Abstract
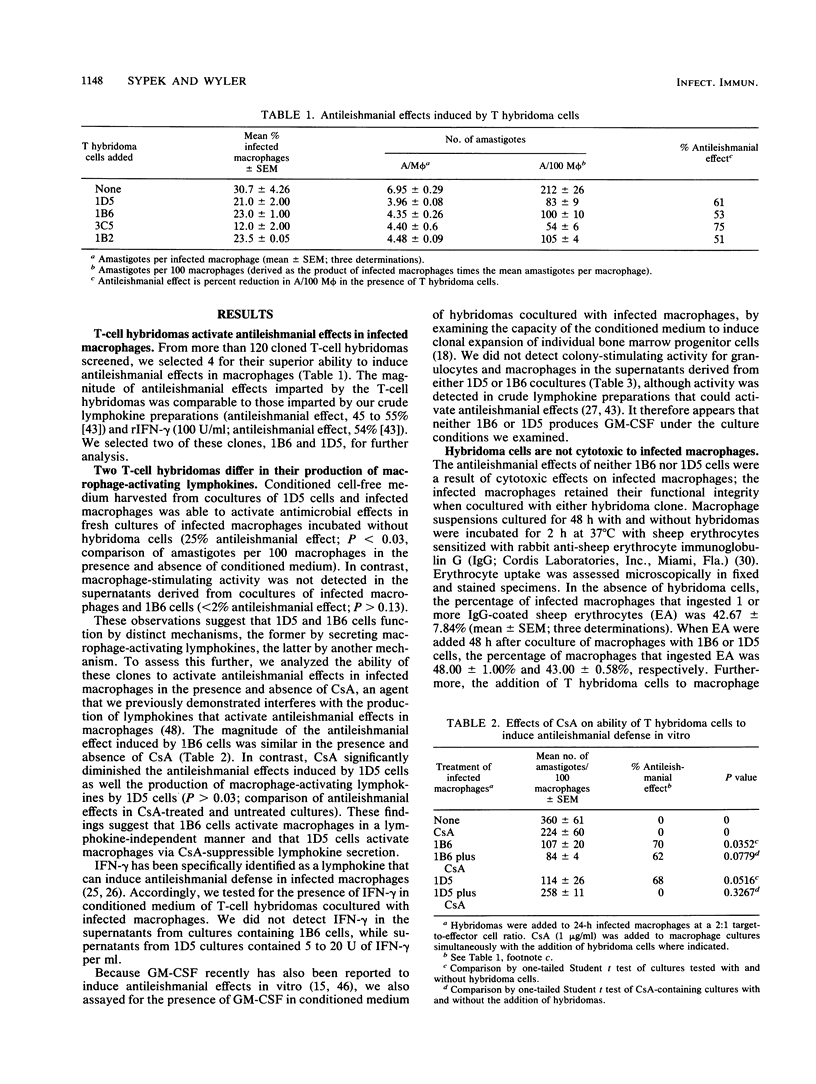
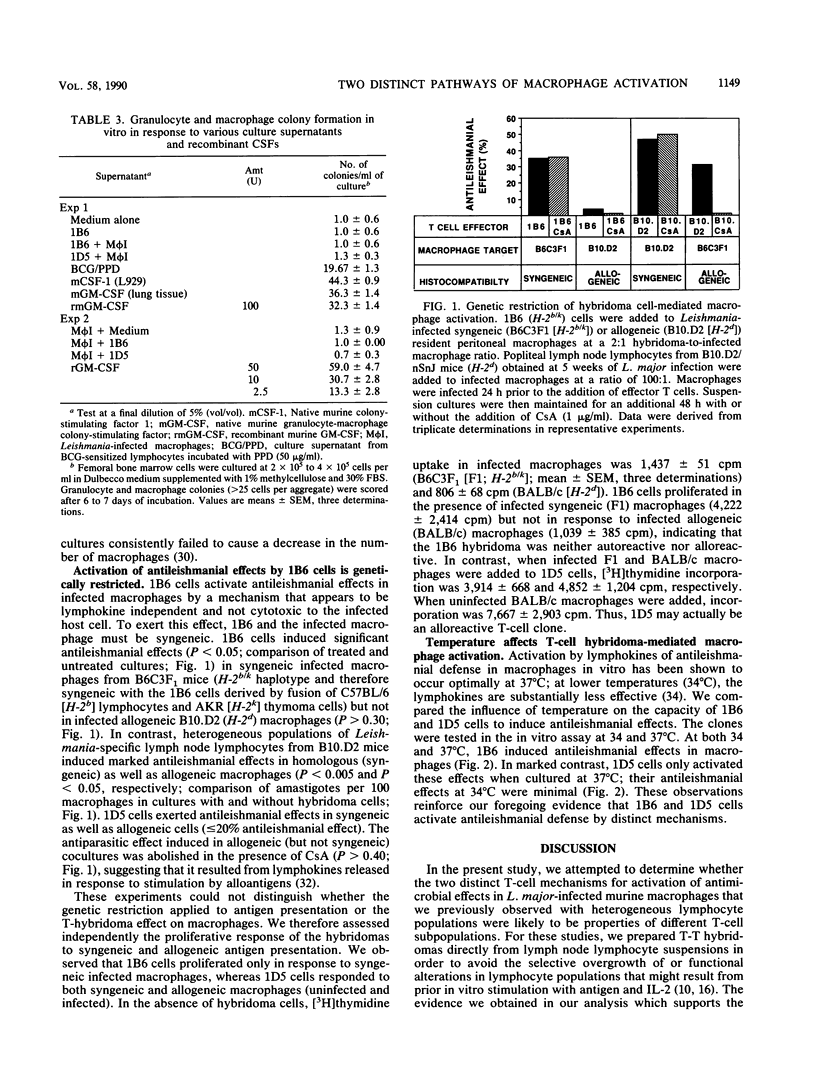
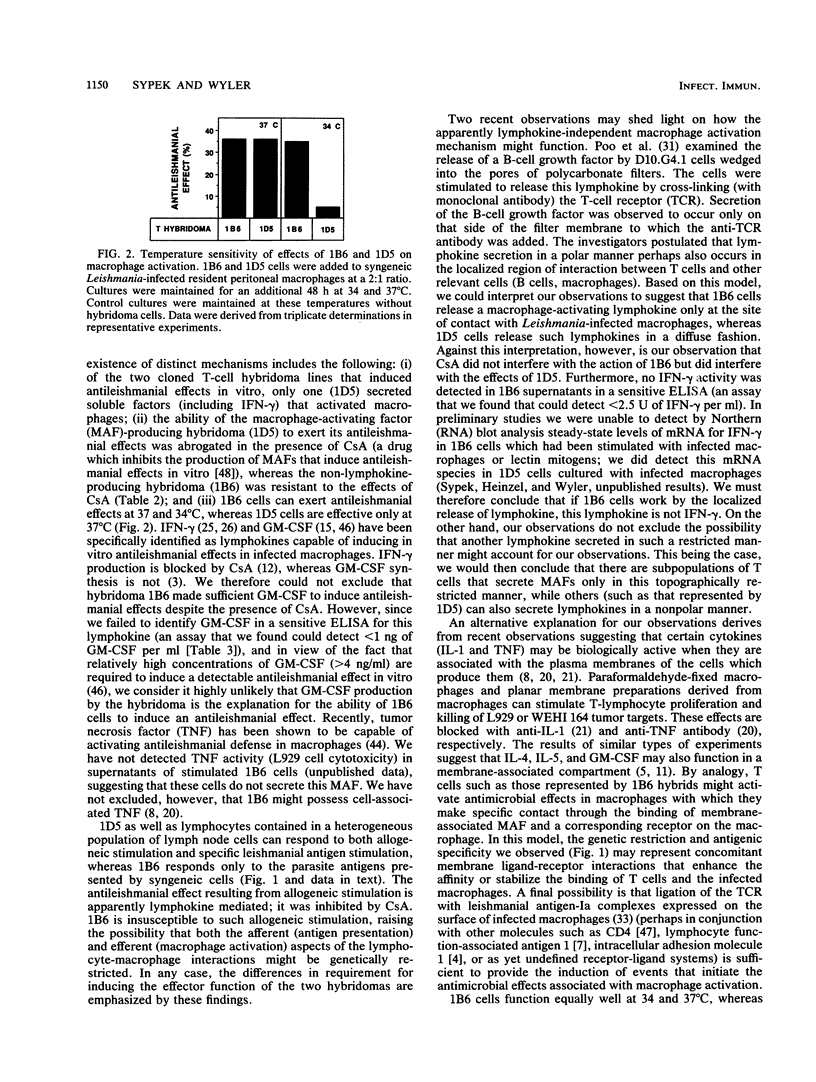
Using lymph node lymphocytes of Leishmania major-infected mice, we constructed and cloned two T-cell hybridomas that could activate macrophages to exert antileishmanial defense in vitro. One clone, 1D5, produced lymphokines (including gamma interferon) that induced these effects. Production of the macrophage-activating lymphokines and the protective effect of 1D5 were suppressed by the addition of cyclosporine A to cultures. The other clone, 1B6, produced no detectable macrophage-activating lymphokines, and its protective ability was not suppressed by cyclosporine A. Granulocyte-macrophage colony-stimulating factor (a lymphokine also known to induce antileishmanial effects in macrophages) was not detectable in culture supernatants of either clone. Furthermore, neither clone was cytotoxic to infected macrophages. Antileishmanial defense induced by 1B6 was genetically restricted; that is, infected macrophages and hybridoma cells had to be syngeneic for an antileishmanial effect to occur. In contrast, such restriction was not a property of clone 1D5, a clone that was responsive to alloantigens as well as leishmanial antigens. When incubated at a temperature (34 degrees C) at which lymphokines are relatively ineffective for antileishmanial defense, 1B6 but not 1D5 retained its antileishmanial properties. These observations provide clear evidence for the existence of two distinct mechanisms of macrophage activation: one that is lymphokine dependent, and one that is apparently lymphokine independent. The expression of these two mechanisms by cloned cells strongly suggests that they are properties of different T-cell subpopulations, extending our prior conclusions based on studies of heterogeneous T-cell populations. We hypothesize that the latter macrophage activation process involves a cell contact-dependent mechanism which might involve the interaction of a lymphocyte membrane-associated macrophage-activating factor (such as tumor necrosis factor) with its receptor on the macrophage, resulting in activation of antileishmanial effects but not host cell cytotoxicity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Finbloom D. S., Van Der Meide P. H., Slayter M. V., Nacy C. A. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989 Jul 1;143(1):266–274. [PubMed] [Google Scholar]
- Bickel M., Tsuda H., Amstad P., Evequoz V., Mergenhagen S. E., Wahl S. M., Pluznik D. H. Differential regulation of colony-stimulating factors and interleukin 2 production by cyclosporin A. Proc Natl Acad Sci U S A. 1987 May;84(10):3274–3277. doi: 10.1073/pnas.84.10.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A. W., Wawryk S. O., Burns G. F., Fecondo J. V. Intercellular adhesion molecule 1 (ICAM-1) has a central role in cell-cell contact-mediated immune mechanisms. Proc Natl Acad Sci U S A. 1988 May;85(9):3095–3099. doi: 10.1073/pnas.85.9.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian A. A. Stimulation of B-cell proliferation by membrane-associated molecules from activated T cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):564–568. doi: 10.1073/pnas.85.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davignon D., Martz E., Reynolds T., Kürzinger K., Springer T. A. Monoclonal antibody to a novel lymphocyte function-associated antigen (LFA-1): mechanism of blockade of T lymphocyte-mediated killing and effects on other T and B lymphocyte functions. J Immunol. 1981 Aug;127(2):590–595. [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L., Gifford G. E. Cell-associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J Immunol. 1987 Feb 1;138(3):957–962. [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Gordon M. Y., Riley G. P., Watt S. M., Greaves M. F. Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. 1987 Mar 26-Apr 1Nature. 326(6111):403–405. doi: 10.1038/326403a0. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Inaba K., Steinman R. M. Stimulation of lymphokine release from T lymphoblasts. Requirement for mRNA synthesis and inhibition by cyclosporin A. J Exp Med. 1984 Dec 1;160(6):1792–1802. doi: 10.1084/jem.160.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Handman E., Burgess A. W. Stimulation by granulocyte-macrophage colony-stimulating factor of Leishmania tropica killing by macrophages. J Immunol. 1979 Mar;122(3):1134–1137. [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R. Phenotypic and functional alteration of CD4+ T cells after antigen stimulation. Resolution of two populations of memory T cells that both secrete interleukin 4. J Exp Med. 1989 Jun 1;169(6):2245–2250. doi: 10.1084/jem.169.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover D. L., Nacy C. A. Macrophage activation to kill Leishmania tropica: defective intracellular killing of amastigotes by macrophages elicited with sterile inflammatory agents. J Immunol. 1984 Mar;132(3):1487–1493. [PubMed] [Google Scholar]
- Hämmerling G. J. T lymphocyte tissue culture lines produced by cell hybridization. Eur J Immunol. 1977 Oct;7(10):743–746. doi: 10.1002/eji.1830071018. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F. Erythroid progenitors in mouse bone marrow detected by macroscopic colony formation in culture. Exp Hematol. 1975 Jan;3(1):32–43. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kriegler M., Perez C., DeFay K., Albert I., Lu S. D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Beller D. I., Mizel S. B., Unanue E. R. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Hamberg S., Ohara J., Paul W. E., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. I. Lymphokine production and lymphokine responsiveness. J Exp Med. 1987 Dec 1;166(6):1774–1787. doi: 10.1084/jem.166.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Nacy C. A., Fortier A. H., Meltzer M. S., Buchmeier N. A., Schreiber R. D. Macrophage activation to kill Leishmania major: activation of macrophages for intracellular destruction of amastigotes can be induced by both recombinant interferon-gamma and non-interferon lymphokines. J Immunol. 1985 Nov;135(5):3505–3511. [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S., Leonard E. J., Wyler D. J. Intracellular replication and lymphokine-induced destruction of Leishmania tropica in C3H/HeN mouse macrophages. J Immunol. 1981 Dec;127(6):2381–2386. [PubMed] [Google Scholar]
- Nacy C. A., Oster C. N., James S. L., Meltzer M. S. Activation of macrophages to kill rickettsiae and Leishmania: dissociation of intracellular microbicidal activities and extracellular destruction of neoplastic and helminth targets. Contemp Top Immunobiol. 1984;13:147–170. doi: 10.1007/978-1-4757-1445-6_8. [DOI] [PubMed] [Google Scholar]
- Neva F. A., Wyler D., Nash T. Cutaneous leishmaniasis--a case with persistent organisms after treatment in presence of normal immune response. Am J Trop Med Hyg. 1979 May;28(3):467–471. [PubMed] [Google Scholar]
- Panosian C. B., Sypek J. P., Wyler D. J. Cell contact-mediated macrophage activation for antileishmanial defense. I. Lymphocyte effector mechanism that is contact dependent and noncytotoxic. J Immunol. 1984 Dec;133(6):3358–3365. [PubMed] [Google Scholar]
- Poo W. J., Conrad L., Janeway C. A., Jr Receptor-directed focusing of lymphokine release by helper T cells. Nature. 1988 Mar 24;332(6162):378–380. doi: 10.1038/332378a0. [DOI] [PubMed] [Google Scholar]
- Puré E., Inaba K., Metlay J. Lymphokine production by murine T cells in the mixed leukocyte reaction. J Exp Med. 1988 Aug 1;168(2):795–800. doi: 10.1084/jem.168.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Scott P. Impaired macrophage leishmanicidal activity at cutaneous temperature. Parasite Immunol. 1985 May;7(3):277–288. doi: 10.1111/j.1365-3024.1985.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Scott P., Sacks D., Sher A. Resistance to macrophage-mediated killing as a factor influencing the pathogenesis of chronic cutaneous leishmaniasis. J Immunol. 1983 Aug;131(2):966–971. [PubMed] [Google Scholar]
- Sheridan J. W., Metcalf D. A low molecular weight factor in lung-conditioned medium stimulating granulocyte and monocyte colony formation in vitro. J Cell Physiol. 1973 Feb;81(1):11–23. doi: 10.1002/jcp.1040810103. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Cellular immunity in vitro. I. Immunologically mediated enhancement of macrophage bactericidal capacity. J Exp Med. 1971 Jun 1;133(6):1377–1389. doi: 10.1084/jem.133.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Enhancement of macrophage bactericidal capacity by antigenically stimulated immune lymphocytes. Cell Immunol. 1972 Jun;4(2):163–174. doi: 10.1016/0008-8749(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Sypek J. P., Panosian C. B., Wyler D. J. Antigen recognition by effector T cells in antileishmanial defense. J Infect Dis. 1985 Nov;152(5):1057–1063. doi: 10.1093/infdis/152.5.1057. [DOI] [PubMed] [Google Scholar]
- Sypek J. P., Panosian C. B., Wyler D. J. Cell contact-mediated macrophage activation for antileishmanial defense. II. Identification of effector cell phenotype and genetic restriction. J Immunol. 1984 Dec;133(6):3351–3357. [PubMed] [Google Scholar]
- Sypek J. P., Wyler D. J. Cell contact-mediated macrophage activation for antileishmanial defence: mapping of the genetic restriction to the I region of the MHC. Clin Exp Immunol. 1985 Dec;62(3):449–457. [PMC free article] [PubMed] [Google Scholar]
- Sypek J. P., Wyler D. J. Susceptibility of lymphokine-resistant Leishmania to cell contact-mediated macrophage activation. J Infect Dis. 1988 Aug;158(2):392–397. doi: 10.1093/infdis/158.2.392. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Sherry B., Cerami A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med. 1989 Dec 1;170(6):2097–2104. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser W. Y., Van Niel A., Clark S. C., David J. R., Remold H. G. Recombinant human granulocyte/macrophage colony-stimulating factor activates intracellular killing of Leishmania donovani by human monocyte-derived macrophages. J Exp Med. 1987 Nov 1;166(5):1436–1446. doi: 10.1084/jem.166.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]
- Wyler D. J., Beller D. I., Sypek J. P. Macrophage activation for antileishmanial defense by an apparently novel mechanism. J Immunol. 1987 Feb 15;138(4):1246–1249. [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A., Adler B., Blanden R. V., Davidson W. F., Kees U., Dunlop M. B., Shreffler D. C. H-2 restriction of cell-mediated immunity to an intracellular bacterium: effector T cells are specific for Listeria antigen in association with H-21 region-coded self-markers. J Exp Med. 1977 May 1;145(5):1353–1367. doi: 10.1084/jem.145.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meide P. H., Dubbeld M., Vijverberg K., Kos T., Schellekens H. The purification and characterization of rat gamma interferon by use of two monoclonal antibodies. J Gen Virol. 1986 Jun;67(Pt 6):1059–1071. doi: 10.1099/0022-1317-67-6-1059. [DOI] [PubMed] [Google Scholar]



