Abstract
Recently, we reported the presence of a local renal aldosterone production. In the present study, we tested the hypothesis that local aldosterone production in the kidney contributes to renal inflammation, matrix formation and albuminuria associated with diabetes. We evaluated changes in renal aldosterone content (RAC), aldosterone synthase expression, nuclear factor κB (NFκB), tumour necrosis factor α (TNFα), interleukin-6 (IL-6), transforming growth factor β (TGFβ), glomerular fibronectin, collagen type IV and urinary albumin extraction (UAE) in response to the aldosterone synthase inhibitor FAD286. Studies were conducted in adrenalectomized, normoglycaemic (control) or diabetic rats for 14 weeks. The FAD286 was administered during the last 10 weeks of the study. Plasma aldosterone levels were not detectable in any of the study groups. Compared with control rats, diabetic rats had higher levels of RAC by 488% (P < 0.01), NFκ B by 293% (P < 0.01), TNFα by 356% (P < 0.01), IL-6 by 378% (P < 0.01), TGFβ by 337% (P < 0.01) and UAE by 1122% (P < 0.01), and increased glomerular fibronectin and collagen type IV immunostaining. In diabetic rats, FAD286 reduced RAC (P < 0.01), UAE (P < 0.05), NFκ B mRNA, TNFα mRNA, IL-6 mRNA and TGFβ mRNA by 51, 41, 41 and 52% and also their proteins and decreased glomerular fibronectin and collagen type IV immunostaining. In conclusion, diabetes increases local aldosterone production in the kidney, which contributes to development of renal inflammation, matrix formation and albuminuria. Inhibition of aldosterone production in the kidney could be helpful in management of diabetic nephropathy.
Diabetes is a major contributing factor to development of end-stage renal disease and to increased cardiovascular morbidity and mortality (Schiffrin et al. 2007). Diabetic nephropathy is associated with increased urinary albumin excretion (UAE) and progressive accumulation of extracellular matrix (Mauer et al. 1984; Wolf & Ziyadeh, 2007). Despite the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, the progression of diabetic nephropathy continues, leading to development of end-stage renal disease (Nakao et al. 2003).
Growing evidence suggests that aldosterone contributes to development of diabetic complications in the kidney (Miric et al. 2001; Sato et al. 2003; Fujisawa et al. 2004). The mechanisms by which aldosterone contributes to the development of diabetic nephropathy are not well elucidated. Candidates which may mediate the effects of aldosterone in diabetic nephropathy include several inflammatory and growth factors. Nuclear factor κ B (NFκ B) mediates inflammatory responses by enhancing the release of tumour necrosis factor α (TNFα ) and interleukin-6 (IL-6) in the kidney (Sharma & Ziyadeh, 1994; Barnes & Karin 1997; Siragy et al. 2003; Kalantarinia et al. 2003; Bacher & Schmitz, 2004; Schott-Ohly et al. 2004). Similarly, production of transforming growth factor β (TGFβ ) is upregulated in diabetes, leading to an increase in synthesis of extracellular matrix (Nakamura et al. 1993; Young et al. 1995; Pfeiffer et al. 1996; Park et al. 1997; Schott-Ohly et al. 2004). Recently, we presented evidence for local aldosterone production in the kidney (Xue & Siragy, 2005). In the present study, we hypothesized that aldosterone production in the kidney contributes to development of diabetic nephropathy via enhancement of renal production of inflammatory and growth factors. Our data suggest that in presence of diabetes, reduction of the local renal aldosterone production with the aldosterone synthase inhibitor FAD286 (Fiebeler et al. 2005) ameliorates renal inflammation, matrix formation and albuminuria.
Methods
Animal preparation
Study protocols were approved by the University of Virginia Animal Care and Use Committee. Sprague–Dawley rats (Harlan Teklad, Madison, WI, USA) weighing 245–255 g were housed in a well ventilated room (21 ± 1° C, 12 h–12 h light–dark cycle). The animals were randomly divided into a normoglycaemic control group (n = 8) and a diabetic group (n = 16). Under general anaesthesia (ketamine 60 mg kg−1, i.p.), all animals underwent bilateral adrenalectomy (ADX) according to a previously published method (Kalantarinia et al. 2003). After adrenalectomy, rats received dexamethasone (12 μ g kg−1 day−1, s.c.; Sigma, St Louis, MO, USA) in sesame oil. Following adrenalectomy diabetes was induced by streptozotocin (65 mg kg−1, i.p.; Xue & Siragy, 2005); in the control group, the same volume of saline was injected intraperitoneally. Studies were conducted for 14 weeks. At the beginning of week 5 after development of diabetes, the aldosterone synthase inhibitor FAD286 (4 mg kg−1 day−1, gavage; Novartis Pharmaceuticals, East Hanover, NJ, USA) or normal saline (n = 8 per group) was given to the diabetic rats for 10 weeks. Blood glucose and urinary albumin were monitored weekly throughout the study. At the end of the experiments, animals were killed by overdose of anaesthetic (Ketamine, 60 mg kg−1, i.p.), and plasma and kidneys were harvested and stored at −80° C for aldosterone measurements and for renal molecular and immunostaining studies.
Blood pressure measurement
At the beginning and at the end of the experiments, the systolic blood pressure (BP) was measured three times at 10 min intervals using SC1000 BP Analysis System (Hatteras, Cary, NC, USA). The mean values of the recorded BP were calculated.
Urine albumin excretion and aldosterone assays
Urine albumin excretion was determined by enzyme-linked immunosorbent assay (Nephrat(tm) kit, Exocell, Inc., Philadelphia, PA, USA) according to manufacturer’s instructions (Kalantarinia et al. 2003). Aldosterone assay was performed as previously described (Xue & Siragy, 2005). Briefly, each sample of plasma (50 μ l) and homogenized kidney tissue (79–94 mg) was extracted by methylene chloride (1:2 v/v). After evaporation of the methylene chloride using a vacuum centrifuge, the extract was dissolved into enzyme immunoassay (EIA; Cayman, Ann Arbour, MI, USA) buffer (1:1 v/v) and added to the assay wells (50 μ l per well). Each well was coated with aldosterone AChE Tracer (50 μ l; Cayman) and antiserum (50 μ l; Cayman) and incubated at 4°C overnight. Finally, the plate was developed by Ellman’s Reagent (Cayman) and read at a wavelength 405 nm (Xue & Siragy, 2005).
Quantitative real time reverse transcriptase-polymerase chain reaction (RT-PCR) and Western blot
The procedures for mRNA measurement were performed as previously described (Xue & Siragy, 2005). The frozen kidneys were thawed and homogenized on ice. Gene-specific primers for NFκ B, TGFβ 1 and β -actin were designed using the Genbank. The exon–intron boundaries were determined using the University of California Santa Cruz (UCSC, CA, USA) Genome Bioinformatics Site (http://genome.ucsc.edu). The corresponding cDNA primers were selected from AF079314, X66539, M26744, AY550025, NM012538 and BC063166, the gene codes for rat NFκ B, TNFα , IL-6, TGFβ 1, aldosterone synthase (CYP11B2) and β -actin sequences, respectively. The specificity of the primers was verified by melting curves (iCycler, Bio-Rad, Hercules, CA, USA) and amplified product size using agarose gel electrophoresis. Quantitative real-time RT-PCR was performed using iCycler, and threshold cycle number was determined using iCycler software version 3.1 (Bio-Rad).
The primers were as follows. For NFκ B: forward sequence, 5′-TCTGGGCCATATGTGGAGAT-3′; reverse sequence, TGCTTCTCTCCCCAGGAATA; length 106 bp. For TNFα : forward sequence, TGCCTCAGCCTCTT-CTCATT; reverse sequence, TTGGGAACTTCTCCTCC-TTG; length 103 bp. For IL-6: forward sequence, GCCCTTCAGGAACAGCTATG; reverse sequence, TGA-AGTAGGGAAGGCAGTGG; length 101 bp. For TGFβ 1: forward sequence, TGAGTGGCTGTCTTTTGACG; reverse sequence, TGGGACTGATCCCATTGATT; length 146 bp. For CYP11B2: forward sequence, TGAGACGTG-GTGTGTTCTTGC; reverse sequence, GGCCTCCAA-GAAGTCCCTTGC; Length 126 bp. For β -actin: forward sequence, AGCCATGTACGTAGCCATCC; reverse sequence, ACCCTCATAGATGGGCACAG; length 115 bp.
The Western blot analysis was performed as previously described (Xue & Siragy, 2005). Antibodies for NFκ B (1:1000 dilution), TGFβ (1:2000 dilution), fibronectin (1:500 dilution), collagen type IV (1:500 dilution; Santa Cruz, CA, USA), TNFα (1 μ g ml−1; R&D Systems, Minneapolis, MN, USA), IL-6 (1 μ g ml−1; R&D Systems) or CYP11B2 (CHEMICON International, Inc., Temecula, CA, USA) were used for measurement of respective proteins. Each band density was normalized to the corresponding density of β -actin.
Immunohistochemistry
This method was performed as previously described (Xue & Siragy, 2005). The kidney sections were incubated overnight with NFκ B (1:100 dilution), TGFβ (1:500 dilution), fibronectin (1:50 dilution) or collagen IV (1:50 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) primary antibodies at −4° C, followed by 1 h of incubation with secondary antibody (IgG) conjugated with biotin (Sigma) at room temperature. Immunoreactive signals were detected with an avidin–biotin immunoperoxidase reaction (Sigma) and visualized by exposure to diaminobenzidine (Sigma). The non-specific binding was controlled by using the same species immunoglobulin G isotypes (Sigma) or omitting the primary antibody for negative comparison. The immunostaining was evaluated by light microscopy.
Periodic acid-Schiff (PAS) staining
The kidney sections were deparaffinized and sequentially treated with 1% periodic acid for 10 min (Sigma), Schiff’s reagent for 10 min (Sigma), followed by Carazzi Haematoxylin for 2 min (Sigma) and differentiated in acid alcohol. Sections were well washed between treatments with tap water. Finally, the sections were mounted and examined by light microscopy.
Statistical analysis
Comparisons among different treatment groups were made by two-way ANOVA. Data are expressed as means ± S.E.M. Statistical significance was identified at P < 0.05.
Results
Plasma and renal aldosterone content, renal aldosterone synthase expression, blood glucose and urine albumin excretion in diabetic rats and response to aldosterone synthase inhibition
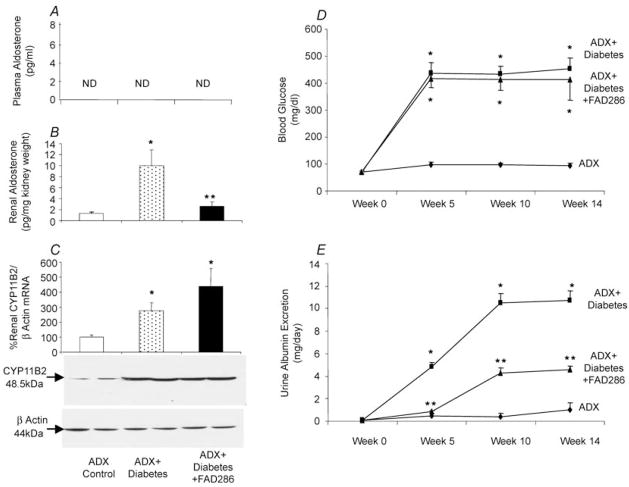
At the start of the study, there were no differences in body weight, BP, 24 h urine volume, blood glucose or UAE between adrenalectomized normoglycaemic (control) rats, diabetic rats and diabetic rats treated with FAD286. Throughout the study, there were no significant differences in BP between different animal groups (102 ± 2.8 mmHg for adrenalectomized control group, 102 ± 4.3 mmHg for adrenalectomized diabetic rats and 101 ± 6.1 mmHg for adrenalectomized diabetic rats treated with FAD286). In adrenalectomized normoglycaemic rats, there were no significant changes in 24 h urine volume, blood glucose or UAE rate (Fig. 1), while their body weight increased from 249 ± 6 g at baseline to 450 ± 19 g at end of week 14 of the study (P < 0.01). Similarly, diabetic rats and diabetic rats treated with FAD286 increased in their body weight from 247 ± 5 and 247 ± 4 g, respectively, at baseline to 436 ± 22 and 437 ± 25 g, respectively, at the end of week 14 of the study (P < 0.01). Plasma aldosterone levels were not detectable in any of the studied groups (Fig. 1A). Compared with the adrenalectomized normoglycaemic control group, adrenalectomized diabetic rats had higher 24 h urine volume (105 ± 11 versus 20 ± 4 ml, P < 0.01), renal aldosterone content (Fig. 1B), aldosterone synthase (CYP11B2) mRNA and protein expression (Fig. 1C), blood glucose (Fig. 1D) and UAE (Fig. 1E). In adrenalectomized diabetic rats, FAD286 treatment did not influence blood glucose (Fig. 1D) but increased urine volume to 123 ± 12.1 ml (P < 0.01) and caused significant reduction in renal aldosterone content (Fig. 1B) and UAE (Fig. 1E). The FAD286 caused a slight but not significant increase in renal aldosterone synthase mRNA and protein expression compared to adrenalectomized diabetic rats (Fig. 1C).
Figure 1. Plasma aldosterone (A), renal aldosterone (B), representative renal aldosterone synthase (CYP11B2) mRNA (C, upper panel) and protein (C, lower panel), blood glucose (D) and urinary albumin excretion (E) in adrenalectomized (ADX) normoglycaemic (ADX control), adrenalectomized diabetic (ADX + Diabetes) and adrenalectomized diabetic rats treated with the aldosterone synthase inhibitor FAD286 (ADX + Diabetes + FAD286).
* P < 0.01 from control; **P < 0.01 from control or ADX + Diabetes. n = 8 each group.
Renal expression of NFκ B, TNFα , IL-6, TGFβ , fibronectin and collagen type IV in diabetes and in response to aldosterone synthase inhibition
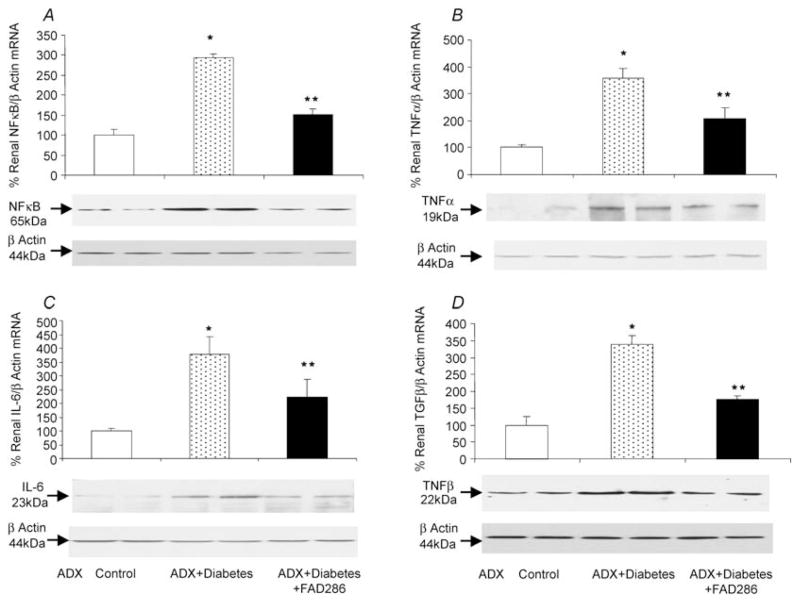
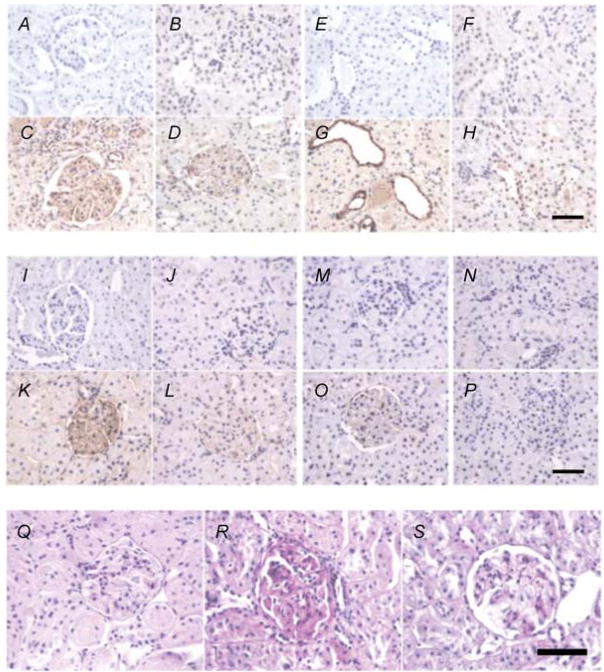
Compared with the adrenalectomized normoglycaemic control group, the diabetic rats had increased renal mRNA expression of NFκ B by 293% (Fig. 2A), TNFα by 356% (Fig. 2B), IL-6 by 378% (Fig. 2C) and TGFβ by 337% (Fig. 2D) and also their respective proteins. Immunostaining for TGFβ , fibronectin and collagen type IV (Fig. 3) was absent in kidney sections when immunoglobulin G pre-immune serum was used as a control (Fig. 3A, E, I and M respectively). In the adrenalectomized normoglycaemic control group, renal immunostaining of TGFβ , fibronectin and collagen type IV is minimal in glomeruli and tubules, respectively (Fig. 3B, F, J and N ). Compared with the control group, kidney immunostaining of TGFβ in glomeruli and tubules (Fig. 3D and G), and fibronectin (Fig. 3K ) and collagen type IV (Fig. 3O), and PAS staining (Fig. 3R) in glomeruli are enhanced in adrenalectomized diabetic rats. Treatment with FAD286 reduced renal mRNA expression of NFκ B, TNFα , IL-6 and TGFβ by 51, 41, 41 and 52%, respectively, and also their corresponding proteins (Fig. 2). The FAD286 also reduced renal TGFβ (Fig. 3D and H ), fibronectin (Fig. 3K ), collagen type IV (Fig. 3P) and PAS staining (Fig. 3S).
Figure 2. Representative renal NFκB (A), TNFα (B), IL-6 (C) and TGFβ (D) mRNA (upper panels) and their protein expression (lower panels) in adrenalectomized normoglycaemic (ADX control), adrenalectomized diabetic (ADX + Diabetes) and adrenalectomized diabetic rats treated with FAD286 (ADX + Diabetes + FAD286).
* P < 0.001 from control; **P < 0.05 from control or ADX + Diabetes. n = 8 each group.
Figure 3. Rat kidney immunostaining of glomeruli and tubules in adrenalectomized normoglycaemic, adrenalectomized diabetic adrenalectomized diabetic rats treated with FAD286.
Immunoglobulin G immunostaining was used as negative control for TGFβ (A and E), fibronectin (I) and collagen type IV (M) in normoglycaemic adrenalectomized rats. Renal immunostaining (brown) in normoglycaemic adrenalectomized rats is presented for TGFβ (B and F), fibronectin (J), collagen type IV (N) and PAS (Q). Renal immunostaining (brown) is increased in diabetic adrenalectomized rats for TGFβ (C and G), fibronectin (K), collagen type IV (O) and PAS (red staining, R). Treatment of adrenalectomized diabetic rats with the aldosterone synthase inhibitor FAD286 reduced renal immunostaining (brown) for TGFβ (D and H), fibronectin (L), collagen type IV (P) and PAS (red staining, S). Scale bars represent 50 μm.
Discussion
Aldosterone is known to play a role in the development of cardiovascular and renal diseases. However, the mechanisms by which aldosterone contributes to the pathophysiology of these diseases are still unclear. In this study, we hypothesized that diabetes increases local aldosterone production in the kidney, which contributes to the development of diabetic nephropathy via stimulation of renal inflammation and matrix formation. Our study demonstrates that diabetes increases local renal aldosterone synthase expression and aldosterone production. These results confirm and extend our recent report (Xue & Siragy, 2005) demonstrating the presence of a local renal aldosterone production that is upregulated by hyperglycaemia. Aldosterone receptors are present in the kidney (Nishiyama et al. 2005), and the presence of local aldosterone production is confirmed in our study by the presence of aldosterone synthase and detectable aldosterone levels in the kidneys 14 weeks post-adrenalectomy, despite the complete absence of detectable levels of aldosterone in the circulation. The production of this hormone in close proximity to its receptors at target sites of action supports the concept of the presence of a local renal aldosterone paracrine system.
The observed increase in renal aldosterone production in diabetes is most likely to be related to increased intrarenal angiotensin II production (Siragy et al. (2003), which is supported by the fact that blockade of the angiotensin subtype 1 (AT1) receptor decreases aldosterone synthase expression and aldosterone levels in the kidney (Xue & Siragy, 2005). In the present study, concomitant with the increase in aldosterone production, there was an increase in expression of NFκ B, TNFα , IL-1, TGFβ and fibronectin, and fibrosis in the kidneys of the diabetic animals. These results suggest that renal inflammation and matrix formation contribute to the development of diabetic kidney disease as evidenced by the presence of albuminuria. Previously, we demonstrated increased renal inflammation in diabetes (Kalantarinia et al. 2003; Siragy et al. 2003), a process that precedes development of albuminuria (Kalantarinia et al. 2003). Inhibition of renal aldosterone synthase with FAD286 in diabetic animals reduced renal aldosterone tissue content, NFB, TNFα , IL-6, TGFβ , fibronectin, collagen type IV and albuminuria despite a lack of influence on blood glucose. These results suggest a direct contribution of aldosterone synthase to renal inflammation and albuminuria independent of changes in blood glucose. Similarly, an absence of reduction in BP during FAD286 treatment confirms the direct renoprotective effects of aldosterone synthase inhibition. In addition, our study shows that inhibition of aldosterone synthase minimizes the development of albuminuria in diabetic rats. These results are consistent with previous reports showing that the development of proteinuria (Abbate et al. 1998) and increased matrix formation (Wolf et al. 1992) in diabetes is related to enhanced stimulation of TGFβ (Kagami et al. 1994).
Previous studies demonstrated that blockade of aldosterone receptors leads to improvement of renal function in diabetic patients (Sato et al. 2003). However, conflicting reports exist concerning circulating aldosterone levels in diabetes (Beretta-Piccoli et al. 1983; Cronin et al. 1995; Perez et al. 1997; Luik et al. 2003), ranging from normal to low levels. In the present study, depletion of circulating aldosterone by adrenalectomy in diabetic animals did not abolish renal aldosterone or totally prevent the development of renal inflammation, matrix formation or albuminuria, indicating the importance of the locally produced aldosterone in the kidney in development of diabetic nephropathy.
Besides the adrenal glands, biosynthesis of aldosterone has been reported in blood vessels and brain, although the pathophysiological relevance of these findings is still not well elucidated (Young et al. 1995; Takeda et al. 1997; Silvestre et al. 1998; Ahmad et al. 2004; Gomez-Sanchez et al. 1997, 2005). Our findings are consistent with a recent report that FAD286 (Fiebeler et al. 2005) decreases albuminuria in a human renin and angiotensinogen double transgenic rat model. The major difference between this report (Fiebeler et al. 2005) and our present study is that in the former study animals developed albuminuria secondary to severe hypertension, while in our study there were no significant differences in BP levels between adrenalectomized normoglycaemic control, adrenalectomized diabetic and FAD286-treated adrenalectomized diabetic animals. Absence of elevated BP did not prevent development of albuminuria in the diabetic rats. It is possible that the mechanisms contributing to development of albuminuria in diabetes are different from those in hypertension. Furthermore, absence of circulating aldosterone in the present study did not prevent development of proteinuria and provides evidence for the importance of local renal aldosterone production in the development of diabetic nephropathy.
In conclusion, there is a local aldosterone system in the kidney and its activity is increased in diabetes. Increased renal production of aldosterone contributes to development of renal inflammation, matrix formation and albuminuria. Inhibition of aldosterone synthase is a potential therapeutic tool in management of diabetic kidney disease via reducing renal inflammation and matrix formation.
Acknowledgments
This study was supported by grants DK078757 and HL091535 from the National Institutes of Health to Helmy M. Siragy, MD. Dr Siragy was the recipient of Research Career Development Award K04-HL-03006 from the National Institutes of Health.
References
- Abbate M, Zoja C, Corna D, Capitanio M, Bertani T, Remuzzi G. In progressive nephropathies, overload of tubular cells with filtered proteins translates glomerular permeability dysfunction into cellular signals of interstitial inflammation. J Am Soc Nephrol. 1998;9:1213–1224. doi: 10.1681/ASN.V971213. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Romero DG, Gomez-Sanchez EP, Gomez-Sanchez CE. Do human vascular endothelial cells produce aldosterone? Endocrinology. 2004;145:3626–3629. doi: 10.1210/en.2004-0081. [DOI] [PubMed] [Google Scholar]
- Bacher S, Schmitz ML. The NF-κ B pathway as a potential target for autoimmune disease therapy. Curr Pharm Des. 2004;10:2827–2837. doi: 10.2174/1381612043383584. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Karin M. Nuclear factor-κ B: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Beretta-Piccoli C, Weidmann P, Fraser R. Responsiveness of plasma 18-hydroxycorticosterone and aldosterone to angiotensin II or corticotropin in nonazotemic diabetes mellitus. Diabetes. 1983;32:1–5. doi: 10.2337/diab.32.1.1. [DOI] [PubMed] [Google Scholar]
- Cronin CC, Barry D, Crowley B, Ferriss JB. Reduced plasma aldosterone concentrations in randomly selected patients with insulin-dependent diabetes mellitus. Diabet Med. 1995;12:809–815. doi: 10.1111/j.1464-5491.1995.tb02084.x. [DOI] [PubMed] [Google Scholar]
- Fiebeler A, Nussberger J, Shagdarsuren E, Rong S, Hilfenhaus G, Al-Saadi N, Dechend R, Wellner M, Meiners S, Maser-Gluth C, Jeng AY, Webb RL, Luft FC, Muller DN. Aldosterone synthase inhibitor ameliorates angiotensin II-induced organ damage. Circulation. 2005;111:3087–3094. doi: 10.1161/CIRCULATIONAHA.104.521625. [DOI] [PubMed] [Google Scholar]
- Fujisawa G, Okada K, Muto S, Fujita N, Itabashi N, Kusano E, Ishibashi S. Spironolactone prevents early renal injury in streptozotocin-induced diabetic rats. Kidney Int. 2004;66:1493–1502. doi: 10.1111/j.1523-1755.2004.00913.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez CE, Zhou MY, Cozza EN, Morita H, Foecking MF, Gomez-Sanchez EP. Aldosterone biosynthesis in the rat brain. Endocrinology. 1997;138:3369–3373. doi: 10.1210/endo.138.8.5326. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Is aldosterone synthesized within the rat brain? Am J Physiol Endocrinol Metab. 2005;288:E342–E346. doi: 10.1152/ajpendo.00355.2004. [DOI] [PubMed] [Google Scholar]
- Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-β expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantarinia K, Awad AS, Siragy HM. Urinary and renal interstitial concentrations of TNF-α increase prior to the rise in albuminuria in diabetic rats. Kidney Int. 2003;64:1208–1213. doi: 10.1046/j.1523-1755.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- Luik PT, Kerstens MN, Hoogenberg K, Navis GJ, Dullaart RP. Low plasma aldosterone despite normal plasma renin activity in uncomplicated type 1 diabetes mellitus: effects of RAAS stimulation. Eur J Clin Invest. 2003;33:787–793. doi: 10.1046/j.1365-2362.2003.01215.x. [DOI] [PubMed] [Google Scholar]
- Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural–functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miric G, Dallemagne C, Endre Z, Margolin S, Taylor SM, Brown L. Reversal of cardiac and renal fibrosis by pirfenidine and spironolactone in streptozotocin-diabetic rats. Br J Pharmacol. 2001;133:687–694. doi: 10.1038/sj.bjp.0704131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Fukui M, Ebihara I, Osada S, Nagaoka I, Tomino Y, Koide H. mRNA expression of growth factors in glomeruli from diabetic rats. Diabetes. 1993;42:450–456. doi: 10.2337/diab.42.3.450. [DOI] [PubMed] [Google Scholar]
- Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting enzyme inhibitor on non-diabetic renal disease (COOPERATE): a randomized controlled trial. Lancet. 2003;361:117–124. doi: 10.1016/S0140-6736(03)12229-5. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yao L, Fan Y, Kyaw M, Kataoka N, Hashimoto K, Nagai Y, Nakamura E, Yoshizumi M, Shokoji T, Kimura S, Kiyomoto H, Tsujioka K, Kohno M, Tamaki T, Kajiya F, Abe Y. Involvement of aldosterone and mineralocorticoid receptors in rat mesangial cell proliferation and deformability. Hypertension. 2005;45:710–716. doi: 10.1161/01.HYP.0000154681.38944.9a. [DOI] [PubMed] [Google Scholar]
- Park IS, Kiyomoto H, Abboud SL, Abboud HE. Expression of transforming growth factor-β and type IV collagen in early streptozotocin-induced diabetes. Diabetes. 1997;46:473–480. doi: 10.2337/diab.46.3.473. [DOI] [PubMed] [Google Scholar]
- Perez GO, Lespier L, Jacobi J, Oster JR, Katz FH, Vaamonde CA, Fishman LM. Hyporeninemia and hypoaldosteronism in diabetes mellitus. Arch Intern Med. 1997;137:852–855. [PubMed] [Google Scholar]
- Pfeiffer A, Middelberg-Bisping K, Drewes C, Schatz H. Elevated plasma levels of transforming growth factor-β 1 in NIDDM. Diabetes Care. 1996;19:1113–1117. doi: 10.2337/diacare.19.10.1113. [DOI] [PubMed] [Google Scholar]
- Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension. 2003;41:64–68. doi: 10.1161/01.hyp.0000044937.95080.e9. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- Schott-Ohly P, Lgssiar A, Partke HJ, Hassan M, Friesen N, Gleichmann H. Prevention of spontaneous and experimentally induced diabetes in mice with zinc sulfate-enriched drinking water is associated with activation and reduction of NF-κ B and AP-1 in islets, respectively. Exp Biol Med. 2004;229:1177–1185. doi: 10.1177/153537020422901113. [DOI] [PubMed] [Google Scholar]
- Sharma K, Ziyadeh FN. Renal hypertrophy is associated with upregulation of TGF-β 1 gene expression in diabetic BB rat and NOD mouse. Am J Physiol Renal Physiol. 1994;267:F1094–F1101. doi: 10.1152/ajprenal.1994.267.6.F1094. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Robert V, Heymes C, Aupetit-Faisant B, Mouas C, Moalic JM, Swynghedauw B, Delcayre C. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J Biol Chem. 1998;273:4883–4891. doi: 10.1074/jbc.273.9.4883. [DOI] [PubMed] [Google Scholar]
- Siragy HM, Awad A, Abadir P, Webb R. The angiotensin II type 1 receptor mediates renal interstitial content of tumor necrosis factor- α in diabetic rats. Endocrinology. 2003;144:2229–2233. doi: 10.1210/en.2003-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Miyamori I, Inaba S, Furukawa K, Hatakeyama H, Yoneda T, Mabuchi H, Takeda R. Vascular aldosterone in genetically hypertensive rats. Hypertension. 1997;29:45–48. doi: 10.1161/01.hyp.29.1.45. [DOI] [PubMed] [Google Scholar]
- Wolf G, Haberstroh U, Neilson EG. Angiotensin II stimulates the proliferation and biosynthesis of type I collagen in cultured murine mesangial cells. Am J Pathol. 1992;140:95–107. [PMC free article] [PubMed] [Google Scholar]
- Wolf G, Ziyadeh FN. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron Physiol. 2007;106:26–31. doi: 10.1159/000101797. [DOI] [PubMed] [Google Scholar]
- Xue C, Siragy HM. Local renal aldosterone system and its regulation by salt, diabetes, and angiotensin II type 1 receptor. Hypertension. 2005;46:584–590. doi: 10.1161/01.HYP.0000175814.18550.c0. [DOI] [PubMed] [Google Scholar]
- Young BA, Johnson RJ, Alpers CE, Eng E, Gordon K, Floege J, Couser WG, Seidel K. Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int. 1995;47:935–944. doi: 10.1038/ki.1995.139. [DOI] [PubMed] [Google Scholar]