Abstract
Adenosine is a signaling molecule produced during conditions that cause cellular stress or damage. This signaling pathway is implicated in the regulation of pulmonary disorders through the selective engagement of adenosine receptors. The goal of this study was to examine the involvement of the A3 adenosine receptor (A3R) in a bleomycin model of pulmonary inflammation and fibrosis. Results demonstrated that A3R-deficient mice exhibit enhanced pulmonary inflammation that included an increase in eosinophils. Accordingly, there was a selective up-regulation of eosinophil-related chemokines and cytokines in the lungs of A3R-deficient mice exposed to bleomycin. This increase in eosinophil numbers was accompanied by a decrease in the amount of extracellular eosinophil peroxidase activity in lavage fluid from A3R-deficient mice exposed to bleomycin, an observation suggesting that the A3R is necessary for eosinophil degranulation in this model. Despite an increase in inflammatory metrics associated with A3R-deficient mice treated with bleomycin, there was little difference in the degree of pulmonary fibrosis. Examination of fibrotic mediators demonstrated enhanced transforming growth factor (TGF)-β1 expression, but not a concomitant increase in TGF-β1 activity. This was associated with the loss of expression of matrix metalloprotease 9, an activator of TGF-β1, in alveolar macrophages and airway mast cells in the lungs of A3R-deficient mice. Together, these results suggest that the A3R serves antiinflammatory functions in the bleomycin model, and is also involved in regulating the production of mediators that can impact fibrosis.
Keywords: pulmonary fibrosis, adenosine receptors, inflammation, eosinophil, extracellular matrix
CLINICAL RELEVANCE
Pulmonary fibrosis is associated with many interstitial lung diseases. The current study suggests that A3 adenosine receptor signaling regulates key aspects of pulmonary inflammation and fibrosis.
Pulmonary fibrosis is a component of many interstitial lung diseases (1). These disorders are characterized by varying degrees of inflammation, aberrant fibroblast proliferation, and extracellular matrix deposition that result in distortion of lung architecture and compromised pulmonary function (1–4). Fibrosis is also a major component of airway remodeling responses seen in asthma (5). Although the various signaling pathways that mediate pulmonary inflammation and fibrosis have not been completely elucidated, emerging evidence suggests that pulmonary fibrosis likely results from abnormal repair responses in the damaged lung, including deregulated inflammation, angiogenesis, and matrix metabolism (1, 6). In this regard, examining the regulation of inflammation and the production of fibrotic mediators by inflammatory cells has become important for understanding the progression and chronicity of pulmonary diseases where fibrosis is a detrimental component.
Adenosine is a signaling molecule that is produced in excess at times of cellular stress and/or damage. Consistent with this, adenosine levels are elevated in the lungs of patients with asthma and patients with chronic obstructive pulmonary disease (COPD) (7, 8), as well as in the lungs of mouse models exhibiting inflammation and remodeling characteristic of lung diseases such as asthma, COPD, and idiopathic pulmonary fibrosis (9–11). Extracellular adenosine regulates numerous cellular responses by engaging specific G protein–coupled adenosine receptors on the surface of target cells (12). Among these responses is the activation of pathways that are important in the regulation of wound healing and fibrosis (13–16). Moreover, adenosine signaling pathways are associated with both pro- and antiinflammatory actions that are dictated by specific adenosine receptor subtypes and their downstream signaling components (17–19). Accordingly, adenosine signaling is emerging as a major pathway involved in regulating the balance between tissue repair and/or fibrosis.
The A3 adenosine receptor (A3R) has emerged as an important regulator of pulmonary inflammation and airway remodeling. Its levels are elevated in patients with chronic lung disease (20), and in numerous models of pulmonary disease (9, 21, 22). In addition, there is evidence that A3R signaling can influence inflammatory cell functions relevant to pulmonary disease. For example, engagement of the A3R on mouse mast cells promotes mediator release (23, 24), and engagement of the A3R on eosinophils exhibits both pro- (25) and antiinflammatory (20, 21, 26) activities. Similarly, there is evidence that A3R engagement can serve pro- and antiinflammatory roles on macrophages and neutrophils (27–29). Thus, there is evidence for both antiinflammatory and proinflammatory effects of A3R signaling that may be relevant to lung disease.
The ability of adenosine to regulate both inflammation and fibrosis, and the pleotrophic effects of A3R on immune regulation, led us to hypothesize that the A3R is involved in regulating aspects of inflammation and fibrosis after lung injury. To test this hypothesis, we used a model of bleomycin-induced lung injury to examine the involvement of A3R signaling in pulmonary inflammation and fibrosis. A3R-deficient mice exhibited enhanced inflammation in response to bleomycin exposure as compared with wild-type mice, suggesting an anti-inflammatory role of the A3R in this model. Surprisingly, despite the increased inflammation, there was little difference in the degree of fibrosis seen in the lungs of wild-type and A3R-deficient mice exposed to bleomycin. Examination of fibrotic mediators revealed enhanced levels of transforming growth factor (TGF)-β1 production; however, there was not a concomitant increase in TGF-β1 activity. This was associated with diminished expression of matrix metalloproteinase (MMP)-9, an important activator of latent TGF-β1, in alveolar macrophages and masts cells. Results also revealed for the first time that the A3R is needed for eosinophil degranulation in this model, which may also contribute to the effects seen on fibrosis. Collectively, these studies indicate that the A3R serves antiinflammatory functions in the bleomycin model. In addition, this receptor plays an important role in regulating the levels of fibrotic mediators that may help to influence the severity of ensuing fibrosis.
MATERIALS AND METHODS
Mice
A3R-deficient (A3R−/−) mice congenic on a C57Blk6 background were obtained from Marlene A. Jacobson (Merck Research Labs, West Point, PA) (23), and wild-type C57Blk6 mice (A3R+/+) were obtained from Harlan (Indianapolis, IN). Animal care was in accordance with institutional and NIH guidelines, and all procedures were approved by the University of Texas Health Science Center at Houston Animal Welfare Committee. Mice were housed in ventilated cages equipped with microisolator lids and maintained under strict containment protocols. No evidence of bacterial, parasitic, or fungal infection was found, and serology on cage littermates was negative for 12 of the most common murine viruses.
Bleomycin Exposures
Eight- to 10-week old female A3R+/+ or A3R−/− mice were anesthetized with avertin (250 mg/kg, intraperitoneally), and 3.5 U/kg bleomycin (Blenoxane; Bristol-Myers Squibb, Princeton, NJ) diluted in 50 μl sterile saline or saline alone was instilled intratracheally. Endpoints were examined at 1, 3, 7, 14 and 21 days after challenge.
Histology and Immunostaining
Mice were anesthetized, and lungs lavaged three times with 0.4 ml PBS. Total cell counts were determined using a hemocytometer, and cellular differentials (300 cells/sample) were conducted on cells cytospun onto microscope slides and stained with Diff-Quick (Dade Behring, Newark, DE). Lungs were then infused with 10% buffered formalin at 25 cm of pressure and fixed overnight at 4°C. Fixed lungs were dehydrated and embedded in paraffin. Sections (5 μm) were collected on microscope slides, rehydrated through graded ethanols to water then stained with hematoxylin and eosin (H&E; Shandon-Lipshaw, Pittsburgh, PA), Masson's trichrome or 0.1% tolidine blue.
For immunostaining, rehydrated slides were quenched with 3% hydrogen peroxide, antigen retrieval performed (Dako Corp. [Glostrup, Denmark] or Zymed pepsin [Zymed, San Francisco, CA]), and endogenous avidin and biotin blocked with a Biotin Blocking System (Dako Corp). Slides were incubated with primary antibody: α-smooth muscle actin (α -sma, 1:1,000 dilution [Sigma, St. Louis, MO] overnight at 4°C after processing sections with Mouse on Mouse Kit [Vector Laboratories, Burlingame, CA]), rabbit anti-mouse major basic protein 1 (mMBP-1, 1:1,000 dilution, 1 h at room temperature), or goat anti-mouse MMP-9 (1:50 dilution, 1 h at room temperature; R&D Systems, Minneapolis, MN). Sections were incubated with ABC Elite Streptavidin reagents and appropriate secondary antibodies, then developed with 3,3′-diaminobenzidine (Sigma-Aldrich) and counterstained with methyl green. Quantification of peribronchial eosinophils was assessed by analysis of 10 fields at ×20 magnification. Eosinophils were counted using Image Pro Plus 4.0 image analysis software (Media Cybernetics, Silver Spring, MD). Immunofluorescence was conducted using goat anti-mouse MMP-9 (1:50 dilution, 1 h at room temperature; R&D Systems) followed by Alexa Fluor 488 (1:1,000, 1 h at room temperature; Invitrogen, Carlsbad, CA). Slides were coverslipped with Vectashield (Vector Laboratories) mounting medium containing DAPI to visualize nuclei.
Eosinophil Peroxidase Assay
Eosinophil peroxidase (EPO) activity present in 75 μl of undiluted lavage fluid samples was detected after incubation in a 96-well plate with equal volumes of substrate solution (50 mM Tris-HCl, pH 8, 0.1% Triton X-100, 8.8 mM H2O2, 10 mM o-phenylenediamine [OPD], 6 m M KBr) at 37°C for 30 minutes. Duplicate samples also containing 10 mM of the peroxidase inhibitor resorcinol (1,3-Benzenediol; Sigma-Aldrich) were used as controls for the specificity of these reactions. The reaction was stopped with 50 μl 4N H2SO4, and OD values were read at 490 nm and EPO content given as OD/ml.
Analysis of mRNA
Mice were killed and the lungs were rapidly removed and frozen in liquid nitrogen. RNA was isolated from frozen lung tissue using TRIzol Reagent (Life Technologies, Inc., Gaithersburg, MD). RNA samples were then DNase-treated (DNaseI; Invitrogen) and subjected to quantitative RT-PCR. The primers, probes and procedures for the PCR reactions were as described previously (30, 31). Values were normalized to 18S ribosomal RNA and are presented as mean normalized transcript levels using the comparative Ct method (2ΔΔCt) (32).
MMP, Tissue Inhibitor of Metalloproteinase-1, and TGF-β1 Assays
Gelatinolytic activity in bronchoalveolar lavage (BAL) fluid was assessed by zymography based on standard methods (33). Briefly, BAL fluid was subjected to 10% SDS-PAGE on gels containing 0.02% gelatin. After electrophoresis, SDS was removed by washes in 2.5% Triton X-100, before incubation at 37°C for 24 hours in developing buffer (50 mM Tris HCl, pH 8, containing 5 mM CaCl2 and 0.02%NaN3) with or without the MMP inhibitor 10 mM of 1′10-phenantroline (Sigma). Gels were fixed and stained with 50% methanol and 10% acetic acid that contained 0.3% wt/vol Coomassie blue R-250. MMP-9 and MMP-2 bands were identified by their molecular weight, and 10 μl plasma from wild-type C57Bl/6 mice served as positive controls. Protein levels for MMP-9 and tissue inhibitor of metalloproteinase (TIMP)-1 were assessed in BAL fluid using Biotrak activity assay (MMP-9; GE Healthcare Life Sciences, Piscataway, NJ) and enzyme-linked immunosorbent assay (TIMP-1, R&D Systems). Active TGF-β1 was measured using a mouse immunoassay kit (R&D Systems).
Fibrosis Assessment
The Sircol assay (Biocolor Ltd., Carrick, UK) was used to measure collagen in whole lung homogenates. Snap-frozen lungs were homogenized in 2 ml 0.5 M acetic acid containing pepsin (1:10 pepsin/tissue; Sigma-Aldrich) and then incubated overnight shaking at room temperature. Homogenates were spun at 10,000 rpm, and supernatant assayed for pepsin-soluble collagen. Ashcroft scores were determined on Masson's Trichrome–stained lung sections using a minor modification of the method outlined by Ashcroft and coworkers (34). Twenty fields per slide were examined using a two-person randomized blind study. At least six mice per group were examined.
Statistics
Values are expressed as means ± SEM. As appropriate, groups were compared by ANOVA, with follow-up comparisons between groups being conducted using Student's t test with a P value of < 0.05 denoting significant differences.
RESULTS
Pulmonary Inflammation in A3R−/− Mice Treated with Bleomycin
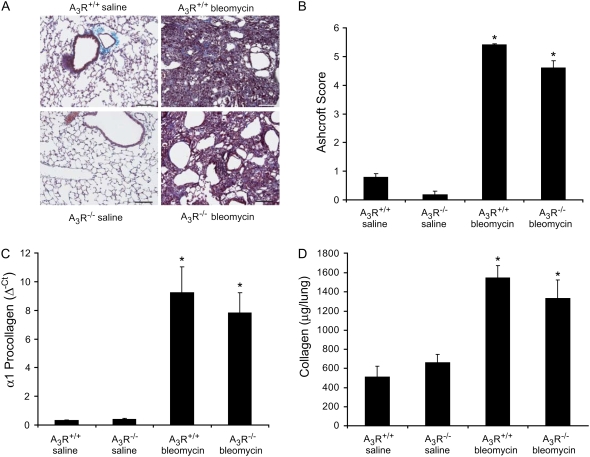
A3R−/− mice were exposed to bleomycin, and pulmonary inflammation was assessed 14 days later to examine the contribution of A3R signaling to the pulmonary inflammation seen after bleomycin injury. There was no difference in appearance of the lungs of A3R+/+ and A3R−/− mice treated with saline (Figures 1A and 1B), suggesting no obvious basal phenotype in the lungs of A3R−/− mice. There was pronounced reorganization of the alveolar airways of A3R+/+ mice exposed to bleomycin (Figure 1C), with extensive fibrosis and inflammatory cell accumulation in the pulmonary interstitial spaces. A3R−/− mice exposed to bleomycin also exhibited severe reorganization of the alveolar spaces, with fibrosis and what appeared to be an enhancement of inflammatory cell foci (Figure 1D). These findings suggest that A3R−/− mice exhibit enhanced pulmonary inflammation after bleomycin exposure.
Figure 1.
Pulmonary histopathology and inflammation. Mice were killed 14 days after saline or bleomycin exposure, and the lungs were lavaged and processed for hematoxylin and eosin staining. (A) Lung section from an A3R+/+ mouse exposed to saline. (B) Lung section from an A3R−/− mouse exposed to saline. (C) Lung section from an A3R+/+ mouse exposed to bleomycin. (D) Lung section from an A3R−/− mouse exposed to bleomycin. Photographs are representative of six to eight mice from each condition; scale bars = 100 μm. (E) Total bronchoalveolar lavage (BAL) cell counts presented as mean cell counts × 104 ± SEM. *Significant difference compared with saline treatment, #significant difference compared with bleomycin-treated A3R+/+ mice (P < 0.05, n = 6–8).
Mice were lavaged on Day 14 after exposure, and the number of inflammatory cells was quantified in BAL fluid to better characterize the pulmonary inflammation seen after bleomycin exposure. There was an 8-fold increase in the number of inflammatory cells recovered from the airways of A3R+/+ mice exposed to bleomycin relative to that seen in A3R+/+ mice exposed to saline (Figure 1E). Interestingly, the number of airway inflammatory cells was significantly higher in A3R−/− mice exposed to bleomycin. Analysis of cellular differentials demonstrated enhanced elevations in macrophages, neutrophils, and eosinophils in the airways of A3R−/− mice exposed to bleomycin (see Figure E1A in the online supplement). Examination of inflammatory mediators demonstrated enhanced expression of key cytokines and chemokines, including CCL2, IL-6, and CXCL1 (Table E1). These findings demonstrate that there is enhanced inflammation in the airways of A3R−/− mice 14 days after bleomycin exposure.
Eosinophil Trafficking and Activation in A3R−/− Mice Treated with Bleomycin
Mice were lavaged at various times after bleomycin exposure, and cellular differentials were determined to examine the ontogeny of the pulmonary inflammation. There were no significant differences in the number of inflammatory cells recovered from the BAL of A3R+/+ and A3R−/− on Day 1 after bleomycin exposure (Figures 2A and E1). There was a trend toward a developmental increase in neutrophils and macrophages in the airways of A3R−/− mice treated with bleomycin; however, only eosinophil numbers exhibited a significant increase during the later stages examined (Figure 2A). The enrichment of airway eosinophils in A3R−/− mice exposed to bleomycin prompted us to examine the levels of eosinophils within the lung parenchyma. Lung sections were stained with an antibody against mMBP-1 to identify tissue-infiltrating eosinophils (Figure 2B). More mMBP-1–positive cells were present within the interstitial spaces of lungs from bleomycin-treated A3R−/− mice, a feature that was verified by quantifying mMBP-1–positive cells in multiple tissue sections (Figure 2C).
Figure 2.
Pulmonary eosinophil recruitment. (A) BAL was collected on Days 1, 3, 7, 14, and 21 after bleomycin exposure, and cells were cytospun onto slides, stained with Diff-Quick, and eosinophils counted using a hemocytometer. Triangles depict eosinophils from A3R+/+ mice exposed to bleomycin, while squares depict eosinophils from A3R−/− mice exposed to bleomycin. Data are presented as mean cell counts × 104 ± SEM. *Significant difference compared with A3R+/+ mice (P < 0.05, n = 3–8). (B) Lung sections from A3R+/+ and A3R−/− mice 14 days after exposure to bleomycin were stained with mMBP-1 to mark eosinophils; scale bar = 100 μm. (C) The number of mMBP-1–positive cells per mm2 was quantified in lung sections using ImagePro software. Data are presented as mean eosinophils/mm2 ± SEM. (D) Total cellular RNA was isolated from the lungs of wild-type (A3R +) or A3R−/− (A3R −) mice 14 days after exposure to saline (open bars) or bleomycin (solid bars). Quantitative rtPCR was used to quantify the levels of key eosinophil related cytokines and chemokines. Values were normalized to 18S ribosomal RNA and are presented as mean normalized transcript levels (Δ−Ct) ± SEM. *Significant difference from saline-treated A3R+/+ mice, #significant difference from bleomycin-treated A3R+/+ mice (P < 0.05, n = 6–8).
To investigate the mechanisms leading to enhanced eosinophil recruitment to the lungs of A3R−/− mice treated with bleomycin, we measured the levels of eosinophil-related cytokine and chemokine transcripts in whole lung extracts (Figure 2D). Levels of CCL17 (TARC) and IL-5 were found to be elevated in the lungs of A3R+/+ mice treated with bleomycin; however, their levels were not enhanced in the lungs of A3R−/− mice. However, transcript levels for CCL11 (eotaxin 1) and GM-CSF were significantly increased in the lungs of A3R−/− mice treated with bleomycin. Similar increases in protein levels of CCL11 were seen in the BAL fluid of these mice (Table E1). These results suggest that in the absence of A3R signaling, there is increased production of key mediators that lead to the enhanced recruitment of eosinophils to the lung after bleomycin exposure.
The enhanced recruitment of eosinophils prompted us to examine eosinophil activation. There was a marked increase in EPO activity in the BAL fluid collected from A3R+/+ mice exposed to bleomycin (Figure 3A), suggesting substantial eosinophil activation. However, despite the increase in eosinophil numbers seen, there was no increase in EPO activity in the BAL fluid of A3R−/− mice exposed to bleomycin. BAL cell pellets were disrupted and EPO activity monitored in homogenates to determine whether the lack of EPO activity in the BAL fluid of A3R−/− mice was due to the absence of EPO protein in eosinophils. Results demonstrated that EPO activity was equally abundant in disrupted eosinophils from the airways of A3R+/+ and A3R−/− mice exposed to bleomycin (Figure 3B), suggesting that the lack of EPO activity in the BAL of A3R−/− mice is due to defects in eosinophil degranulation. These findings demonstrate that the eosinophil degranulation associated with bleomycin treatment is A3R dependent.
Figure 3.
Eosinophil activity. (A) Eosinophil peroxidase activity was measured in the BAL fluid of mice 14 days after exposure to saline or bleomycin. (B) Eosinophil peroxidase activity was measured in the BAL cell pellets from mice 14 days after exposure to saline or bleomycin. Eosinophil peroxidase activity is presented as mean optical density (OD) at 490 nm/ml ± SEM. *Significant difference from saline-treated A3R+/+ mice, #significant difference from bleomycin-treated A3R+/+ mice (P < 0.05, n = 6–8).
Pulmonary Fibrosis in A3R−/− Mice Treated with Bleomycin
A3R−/− mice were exposed to bleomycin, and fibrosis endpoints were examined 14 days later to assess the contribution of A3R signaling to pulmonary fibrosis. Masson's trichrome staining revealed extensive collagen deposition throughout the lungs of A3R+/+ mice exposed to bleomycin (Figure 4A). Extensive collagen deposition was also seen in the lungs of A3R−/− mice exposed to bleomycin. There were substantial increases in the levels of collagen transcripts and protein in the lungs of A3R+/+ mice challenged with bleomycin (Figures 4C and 4D). Collagen transcripts and protein levels were also elevated in the lungs of A3R−/− mice exposed to bleomycin; however, the levels were slightly lower than those measured in A3R+/+ mice. Fibrosis was also assessed using α-smooth muscle actin (α-SMA) immunostaining for myofibroblasts. Results demonstrated a similar increase in α-SMA–positive myofibroblasts in the airways of A3R+/+ and A3R−/− mice exposed to bleomycin (data not shown). Finally, Ashcroft scoring was used as a means of assessing the overall fibrosis seen. Consistent with our measurements of collagen, the degree of fibrosis in the lungs of A3R+/+ and A3R−/− mice were equivalent (Figure 4B). Collectively, these results demonstrate that there is no substantial difference in the degree of bleomycin-induced pulmonary fibrosis in A3R+/+ and A3R−/− mice.
Figure 4.
Pulmonary fibrosis in A3R+/+ and A3R−/− mice. (A) A3R+/+ and A3R−/− mice were killed 14 days after saline or bleomycin exposure, and the lungs were processed for Masson's trichrome staining. Photographs are representative of six to eight mice from each condition; scale bars = 100 μm. (B) Ashcroft scores were determined by examining multiple sections from lungs of mice from each condition. Data are presented as mean Ashcroft score ± SEM (n = 6–8 lungs per condition). (C) RNA was extracted from the lungs of mice 14 days after saline or bleomycin exposure, and α1 procollagen levels were quantified using quantitative rtPCR. Values were normalized to 18S ribosomal RNA and are presented as mean normalized transcript levels (Δ−Ct) ± SEM (n = 6–8). (D) Collagen protein levels were measured in whole lungs 14 days after saline or bleomycin exposure using the Sircol assay. Data are presented as mean μg collagen per lung ± SEM (n = 6–8). *Significant difference from saline-treated A3R+/+ mice (P < 0.05).
TGF-β1 Regulation in A3R−/− Mice Treated with Bleomycin
Given the enhanced inflammation seen in the lungs of A3R−/− mice exposed to bleomycin, it was surprising to find little difference in the degree of pulmonary fibrosis in these mice. The levels of the profibrotic mediator TGF-β1 were examined to address the mechanisms involved in this discrepancy. As expected, levels of TGF-β1 mRNA were elevated in lung extracts of A3R+/+ mice treated with bleomycin (Figure 5A). Interestingly, levels of TGF-β1 mRNA were found to be hyperelevated in whole lung RNA extracts (Figure 5A) and RNA extracts from BAL cell pellets (Figure 5B) of A3R−/− mice treated with bleomycin. However, there was no difference in the levels of active TGF-β1 protein in the BAL fluid of A3R+/+ and A3R−/− mice exposed to bleomycin (Figure 5C). These findings suggest that there is enhanced production of latent TGF-β1 in the lungs of A3R−/− mice treated with bleomycin, but no increase in the active form of this mediator.
Figure 5.
TGF-β1 expression and activity. Total cellular RNA was isolated from the lungs (A) or BAL cell pellets (B) of wild-type (A3R +) or A3R−/− (A3R −) mice 14 days after exposure to saline (open bars) or bleomycin (solid bars). Quantitative rtPCR was used to measure TGF-β1 transcript levels. Values were normalized to 18S ribosomal RNA and are presented as mean normalized transcript levels (Δ−Ct) ± SEM (n = 6–8). (C) Active TGF-β1 protein levels were determined in BAL fluid from mice treated the same as in A and B. Values are presented as mean pg/ml TGF-β1 ± SEM (n = 4–6). *Significant difference from saline-treated A3R+/+ mice, #significant difference from bleomycin-treated A3R+/+ mice (P < 0.05).
Studies have shown that MMP-9 is an important regulator of active TGF-β1 production in pulmonary fibrosis (35). We therefore hypothesized that MMP-9 production is diminished in the lungs of A3R−/− mice, and this reduction may account for the lack of enhanced active TGF-β1. To address this, MMP-9 levels were measured. MMP-9 whole lung mRNA levels (Figure 6A), whole lung protein activity (Figure 6B), and BAL fluid activity (Figure 6C) were all elevated in the lungs of A3R+/+ mice treated with bleomycin. Levels of MMP-9 RNA and activity were diminished in the lungs of A3R−/− mice treated with bleomycin, suggesting that the A3R regulates the production of this protease. MMP-9 activity is also regulated by the levels of protease inhibitors such as TIMP-1. TIMP-1 protein levels were found to be elevated in the BAL fluid of A3R+/+ mice treated with bleomycin, while levels were significantly reduced in the BAL fluid of A3R−/− mice (Figure 6D). Consitent with these findings, there was a reduction in the ratio of MMP-9 to TIMP-1 activity in the BAL fluid of A3R−/− mice (Figure 6E), further indicating a reduction in MMP-9 activity.
Figure 6.
Matrix metalloproteinase (MMP)-9 and tissue inhibitor of metalloproteinase (TIMP)-1 expression and activity. (A) Total cellular RNA was isolated from the lungs of wild-type (A3R +) or A3R−/− (A3R −) mice 14 days after exposure to saline (open bars) or bleomycin (solid bars). Quantitative rtPCR was used to quantify the levels MMP-9 transcripts. Values were normalized to 18S ribosomal RNA and are presented as mean normalized transcript levels (Δ−Ct) ± SEM (n = 6–8). (B) BAL fluid (20 μl from saline-, and 10 and 5 μl from bleomycin-treated samples) were subjected to 10% SDS-PAGE in gels containing 0.02% gelatin. Gels were fixed and stained with 50% methanol and 10% acetic acid that contained 0.3% wt/vol Coomassie blue. MMP-9 and MMP-2 bands were identified by their molecular weight. MMP-9 (C) and TIMP-1 (D) activities were determined in BAL fluid using specific immunoassays. (E) MMP-9 and TIMP-1 BAL fluid activities were used to determine a ratio of MMP-9 to TIMP-1. Data are presented as means ± SEM. *Significant difference from saline-treated A3R+/+ mice, #significant difference from bleomycin-treated A3R+/+ mice (P < 0.05, n = 6–8).
Examination of the cellular source of MMP-9 in this model using immune detection methods revealed that MMP-9 production is limited to inflammatory components in the lungs of both A3R+/+ and A3R−/− mice treated with bleomycin (Figures 7A and 7B). Examination of isolated BAL cells revealed that all neutrophils and a subset of alveolar macrophages produced MMP-9 in the lungs of bleomycin-treated A3R+/+ mice (Figure 7C). In contrast, MMP-9 production was detected in neutrophils isolated from the airways of A3R−/− mice treated with bleomycin; however, alveolar macrophages isolated from these mice did not produce MMP-9 (Figure 7D). MMP-9 expression was not detected in eosinophils in either group of mice (data not shown). MMP-9 expression was abundant in mast cells found in the bronchi of A3R+/+ mice treated with bleomycin (Figures 7E and 7F), but was absent from mast cells in the bronchi of A3R−/− mice (Figures 7G and 7H). Collectively, these findings demonstrate that MMP-9 levels are diminished in the lungs of A3R−/− mice and that A3R-dependent expression of MMP-9 in mast cells and alveolar macrophages likely accounts for this reduction.
Figure 7.
MMP-9 immunolocalization. Mice were killed 14 days after saline or bleomycin exposure, and the lungs were lavaged and processed for MMP-9 immunolocalization. Immunoperoxidase detection using a MMP-9 antibody was used to localize MMP-9 (brown stain) to inflammatory cells in the lungs of (A) A3R+/+ mice exposed to bleomycin and (B) A3R−/− mice exposed to bleomycin. The same antibody was used in an immunofluorecence assay to examine MMP-9 expression (green) in BAL cells from (C) A3R+/+ mice exposed to bleomycin and (D) A3R−/− mice exposed to bleomycin. Blue staining denotes DAPI-stained nuclei. Mast cells were localized in bronchi of (E) A3R+/+ mice exposed to bleomycin and (G) A3R−/− mice exposed to bleomycin using tolidine blue staining. MMP-9 immunoperoxidase detection (brown stain) was used to localize MMP-9 to mast cells in the bronchi of (F) A3R+/+ mice exposed to bleomycin and (G) A3R−/− mice exposed to bleomycin. n, neutrophils; Φ, macrophages; arrows denote mast cells. Scale bars: A and B, 50 μm; C and D, 10 μm; H, 10 μm (also applies to E–G).
DISCUSSION
Regulated inflammation and matrix production are critical components of wound healing processes. An emerging model for the development of pulmonary fibrosis is that abnormal inflammation and matrix regulation after lung injury progresses to an overactive wound healing response that culminates in fibrosis. In this context, understanding the factors produced after injury and how they influence the normal or abnormal wound healing response could provide important mechanistic information. Adenosine is produced in response to tissue damage, where it is thought to regulate key features of the injury response. Antiinflammatory and tissue-protective activities of adenosine are well documented (17, 18, 36), and adenosine signaling pathways can promote wound healing (13). In addition, recent studies demonstrate that adenosine has profibrotic activities in disease models including the lung (14, 15, 37). Thus, adenosine production after lung injury may access pathways that regulate the balance of inflammation and matrix production that influence lung repair or the progression to fibrosis. Adenosine elicits many of its activities by engaging cell surface adenosine receptors (12). The goal of this study was to examine the contribution of the A3R to inflammatory and fibrotic processes after injury in the lung. This was done by subjecting A3R−/− mice to bleomycin-induced pulmonary injury. The results of these studies demonstrate that A3R signaling serves a largely antiinflammatory role in the bleomycin model; however, it also serves to increase the levels of MMP-9 that may promote TGF-β1 activation and downstream fibrotic pathways. These findings suggest that A3R signaling may be an important pathway linking inflammation to the progression of pulmonary fibrosis.
A major finding of this study was that mice deficient in the A3R develop enhanced pulmonary inflammation after bleomycin exposure. This was characterized by an increase in the numbers of macrophages, neutrophils, and particularly eosinophils in A3R−/− mice exposed to bleomycin. These findings suggest that the A3R plays an antiinflammatory role in the bleomycin model. Similar findings were reported in a study that demonstrated increased inflammation in A3R−/− mice subjected to an air-pouch model of acute inflammation (38). In addition, our observations are consistent with findings in in vivo models of injury, including lipopolysaccharide-induced endotoxemia (39), adjuvant-induced arthritis (40), colitis (41), and ischemic injury in the lung (42), where A3R activation has been shown to suppress inflammatory processes. Thus, our findings strengthen the notion that the A3R plays an important role in regulating the degree of inflammation after injury.
We also demonstrate for the first time that the A3R is required for the release of EPO from eosinophils recruited to the lung. Despite the enhanced numbers of eosinophils recruited to the lungs of A3R−/− after bleomycin exposure, there was no evidence of EPO release from these cells, leading us to conclude that the A3R is needed for eosinophil degranulation. A3R expression is abundant on mouse (21) and human eosinophils (43), and engagement of this receptor on eosinophils increases intracellular Ca2+ levels (43). However, the role of the A3R in eosinophil degranulation is controversial. In vitro studies have demonstrated that engagement of the A3R on guinea pig pulmonary eosinophils can induce superoxide production (26). In contrast, studies on cultured human peripheral blood eosinophils do not support a role for A3R engagement in stimulating eosinophil degranulation (43, 44), and there is evidence that it may even prevent degranulation (25). These discrepancies may arise in part from the inability of the in vitro setting to accurately capture the environment of the diseased lung where degranulation occurs. In support of this, we isolated peripheral blood eosinophils as well as allergen-recruited BAL eosinophils from transgenic mice overexpressing IL-5 (45), and we were not able to directly stimulate EPO release in vitro with an A3R agonist (data not shown). We thus conclude that important co-stimulators or cellular interactions necessary for A3R-mediated eosinophil degranulation are not present in culture situations. A role for the A3R in eosinophil degranulation in vivo may have important implications for diseases such as asthma, in which both elevations in eosinophils and adenosine are thought to be involved in disease progression (5, 46). Indeed, a recent expression study assessing transcripts from the lungs of allergen-treated wild-type and eosinophil-less mice demonstrated that A3R transcripts were significantly down-regulated in the lungs of mice lacking eosinophils (47).
In the bleomycin model, pulmonary inflammation peaks between Days 7 and 10 after exposure and then diminishes, while fibrotic changes appear around Day 7 and progressively increase (48). Work from our laboratory and others has demonstrated that perturbations that lead to enhanced inflammation in this model are associated with enhanced fibrosis (11, 49, 50). Interestingly, we found that despite enhanced pulmonary inflammation in A3R−/− mice exposed to bleomycin, there was not an increase in standard measures of pulmonary fibrosis, including collagen production and deposition and α-SMA staining. This observation suggests that removing the A3R uncouples amplification pathways that link inflammation and the severity of fibrosis. One mechanism that may be responsible for this is the regulation of MMP-9 expression. This protease is known to be elevated in fibrotic lungs diseases (51) and can contribute to fibrosis by promoting the processing of latent TGF-β1 into active TGF-β1 (35, 52). Our results demonstrate that MMP-9 expression is decreased in the lungs of A3R−/− mice exposed to bleomycin and that loss of expression in alveolar macrophages and mast cells is responsible for this decrease. In addition, we found that TGF-β1 expression levels were elevated in A3R−/− mice treated with bleomycin; however, there was not a concomitant increase in the levels of active TGF-β1. These findings have led us to propose a model in which adenosine generated during bleomycin injury (11) can engage the A3R on alveolar macrophages and mast cells to promote the production of MMP-9 that in turn can lead to the activation of TGF-β1 and contribute to the amplification of pulmonary fibrosis. This mechanism could account for the observation that despite increased inflammation in A3R−/− mice exposed to bleomycin, there is not an increase in the degree of fibrosis seen. This provides a novel route by which a factor generated in response to damage (namely, adenosine) can impact inflammation and subsequent fibrosis.
Another consideration for the disconnection of inflammation and fibrosis is the lack of eosinophil degranulation. Eosinophils are present in the lungs of patients with idiopathic lung fibrosis and are elevated in models of bleomycin-induced fibrosis (53, 54). Moreover, eosinophil-derived cationic proteins such as EPO have been shown to influence the production of mediators such as MMP-9 and TGF-β1 from pulmonary cells (55), suggesting that eosinophil degranulation may play an important role in regulating fibrosis. Although we found increased numbers of eosinophils in the lungs of A3R−/− mice exposed to bleomycin, they did not appear to be degranulating as evidenced by the lack of EPO release. Thus, the lack of release of eosinophil granule proteins may contribute to the lack of enhanced fibrosis in A3R−/− mice exposed to bleomycin.
In conclusion, this study has characterized the contribution of the A3R in the inflammation and fibrosis that results from bleomycin injury. The roles of this receptor in this model are complex. There was clear evidence for antiinflammatory influences in that A3R−/− mice exhibited exaggerated expression of certain cytokines and chemokines in association with an excessive influx of multiple inflammatory cell types. There was essentially no difference in the degree of fibrosis seen in A3R+/+ and A3R−/− exposed to bleomycin, suggesting that the A3R is not necessary for the development of fibrosis in this model. However, investigation of TGF-β1 activation suggests that the A3R may contribute to the amplification of pulmonary fibrosis by regulating MMP-9, and/or by regulating eosinophil activation. Thus, signaling through the A3R may contribute to inflammatory and fibrotic processes that regulate the balance between normal wound healing and the unremitting fibrosis seen in interstitial lung diseases.
Supplementary Material
Acknowledgments
The authors thank Marlene Jacobson for providing A3R-deficient mice, and Hope Northrop for her assistance in obtaining Blenoxane for use in this study.
This work was funded by NIH Grants HL70952 (M.R.B.), AI43572 (M.R.B.), and U19A1070973 (F.K.), and by the Mayo Foundation. E.M. was supported by an American Academy of Allergy, Asthma and Immunology Strategic Training in Allergy Research (ST*AR) Award.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2007-0419OC on June 27, 2008
Conflict of Interest Statement: M.R.B. is a paid consultant for CV Therapeutics for work on the A2B adenosine receptor. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Thannickal VJ, Toews GB, White ES, Lynch JP III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 2004;55:395–417. [DOI] [PubMed] [Google Scholar]
- 2.Panos RJ, Mortenson RL, Niccoli SA, King TE Jr. Clinical deterioration in patients with idiopathic pulmonary fibrosis: causes and assessment. Am J Med 1990;88:396–404. [DOI] [PubMed] [Google Scholar]
- 3.Lynch J, Toews G. Idiopathic pulmonary fibrosis. In: Fishman AP, Elias JA, Fishman JA, Grippi MA, Kaiser LR, Senior RM, editors. Pulmonary diseases and disorders, 3rd ed. New York: McGraw-Hill; 1998. pp. 1069–1084.
- 4.Sime PJ, O'Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol 2001;99:308–319. [DOI] [PubMed] [Google Scholar]
- 5.Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest 2003;111:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheppard D. Pulmonary fibrosis: a cellular overreaction or a failure of communication? J Clin Invest 2001;107:1501–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis 1993;148:91–97. [DOI] [PubMed] [Google Scholar]
- 8.Huszar E, Vass G, Vizi E, Csoma Z, Barat E, Molnar VG, Herjavecz I, Horvath I. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur Respir J 2002;20:1393–1398. [DOI] [PubMed] [Google Scholar]
- 9.Blackburn MR, Chun GL, Young HWJ, Chunn JL, Banerjee SK, Elias JA. Adenosine mediates IL-13-induced inflammation and remodeling in the lung: evidence for an IL-13-adenosine amplification pathway. J Clin Invest 2003;112:332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma B, Blackburn MR, Lee CG, Homer RJ, Liu W, Flavell RA, Boyden L, Lifton RP, Sun CX, Young HW, et al. Adenosine metabolism and murine strain-specific IL-4-induced inflammation, emphysema, and fibrosis. J Clin Invest 2006;116:1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volmer JB, Thompson LF, Blackburn MR. Ecto-5′-nucleotidase (CD73)-mediated adenosine production is tissue protective in a model of bleomycin-induced lung injury. J Immunol 2006;176:4449–4458. [DOI] [PubMed] [Google Scholar]
- 12.Fredholm BB, Jzerma IJP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 13.Montesinos MC, Shaw JP, Yee H, Shamamian P, Cronstein BN. Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol 2004;164:1887–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan ES, Fernandez P, Merchant AA, Montesinos MC, Trzaska S, Desai A, Tung CF, Khoa DN, Pillinger MH, Reiss AB, et al. Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum 2006;54:2632–2642. [DOI] [PubMed] [Google Scholar]
- 15.Chunn JL, Molina JG, Mi T, Xia Y, Kellems RE, Blackburn MR. Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J Immunol 2005;175:1937–1946. [DOI] [PubMed] [Google Scholar]
- 16.Chunn JL, Mohsenin A, Young HW, Lee CG, Elias JA, Kellems RE, Blackburn MR. Partially adenosine deaminase-deficient mice develop pulmonary fibrosis in association with adenosine elevations. Am J Physiol Lung Cell Mol Physiol 2006;290:L579–L587. [DOI] [PubMed] [Google Scholar]
- 17.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol 2004;25:33–39. [DOI] [PubMed] [Google Scholar]
- 18.Sitkovsky MV, Ohta A. The ‘danger’ sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol 2005;26:299–304. [DOI] [PubMed] [Google Scholar]
- 19.Blackburn MR. Too much of a good thing: adenosine overload in adenosine-deaminase-deficient mice. Trends Pharmacol Sci 2003;24:66–70. [DOI] [PubMed] [Google Scholar]
- 20.Walker BA, Jacobson MA, Knight DA, Salvatore CA, Weir T, Zhou D, Bai TR. Adenosine A3 receptor expression and function in eosinophils. Am J Respir Cell Mol Biol 1997;16:531–537. [DOI] [PubMed] [Google Scholar]
- 21.Young HW, Molina JG, Dimina D, Zhong H, Jacobson M, Chan L-NL, Chan T-S, Lee JJ, Blackburn MR. A3 adenosine receptor signaling contributes to airway inflammation and mucus production in adenosine deaminase-deficient mice. J Immunol 2004;173:1380–1389. [DOI] [PubMed] [Google Scholar]
- 22.Young HW, Sun CX, Evans CM, Dickey BF, Blackburn MR. A3 adenosine receptor signaling contributes to airway mucin secretion after allergen challenge. Am J Respir Cell Mol Biol 2006;35:549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA. Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem 2000;275:4429–4434. [DOI] [PubMed] [Google Scholar]
- 24.Zhong H, Sergiy S, Molina JG, Sanborn BM, Jacobson MA, Tilley SL, Blackburn MR. Activation of murine lung mast cells by the adenosine A3 receptor. J Immunol 2003;170:338–345. [DOI] [PubMed] [Google Scholar]
- 25.Ezeamuzie CI, Philips E. Adenosine A3 receptors on human eosinophils mediate inhibition of degranulation and superoxide anion release. Br J Pharmacol 1999;127:188–194. (In Process Citation). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker BA. Effects of adenosine on guinea pig pulmonary eosinophils. Inflammation 1996;20:11–21. [DOI] [PubMed] [Google Scholar]
- 27.Martin L, Pingle SC, Hallam DM, Rybak LP, Ramkumar V. Activation of the adenosine A3 receptor in RAW 264.7 cells inhibits lipopolysaccharide-stimulated tumor necrosis factor-alpha release by reducing calcium-dependent activation of nuclear factor-kappaB and extracellular signal-regulated kinase 1/2. J Pharmacol Exp Ther 2006;316:71–78. [DOI] [PubMed] [Google Scholar]
- 28.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther 2006;317:172–180. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 2006;314:1792–1795. [DOI] [PubMed] [Google Scholar]
- 30.Chunn JL, Young HW, Banerjee SK, Colasurdo GN, Blackburn MR. Adenosine-dependent airway inflammation and hyperresponsiveness in partially adenosine deaminase-deficient mice. J Immunol 2001;167:4676–4685. [DOI] [PubMed] [Google Scholar]
- 31.Sun CX, Young HW, Molina JG, Volmer JB, Schnermann J, Blackburn MR. A protective role for the A(1) adenosine receptor in adenosine-dependent pulmonary injury. J Clin Invest 2005;115:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 33.Corry DB, Rishi K, Kanellis J, Kiss A, Song Lz LZ, Xu J, Feng L, Werb Z, Kheradmand F. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol 2002;3:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 1988;41:467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med 2001;194:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol 2001;41:775–787. [DOI] [PubMed] [Google Scholar]
- 37.Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest 2006;116:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montesinos MC, Desai A, Delano D, Chen JF, Fink JS, Jacobson MA, Cronstein BN. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis Rheum 2003;48:240–247. [DOI] [PubMed] [Google Scholar]
- 39.Hasko G, Nemeth ZH, Vizi ES, Salzman AL, Szabo C. An agonist of adenosine A3 receptors decreases interleukin-12 and interferon-gamma production and prevents lethality in endotoxemic mice. Eur J Pharmacol 1998;358:261–268. [DOI] [PubMed] [Google Scholar]
- 40.Baharav E, Bar-Yehuda S, Madi L, Silberman D, Rath-Wolfson L, Halpren M, Ochaion A, Weinberger A, Fishman P. Antiinflammatory effect of A3 adenosine receptor agonists in murine autoimmune arthritis models. J Rheumatol 2005;32:469–476. [PubMed] [Google Scholar]
- 41.Mabley J, Soriano F, Pacher P, Hasko G, Marton A, Wallace R, Salzman A, Szabo C. The adenosine A3 receptor agonist, N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide, is protective in two murine models of colitis. Eur J Pharmacol 2003;466:323–329. [DOI] [PubMed] [Google Scholar]
- 42.Rivo J, Zeira E, Galun E, Matot I. Activation of A3 adenosine receptor provides lung protection against ischemia-reperfusion injury associated with reduction in apoptosis. Am J Transplant 2004;4:1941–1948. [DOI] [PubMed] [Google Scholar]
- 43.Kohno Y, Ji X, Mawhorter SD, Koshiba M, Jacobson KA. Activation of A3 adenosine receptors on human eosinophils elevates intracellular calcium. Blood 1996;88:3569–3574. [PMC free article] [PubMed] [Google Scholar]
- 44.Reeves JJ, Harris CA, Hayes BP, Butchers PR, Sheehan MJ. Studies on the effects of adenosine A3 receptor stimulation on human eosinophils isolated from non-asthmatic or asthmatic donors. Inflamm Res 2000;49:666–672. [DOI] [PubMed] [Google Scholar]
- 45.Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol 1997;158:1332–1344. [PubMed] [Google Scholar]
- 46.Fozard JR. The case for a role for adenosine in asthma: almost convincing? Curr Opin Pharmacol 2003;3:264–269. [DOI] [PubMed] [Google Scholar]
- 47.Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci USA 2006;103:16418–16423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Izbicki G, Segel MJ, Christensen TG, Conner MW, Breuer R. Time course of bleomycin-induced lung fibrosis. Int J Exp Pathol 2002;83:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawson WE, Polosukhin VV, Stathopoulos GT, Zoia O, Han W, Lane KB, Li B, Donnelly EF, Holburn GE, Lewis KG, et al. Increased and prolonged pulmonary fibrosis in surfactant protein C-deficient mice following intratracheal bleomycin. Am J Pathol 2005;167:1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore BB, Coffey MJ, Christensen P, Sitterding S, Ngan R, Wilke CA, McDonald R, Phare SM, Peters-Golden M, Paine R III, et al. GM-CSF regulates bleomycin-induced pulmonary fibrosis via a prostaglandin-dependent mechanism. J Immunol 2000;165:4032–4039. [DOI] [PubMed] [Google Scholar]
- 51.Hoshino M, Nakamura Y, Sim J, Shimojo J, Isogai S. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. J Allergy Clin Immunol 1998;102:783–788. [DOI] [PubMed] [Google Scholar]
- 52.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 53.Hallgren R, Bjermer L, Lundgren R, Venge P. The eosinophil component of the alveolitis in idiopathic pulmonary fibrosis. Signs of eosinophil activation in the lung are related to impaired lung function. Am Rev Respir Dis 1989;139:373–377. [DOI] [PubMed] [Google Scholar]
- 54.Huaux F, Liu T, McGarry B, Ullenbruch M, Xing Z, Phan SH. Eosinophils and T lymphocytes possess distinct roles in bleomycin-induced lung injury and fibrosis. J Immunol 2003;171:5470–5481. [DOI] [PubMed] [Google Scholar]
- 55.Pegorier S, Wagner LA, Gleich GJ, Pretolani M. Eosinophil-derived cationic proteins activate the synthesis of remodeling factors by airway epithelial cells. J Immunol 2006;177:4861–4869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.