Abstract
Little is known about collagen XI expression in normal and malignant breast tissue. Tissue microarrays, constructed from 72 patients with breast carcinoma and matched normal tissue, were immunohistochemically stained with five antisera against isoform-specific regions of collagen α1(XI) N-terminal domain. Staining intensity was graded on a 0–3 scale in epithelial cytoplasm, stroma, and endothelial staining of the vasculature of each tissue core. The staining was compared to known pathologic parameters: age, tumor size, overall tumor grade, nuclear grade, tubule formation, mitotic counts, angiolymphatic invasion, node status, estrogen receptor status, progesterone receptor status, and HER-2/neu status. Estrogen and progesterone receptor status were used as a control for comparison. With antisera V1a and amino propeptide (Npp), stroma surrounding cancerous cells was found to have decreased collagen α1(XI) staining compared to stroma adjacent to normal epithelium (P=0.0006, P<0.0001). Collagen α1(XI) staining with V1a antiserum in cytoplasm of cancer cells demonstrated decreased intensity in metastasized primary tumors when compared to nonmetastasized primary tumors (P=0.009). Cytoplasmic staining with Npp antiserum in cancer demonstrated an inverse relationship to positive estrogen receptor status in cancer (P=0.012) and to progesterone receptor status (P=0.044). Stromal staining for Npp in cancerous tissue demonstrated an inverse relationship with tubule formation score (P=0.015). This is the first study to localize collagen XI within normal and malignant breast tissue. Collagen α1(XI) appears to be downregulated in stroma surrounding breast cancer. Detection of collagen XI in breast tissue may help predict women who have lymph node metastases.
Keywords: collagen type XI, breast cancer, tissue microarray, immunohistochemistry, node status, metastasis
Approximately 180 000 US women will be diagnosed with breast cancer in the year 2007.1 Sentinel lymph node biopsy has become the favored method for the nodal staging of breast cancer. Looking for alternatives to invasive procedures such as sentinel lymph node biopsy is imperative for improving patient care. Known predictors of metastasis have been studied extensively: age, race, menopausal status, palpability, tumor size, positive margin on initial excision, histopathologic diagnosis, tumor grade, mitotic counts, nuclear polymorphism, tubule formation as an indication of tumor differentiation, lymphatic and vascular invasion, estrogen receptor status, progesterone receptor status, and HER-2/neu status.2-8
Collagen XI, a minor collagen found in many tissues, but characterized most thoroughly in cartilage, nucleates, and regulates formation of thin fibrils of developing or remodeling tissues.9-13 It is a heterotrimeric protein, consisting of three α-chains organized into a triple helix. Both α1(XI) and α2(XI) are unique gene products, and α3(XI) is a hypergly-colsylated version of the collagen (α1)II chain.14,15 Originally synthesized as procollagen, collagen XI has its C and N termini proteolytically removed after secretion from the cell.16,17 An N-terminal domain (NTD), consisting of a variable region and an amino propeptide (Npp) exists in α1(XI) and α2(XI). Alternative splicing of three separate exons (6a, 6b, and 8) may produce eight unique spliceforms encoding the corresponding protein regions p6a (V1a), p6b (V1b), and p8 (V2; Figure 1).18,19 Common to all spliceforms are the Npp encoded by exons 2–5, and the C2 region, encoded by exon 7 (Figure 1 and Table 1).
Figure 1.
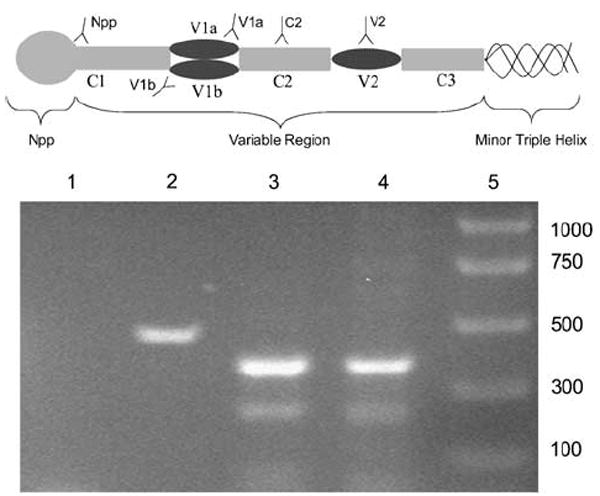
Schematic illustration of collagen α1(XI) N-terminal domain. Positions of target sequences are labeled for each of the antisera used in this study. The V1a region is recognized by antiserum V1a, the C2 region is recognized by antiserum C2, and the Npp is recognized by antiserum Npp. Col11a1 fragment was amplified by RT–PCR. (Lane 1) PCR control with no reverse transcriptase added; (lane 2) mRNA isolated from human mammary epithelial cells (HMEC), amplified with primers specific for the housekeeping gene GAPDH; (lane 3) mRNA isolated from HMEC, amplified with primers specific for Col11a1; (lane 4) mRNA isolated from human mammary cells, amplified with Col11a1 primers; (lane 5) size of markers specified in # basepairs.
Table 1.
Reactivity of antisera for spliceforms of Collagen α1(XI)
| Spliceform of Col11a1 | Antisera
|
||||
|---|---|---|---|---|---|
| V1a | C2 | Npp | V2 | V1b | |
| Col11a1p7 | — | X | X | — | — |
| Col11a1p6a7 | X | X | X | — | — |
| Col11a1p6b7 | — | X | X | — | X |
| Col11a1p78 | — | X | X | X | — |
| Col11a1p6a78 | X | X | X | X | — |
| Col11a1p6b78 | — | X | X | X | X |
| Col11a1p6a6b7 | X | X | X | — | X |
| Col11a1p6a6b78 | X | X | X | X | X |
Antisera used for this study have overlapping and/or complementary specificity as indicated by X. The eight potential spliceforms are listed with an indication of which antisera will recognize the spliceforms.
While the major triple helix of collagens may be difficult to access in immunohistochemical procedures, the NTD may be a more useful target. Previous studies have demonstrated the surface location and its persistence following biosynthesis.20,21
It has been found that several genes encoding extracellular matrix components including COL6A3 and COL8A1 were upregulated in cancer cells when compared with normal tissue.22 A previous study evaluated the expression of the gene COLXIA1 coding collagen α1(XI) between primary cancer and paired lymph node metastases as a predictor of clinical outcome of node-positive breast cancer patients. It was found that there was decreased expression of COLXIA1 in lymph node metastatic tissue as confirmed by reverse transcription (RT)–PCR.23 Finally, downregulation of all collagen has been shown in breast cancer tissue when compared with normal tissue.24 Modification in cancerous breast tissue collagen metabolism may reflect tissue remodeling, which is typical for the invasive phenotype of malignant cells.24
Collagen is known to play an active role in numerous biological processes such as cell morphogenesis, proliferation, migration, differentiation, apoptosis as well as carcinogenesis. Upregulation of collagen in carcinomas has been demonstrated in a variety of organs including colorectal tumors, non-small-cell lung cancer, and high-grade prostate carcinoma.25-28 It was found that colon mucosal stroma in the majority of colorectal cancers expressed COLXIA1, whereas it was rarely expressed in normal colon mucosa.25 In a study involving non-small-cell lung cancer, collagen α1(XI) was overexpressed at high frequency among both adenocarcinoma and squamous cell carcinoma.29 This study aims to analyze the presence of collagen α1(XI) spliceforms in normal and malignant human breast tissue while also evaluating the relationships to clinical/pathologic parameters including its ability to predict lymph node metastases.
Materials and methods
Five antisera were raised against specific portions of the collagen α1(XI) molecule. A purified 40-aminoacid (AA) synthetic peptide was used to generate a polyclonal antiserum to V1a (exon 6a) that has the potential to recognize spliceforms Col11a1p6a, Col11a1p6a78, Col11a1p6a6b, and Col11a1p6a6b78. A 29-AA synthetic peptide was used to generate a polyclonal antiserum that has the capacity to recognize all possible spliceforms through interaction with the epitope in the constitutively expressed exon 7 region (referred to as C2). An 11-AA synthetic peptide designed from the C terminus of the cleaved Npp was used to generate a polyclonal antiserum to α1(XI) Npp that has the capacity to recognize all possible spliceforms.20,30,31 A 20-AA synthetic peptide designed from the carboxy portion of V2 was used to generate a polyclonal antiserum to α1(XI) V2 (exon p8) that has the potential to recognize spliceforms Col1a1p8, Col11a1p6a78, Col11a1p6b78, and Col11a1p6a6b78. A purified 51-AA rat recombinant was used to generate a monoclonal antibody to V1b (exon p6b) that has the potential to recognize spliceforms Col11a1p6b, Col11a1p6a6b, Col11a1p6b78, and Col11a1p6a6b78 (Figure 1 and Table 1). The antibody specificity has been formerly characterized for rat, mouse, bovine, and human tissues.19,20,32 RT–PCR was performed to confirm that normal human mammary epithelial cells express Col11a1 at the mRNA level (Figure 1).
Grossly normal and malignant human breast tissue was obtained from pathology archives. Three tissue microarrays containing 1 mm diameter cores from 72 breast cancer cases were prepared using a Quick-Ray arrayer (Woo-Ri Medic, Seattle, WA, USA). Two tissue microarray blocks consisted of nonmetastasized cases containing three cancer cores and one normal core per case. The third tissue microarray block consisted of cases with metastasis and contained three cancer cores, two metastasis cores, and one normal core per case. After completion, the blocks were incubated at 60°C for 45 min, embedded in paraffin, cut to 1 μm thickness, and heat fixed onto glass slides, as formerly described.33
To initiate deparaffinization, tissue microarray slides were incubated for 25 min at 65°C. Sequential washes in xylene (twice for 5 min), absolute ethanol (twice for 3 min), 95% ethanol (twice for 3 min), and distilled water for 5 min were used for complete deparaffinization and rehydration. Throughout the subsequent staining process, the slides were stored in a humidified slide moat (Boekel Scientific, Feasterville, PA, USA) to prevent tissue desiccation. A Pap pen (DakoCytomation, Dako North America Inc., Carpinteria, CA, USA) was used to encircle the tissue to contain the solution.
Hyaluronidase (H3506, Sigma-Aldrich Corporation, St Louis, MO, USA) was diluted to 0.01 mg/ml in Tris buffer saline (TBS), pH 7.5 (0.2 M Tris-HCl, 0.17 M NaCl). This solution (200 μl) was placed on each slide and incubated at 25°C for 45 min. Sequentially, TBS and distilled water were used to rinse slides, which were subsequently placed into 95–99°C Target Retrieval Solution, pH 7.5, (DakoCytomation) for 40 min. The slides were permitted to cool, followed by a 25 min incubation in Dual Endogenous Enzyme Block for Autostainer (200 μl; DakoCytomation) to quench endogenous peroxidases. Slides were rinsed and immersed 3–5 times in Wash Buffer (DakoCytomation).
Antibody diluent (DakoCytomation) was used to dilute each primary antiserum to an optimal concentration, which was determined by serial dilutions prior to this study (V1a=1:200; C2=1:200; Npp=1:200; V2=1:200; V1b=1:25). The tissue was incubated in 200 μl of primary antiserum at 25°C for 1 h. After incubation, slides were rinsed, and 200 μl of an anti-rabbit and anti-mouse secondary antibody, EnVision+ Dual Link System Peroxidase (DakoCytomation), was applied to each slide and incubated for 30 min at 25°C. Following a wash step, each slide was covered with 200 μl of liquid DAB+ Substrate Chromagen System for Autostainer (DakoCytomation) and incubated for 20 min at 25°C. After a distilled water rinse, hematoxylin (7211, Richard-Allan Scientific, Kalamazoo, MI, USA) was applied to each slide for 2 min at 25°C; then the slides were rinsed thoroughly and submerged in distilled water for 5 min. Dehydration of the tissue was accomplished with consecutive ethanol-concentration steps and xylene. A negative control excluding primary antiserum and a positive control using placenta tissue were run with the tissue microarray slides for quality control.34,32
The distribution of collagen α1(XI) in tissue microarray cores was assessed by a pathologist (JDK). Staining with five antisera against specific regions of the NTD was evaluated to determine the primary spliceforms present in breast tissue. For three antisera, V1a, C2, and Npp, cores were assessed to determine if epitopes were present in cancer or normal and a score indicative of staining intensity was given to cytoplasm, stroma, and vasculature (intensity: 0=no staining, 1=weak staining, 2=intermediate staining, 3=strong staining). Staining data were collected for the three antisera with other tumor characteristics and case demographics including: age, tumor size, overall tumor grade (I, II, or III, as described in Elston and Ellis8), nuclear polymorphism, tubule formation, mitotic counts, angiolymphatic invasion, node status, estrogen receptor status, progesterone receptor status, and HER-2/neu status. If a case included multiple cores of cancer or normal, the staining intensities were averaged so that each case had only one data point for cancer and one for normal.
SPSS 15 and the SAS package were used for statistical analysis. The Pearson’s Product Moment Correlation was used to determine correlation between common pathologic parameters and lymph node status. Repeated measures analysis of variance determined cytoplasm, stroma, and vascular staining outcomes for each antiserum between cancer and normal tissue. Finally, univariate analyses were carried out to determine significance between staining of cancerous breast tissue with the three antisera against collagen α1(XI) and standard pathologic parameters.
Results
In this study, 72 cases of breast cancer were evaluated. Of these, 18 women had lymph node metastases. The average age of the patients was 63 years old (range: 34–90). Average tumor size of the cases studied was 1.9 cm with 46 patients with tumor size less than or equal to 2 cm and 25 patients with tumor size greater than 2 cm. Of those, 15 patients had angiolymphatic invasion and 55 did not. The study had 29 patients with an overall tumor grade of I, 29 patients with an overall tumor grade of II, and 10 patients with an overall tumor grade of III. In addition, there were 8 patients with a tubule formation score of 1, 18 with a tubule formation score of 2, and 39 patients with a tubule formation score of 3.
Typical clinical pathologic parameters including tumor size, angiolymphatic invasion, and tubule formation score correlated with node status (Table 2). The presence of angiolymphatic invasion directly correlated with positive node status (P=0.037). Of the patients with axillary lymph node metastases, 39% had angiolymphatic invasion. Furthermore, of the patients without metastasis, only 15% had angiolymphatic invasion. With increasing tubule formation score and tumor size, there was an increase in probability of metastasis in the breast cancer cases assessed in this study (P=0.051, P=0.037). Conversely, overall tumor grade was not found to be a significant predictor of lymph node metastasis in our study.
Table 2.
Correlation of common pathologic parameters and node status in breast carcinomas analyzed by tissue microarrays
| Parameter | Number of cases | Lymph node positive (%) | Lymph node negative (%) | Statistical significance |
|---|---|---|---|---|
| Angiolymphatic invasion | P=0.037 | |||
| Yes | 15 | 7 (39) | 8 (15) | |
| No | 55 | 11 (61) | 44 (85) | |
| Tubule formation score | P=0.051 | |||
| 1 | 8 | 1 (6) | 7 (15) | |
| 2 | 18 | 2 (12) | 16 (33) | |
| 3 | 39 | 14 (82) | 25 (52) | |
| Tumor size | P=0.037 | |||
| ≤2 cm | 46 | 8 (44) | 38 (72) | |
| >2 cm | 25 | 10 (56) | 15 (28) |
Collagen α1(XI) was variably expressed and localized to epithelial cell cytoplasm, stroma, and vascular structures within normal and cancerous breast tissue. Col11a1 mRNA was detected in normal human mammary epithelial cells by RT–PCR (Figure 1). Figure 2 illustrates the lack of staining with antisera against the V1b and V2 regions of the NTD when compared with the V1a antiserum used in this study.Table 3 summarizes the significance of staining in the regions with two antisera relative to cancer vs normal tissue. Downregulation of collagen α1(XI) in the stroma surrounding cancer epithelium compared with normal tissue was evident with V1a and Npp antisera (Figure 3; P=0.0006, P<0.0001, respectively). Antisera directed to the V1b and V2 regions of collagen α1(XI) did not detect any epitope within the tissue analyzed.Table 4 reports the statistically significant findings when staining intensity in cancerous breast tissue was compared to the standard pathological parameters, including node status. There was an inverse relationship observed between V1a antiserum cytoplasmic staining and node status; negative node status cases had mean staining intensity of 2 and positive node status cases had mean staining intensity of 1.5 (Figure 3; P=0.009). Stromal staining with Npp antiserum in cancerous tissue demonstrated significance with tubule formation score in an inverse manner with more differentiated glandular tissue having more staining (P=0.015).
Figure 2.
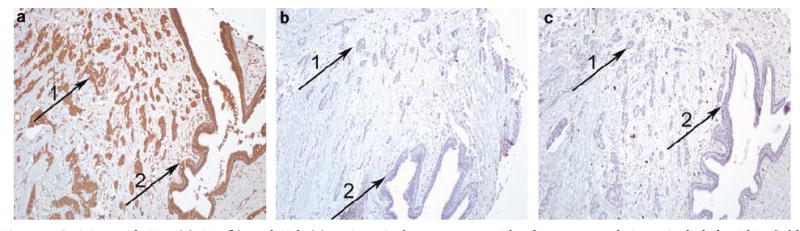
Staining with V1a (a), V2 (b), and V1b (c) antisera in breast cancer with adjacent normal tissue included within field. Immunostaining: original magnification × 100.
Table 3.
Staining intensity and location in normal vs cancerous tissue for the V1a and the Npp antisera to epitopes of Collagen α1(XI) N-terminal domain
| Antiserum and stain location | Tissue type | Mean of staining intensity | Statistical significance |
|---|---|---|---|
| V1a cytoplasm | Normal | 2 | NS |
| Cancer | 2 | ||
| V1a stroma | Normal | 0.5 | P=0.0006 |
| Cancer | 0 | ||
| V1a vascular | Normal | 1.5 | NS |
| Cancer | 1 | ||
| Npp cytoplasm | Normal | 1.5 | NS |
| Cancer | 1.5 | ||
| Npp stroma | Normal | 1 | P<0.0001 |
| Cancer | 0 | ||
| Npp vascular | Normal | 1.0 | NS |
| Cancer | 1.0 |
Npp, amino propeptide; NS, nonsignificant.
Figure 3.
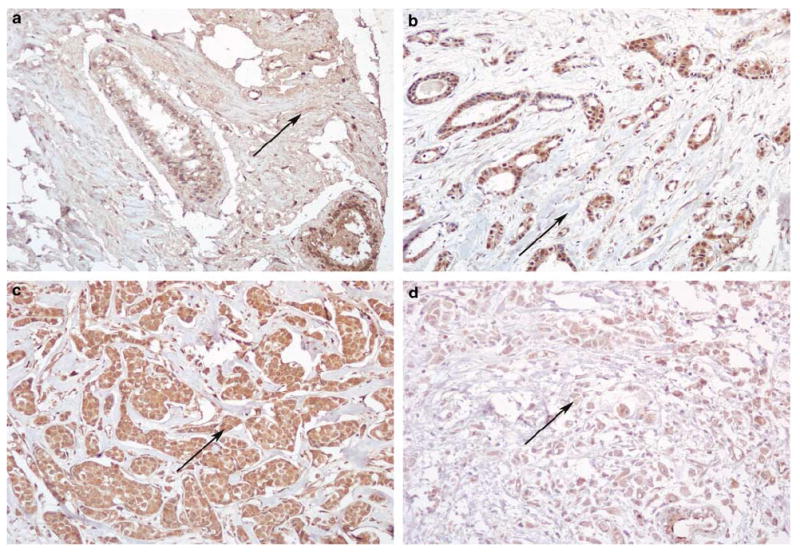
Stromal staining with antiserum Npp in normal (a) and cancerous (b) human breast tissue. Cytoplasmic staining with V1a in primary breast tumors of patients with nonmetastatic (c) and metastatic (d) disease. Immunostaining: original magnification × 200.
Table 4.
Statistically significant relationships between staining intensity and established pathologic parameters
| Antiserum and stain location | Parameter assessed | Number of samples | Mean stain intensity | Statistical significance |
|---|---|---|---|---|
| Npp stroma in cancer | Tubule formation score | P=0.015 | ||
| 1 | 8 | 0.5 | ||
| 2 | 15 | 0 | ||
| 3 | 39 | 0 | ||
| Npp cytoplasm in cancer | Progesterone receptor | P=0.044 | ||
| Positive | 28 | 1 | ||
| Negative | 40 | 1.5 | ||
| Npp cytoplasm in cancer | Estrogen receptor | P=0.012 | ||
| Positive | 35 | 1 | ||
| Negative | 32 | 1.5 | ||
| V1a cytoplasm in cancer | Node status | P=0.009 | ||
| Positive | 17 | 1.5 | ||
| Negative | 52 | 2 |
Npp, amino propeptide.
Collagen XI staining was also compared to progesterone and estrogen receptor status for comparison (Figure 4). Cytoplasmic staining with Npp antiserum exhibited significance and an inverse relationship when assessed relative to positive estrogen receptor status (P=0.012) and to progesterone status (P=0.044).
Figure 4.
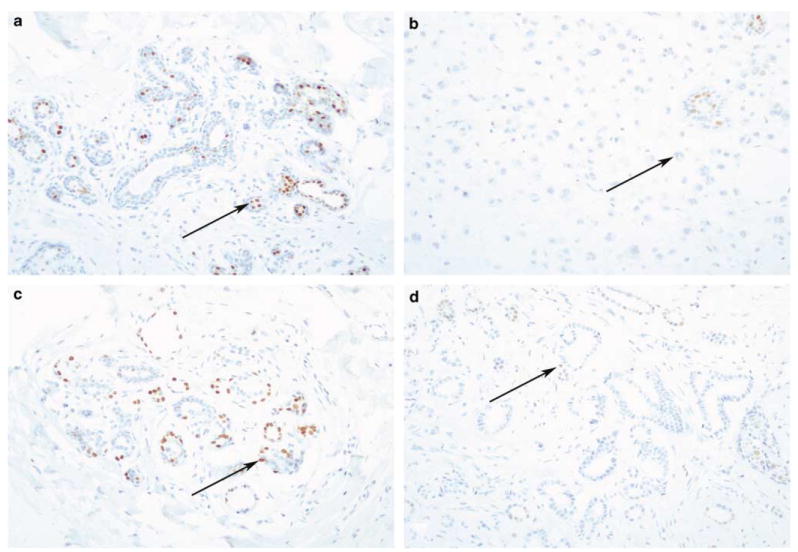
Estrogen and progesterone receptor staining for comparison. Estrogen receptor positivity in normal (a) and cancerous (b) human breast tissue. Progesterone receptor positivity in normal (c) and cancerous (d) human breast tissue. Immunostaining: original magnification × 200.
Discussion
Collagen XI is a critical regulatory protein primarily associated with fibril formation and limitation of fibril growth. It exists in several spliceforms, which have not been studied previously in breast cancer. The aim of this study was to determine if a correlation exists between collagen α1(XI) and the progression stage of breast cancer. Collagens have been shown to change in neoplasms, and detection of change in expression may lead to the development of novel diagnostic and/or prognostic tools.
To confirm the validity of this study, known predictors of node status in breast cancer patients were evaluated. It was found that the presence of angiolymphatic invasion correlated with positive node status suggesting the two are directly related and indicate poor prognosis. Previous studies corroborated our results.2-7 Other pathologic clinical parameters have been evaluated in earlier studies, which suggest direct relationships between node status and tubule formation score, tumor size, and angiolymphatic invasion.3,5,6 Although overall tumor grade was not found to be a significant predictor of lymph node metastasis in our study, it has been proven in earlier studies to have significance in relation to node status.3,5-7 It is possible that significance was not found due to small sample size of 72 patients.
Cytoplasmic, stromal, and vascular staining was observed with three Col11a1 antisera. Collagen XI is an expected component of stroma. Current studies are underway in our laboratory to understand the cytoplasmic localization that has been observed in some cases. It is possible that the NTD region once cleaved from the major triple helix, may be involved in other cellular processes or alternatively, may be taken up by cells and targeted for degradation and turnover. Alternatively, the data may indicate that the protein accumulates within the cell rather than being properly secreted. Previous studies have identified a type V/XI hybrid molecule in cartilage canals, perichondral invaginations of blood vessels, and blood vessels (36 and unpublished observations).
A relationship between the staining results of cancer vs normal tissue demonstrated significance with two antisera staining the stroma. Staining intensity of stroma with V1a and Npp antisera was significant relative to cancer vs normal, with stroma surrounding normal epithelium consistently staining more intense than that surrounding cancerous epithelium. This result is consistent with previous findings that suggest modification in collagen metabolism of cancerous breast tissue may be a result of tissue remodeling in invasive malignancies.24 On the contrary, various other cancers fail to exhibit this same pattern of downregulation. Studies of prostate cancer, lung cancer, and colorectal cancer have reported upregulation of collagen in cancerous tissue when compared with normal tissue.25-29 However, collagen XI was specifically considered only in colon cancer and non-small-cell lung cancer.25,29 Other collagens studied in relation to cancer include collagen XVIII, a precursor to angiogenesis inhibitor endostatin, which was shown to indicate poor prognosis and progression of non-small-cell lung cancer when overexpressed in the malignancy.26 In addition, collagen XXIII, was suggested as a biomarker of prostate cancer progression and metastasis.27 Increased collagen XXIII expression in prostate cancer tissue was demonstrated, and it was suggested that the amount of expression is a significant independent predictor of prostate-specific antigen defined disease recurrence.27 However, collagen expression in cancer decreased with increased Gleason sum in a prostate cancer study, which indicated that collagen content in the cancer decreased whereas the collagen levels in the adjacent normal tissue was dramatically upregulated when compared with control normal prostate from a patient without cancer.28 The increased collagen content in surrounding tissue was hypothesized to be due to a host response against the tumor. Clinically, it was suggested to be a predictor of patient survival.28
In prior breast cancer studies, collagen was downregulated and in an earlier prostate cancer study similar findings were demonstrated.24,28 These findings support our results that collagen α1(XI) staining in stroma surrounding carcinoma is decreased as compared to stroma surrounding normal epithelium.
Not only were staining results of cancer vs normal tissue assessed, but we also looked at staining intensity as a predictor of common pathologic parameters including node status. Collagen α1(XI) staining with V1a antiserum in epithelial cytoplasm demonstrated decreased intensity in primary tumors that had metastasized when compared with tumors that did not metastasize. These results suggest a downregulation of collagen α1(XI) in more advanced tumors. This exhibits potential for further study that may lead to developing collagen XI as a marker for metastasis. The inverse relationship between intensity of cytoplasmic staining in cancerous tissue with V1a antiserum and tumor size may indicate that as a tumor progresses the collagen XI content decreases.
Because cytoplasmic staining with Npp antisera in cancerous tissue demonstrated an inverse relationship to positive estrogen receptor status and cytoplasmic staining with Npp antiserum exhibited significance relative to progesterone status, it may indicate that well-differentiated tumors with more positive estrogen and progesterone receptors have a relatively high expression of collagen XI. It appears that as the tumor becomes less differentiated, collagen XI expression declines. The lack of collagen XI, a regulatory molecule, may contribute to the uncontrolled growth of the tumor. Stromal staining with Npp antiserum in cancerous tissue demonstrated significance with tubule formation score demonstrating a trend with well-differentiated cancers of tubule formation score of 1 having more staining than less differentiated tumors of tubule formation score of 2 and 3. This inverse relationship between differentiation and collagen XI expression may indicate that collagen XI must be downregulated for tumors to become less differentiated.
In the future, it would also be valuable to stain normal breast tissue from patients without breast cancer for comparison. In a previous study, normal prostate from cancer-free patients was assessed in addition to cancer and normal tissue from a patient with prostate cancer.28 In addition, further study is warranted to determine the mechanism underlying downregulation of collagen XI in cancer when compared with normal breast tissue. Because collagen XI appears to be present in normal tissue at higher levels, this suggests downregulation in cancerous tissue as a desmoplastic response that although significant statistically, its biological significance is unknown. It may also be valuable to look at collagen α1(XI) content of intraductal carcinoma, the precursor to breast carcinoma, in normal and cancer tissue.
In conclusion, collagen α1(XI) is expressed in epithelial cells, stroma, and vessels of normal and cancerous breast tissue. The spliceform containing the region encoded by exon 6a, but excluding regions encoded by exon 6b and exon 8 appears to be the most prevalent form expressed in breast tissue. Collagen α1(XI) stromal expression is markedly decreased in breast carcinoma as compared to patient matched normal tissue suggesting the possibility of use as a future diagnostic tool. Additionally, quantification of cytoplasmic and stromal expression of collagen XI may also be a valuable tool in diagnosing lymph node status in patients providing an alternative to invasive techniques such as sentinel node biopsies. Further studies will be necessary to validate our findings.
Acknowledgments
We thank Dorthyann Isaackson, Patrick Aranda and Raquel Brown for their technical support. This publication was made possible by NIH Grant no. P20RR016454 from the INBRE Program of the National Center for Research Resources and support from the Boise State University Lori and Duane Stueckle Deans Distinguished Professorship.
Footnotes
Disclosure/conflict of interest The authors declare no conflict of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Gajdos C, Tartter PI, Bleiweiss IJ. Lymphatic invasion, tumor size, and age are independent predictors of axillary lymph node metastases in women with T1 breast cancers. Ann Surg. 1999;230:692–696. doi: 10.1097/00000658-199911000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markopoulos C, Kouskos E, Gogas H, et al. Factors affecting axillary lymph node metastases in patients with T1 breast carcinoma. Am Surg. 2000;66:1011–1013. [PubMed] [Google Scholar]
- 4.Rivadeneira DE, Simmons RM, Christos PJ, et al. Predictive factors associated with axillary lymph node metastases in T1a and T1b breast carcinomas: analysis in more than 900 patients. J Am Coll Surg. 2000;191:1–6. doi: 10.1016/s1072-7515(00)00310-0. [DOI] [PubMed] [Google Scholar]
- 5.Brenin DR, Manasseh DM, El-Tamer M, et al. Factors correlating with lymph node metastases in patients with T1 breast cancer. Ann Surg Oncol. 2001;8:432–437. doi: 10.1007/s10434-001-0432-7. [DOI] [PubMed] [Google Scholar]
- 6.Barth A, Craig PH, Silverstein MJ. Predictors of axillary lymph node metastases in patients with T1 breast carcinoma. Cancer. 1997;9:1918–1922. [PubMed] [Google Scholar]
- 7.Bader AA, Tio J, Petru E, et al. T1 breast cancer: identification of patients at low risk of axillary lymph node metastases. Breast Cancer Res Treat. 2002;76:11–17. doi: 10.1023/a:1020231300974. [DOI] [PubMed] [Google Scholar]
- 8.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 9.Yoshioka H, Iyama K-I, Inoguchi K, et al. Developmental pattern of expression of the mouse α1(XI) collagen gene (Col11a1) Dev Dyn. 1995;204:41–47. doi: 10.1002/aja.1002040106. [DOI] [PubMed] [Google Scholar]
- 10.Miller EJ, Gay S. The collagens: an overview and update. Methods Enzymol. 1987;144:3–41. doi: 10.1016/0076-6879(87)44170-0. [DOI] [PubMed] [Google Scholar]
- 11.Mendler M, Eich-Bender SG, Vaughan L, et al. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989;108:191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Lacerda DA, Warman ML, et al. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell. 1995;80:423–430. doi: 10.1016/0092-8674(95)90492-1. [DOI] [PubMed] [Google Scholar]
- 13.Wu J-J, Eyre DR. Structural analysis of cross-linking domains in cartilage type XI collagen: insights on polymeric assembly. J Biol Chem. 1995;270:18865–18870. doi: 10.1074/jbc.270.32.18865. [DOI] [PubMed] [Google Scholar]
- 14.Morris NP, Bächinger HP. Type XI collagen is a heterotrimer with the composition (1α,2α,3α) retaining non-triple-helical domains. J Biol Chem. 1987;262:11345–11350. [PubMed] [Google Scholar]
- 15.Burgeson RE, Hollister DW. Collagen heterogeneity in human cartilage: identification of several new collagen chains. Biochem Biophys Res Commun. 1979;87:1124–1131. doi: 10.1016/s0006-291x(79)80024-8. [DOI] [PubMed] [Google Scholar]
- 16.Sussman MD, Ogle RC, Balian G. Biosynthesis and processing of collagens in different cartilaginous tissues. J Orthop Res. 1984;2:134–142. doi: 10.1002/jor.1100020204. [DOI] [PubMed] [Google Scholar]
- 17.Thom JR, Morris NP. Biosynthesis and proteolytic processing of type XI collagen in embryonic chick sterna. J Biol Chem. 1991;266:7262–7269. [PubMed] [Google Scholar]
- 18.Zhidkova NI, Justice SK, Mayne R. Alternative mRNA processing occurs in the variable region of the pro-α1(XI) and pro-α2(XI) collagen chains. J Biol Chem. 1995;270:9486–9493. doi: 10.1074/jbc.270.16.9486. [DOI] [PubMed] [Google Scholar]
- 19.Oxford JT, Doege KJ, Morris NP. Alternative exon splicing within the amino-terminal nontriple-helical domain of the rat pro-α1(XI) collagen chain generates multiple forms of the mRNA transcript which exhibit tissue-dependent variation. J Biol Chem. 1995;270:9478–9485. doi: 10.1074/jbc.270.16.9478. [DOI] [PubMed] [Google Scholar]
- 20.Keene DR, Oxford JT, Morris NP. Ultrastructural localization of collagen types II, IX, and XI in the growth plate of human rib and fetal bovine epiphyseal cartilage: type XI collagen is restricted to thin fibrils. J Histochem Cytochem. 1995;43:967–979. doi: 10.1177/43.10.7560887. [DOI] [PubMed] [Google Scholar]
- 21.Gregory KE, Oxford JT, Chen Y, et al. Structural organization of distinct domains within the non-collagenous N-terminal region of collagen type XI. J Biol Chem. 2000;275:11498–11506. doi: 10.1074/jbc.275.15.11498. [DOI] [PubMed] [Google Scholar]
- 22.Turashvili G, Bouchal J, Baumforth K, et al. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdis-section and microarray analysis. BMC Cancer. 2007;7:55. doi: 10.1186/1471-2407-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Y, Sun B, Li X, et al. Differentially expressed genes between primary cancer and paired lymph node metastases predict clinical outcome of node-positive breast cancer patients. Breast Cancer Res Treat. 2007;103:319–329. doi: 10.1007/s10549-006-9385-7. [DOI] [PubMed] [Google Scholar]
- 24.Cechowska-Pasko M, lstrok;ka J, Wojtukiewicz MZ. Enhanced prolidase activity and decreased collagen content in breast cancer tissue. Int J Exp Pathol. 2006;87:289–296. doi: 10.1111/j.1365-2613.2006.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer H, Stenling R, Rubio C, et al. Colorectal carcinogenesis is associated with stromal expression of COL11A1 and COL5A2. Carcinogenesis. 2001;22:875–878. doi: 10.1093/carcin/22.6.875. [DOI] [PubMed] [Google Scholar]
- 26.Chang H, Iizasa T, Shibuya K, et al. TI: increased expression of collagen XVIII and its prognostic value in nonsmall cell lung carcinoma. Cancer. 2004;100:1665–1672. doi: 10.1002/cncr.20156. [DOI] [PubMed] [Google Scholar]
- 27.Banyard J, Bao L, Hofer MD, et al. Collagen XXIII expression is associated with prostate cancer recurrence and distant metastases. Clinical Cancer Res. 2007;13:2634–2642. doi: 10.1158/1078-0432.CCR-06-2163. [DOI] [PubMed] [Google Scholar]
- 28.Burns-Cox N, Avery NC, Gingell JC, et al. Changes in collagen metabolism in prostate cancer: a host response that may alter progression. J Urol. 2001;166:1698–1701. doi: 10.1016/s0022-5347(05)65656-x. [DOI] [PubMed] [Google Scholar]
- 29.Wang KK, Liu N, Tadulovich N, et al. Novel candidate tumor marker genes for lung adenocarcinoma. Oncogene. 2002;21:7598–7604. doi: 10.1038/sj.onc.1205953. [DOI] [PubMed] [Google Scholar]
- Morris NP, Oxford JT, Gillian BM, et al. Developmentally regulated alternative splicing of the α1(XI) collagen chain: spatial and temporal segregation of isoforms in the cartilage of fetal rat long bones. J Histochem Cytochem. 2000;48:725–741. doi: 10.1177/002215540004800601. [DOI] [PubMed] [Google Scholar]
- 31.Oxford JT, Doege KJ, Horton WE, Jr, et al. Characterization of type II and type XI collagen synthesis by an immortalized rat chondrocite cell line (IRC) having a low level of type II collagen mRNA expression. Exp Cell Res. 1994;213:28–36. doi: 10.1006/excr.1994.1169. [DOI] [PubMed] [Google Scholar]
- 32.Davies GBM, Oxford JT, Hausafus LC, et al. Temporal and spatial expression of alternative splice-forms of the α1(XI) collagen gene in fetal rat cartilage. Dev Dyn. 1998;213:12–26. doi: 10.1002/(SICI)1097-0177(199809)213:1<12::AID-AJA2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 33.Fyffe WE, Kronz JD, Edmonds PA, et al. Effect of high-level oxygen exposure on the peroxidase activity and the neuromelanin-like pigment content of the nerve net in the earthworm, Lumbricus terrestris. Cell Tissue Res. 1999;295:349–354. doi: 10.1007/s004410051241. [DOI] [PubMed] [Google Scholar]
- 34.Bernard M, Yoshioka H, Rodriguez E, et al. Cloning and sequencing of pro-α1(XI) collagen cDNA demonstrates that type XI belongs to the fibrillar class of collagens and reveals that the expression of the gene is not restricted to cartilaginous tissue. J Biol Chem. 1988;263:17159–17166. [PubMed] [Google Scholar]


