were analyzed with a standardized protocol as explained in the text. Note the positive blue-purple (pseudocolor) signal in the left femur containing BC, indicating accumulation of macrophages. Images at day 12 and 14 were not included in this figure since they have higher signals at operated left femora but also with higher background signals from the kidney and skull. Except for day 0 and day 2 (their scale bar Max = 50,000), the other scale bar have a Max = 1.0 × 106 p/sec/cm2/sr as shown in the right of the figure.
3.3 Immunohistology
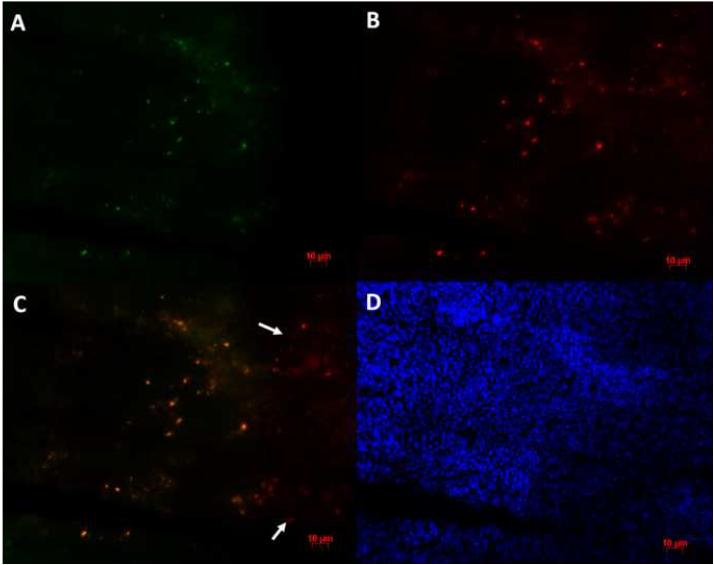
After completion of all of the imaging experiments, animals were euthanized and femora were collected for histological analysis. Double-fluorescence was used to detect both the macrophage marker MOMA2 and the GFP marker expressed by the macrophages. As shown in Figure 4 (A and B), both reporter gene GFP and macrophage antigen could be detected in the femoral canals of BC particle injected femora. If the images of the two macrophage markers are overlapped using Adobe Photoshop, the staining was similar for both markers (Figure 4 A, B, C). In contrast, there were very few reporter macrophages found in the PBS injected or non-operated femora.
Figure 4.
Immunofluorescent staining of macrophages in the marrow of a femur injected with 10% BC particles. (A) Mouse anti-GFP monoclonal antibody is the primary antibody and Alexa Fluor 488 conjugated goat anti-mouse IgG is the secondary antibody; (B) Rat anti-MOMA monoclonal antibody is the primary antibody and Alexa Fluor 594 conjugated goat anti-rat IgG is the secondary antibody; (C) Image A overlaped with image B using Adobe Photoshop. Note the co-localization of the two antibodies. Arrows indicate the spots with only MOMA-2 immunostaining signal. (D) DAPI was used for nuclear staining. All three images were taken from the same field of vision. Scale bar is 10 microns.
4. Discussion
Wear particles, periprosthetic osteolysis and implant loosening jeopardize the longevity of joint replacements. Macrophages play an important role in these biological processes [8, 15, 17, 21-25]. Macrophages release pro-inflammatory cytokines, chemokines and other factors, and can differentiate into osteoclasts that resorb bone [8, 15, 17, 21-27]. The precursor cells for activated macrophages, foreign body giant cells and osteoclasts are monocytes/macrophages. Systemic trafficking of mature macrophages to remote sites that contain orthopaedic particles has not been previously demonstrated using advanced imaging techniques in vivo.
In this study, we used sequential in vivo imaging using bioluminescence, and immunohistochemistry to clearly demonstrate systemic trafficking of intravenously injected mouse macrophages, in which fluc and gfp reporter genes are expressed, to BC particles implanted in the femoral canal of nude mice. Simplex bone cement particles were chosen for these studies because they are easily acquired, relatively inexpensive, of known size and shape, are packaged sterilely, and are easily injected into the mouse femur. Nude mice were used for two reasons. First, these mice are immunodeficient and therefore could not mount an immune response against the foreign RAW264.7 macrophages; thus this design permitted the undeterred migration and behavior of the exogenous macrophages. Second, these mice are hairless and lack skin pigment, which facilitates propagation of the light emission from optical reporters and imaging of the bioluminescent signal [28, 29]. Intravenous injection of macrophages was performed 7 days post particle injection to avoid the immediate inflammatory phase associated with the surgical trauma.
The tagged macrophage cell line RAW264.7 used in this experiment is immortal. We used data collected for 14 days post-macrophage infusion to minimize the potential longer-term adverse effects of systemic growth of a macrophage tumor cell line. Thus with the protocol outlined above, imaging for prolonged periods is not recommended if the animals manifest weight loss or other systemic signs of malignancy.
As shown in Figure 3, at day 0 the strongest signals were located at the lung; this pulmonary signal was used as an indicator of successful intravenous injection of reporter cells [30- 34]. Intravenous tail vein injections go to the lung via the inferior vena cava and pulmonary artery. The labeled cells could be detected in the lung as early as 5 minutes post-injection, and start to leave the lung and migrate systemically to liver, spleen, and kidney etc. at approximately 2 hours post-injection. Cells might remain in the lung for a prolonged time at the first stage of distribution due to the size of the cells relative to the pulmonary capillaries, which have an average diameter of 14 μm [35]. At day 2, the majority of injected cells are widely distributed throughout the body and the signal from these cells was not strong enough to be detected in our studies (Figure 3). As seen in the images in Figure 3, there are also bioluminescent signals localized in the vicinity of the kidney, spine, and skull area. In general, the distribution of intravenous administered cells to the long bones is much lower than with viscera such as liver, lung, kidney, and spleen in normal rodents [30-34]. Based on the distribution of infused macrophages using bioluminescence, the femora containing BC particles in our study stimulated a robust systemic migratory response.
There is an unexpected high bioluminescence signal from the non-operated femora in 3 of the 11 animals in the BC group, much higher than those from the other 8 animals in this group. However, the femora that contained bone cement particles in those same three animals had, on average, 7.3±2.3 times higher signal than the non-operated contralateral limbs. Furthermore, the average ratio of bioluminescence for operated divided by non-operated limbs in the other 8 animals for this BC particle group was 8.0±3.7. The reason for the high amount of bioluminescent signal in the non-operated femora of these three animals is unclear, but this phenomenon has been observed in other inflammatory animal models and human conditions, such as rheumatoid arthritis [36-38]. Kumagai et al. [39] also reported the systemic migration of GFP-labeled osteogenic connective tissue progenitor cells to the uninjured femur of the wild-type partner using a murine parabiotic model.
The presence of BC particles in the femoral medullary canal was confirmed histologically and by birefringence using polarized light. Furthermore, analysis using immunohistochemistry confirmed that systemic macrophages had migrated to the BC particles. Co-localization of the two macrophage markers was observed, indicating that the exogenous macrophages recruited to the particles exhibited the bioluminescent signal. In contrast, no such immunostaining was found in femora in which the saline carrier had been placed, or in non-operated femora. Thus, the results from in vivo imaging and immunohistochemistry support our hypothesis that the exogenous tagged macrophages introduced from a distant site were recruited to the area in which the cement particles had been implanted.
5. Conclusion
This study employing the techniques of sequential in vivo imaging using bioluminescence, and immunohistochemistry clearly demonstrates that exogenous reporter macrophages injected into the tail vein of nude mice are systemically recruited to a distant site, the femur that contains bone cement particles. Modulation of the signaling mechanisms that regulate systemic macrophage recruitment and homing may provide a new strategy for mitigating the chronic inflammatory response associated with wear debris.
Acknowledgements
We gratefully acknowledge Dr. Gobalakrishnan Sundaresan who supplied the fluc and gfp expressing RAW264.7 macrophage cell line. This study was supported in part by Grants R21 AR053189 and R01 AR055650-01 from the National Institute of Health, the Ellenburg Chair in Surgery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jones LC, Hungerford DS. Cement disease. Clin Orthop Relat Res. 1987;225:192–206. [PubMed] [Google Scholar]
- [2].Schmalzried TP, Jasty M, Harris WH. Periprosthetic bone loss in total hip arthroplasty. J Bone Joint Surg Am. 1992;74:849–863. [PubMed] [Google Scholar]
- [3].Maloney WJ, Smith RL. Periprosthetic osteolysis in total hip arthroplasty: the role of particulate wear debris. J Bone Joint Surg Am. 1995;77:1448–1461. [PubMed] [Google Scholar]
- [4].Akisue T, Bauer TW, Farver CF, Mochida Y. The effect of particle wear debris on NFkappaB activation and pro-inflammatory cytokine release in differentiated THP-1 cells. J Biomed Mater Res. 2002;59(3):507–515. doi: 10.1002/jbm.1264. [DOI] [PubMed] [Google Scholar]
- [5].Wang ML, Sharkey PF, Tuan RS. Particle bioreactivity and wear-mediated osteolysis. J Arthroplasty. 2004;19(8):1028–1038. doi: 10.1016/j.arth.2004.03.024. [DOI] [PubMed] [Google Scholar]
- [6].Goodman SB, Trindade M, Ma T, Genovese M, Smith RL. Pharmacologic modulation of periprosthetic osteolysis. Clin Orthop Relat Res. 2005;430:39–45. doi: 10.1097/01.blo.0000149998.88218.05. [DOI] [PubMed] [Google Scholar]
- [7].Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. 2007;454:251–261. doi: 10.1097/01.blo.0000238813.95035.1b. [DOI] [PubMed] [Google Scholar]
- [8].Glant TT, Jacobs JJ, Molnár G, Shanbhag AS, Valyon M, Galante JO. Bone resorption activity of particulate-stimulated macrophages. J Bone Miner Res. 1993;8(9):1071–1079. doi: 10.1002/jbmr.5650080907. [DOI] [PubMed] [Google Scholar]
- [9].Nakashima Y, Sun DH, Maloney WJ, Goodman SB, Schurman DJ, Smith RL. Induction of matrix metalloproteinase expression in human macrophages by orthopaedic particulate debris in vitro. J Bone Joint Surg Br. 1998;80:694–700. doi: 10.1302/0301-620x.80b4.8374. [DOI] [PubMed] [Google Scholar]
- [10].Nakashima Y, Sun DH, Trindade MC, Maloney WJ, Goodman SB, Schurman DJ, et al. Signaling pathways for tumor necrosis factor-alpha and interleukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro. J Bone Joint Surg Am. 1999;81:603–615. doi: 10.2106/00004623-199905000-00002. [DOI] [PubMed] [Google Scholar]
- [11].Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL. Tumor necrosis factor-α mediates orthopedic implant osteolysis. Am J Pathol. 1999;154(1):203–210. doi: 10.1016/s0002-9440(10)65266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kinov P, Leithner A, Radl R, Bodo K, Khoschsorur GA, Schauenstein K, et al. Role of free radicals in aseptic loosening of hip arthroplasty. J Orthop Res. 2006;24(1):55–62. doi: 10.1002/jor.20013. [DOI] [PubMed] [Google Scholar]
- [13].Talmo CT, Shanbhag AS, Rubash HE. Nonsurgical management of osteolysis: challenges and opportunities. Clin Orthop Relat Res. 2006;453:254–264. doi: 10.1097/01.blo.0000246531.59876.a8. [DOI] [PubMed] [Google Scholar]
- [14].Drees P, Eckardt A, Gay RE, Gay S, Huber LC. Mechanisms of disease: Molecular insights into aseptic loosening of orthopedic implants. Nat Clin Pract Rheumatol. 2007;3(3):165–171. doi: 10.1038/ncprheum0428. [DOI] [PubMed] [Google Scholar]
- [15].Athanasou NA, Quinn J, Bulstrode CJK. Resorption of bone by inflammatory cells derived from the joint capsule of hip arthroplasties. J Bone Joint Surg Br. 1992;74B:57–62. doi: 10.1302/0301-620X.74B1.1732267. [DOI] [PubMed] [Google Scholar]
- [16].Horowitz SM, Purdon MA. Mediator interactions in macrophage/particulate bone resorption. J Biomed Mater Res. 1995;29:477–484. doi: 10.1002/jbm.820290407. [DOI] [PubMed] [Google Scholar]
- [17].Miyanishi K, Trindade MC, Ma T, Goodman SB, Schurman DJ, Smith RL. Periprosthetic osteolysis: induction of vascular endothelial growth factor from human monocyte/macrophages by orthopaedic biomaterial particles. J Bone Miner Res. 2003;18(9):1573–1583. doi: 10.1359/jbmr.2003.18.9.1573. [DOI] [PubMed] [Google Scholar]
- [18].Epstein NJ, Warme BA, Spanogle J, Ma T, Bragg B, Smith RL, et al. Interleukin-1 modulates periprosthetic tissue formation in an intramedullary model of particle-induced inflammation. J Orthop Res. 2005;23:501–510. doi: 10.1016/j.orthres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- [19].De A, Zhou X-M, Gambhir SS. Noninvasive imaging of lentiviral-mediated reporter gene expression in living mice. Molecular Therapy. 2003;7(5):681–691. doi: 10.1016/s1525-0016(03)00070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zilber S, Epstein NJ, Lee S-W, Larsen M, Ma T, Smith RL, et al. Mouse femoral intramedullary injection model: technique and micro CT scan validation. J Biomed Mater Res B. 2008;84(1):286–290. doi: 10.1002/jbm.b.30872. [DOI] [PubMed] [Google Scholar]
- [21].Davis RG, Goodman SB, Smith RL, Lerman JA, Williams RJ., III The effects of bone cement powder on human adherent monocytes/macrophages in vitro. J Biomed Mater Res A. 1993;27:1039–1046. doi: 10.1002/jbm.820270809. [DOI] [PubMed] [Google Scholar]
- [22].Archibeck MJ, Jacobs JJ, Roebuck KA, Glant TT. The basic science of periprosthetic osteolysis. Instr Course Lect. 2001;50:185–195. [PubMed] [Google Scholar]
- [23].Linder L, Lindberg L, Carlsson A. Aseptic loosening of hip prostheses: A histologic and enzyme histochemical study. Clin Orthop. 1983;175:93–104. [PubMed] [Google Scholar]
- [24].Kim KJ, Rubash H, Wilson SC, D'Antonio JA, McClain EJ. A histologic and biochemical comparison of the interface tissues in cementless and cemented hip prostheses. Clin Orthop Relat Res. 1993;287:142–152. [PubMed] [Google Scholar]
- [25].Quinn J, Neale S, Fujikawa Y, McGee J, Athanasou NA. Human osteoclast formation from blood monocytes, peritoneal macrophages, and bone marrow cells. Calcif Tissue Int. 1998;62:527–531. doi: 10.1007/s002239900473. [DOI] [PubMed] [Google Scholar]
- [26].Quinn J, Joyner C, Triffitt JT, Athanasou NA. Polymethylmethacrylate-induced inflammatory macrophages resorb bone. J Bone Joint Surg Br. 1992;74B:652–658. doi: 10.1302/0301-620X.74B5.1527108. [DOI] [PubMed] [Google Scholar]
- [27].Sabokbar A, Pandey R, Quinn JMW, Athanasou NA. Osteoclastic differentiation by mononuclear phagocytes containing biomaterial particles. Arch Orthop Trauma Surg. 1998;117:136–140. doi: 10.1007/s004020050213. [DOI] [PubMed] [Google Scholar]
- [28].Rocchetta HL, Boylan CJ, Foley JW, Iversen PW, LeTourneau DL, McMillian CL. Validation of a noninvasive, real-time imaging technology using bioluminescent Escherichia coli in the neutropenic mouse thigh model of infection. Antimicrob Agents Chemother. 2001;45:129–137. doi: 10.1128/AAC.45.1.129-137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng. 2002;4:235–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- [30].Lotze MT, Line BR, Mathisend J, Rosenberg SA. The in vivo distribution of autologous human and murine lymphoid cells grown in T cell growth factor (TCGF): implication for the adoptive immunotherapy of tumors. J Immunol. 1980;125:1487–1493. [PubMed] [Google Scholar]
- [31].Maghazachi AA, Herbermarn B, Vujanovinc L, Hiseodt JC. In vivo distribution and tissue localization of highly purified rat lymphokine-activated killer (LAK) cells. Cell Immunol. 1988;115:179–194. doi: 10.1016/0008-8749(88)90172-4. [DOI] [PubMed] [Google Scholar]
- [32].Felgar E, Hiserodt JC. In vivo migration and tissue localization of highly purified lymphokine-activated killer cells (ALAK cells) in tumor-bearing rats. Cell Immunol. 1990;129:288–298. doi: 10.1016/0008-8749(90)90205-6. [DOI] [PubMed] [Google Scholar]
- [33].Kuppen PJ, Marinelli A, Camps JA, Pauwels EK, van de Velde CJ, Fleuren GJ, et al. Biodistribution of lymphokine-activated killer (LAK) cells in Wag rats after hepatic-artery or jugular-vein infusion. Int J Cancer. 1992;52(2):266–270. doi: 10.1002/ijc.2910520219. [DOI] [PubMed] [Google Scholar]
- [34].Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- [35].Richardson DR. The microcirculation and regulation of blood flow. In: Richardson DR, editor. Basic Circulatory Physiology. Little, Brown; Boston: 1976. pp. 101–136. [Google Scholar]
- [36].Kelly S, Dunham JP, Donaldson LF. Sensory nerves have altered function contralateral to a monoarthritis and may contribute to the symmetrical spread of inflammation. Eur J Neurosci. 2007;26(4):935–942. doi: 10.1111/j.1460-9568.2007.05737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Decaris E, Guingamp C, Chat M, Philippe L, Grillasca JP, Abid A, et al. Evidence for neurogenic transmission inducing degenerative cartilage damage distant from local inflammation. Arthritis Rheum. 1999;42:1951–1960. doi: 10.1002/1529-0131(199909)42:9<1951::AID-ANR22>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- [38].Donaldson LF, Seckl JR, McQueen DS. A discrete adjuvant-induced monoarthritis in the rat: effects of adjuvant dose. J Neurosci Meth. 1993;49:5–10. doi: 10.1016/0165-0270(93)90103-x. [DOI] [PubMed] [Google Scholar]
- [39].Kumagai K, Vasanji A, Drazba JA, Butler RS, Muschler GF. Circulating cells with osteogenic potential are physiologically mobilized into the fracture healing site in the parabiotic mice model. J Orthopaedic Research. 2008;26(2):165–175. doi: 10.1002/jor.20477. [DOI] [PubMed] [Google Scholar]