Abstract
Recent studies have demonstrated the presence of the (pro)renin receptor (PRR) in the glomerular mesangium and the subendothelial layer of the renal arteries. We hypothesized that diabetes upregulates PRR expression through enhanced angiotensin subtype 1 (AT1) receptor–NADPH oxidase cascade activity. Using real-time polymerase chain reaction, Western blot analysis and immunostaining, we studied renal localization of the PRR in the streptozotocin-induced diabetic rat model and in response to 1 week of treatment with the AT1 receptor blocker valsartan (10 mg kg−1 day−1), the angiotensin AT2 receptor blocker PD123319 (0.5 mg kg−1 day−1) or the NADPH oxidase inhibitor diphenylene iodonium (DPI; 0.5 mg kg−1 day−1) 6 weeks post-induction of diabetes. Both PRR mRNA and protein were expressed constitutively in the kidneys of normal rat renal cortex and medulla, mainly in glomerular mesangium, proximal, distal and collecting tubules. Compared with normal rats (100%), diabetic rats demonstrated an increase in renal PRR mRNA (184%), protein (228%) and immunostaining. Valsartan and DPI prevented the increase in the PRR mRNA (106 and 126%, respectively), protein (97 and 140%, respectively) and immunostaining that was seen in the kidneys of diabetic rats. The AT2 blocker PD123319 did not have significant effects on PRR mRNA (157%) or protein expression (200%) in the kidneys of diabetic rats. These results demonstrate that the PRR is constitutively expressed in renal glomeruli and tubules. Expression of the PRR is upregulated in diabetes via enhancement of AT1 receptor–NADPH oxidase activity.
All components of the renin–angiotensin system (RAS) are present within the kidney (Siragy, 2006). Previous studies have demonstrated the contribution of this system to the development of kidney disease in diabetes (Andersen et al. 2000; Chan et al. 2000; Brenner et al. 2001; Lewis et al., 2001; Carey & Siragy, 2003). However, the mechanisms involved in the pathophysiology of this clinically relevant problem are still not well elucidated.
Recently, a (pro)renin receptor (PRR) was discovered as a new component of the RAS (Nguyen et al. 2002). This receptor has been associated with prorenin/renin uptake, non-proteolytic activation of prorenin and local production of angiotensin I (Nguyen et al. 2002; Nabi et al. 2006; Nguyen, 2007). The PRR was reported to be expressed in the glomerular mesangial cells and subendothelial space of the renal vasculature (Nguyen et al. 2002) but not in renal tubules.
Angiotensinogen and angiotensin-converting enzyme are present in the renal tubular cells (Tang et al. 1995; Imig et al. 1999; Ingelfinger et al. 1999; Harrison-Bernard et al. 2002). The presence of PRR in renal tubules may contribute to tubular generation of angiotensin II through enhanced conversion of locally produced angiotensinogen to angiotensin I (Kobori et al. 2007). Angiotensin subtype 1 receptors (AT1 receptors) are also present in renal glomeruli and tubules (Siragy, 2006) and in close proximity to sites of angiotensin II formation. Excess of AT1 receptors and oxidative stress activities contribute to development of renal and cardiac diseases (Onozato et al. 2002; Privratsky et al. 2003).
A recent study demonstrated that PRR blockade improves kidney disease in diabetes (Ichihara et al. 2006). However, the interaction between diabetes and renal expression of the PRR is not known. Similarly, it is not known whether oxidative stress associated with diabetes influences PRR expression. It is plausible that both AT1 receptors and oxidative stress lead to increased expression of the PRR in different renal regions, such as glomeruli and tubules, a process that could further enhance the development of renal disease in diabetes. In the present study, we hypothesized that in addition to glomerular mesangium and renal vasculature, PRR is expressed in the renal tubules and is upregulated in diabetes via enhanced angiotensin AT1 receptor and oxidative stress activities. Our results confirm that PRR is expressed not only in glomerular mesangium but also in renal tubules. The PRR expression is upregulated in diabetes, a process that can be suppressed by angiotensin AT1 receptor blockade and inhibition of NADPH oxidase activity.
Methods
Animal preparation and treatment
The experiments were approved by the University of Virginia Animal Research Committee and conducted in accordance with institutional guidelines. Sprague–Dawley rats were divided randomly into control and diabetic groups. Diabetes was induced by streptozotocin (STZ; 65 mg/kg i.p.). Studies were conducted for 6 weeks. At the end of the fifth week, an osmotic mini-pump filled with vehicle (0.9% NaCl; control group, n = 8), valsartan (10 mg kg−1 day−1; diabetes + valsartan group, n = 8), PD123319 (0.5 mg kg−1 day−1; diabetes + PD123319 group, n = 8) or diphenylene iodonium (DPI; 0.5 mg kg−1 day−1; diabetes + DPI group, n = 8) was implanted into the peritoneal cavities of the studied rats. At the end of the sixth week of the study, rats’ body weight was recorded and blood samples were collected. The kidneys were removed under anesthesia (Ketamine 60 mg kg−1, I.P.) and stored in −80°C for quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR), Western blot analysis and immunostaining.
Quantitative real-time RT-PCR
After removal of the kidneys, the renal tissue was weighed promptly and homogenized on ice. The total renal RNA was extracted using RNeasy Kit (Qiagen, Valencia, CA, USA). The quality of RNA was confirmed by ethidium bromide staining in 1% formaldehyde agarose gel. Single-stranded cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Gene-specific primers were as follows: for (pro)renin receptor, forward sequence 5′-GAGGCAGTGACCCTCAACAT-3′ and reverse sequence 5′-CCCTCCTCACACAACAAGGT-3′; and for β-actin, forward sequence 5′-AGCCATGTACGTAGCCATCC-3′ and reverse sequence 5′-ACCCTCATAGATGGGCACAG-3′. The specificities of the primers were verified by melting curves (iCycler; Bio-Rad) and amplified product size using agarose gel electrophoresis. Quantitative real-time RT-PCR was performed using iCycler (Bio-Rad), and threshold cycle number was determined using iCycler software version 3.0 (Bio-Rad). Reactions were performed in triplicate, and threshold cycle numbers were averaged. Non-template control was used as negative control. The mRNA results were normalized to β-actin mRNA. Fold-downexpression or -upexpression was calculated according to the formula 2(Rt − Et)/2(Rn − En), where Rt is the threshold cycle number for the reference gene observed in the test sample, Et is the threshold cycle number for the experimental gene observed in the test sample, Rn is the threshold cycle number for the reference gene observed in the control sample and En is the threshold cycle number for the experimental gene observed in the test sample.
Western blot analysis
Kidney tissue (n = 8) was homogenized in lysis buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 2 mM EDTA, 0.1% sodium dodecyl sulphate, 1% IGEPAL CA-630, 0.5% deoxycholate sodium, 20 μM MG132 (CalBiochem, La Jolla, CA, USA), 50 mM sodium fluoride (NaF), 2 mM sodium orthovanadate, 1 mM phenylmethylsulphonic fluoride (PMSF) and 1 × dilution protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA)] to form a crude homogenate. Crude lysates were cleared by centrifugation at 22 000 g, 4°C for 20 min. Protein quantification of whole cell lysate was performed using the BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA). A total of 50 μg of cell lysate was loaded for each sample and separated on a 4–20% Tris-HCl Criterion precast gel (Bio-Rad, Hercules, CA, USA), followed by blotting of the proteins on polyvinylidene fluoride membrane (Bio-Rad). Antibody to PRR (Abcam, Cambridge, MA, USA) was used in the Western blot (Kaneshiro, 2007). Signal detection was carried out by using Super Signal West Pico Chemiluminescent Subtract (Pierce Biotechnology, Rockford, IL, USA). The blot was treated with Restore Western Blot Stripping Buffer (Pierce Biotechnology) according to the manufacturer’s recommendation, followed by reprobing with a monoclonal antibody against β-actin (Sigma, St Louis, MO, USA). Densitometry of the bands was done using Image MasterTM TotalLab version 2.0 (Amersham, Piscataway, NJ, USA). The band density of PRR was normalized to the corresponding density of β-actin. The arbitrary unit of band densities was represented as the expression level of PRR.
Immunostaining for (pro)renin receptor
Immunostaining was performed for PRR localization in the kidney. Briefly, the frozen kidneys were taken out from −80°C storage and placed into a −18°C cryostat chamber for 1 h. Each tissue sample was mounted with OCT (an embedding medium) on a microtome block with designed cutting orientation. The block was held firmly in the cryostat microtome and 2–4-μm-thick sections were cut. Sections were then picked up on histological slides and immediately dipped into Streck Tissue Fixative (NE68128; Streck Laboratories, La Vista, NE, USA) at −4°C for 15 min, transferred into phosphate-buffered saline at −4°C for 30 min, and preabsorbed in 5% goat serum for 20 min before the immunostaining process. The immunostaining was performed by incubating with polyclonal rabbit anti-(pro)renin receptor antibody at 4°C overnight, followed by 1 h of incubation with secondary antibody conjugated with biotin at room temperature (Sigma). Immunoreactive signal was detected with an avidin–biotin immunoperoxidase reaction (Sigma) and visualized by exposure to diaminobenzidine (Sigma). The non-specific binding was controlled by using the mouse immunoglobulin G isotype (Sigma) as a primary antibody for negative control.
Statistical analysis
Comparisons among different treatment groups were examined by ANOVA, including a repeated-measures term, using the general linear models procedure of the Statistical Analysis System (SAS Software, SAS Institute, Cary, NC, USA). Data are expressed as means ± S.E.M. P < 0.05 was considered statistically significant.
Results
Body weight and blood glucose levels
After 6 weeks of STZ-induced diabetes, body weights of rats in the diabetes groups (227.4 ± 21.37 g) were significantly less (P < 0.01) compared with normal control rats (392.3 ± 16.31 g). There were no significant differences in body weight among different treatments of the diabetes groups: the AT1 receptor blocker valsartan (230.9 ± 17.28 g); the AT2 receptor blocker PD123319 (219.8 ± 23.78 g); and the NADPH oxidase inhibitor DPI (231.6 ± 17.34 g).
The blood glucose levels were 69.4 ± 19.3 mg dl−1 in normal rats, 323.3 ± 134.3 mg dl−1 in diabetic rats (P < 0.01), 312.2 ± 109.8 mg dl−1 in diabetic rats treated with valsartan (P < 0.01), 331.9 ± 124.2 mg dl−1 in diabetic rats treated with PD123319 (P < 0.01) and 309.1 ± 98.39 mg dl−1 in diabetic rats treated with DPI. There were no significant differences in blood glucose levels among diabetic rats treated with valsartan, PD123319 or DPI.
Expression of PRR in the kidney of diabetic rats
The expression of PRR mRNA (Fig. 1A) and protein (Fig. 1B) was significantly higher in diabetic compared with normal rats. Valsartan and DPI significantly decreased PRR mRNA and protein expressions. Treatment with PD123319 had no significant effect on PRR mRNA or protein expressions in diabetic rats.
Figure 1. Expressions of renal PRR mRNA (A) and protein (B) in normal and diabetic rats and in response to AT1 receptor blockade with valsartan (Val), AT2 receptor blockade with PD123319 (PD) or NADPH oxidase inhibition with DPI.

Data represent two rats from each group, while the total number of animals studied is 8 per group. Lower panel represents densometric analysis of the PRR Western blots (n = 8 each group). *P < 0.05 versus normal rats; **P < 0.05 and ***P < 0.01 versus untreated diabetic rats.
Effect of valsartan, PD123319 and DPI on glomerular and tubular expression of PRR
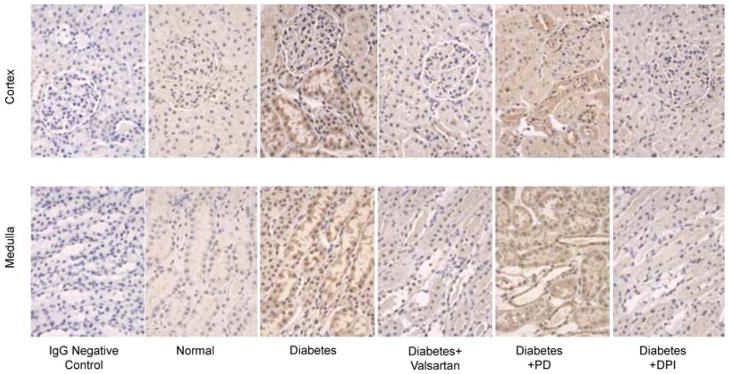
Figure 2 shows a representative immunostaining for PRR in renal cortex and medulla. In normal animals, PRR immunostaining showed a distribution pattern in glomeruli and renal tubules in cortex and medulla. Compared with normal rats, immunostaining for PRR increased significantly in glomeruli and tubules of the diabetic animals. The PRR immunostaining was significantly decreased in the renal cortex and medulla of diabetic rats treated with valsartan or DPI compared with untreated diabetic animals. Treatment of diabetic rats with PD123319 did not influence PRR immunostaining and was not different from PRR immunostaining in untreated diabetic rats.
Figure 2. Representative PRR immunostaining.
Upper panel represents rat renal cortex and lower panel represents renal medulla. Brown color indicates PRR immunostaining in glomerular mesangium and tubules. Diabetes increased PRR immunostaining in the renal cortex and medulla, while valsartan and DPI treatments were associated with reduced immunostaining of this receptor in diabetic rats. There were no significant changes in PRR immunostaining in response to PD123319 (PD) treatment.
Discussion
Our studies demonstrated that in basal conditions, PRR is expressed in renal glomeruli and tubules. This finding was confirmed by RT-PCR, Western blot analysis and immunostaining. The localization of these receptors to specific kidney sites may suggest their involvement in physiological regulation of renal haemodynamic and excretory functions and possible contribution to the development of renal diseases. At the present time, since there are no available PRR knockout animal models, the exact functions of these receptors remain largely unknown. Inhibitors PRR, although not commercially available, can be constructed (Ichihara et al. 2006). Recent studies reported the contribution of the PRR to the conversion of angiotensinogen to angiotensin I at the cell surfaces which express these receptors (Nguyen et al. 2002, 2007; Nabi et al. 2006). This solid-state formation of angiotensin I seems to be more efficient than the process of its formation in the circulation (Danser & Deinum, 2005).
Our data also show an upregulation of PRR in the STZ-induced diabetic rat model. Expression of PRR mRNA and protein was significantly increased 6 weeks after development of diabetes. The renal upregulation of PRR in diabetes suggests that this receptor may play a role in the development of renal complications of this disease. Using a decoy peptide to block the PRR in a diabetic mouse model led to significant reduction in the development of glomerulosclerosis (Ichihara et al. 2006). In the present study, we could not conclude whether the increased blood glucose or the decrease in insulin availability was responsible for the increased expression of the PRR in our STZ-induced diabetic rat model.
Our study demonstrated that AT1 receptor blockade with valsartan significantly inhibits the renal expressions of PRR mRNA and protein in the diabetic rats. The exact mechanisms responsible for the reduction of renal PRR expression by this treatment are unknown at the present time. The AT1 receptor regulates renin production through what is known as the short-loop negative feedback mechanism, with decreased renin production during stimulation of this receptor (Kurtz & Wagner, 1999). Thus, it is possible that the increased production of prorenin/renin during AT1 receptor blockade contributes to the downregulation of PRR expression. Another possibility that could contribute to upregulation of the PRR is related to increased angiotensin II production and expression of AT1 receptors in diabetes (Siragy et al. 2003; Sodhi et al. 2003), leading to enhanced oxidative stress by increasing NADPH oxidase activity (Privratsky et al. 2003; Wilcox, 2003). Blockade of AT1 receptors reduces oxidative stress and superoxide production in the kidney of STZ-induced diabetic rats (Onozato et al. 2002; Onozato & Toji, 2005). Our study clearly demonstrated that blockade of NADPH oxidase activity led to downregulation of PRR expression in the kidney. In contrast to the AT1 receptor, AT2 receptor blockade does not seem to influence PRR expression in our diabetic rat model and could be related to a reduction in expression of this receptor in diabetes (Wehbi et al. 2001).
In conclusion, this study demonstrated that PRR is constitutively expressed in renal glomeruli and tubules. In diabetes, PRR expression is upregulated through enhancement of AT1 receptor and NADPH oxidase activities.
Acknowledgments
This study was supported by grant DK-078757 and HL091535 from the National Institutes of Health to Helmy M. Siragy, MD. Dr Siragy was the recipient of Research Career Development Award K04-HL-03006 from the National Institutes of Health.
References
- Andersen S, Tarnowl L, Rossing P, Hansen BV, Parving HH. Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney Int. 2000;57:601–606. doi: 10.1046/j.1523-1755.2000.00880.x. [DOI] [PubMed] [Google Scholar]
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes mellitus and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- Carey RM, Siragy HM. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab. 2003;14:274–281. doi: 10.1016/s1043-2760(03)00111-5. [DOI] [PubMed] [Google Scholar]
- Chan JC, Ko JG, Leung DH, Cheung RC, Cheung MY, So WY, Swaninathan R, Nicholls MG, Critchley JA, Cockram CS. Long-term effects of angiotensin-converting enzyme inhibition and metabolic control in hypertensive type 2 diabetic patients. Kidney Int. 2000;57:590–600. doi: 10.1046/j.1523-1755.2000.00879.x. [DOI] [PubMed] [Google Scholar]
- Danser AH, Deinum J. Renin, prorenin and the putative (pro)renin receptor. Hypertension. 2005;46:1069–1076. doi: 10.1161/01.HYP.0000186329.92187.2e. [DOI] [PubMed] [Google Scholar]
- Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT1 receptor and ACE binding in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F19–F25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara A, Suzuki F, Nakagawa T, Kaneshiro Y, Takemitsu T, Sakoda M, Nabi AH, Nishiyama A, Sugaya T, Hayashi M, Inagami T. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol. 2006;17:1950–1961. doi: 10.1681/ASN.2006010029. [DOI] [PubMed] [Google Scholar]
- Imig JD, Navar GL, Zou LX, O’Reilly KC, Allen PL, Kaysen JH, Hammond TG, Navar LG. Renal endosomes contain angiotensin peptides, converting enzyme, and AT1A receptors. Am J Physiol Renal Physiol. 1999;277:F303–F311. doi: 10.1152/ajprenal.1999.277.2.F303. [DOI] [PubMed] [Google Scholar]
- Ingelfinger JR, Jung F, Diamant D, Haveran L, Lee E, Brem A, Tang SS. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol Renal Physiol. 1999;276:F218–F227. doi: 10.1152/ajprenal.1999.276.2.F218. [DOI] [PubMed] [Google Scholar]
- Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H. Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol. 2007;18:1789–1795. doi: 10.1681/ASN.2006091062. [DOI] [PubMed] [Google Scholar]
- Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol. 2007;293:F938–945. doi: 10.1152/ajprenal.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A, Wagner C. Regulation of renin secretion by angiotensin II-AT1 receptor. J Am Soc Nephrol. 1999;10(Suppl 11):S162–S168. [PubMed] [Google Scholar]
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- Nabi AH, Kageshima A, Uddin MN, Nakagawa T, Park EY, Suzuki F. Binding properties of rat prorenin and renin to the recombinant rat renin/prorenin receptor prepared by a baculovirus expression system. Int J Mol Med. 2006;18:483–488. [PubMed] [Google Scholar]
- Nguyen G. The (pro)renin receptor: pathophysiological roles in cardiovascular and renal pathology. Curr Opin Nephrol Hypertens. 2007;16:129–133. doi: 10.1097/MNH.0b013e328040bfab. [DOI] [PubMed] [Google Scholar]
- Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onozato ML, Toji A. Role of NADPH oxidase in hypertension and diabetic nephropathy. Curr Hypertens Rev. 2005;1:15–20. [Google Scholar]
- Onozato ML, Toji A, Goto A, Fujita T, Wilcox CS. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int. 2002;61:186–194. doi: 10.1046/j.1523-1755.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- Privratsky JR, Wold LE, Sowers JR, Quinn MT, Ren J. AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension. 2003;42:206–212. doi: 10.1161/01.HYP.0000082814.62655.85. [DOI] [PubMed] [Google Scholar]
- Siragy HM. Angiotensin II compartmentalization within the kidney: effects of salt diet and blood pressure alterations. Curr Opin Nephrol Hypertens. 2006;15:50–53. doi: 10.1097/01.mnh.0000196148.42460.4f. [DOI] [PubMed] [Google Scholar]
- Siragy HM, Awad A, Abadir P, Webb R. The angiotensin II type 1 receptor mediates renal interstitial content of tumor necrosis factor-α in diabetic rats. Endocrinology. 2003;144:2229–2233. doi: 10.1210/en.2003-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi CP, Kanwar YS, Sahai A. Hypoxia and high glucose upregulate AT1 receptor expression and potentiate ANG II-induced proliferation in VSM cells. Am J Physiol Heart Circ Physiol. 2003;284:H846–852. doi: 10.1152/ajpheart.00625.2002. [DOI] [PubMed] [Google Scholar]
- Tang SS, Jung F, Diamant D, Brown D, Bachinsky D, Hellman P, Ingelfinger JR. Temperature-sensitive SV40 immortalized rat proximal tubule cell line has functional renin-angiotensin system. Am J Physiol Renal Physiol. 1995;268:F435–F446. doi: 10.1152/ajprenal.1995.268.3.F435. [DOI] [PubMed] [Google Scholar]
- Wehbi GJ, Zimpelmann J, Carey RM, Levine DZ, Burns KD. Early streptozotocin-diabetes mellitus downregulates rat kidney AT2 receptors. Am J Physiol Renal Physiol. 2001;280:F254–265. doi: 10.1152/ajprenal.2001.280.2.F254. [DOI] [PubMed] [Google Scholar]
- Wilcox CS. Redox regulation of the afferent arteriole and tubuloglomerular feedback. Acta Physiol Scand. 2003;179:217–223. doi: 10.1046/j.0001-6772.2003.01205.x. [DOI] [PubMed] [Google Scholar]