Abstract
Human herpesvirus-8 (HHV-8) is the causative agent of Kaposi's sarcoma and is associated with the angioproliferative disorders primary effusion lymphoma and multicentric Castleman's disease. Evidence of HHV-8 infection within the pulmonary vasculature of patients with idiopathic pulmonary arterial hypertension (IPAH) has been described. We hypothesize that HHV-8 infection of pulmonary microvascular endothelial cells results in an apoptotic-resistant phenotype characteristic of severe pulmonary arterial hypertension. Our objective was to investigate the ability of HHV-8 to infect human pulmonary microvascular endothelial cells in vitro and characterize the phenotypic effect of this infection. Human pulmonary microvascular endothelial cells were exposed to HHV-8 using two methods (direct virus and co-culture technique). The presence of lytic and latent infection was confirmed. Changes in endothelial cell gene and protein expression and effects on cellular apoptosis were measured. HHV-8 can both lytically and latently infect primary human pulmonary microvascular endothelial cells in vitro. HHV-8 infection results in significant changes in gene expression, including alterations of pathways important to cellular apoptosis. HHV-8 infection also alters expression of genes integral to the bone morphogenic protein pathway, including down-regulation of bone morphogenic protein-4. Other genes previously implicated in the development of PAH are affected by HHV-8 infection, and cells infected with HHV-8 are resistant to apoptosis.
Keywords: HHV-8, endothelial cells, pulmonary hypertension
CLINICAL RELEVANCE
This article suggests mechanisms by which human herpesvirus (HHV)-8 may result in pathology in the pulmonary vasculature. It provides insight into the means by which HHV-8 could play a role in the development of some forms of pulmonary hypertension.
Pulmonary arterial hypertension (PAH) is a poorly understood disease associated with proliferation of vascular endothelial cells in the pre-capillary pulmonary arteries (1, 2). Endothelial cells occlude the pulmonary vascular lumen, contributing to the formation of plexiform lesions. The etiology of this cellular proliferation is unclear, though proangiogenic and antiapoptotic mechanisms have been proposed (1–3).
Heterozygous mutations of the bone morphogenic protein receptor-2 (BMPR2), a member of the TGF-β receptor family, have been described in patients with both familial and sporadic idiopathic pulmonary arterial hypertension (IPAH) (4–6). The mechanism by which these mutations contribute to the development of PAH is also unknown, but apoptosis resistance has been implicated (7). Signaling through the BMP receptors requires heterodimerization of the serine/threonine kinase type 1 receptors (BMPR1a or BMPR1b) and the type 2 receptor (BMPR2). The ligand for these receptors (BMPs) initially binds type 2 receptors, which phosphorylate type 1 receptors, which in turn phosphorylate intracellular signaling molecules, mediating transcriptional activation (8). Bone morphogenic proteins-2, -4, and -7 inhibit the proliferation of smooth muscle cells from normal pulmonary arteries and from the arteries of patients with PAH with congenital heart diseases, but fail to suppress proliferation of cells from patients with BMPR2 mutations (9).
While BMPR2 mutations are clearly implicated in the development of some forms of pulmonary hypertension, many patients with PAH lack evidence of these mutations (10–12). However, these patients have phenotypic changes within their pulmonary vasculature similar to those seen in patients with BMPR2 mutations (10, 13, 14). It is therefore possible that anomalies of the BMP signaling cascade other then BMPR2 contribute to the development of PAH. Supporting this hypothesis are observations by Du and coworkers that expression of BMPR1a is severely reduced in lungs of patients with various forms of acquired as well as idiopathic nonfamilial pulmonary hypertension (15).
While the BMP signaling cascade has received much recent attention, a variety of other inflammatory, angiogenic, and proteolytic molecules are also suspected of involvement in PAH. Interleukin 6 (IL-6) is an inflammatory cytokine, elevated in a number of diseases (16). Miyata and colleagues and Golembeski and coworkers have demonstrated that administration of IL-6 in both rat and mouse models results in pulmonary hypertension (17, 18). IL-6 levels are also elevated in the peripheral blood of patients with severe IPAH (19). Matrix metalloproteinases (MMPs) are a family of inducible proteases important in the remodeling of the extracellular matrix (ECM) as well as in smooth muscle and endothelial cell migration (20). Fibroblast accumulation is prominent surrounding the plexiform lesions of PAH, and several animal models of PAH indicate a role for MMP-induced remodeling of the lung parenchyma (21–25). Angiogenic endothelial cells produce MMP-2, which modulates endothelial cell migration and vascular remodeling (26). Lepetit and colleagues reported an increase in MMP-2 in smooth muscle cells cultured from patients with PAH (27).
HHV-8, a recently discovered γ-herpesvirus, is the causative agent of the endothelial cell neoplasia Kaposi's sarcoma (28). HHV-8 is also associated with the angioproliferative disorders multicentric Castleman's disease and primary effusion lymphoma (29, 30). We have previously suggested an association between HHV-8 infection and the development of PAH (31, 32). This association has been challenged based on the lack of serologic evidence of HHV-8 infection in patients with IPAH, as well as on the inability of other investigators to confirm PCR evidence of viral open reading frames (ORFs) within the lung parenchyma of patients with IPAH (33–35). It is notable, however, that immunohistochemical staining for LANA-1 is an accepted means of identifying HHV-8 infection in tissue samples (36). It is also notable that the percentage of LANA-1–positive staining in lung tissue of patients with IPAH (61.5%) reported by other groups was the same as reported by Cool and colleagues (62%) in which both LANA-1 staining and a PCR assay for ORF-72, the viral cyclin gene of HHV-8, was positive (32). Montani and coworkers recently reported the presence of severe PAH in a patient with HIV-1 infection and multicentric Castleman's disease (37). Interestingly, the patient's pulmonary hypertension resolved with successful treatment of the patient's Castleman's disease. One of the two patients described in our initial report of Castleman's disease and PAH also eventually had complete resolution of severe PAH after successful treatment of Castleman's disease (31). Gutierrez and colleagues reported a patient with occult lymphadenopathic Kaposi's sarcoma who developed severe PAH. They speculated on a possible relationship between HHV-8 infection and HIV-associated PAH (38). Finally, Shan and coworkers have recently reported that expression of the HHV-8 gene product viral G protein–coupled-receptor (vGPCR) in human pulmonary artery endothelial cells promoted angiogenesis. The proposed mechanism of this increased angiogenesis was the selective activation of MMP-2 expression in these cells. The authors concluded that vGPCR could serve as an etiologic agent in the development of virus-induced PAH (39).
The conflicting data regarding the role of HHV-8 in patients with IPAH has led to questions regarding a role of this virus in the development of PAH. In this manuscript we explore means by which HHV-8 infection could contribute to the development of PAH using an in vitro model of pulmonary endothelial cell viral infection. Two methods were used to induce cell infection: direct exposure of ECs to concentrated virus preparation, and co-culture of ECs with reservoir B-cells that harbor HHV-8. The effect of HHV-8 infection on these cells was investigated.
MATERIALS AND METHODS
Cell Culture
The HHV8+/EBV− BCBL-1 cell line was obtained through the NIH AIDS Research and Reference Reagent Program (40). The cell line was cultured in RPMI 1640 supplemented with 12% FBS, 5 × 10−5 M 2-mercaptoethanol, 2 mM L-glutamine, 20 mM HEPES buffer, 100 U/ml penicillin, and 100 μg/ml streptomycin. Primary human pulmonary microvascular endothelial cells (HMVEC-L) were obtained from Cambrex Bio Science (Clonetics) (Walkersville, MD). HMVEC-L were cultured in EBM-2 media with Bullet Kit supplement (to create EGM-2 MV media), according to Clonetics' recommendations.
Virus Preparation
BCBL-1 cells (reservoir cells for HHV-8) were seeded at a density of 3 × 105 cells/ml in serum-free media. Twenty-four hours later, 12% FBS was added back to the cell cultures. Sixteen hours later, cells were induced into lytic replication by culturing with tetradecanoyl phorbol acetate (TPA, 20 ng/ml; Sigma, St. Louis, MO) for 5 days. To obtain cell-free virus, cells were centrifuged and supernatant was passed through a 0.45-μm syringe filter. Filtered supernatant was then concentrated for 2 hours at 40,000 × g in an SW28 rotor at 4°C. The resulting pellet was re-suspended at 1:100th the original volume in EGM-2 MV media.
Mock Infection Media Preparation
Mock infection media was used as a control condition for the gene expression experiments. It was composed of EGM-2 MV media with the addition of 20 ng/ml TPA and 1% polyethylene glycol (PEG) and 8 μg/ml polybrene (Sigma). This was used to control for the possible effects of TPA and PEG on cell gene expression.
Conditioned Media Preparation
Conditioned media (supernatant minus whole virus) was used as a control condition in the co-culture apoptosis, gene expression, and enzyme-linked immunosorbent assay (ELISA) experiments. The conditioned media was prepared from supernatant obtained from the purified virus preparation after pelleting the virus via centrifugation for 2 hours at 40,000 × g. The supernatant was diluted 1:10 in EGM-2 MV. Absence of infectious virus was confirmed by immunofluorescence assay (IFA) for LANA and K8.1 performed on the cells to which the media was applied, and by absence of viral gene expression via real-time RT-PCR for vGPCR and T1.1 performed on the conditioned media itself.
Infection of HMVEC-L with HHV-8 (Direct Virus Technique)
One milliliter of concentrated virus or 1 ml of control (mock infection media) was added per 1 × 105 HMVEC-L. Target cells were cultured in EGM-2 MV with the addition of hexamethadrine bromide (polybrene) (8 μg/ml) and 1% PEG (Sigma). After overnight incubation, media was removed and replaced with fresh EGM-2 MV. At 72 hours after infection, cells were detached with 1× trypsin/EDTA (Cellgro; Fisher Scientific, Fair Lawn, NJ) and harvested for IFA, RNA, and protein extraction. Mock-infected cells were processed in an identical fashion, but were exposed to “mock infection media” as opposed to the viral preparation.
B Cell–Mediated Infection (Co-Culture Technique)
This is a variation of a technique initially described by Sakurada and coworkers (41) HMVEC-L were cultured in EGM-2 MV in 96-well tissue culture plates. BCBL-1 cells were induced with 20 ng/ml TPA and were added at a 2:1 effector:target ratio. HMVEC-L grow as an adherent monolayer, while BCBL-1 cells grow in suspension. As controls, HMVEC-L were cultured in the presence of “conditioned media” (see above) from a purified virus preparation, or in media alone (“untreated”). After 24 hours, the media was removed and each well washed vigorously three times with EBM-2 basal media to remove the BCBL-1 cells. The media was replaced with fresh EGM-2 MV and changed every 2 days thereafter. At Days 10, 15, and 20 after exposure, duplicate wells for each condition were harvested by trypsinization, counted, and seeded onto slides for evaluation of infection by IFA as described above. Absence of residual BCBL-1 cells in the culture after washing was confirmed by IFA for CD38 (B cell marker) and by flow cytometry for cell size and by visual inspection by light microscopy for cell morphometry (data not shown). Triplicate wells for each condition were assessed for cellular apoptosis after exposure to camptothecin (see Apoptosis Assay below). At time points of 72 hours, 10 days, 15 days, and 20 days, the supernatant from each well was collected before addition of fresh media for ELISA examination of secreted proteins present in the supernatant.
Immunofluorescence Assay
HMVEC-L were seeded onto charged slides in EGM-2 MV, then incubated overnight at 37°C. Slides were washed three times in phosphate-buffered saline (PBS), air dried, and incubated for 1 hour at 56°C. They were incubated for 2 hours with primary α-ORF73 antibody (Advanced Biotechnologies Inc., Columbia, MD) at 1:1,000, and secondary goat α-rat antibody conjugated to Alexa Fluor 488 (Molecular Probes, Invitrogen Corp., Eugene, OR) at 1:1,000 for detection of LANA-1. Slides were incubated with primary α-K8.1 monoclonal antibody (clone 19B4, a gift to Dr. Campbell from Dr. Bagher Forghani, Viral and Rickettsial Disease Laboratory, Division of Communicable Disease Control, California Department of Health Services) at 1:1,000 and secondary goat α-mouse antibody conjugated to Alexa Fluor 568 (Molecular Probes) at 1:2,000 for detection of the HHV-8 lytic K8.1 proteins. They were counterstained with Hoechst 33258 dye for 10 minutes (Molecular Probes). All antibodies were diluted with 1% normal goat serum (Gibco, Invitrogen, Carlsbad, CA) in 1× PBS.
Microarray Analysis of HHV-8–Infected HMVEC-L
Details regarding the microarray experiments are included in the online supplement and were performed as described previously (42). HG-U133 Plus 2.0 microarrays were used for the array experiments (Affymetrix, Foster City, CA). Tabular gene expression data are published on line in Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=zjgbpoiyuqogwhuandacc=GSE6489) submission GSE6489.
Quantitative PCR
RNA was extracted from the HMVEC-L using the RNeasy kit (Qiagen, Valencia, CA). Primers and probes for the following genes were obtained from Applied Biosystems Assays on Demand using their recommended conditions (Foster City, CA): BMP-4, BMPR1a, MMP-1, IL-6, Noggin, TGFβ-2, FLT-1, ANG-2, and β-actin. All reactions were performed on a Gene Amp 5700 sequence detector (Applied Biosystems) using conditions recommended by the manufacturer. Three separate infections were performed, each sample was run in duplicate, and the results were averaged. Results were standardized to expression of a control gene (β-actin or HPRT). The absence of nonspecific amplification was confirmed by examining PCR products by agarose gel electrophoresis. RT-PCR was performed from the RNA of six samples of cells (three infected and three untreated) used to generate the microarrays as well as on three subsequent samples (from a separate infection and mock infection) to confirm the microarray data. RT-PCR was performed to confirm both lytic and latent infection of HMVEC-L. The genes selected to confirm HHV-8 infection were: LANA-1, ORF50, K8.1, vGPCR, T1.1. The primer sequences for PCR to confirm viral infection are included in the online supplement.
ELISA
IL-6, MMP-1, and BMP-4 levels were measured in the supernatant (400 μl) collected from HHV-8–infected and Mock-treated HMVEC-L at 72 hours after exposure to direct virus. IL-6, MMP-1, BMP-4, and ANG-2 levels were measured in the supernatant of HHV-8 infected HMVEC-L via co-culture, cells exposed to conditioned media, and untreated cells at 72 hours and at Days 10, 15, and 20. EGM-2 media alone was also assayed to confirm reference standard. All samples were run in triplicate (three separate infections and controls). The ELISA reactions were performed per the manufacturer's recommendations (ELISA Tech, Aurora, CO).
Apoptosis Assay
Caspse-Glo assay.
HMVEC-L were infected via co-culture with BCBL-1 cells (see above). The cell conditions for the assessment of cellular apoptosis included: (1) HMVEC-L infected with HHV-8 via co-culture with BCBL-1 cells as described above; (2) HMVEC-L exposed to conditioned media (CM- media aspirated from an active HHV-8 infection but without the presence of infectious virons); and (3) control (untreated) HMVEC-L without exposure to virus or conditioned media. The HMVEC-L were plated into 96-well plates at a density of 1 × 104 cells/well 24 hours before the apoptosis assay. The EGM-2 MV media from each condition was aspirated and replaced with EGM-2 MV media containing camptothecin (1:1,000 × 2 mM; Bio Vision, Mountain View, CA) to induce apoptosis. The cells were assayed for apoptosis 4 hours later via the Caspase-Glo 3/7 assay (Promega, Madison, WI) per the manufacturer's recommendations. Caspase 3/7 luminescence was measured on a Spectrafluor Plus luminometer (Tecan, Research Triangle Park, NC) with Magellan 5.01 software. Latent HHV-8 infection of HMVEC-L was confirmed at these same three time points as described above (IFA for LANA-1).
TUNEL assay.
In a separate assay for apoptosis HMVEC-L were seeded into 8-well chamber slides at 2 × 104cells/well, and B cell–mediated infection (co-culture technique) was employed to infect the target cells. At Days 3 and 20 after exposure, cells were cultured with or without 1:1,000 of 2 mM camptothecin (BioVision, Mountain View, CA) for 6 hours at 37°C. Wells were washed with PBS, and then chambers were removed from the slides. Slides were washed in PBS three times for 5 minutes each, air dried for 30 minutes, and then heat-fixed at 56°C for 1 hour. Slides were washed in PBS with 0.05% Tween-20 (Bio-Rad Laboratories, Hercules, CA), and then in PBS alone. TUNEL staining was accomplished using an In Situ Cell Death Detection Kit (Roche, Indianapolis, IN), following the manufacturer's protocol. Slides were counterstained for 10 minutes with Hoechst 33258 dye (Molecular Probes), diluted at 1:20,000. Cells were examined for evidence of apoptosis via visual inspection for immunofluorescence at Days 3 and 20 after infection or exposure to CM. Two hundred cells per each condition were counted, and the percentage of TUNEL-positive cells was recorded.
Addition of Noggin, IL-6, and BMP-4
Exogenous Noggin, IL-6, and BMP-4 were added to cell culture of HMVEC-L and the cells were assessed for apoptosis resistance. HMVEC-L were trypsinized, resuspended in EGM-2 MV medium, seeded into 96-well plates at a concentration of 6 × 103 cells/well, and then allowed to attach to the wells for 4 hours. The media was then changed and cells were cultured in either complete medium, or in serum-free medium. Cells were treated with either Noggin (250 ng/ml), BMP4 (75 ng/ml), or with both in combination. Additional wells were treated with IL-6 at various concentrations in the range of 10 to 200 pg/ml (concentrations based on recommendations from the manufacturer; R&D Systems, Minneapolis MN). After 24 hours, the EGM-2 MV media from each condition was aspirated and replaced with EGM-2 MV media containing camptothecin (1:1,000 × 2 mM; BioVision) to induce apoptosis. Four hours later, the media was removed from all wells and replaced with 1:1 Caspase-Glo reagent in EGM-2 MV. The cell plates were incubated on a plate shaker for 1 hour at room temperature. Caspase 3/7 luminescence was measured using a Luminoskan Ascent 2.5 luminometer with Ascent software (Thermo Scientific, Waltham, MA).
Statistical Analysis of Data
Statistical analysis of the nonmicroarray data was performed using the Prism version 3.0 for windows (GraphPad software, San Diego, CA). Unpaired Student's t tests were used to compare the 72 hours q-PCR data. Two-way ANOVA with a Bonferroni post test for treatment effect of infection versus conditioned media or versus untreated cells was used to assess effect of gene expression by co-culture and for the ELISA data over the 20-day time period. One-way ANOVA with a Tukey post test analysis was used to asses the apoptosis experiments.
Microarray Data Analysis
Analysis of the microarrays was performed as previously described (43). Microarray data were analyzed using BRB ArrayTools v3.3b (developed by Dr. Richard Simon and Amy Peng Lam). Cluster analysis and class comparison using the univariate two sample t test was performed using the set of 25,866 genes that were reliably detected on two or more arrays. Multivariate permutation tests to determine false discovery rates were based on 1,000 random class label permutations.
RESULTS
HHV-8 Can Infect Primary Human Pulmonary Microvascular Endothelial Cells
To our knowledge, this is the first time that HHV-8 has been shown to infect a pulmonary-derived microvascular endothelial cell in vitro. Both infection techniques described in this manuscript (concentrated virus and co-culture with BCBL-1 cells) were successful in infecting the target cells. LANA-1 expression was detected in approximately 80% of HMVEC-L exposed to the concentrated virus preparation at 72 hours. Both lytic and latent infection was demonstrated by immunofluorescence (IFA) (Figures 1 and 2) and PCR assays (Figure 3). Figure 1 demonstrates IFA evidence of latent infection (LANA-1) of HMVEC-L by HHV-8 virus 72 hours after exposure. Figure 2 shows IFA markers of both latent (LANA-1) and lytic (K8.1) infection of these cells after 72 hours of exposure to the virus. BCBL-1 cells served as a positive control in the IFA and PCR assays. There was no IFA or PCR evidence of viral infection in the negative control condition (mock infection) or in untreated cells. Co-culture of HMVEC-L with BCBL-1 cells also results in successful HHV-8 infection at the three time points assayed (10, 15, and 20 d)(see Figure E1 in the online supplement). LANA-1 expression was detected in approximately 10 to 20% of the HMVEC-L exposed to HHV-8 via co-culture system at the three time points assayed (10, 15, and 20 d) (Figure E2).
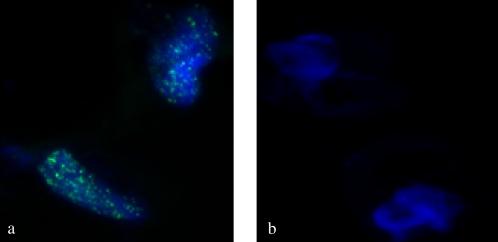
Figure 1.
Immunofluorescence assay (IFA) for latency-associated nuclear antigen-1 (LANA-1) (green) 72 hours after viral exposure (×100 magnification). The cells were counter stained with Hoechst nuclear stain (blue). (a) Human herpesvirus (HHV)-8–infected cells; note the “punctate” staining characteristic of LANA-1 staining. (b) Mock (treated in identical fashion but without addition of HHV-8)-infected cells are negative for LANA-1 staining.
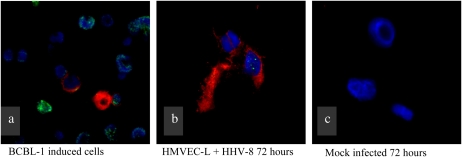
Figure 2.
Demonstration of lytic infection of primary human pulmonary microvascular endothelial cells (HMVEC-L) infected with HHV-8. Green staining indicates LANA-1, red staining indicates K8.1 (a marker of lytic replication), and blue is a Hoechst nuclear stain. (a) BCBl-1 cells (reservoir cells for HHV-8) induced to enter lytic infection. These cells serve as a positive control. (b) HMVEC-L 72 hours after exposure to HHV-8. (c) Mock-infected HMVEC-L (negative control) treated in identical fashion as in b, though without exposure to HHV-8.
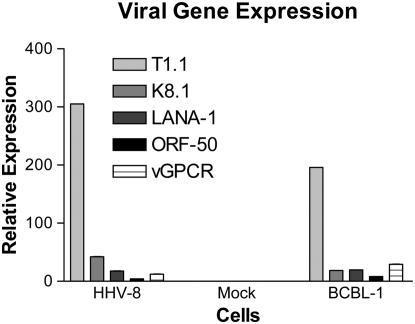
Figure 3.
q-PCR assay for markers of viral infection in HHV-8–infected HMVEC-L, mock-infected cells (negative control), and BCBL-1 cells (positive control). The genes assayed by PCR were T1.1, K8.1, LANA-1, ORF-50, and vGPCR. Both HHV-8–infected HMVEC-L and BCBL-1 cells were positive for HHV-8 gene expression indicating infection. Mock-infected cells were negative for PCR evidence of HHV-8 infection.
HHV-8 Infection of Pulmonary Microvascular Endothelial Cells Significantly Alters Gene Expression
HHV-8 infection of HMVEC-L alters gene expression as measured by microarray and q-PCR. Figure 4 represents a microarray unsupervised cluster analysis of infected versus mock-infected cells. Of the 54,675 genes represented on the microarray, 25,899 passed filtering criteria. A 1,128-gene expression signature (Table E1) distinguished HHV-8–infected cells versus mock-infected cells. This gene expression signature was generated using a two-sample t test and a conservative P value cutoff of 0.001. Perturbation testing of the resulting 1,128 gene list suggests that it contains fewer then 10 false discoveries. A number of genes of interest in relation to the development of PAH were identified by this microarray analysis (Table 1). These included a decrease in the expression of BMP4 and BMPR1a in infected cells, an increased expression of Noggin (an endogenous inhibitor of BMPs), increased expression of IL-6, and increased expression of MMP-1, MMP-2, and MMP-10. A number of genes involved in apoptosis were also altered in their expression in HMVEC-L infected with HHV-8 as compared with control cells (Table 2).
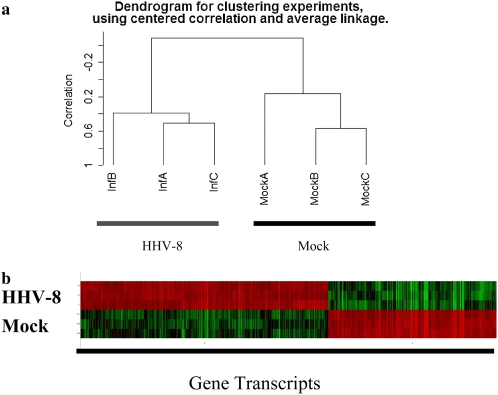
Figure 4.
(a) Dendrogram of HHV-8–infected HMVEC-L versus mock (control)-infected cells. Micorarrays were performed at 72 hours after infection. Unsupervised cluster analysis was performed using centered correlation and average linkage. (b) Representative color display of the gene signature distinguishing HHV-8–infected cells from mock-infected cells. Samples (infected versus mock) are along the y-axis and specific gene transcripts are along the x-axis. Red color indicates a relative increase in gene expression, while green indicates a relative decease. HHV-8 infection of HMVEC-L resulted in significant changes in gene expression compared with mock-infected cells. Compete list of the 1,128 gene expression signature altered by HHV-8 infection is included in the online supplement (Table E1).
TABLE 1.
GENES ALTERED IN THEIR EXPRESSION BY HHV-8 INFECTION OF POTENTIAL INTEREST IN THE PATHOPHYSIOLOGY OF PULMONARY HYPERTENSION
| Gene | Gene Symbol | Fold Change in HHV-8 Infection | Cellular Function/Pathway |
|---|---|---|---|
| Bone morphogenic protein receptor-1a | BMPR1a | 0.5 | TGF-β pathway |
| Bone morphogenic protein 4 | BMP4 | 0.6 | TGF-β pathway |
| Noggin | Nog | 22.6 | TGF-β pathway |
| Interleukin-6 | IL-6 | 5.7 | Inflammation/immunity |
| Matrix metalloproteinase-1 | MMP-1 | 73 | Peptidase/ECM remodeling |
| Matrix metalloproteinase-2 | MMP-2 | 1.4 | Peptidase/ECM remodeling |
| Matrix metalloproteinase-10 | MMP-10 | 5.3 | Peptidase/ECM remodeling |
| Platelet derived growth factor D | PDGFD | 0.5 | Growth factor |
| Fms-Related Tyrosine Kinase-1 | FLT-1 | 6.9 | Angiogenesis |
| Angiopoietin 2 | ANGPT12 | 5.1 | Angiogenesis |
Definition of abbreviations: ECM, extracellular matrix; HHV-8, human herpesvirus-8.
Relative fold change of gene expression of HHV-8–infected cells as compared to mock-infected cells is listed.
TABLE 2.
GENES ALTERED IN THEIR EXPRESSION BY HHV-8 INFECTION RELATED TO CELLULAR APOPTOSIS
| Gene | Gene Symbol | Fold Change in HHV-8 Infection |
|---|---|---|
| BCL2-like 11 (apoptosis facilitator) | BCL2L11 | 0.5 |
| Angiopoietin 2 | ANGPT2 | 5.0 |
| Vascular endothelial growth factor receptor | FLT1 | 6.0 |
| Serine/threonine kinase 17b (apoptosis-inducing) | STK17b | 0.5 |
| Transforming growth factor β1 | TGF-β1 | 0.7 |
| Transforming growth factor β2 | TGF-β2 | 0.29 |
| Bone morphogenic protein receptor 1a | BMPR1a | 0.5 |
| Caspase 1 | CASP1 | 2.0 |
| Caspase 4 | CASP4 | 1.9 |
| TNF receptor superfamily 6 | TNFRSF6 | 2.0 |
Definition of abbreviation: HHV-8, human herpesvirus-8.
Relative fold change of gene expression of HHV-8 infected cells as compared to mock infected cells is listed.
q-PCR Confirms the Findings of the Gene Microarray Studies
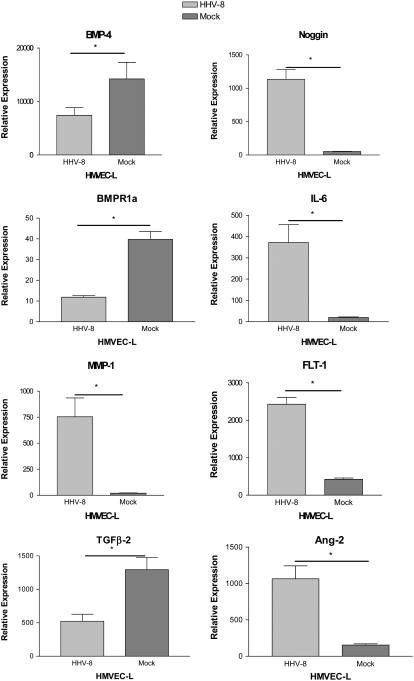
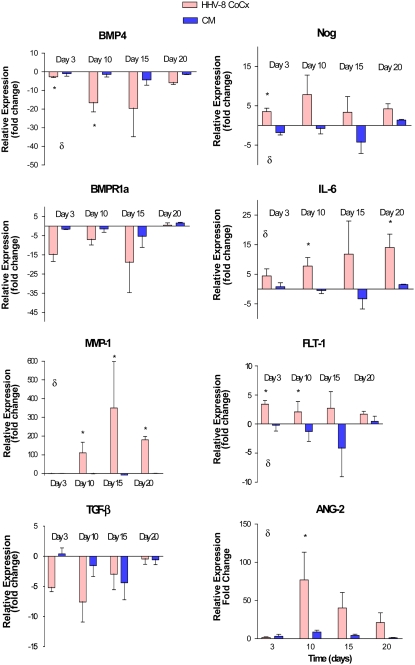
We used q-PCR to confirm the finding of the microarray data. Selected genes of interest identified by microarray analysis were confirmed by quantitative PCR analysis of both the original cell preparations (HHV-8–infected versus mock-infected cells) and on subsequent infections and mock preparations of cells. Genes for confirmation were selected for their perceived biologic interest as pertains to PAH and the pathobiology of HHV-8 infection. These included: bone morphogenic protein 4 (BMP-4), bone morphogenic protein receptor 1a (BMPR1a), Noggin (Nog), matrix metalloproteinase-1 (MMP-1), IL-6, fms-related tyrosine kinase 1 (FLT-1), TGF-β2, and angiopoietin-2 (ANG-2). As predicted by the microarray data, there were significant decreases in the expression of BMP-4, BMPR1a, and TGF-β2 and significant increases in the expression of MMP-1, IL-6, Noggin, FLT-1, and ANG-2 in HMVEC-L infected with HHV-8 as compared with mock-infected cells (Figure 5). We then assayed for these changes in gene expression over a longer period of time in the co-culture HHV-8 infection system comparing the gene expression of cells infected with HHV-8 to cells exposed to conditioned media and untreated cells. The results were standardized to the gene expression of untreated cells (expressed as fold change compared with untreated cell gene expression) (Figure 6). The changes in gene expression were more variable over time though similar trends in gene expression were noted. BMP-4 was significantly decreased in it expression in cells infected with HHV-8 and IL-6 and MMP-1 were increased in expression over this time period.
Figure 5.
q-PCR analysis of genes selected for confirmation of the microarray results. The gene expression of HMVEC-L infected with HHV-8 via direct virus exposure was compared with that of mock-infected cells (see Materials and Methods). Gene expression was assayed 72 hours after exposure. All infections and controls were performed in triplicate. Error bars represent SEM. As predicted by microarray analysis, there was decreased expression of BMP-4, BMPR1a, TGFB-2 and increased expression of Noggin, IL-6, MMP-1, and FLT-1 in cells infected with HHV-8 as compared with mock-infected cells.*P < 0.05.
Figure 6.
q-PCR of genes in HMVEC-L infected with HHV-8 via co-culture technique compared with cells exposed to conditioned media and untreated cells (media alone). The y-axis represents gene expression fold change compared with untreated cell gene expression. Expression was assayed at four time points: 72 hours, Day 10, Day 15, and Day 20. All samples were run in triplicate. Error bars represent SEM. δ indicates P < 0.05 (two-way ANOVA). *P < 0.05 (post-test infection versus conditioned media).
Relevant Proteins Were Also Altered in Expression by HHV-8 Infection
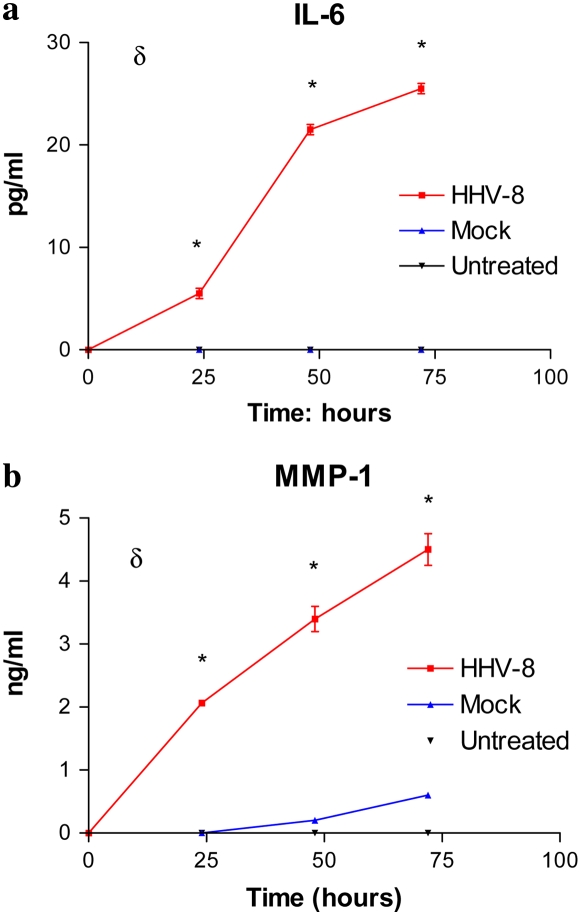
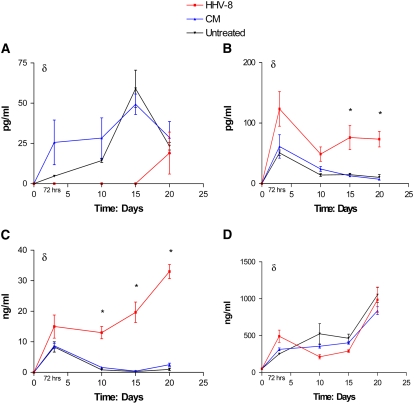
ELISA assays of the cellular supernatant in the directly infected cells as well as cells infected via co-culture were used to assay for changes in relevant proteins. Supernatant aspirated from HMVEC-L cells infected directly with HHV-8 demonstrated increased levels of IL-6 and MMP-1 as compared with mock-infected cells (Figure 7). Cells infected via co-culture also showed increased levels of IL-6 and MMP-1 and decreased levels of BMP4 at the measured time points over a 20-day period (Figure 8).
Figure 7.
Enzyme-linked immunosorbent assay (ELISA) for (a) IL-6 and (b) MMP-1 in the supernatant of HHV-8–infected HMVEC-L as compared with untreated cells (media) and mock-infected cells. δ indicates P < 0.05 (two-way ANOVA). *P < 0.05 (post-test infection versus untreated).
Figure 8.
ELISA for (a) BMP4, (b) IL-6, (c) MMP-1, and (d) ANG-2 in supernatant of HMVEC-L infected with HHV-8 via co-culture technique as compared with cells exposed to conditioned media (CM) and untreated cells (media alone). Time points assayed were 72 hours, Day 10, Day 15, and Day 20 after exposure to virus or CM. δ indicates P < 0.05 (two-way ANOVA) *P < 0.05 (post-test infection versus untreated).
HHV-8 Infection Results in an Apoptosis-Resistant Phenotype
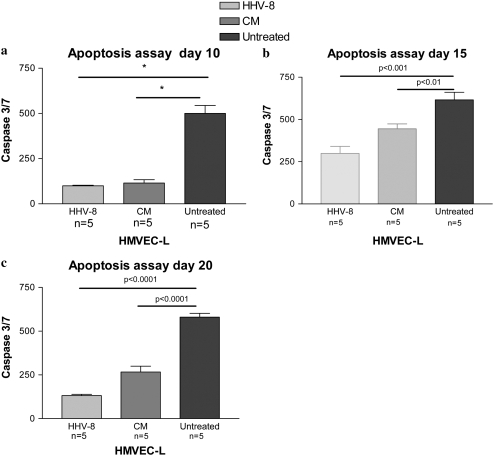
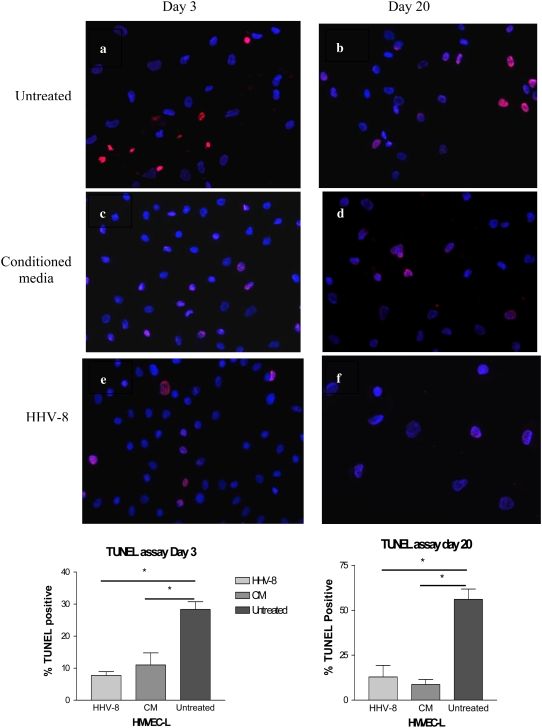
To examine the effect of HHV-8 infection on cellular apoptosis, we used the co-culture system, incubating BCBL-1 cells with the HMVEC-L as described above. HMVEC-L were successfully infected in this system, demonstrating evidence of latent infection at all subsequent time points investigated (Days 3, 10, 15, and 20) (Figures E1 and E2). Both HMVEC-L infected with HHV-8 and cells exposed to conditioned media were resistant to camptothecin-induced apoptosis as compared with untreated cells. Figure 9 shows the degree of cellular apoptosis present as assessed by the Caspase-Glo 3/7 assay at Days 10, 15, and 20. Both cells infected with HHV-8 and cells exposed to conditioned media were resistant to camptothecin-mediated apoptosis as compared with untreated cells at these time points. TUNEL assay also demonstrated that HHV-8 infection of HMVEC-L results in cellular resistance to apoptosis. Figure 10a shows a representative high-powered field of the TUNEL assay at 3 days after infection via co-culture technique and exposure to camptothecin, and again at Day 20 after infection and exposure to camptothecin as compared with cells exposed to conditioned media and untreated cells at the same time points. Cells infected with HHV-8 via co-culture and cells exposed to conditioned media demonstrate a lower percentage of apoptotic cells after camptothecin exposure as compared with untreated cells. This is demonstrated graphically in Figure 10b.
Figure 9.
Measurement of apoptosis by caspase 3/7 assay in HMVEC-L infected with HHV-8 as compared with cells exposed to conditioned media (CM) and untreated cells. The HMVEC-L were infected via co-culture with BCBL-1 cells. The cells were assessed at three time points after infection: (a) Day 10, (b) Day 15, and (c) Day 20. On the day of the selected time point the cells were exposed to camptothecin for 4 hours to induce apoptosis. Caspase 3/7 levels were then measured via luminescence. There were five replicate wells for each condition (n = 5). IFAs were performed to confirm HHV-8 infection of the HMVEC-L cells and lack of infection in the cells exposed to CM. Approximately 10 to 20% of the cells were HHV-8 infected (LANA-1 positive). *P < 0.05.
Figure 10.
Measurement of apoptosis by TUNEL staining of HMVEC-L infected with HHV-8 as compared with cells exposed to conditioned media (CM) and untreated cells. The HMVEC-L were infected via co-culture with BCBL-1 cells. The cells were assayed at two time points (Day 3 and Day 20) after infection or exposure to CM. At these time points the cells were exposed to camptothecin for 4 hours to induce apoptosis, and then TUNEL staining was performed. The percent of TUNEL-positive cells were quantified by light microscopy and compared between the three groups at each time point. Three separate infections and three controls were performed for each time point. The first row represents untreated cells at (a) Day 3 and (b) Day 20. The second row demonstrates cells exposed to CM at (c) Day 3 and (d) Day 20. The third row demonstrates cells infected with HHV-8 via co-culture at (e) Day 3 and (f) Day 20. Figures are at ×40 magnification. The percent of TUNEL-positive cells are represented graphically below the figures. *P < 0.05.
Addition of Exogenous Noggin, IL-6, and BMP-4 Does Not Result in Apoptosis Resistance
To investigate potential mediators of apoptosis resistance in the HHV-8–infected HMVEC-L, we added the exogenous proteins Noggin, BMP-4, and IL-6 to cell culture. The addition of these individual proteins did not result in apoptosis resistance as measured by Caspase-Glo assay (no difference from control cells exposed to media only; data not shown).
DISCUSSION
The present study demonstrates the ability of HHV-8 to infect human primary pulmonary microvascular endothelial cells in vitro. While in vitro infection of other endothelial cell lines (i.e., human umbilical vein endothelial cells, lymphatic endothelial cells, and dermal microvascular endothelial cells) has been demonstrated previously, to our knowledge this is the first study reporting HHV-8 infection of a primary cell line of pulmonary vascular origin (44–48). HHV-8 infection results in marked alteration of the gene expression pattern of HMVEC-L, impacting a number of pathways involved in angiogenesis, inflammation, and apoptosis. Infection of HMVEC-L with HHV-8 also alters the expression of genes perceived important in the pathophysiology of PAH. These changes include alterations of BMP pathway genes (decreased BMP4 and increased Noggin expression) and a variety of mediators that may be relevant to the development of PAH, including IL-6 and MMP-1. Finally, infection of HMVEC-L with HHV-8 results in an apoptosis-resistant phenotype. This finding is of particular interest as apoptosis resistance may be important in the development of the plexiform lesions characteristic of patients with PAH (2, 49). Conditioned media (media aspirated from a purified virus preparation but without infectious virions) also resulted in HMVEC-L apoptosis resistance. This observation suggests that a secreted factor may be responsible for some of the phenotypic changes conferred to the cultured endothelial cells. The potential list of factors that could induce these changes is long. IL-6 is an attractive candidate, as this cytokine, or a similar viral cytokine produced by HHV-8 (v-IL6), is hypothesized to induce proliferative changes present in multicentric Castleman's disease (50). Increased IL-6 expression is also hypothesized to play a role in HIV-1–associated PAH (51). We documented increased levels of IL-6 in the supernatant of our HHV-8 infected cells by ELISA. These levels were maintained in the infected cells, but decreased over time to baseline in the cells exposed to conditioned media. The decrease in BMP-4 levels induced by HHV-8 infection also has interesting implications for the observation of apoptosis resistance in the HHV-8–infected cells. The signaling of BMP-4 through the receptor complex of BMPR2 and BMPR1a has been previously shown to inhibit cellular proliferation in pulmonary artery smooth muscle cells (9). BMP-4 has also been demonstrated to be decreased by HHV-8 infection in other endothelial cells models (52). However, addition of exogenous IL-6 and Noggin (which competitively binds to BMP-4) did not induce a similar apoptosis-resistant phenotype (data not shown). We hypothesize that the HHV-8 infection induced phenotypic changes in apoptosis observed are more complicated than an increase or decrease of any one secreted protein. Ultimately, the mechanism behind the development of apoptosis resistance in these cells will require further investigation.
Our manuscript describes two different means of infecting pulmonary artery endothelial cells with HHV-8. The first method involves exposing target cells directly to isolated virus and examining the impact of a high-level (∼ 80%) viral infection on endothelial cell gene expression. For these experiments we were specifically interested in the interaction of the herpesvirus and the cultured endothelial cells, and wished to avoid the confounding effects of other contaminating cells on gene expression. For the apoptosis experiments, we hoped to generate conditions more analogous to the in vivo scenario, in which B cells are thought to harbor virus which then infects surrounding endothelial cells (41, 53, 54). The co-culture experiments resulted in a less robust infection (∼ 10–20%), but this may more closely mirror the in vivo scenario. Importantly, many of the same genes altered by direct viral infection were similarly altered in cells infected by the co-culture technique as demonstrated by qPCR and ELISA.
The findings of this manuscript do not prove an association between HHV-8 infection and PAH. Instead, this work demonstrates the ability of HHV-8 to infect cells relevant to the disease process of PAH and to induce changes consistent with our current understanding of the pathobiology of PAH. We believe further investigation of HHV-8 as a contributing factor in the development of some forms of pulmonary hypertension is warranted. Finally, this study has implications toward the disease entity of pulmonary Kaposi's sarcoma (KS). Pulmonary KS results in significant morbidity and mortality in patients infected with HIV-1 (55). The mechanism of HHV-8 infection and propagation in cutaneous KS has been the subject of much investigative effort, but little is known regarding the effect of the virus in the pulmonary vasculature. Our findings may point to mechanisms relevant to the pathobiology of pulmonary KS.
Supplementary Material
This work has been facilitated by the infrastructure and resources provided by the Colorado Center for AIDS Research Grant P30 AI054907. This work was supported by the American Thoracic Society/Pulmonary Hypertension Association Partnership Grant (ATS Reference PH-05-013) to T.M.B. and by a K08 award from the NHLBI (5 KO8 HLO728558-01A2) to T.M.B.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0368OC on June 27, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 2.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 2004;43:13S–24S. [DOI] [PubMed] [Google Scholar]
- 3.Voelkel NF, Cool C, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest 1998;114:225S–230S. [DOI] [PubMed] [Google Scholar]
- 4.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA III, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet 2000;26:81–84. [DOI] [PubMed] [Google Scholar]
- 5.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 2000;67:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, Ward K, Yacoub M, Mikhail G, Rogers P, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet 2000;37:741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, Atkinson C, Chen H, Trembath RC, Morrell NW. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res 2005;96:1053–1063. [DOI] [PubMed] [Google Scholar]
- 8.De Caestecker M, Meyrick B. Bone morphogenetic proteins, genetics and the pathophysiology of primary pulmonary hypertension. Respir Res 2001;2:193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation 2001;104:790–795. [DOI] [PubMed] [Google Scholar]
- 10.Morse J, Barst R, Horn E, Cuervo N, Deng Z, Knowles J. Pulmonary hypertension in scleroderma spectrum of disease: lack of bone morphogenetic protein receptor 2 mutations. J Rheumatol 2002;29:2379–2381. [PubMed] [Google Scholar]
- 11.Tew MB, Arnett FC, Reveille JD, Tan FK. Mutations of bone morphogenetic protein receptor type II are not found in patients with pulmonary hypertension and underlying connective tissue diseases. Arthritis Rheum 2002;46:2829–2830. [DOI] [PubMed] [Google Scholar]
- 12.Therrien J, Rambihar S, Newman B, Siminovitch K, Langleben D, Webb G, Granton J. Eisenmenger syndrome and atrial septal defect: nature or nurture? Can J Cardiol 2006;22:1133–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman JH, Trembath RC, Morse JA, Grunig E, Loyd JE, Adnot S, Coccolo F, Ventura C, Phillips JA III, Knowles JA, et al. Genetic basis of pulmonary arterial hypertension: current understanding and future directions. J Am Coll Cardiol 2004;43:33S–39S. [DOI] [PubMed] [Google Scholar]
- 14.Rubin LJ, Galie N. Pulmonary arterial hypertension: a look to the future. J Am Coll Cardiol 2004;43:89S–90S. [DOI] [PubMed] [Google Scholar]
- 15.Du L, Sullivan CC, Chu D, Cho AJ, Kido M, Wolf PL, Yuan JX, Deutsch R, Jamieson SW, Thistlethwaite PA. Signaling molecules in nonfamilial pulmonary hypertension. N Engl J Med 2003;348:500–509. [DOI] [PubMed] [Google Scholar]
- 16.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol 2006;2:619–626. [DOI] [PubMed] [Google Scholar]
- 17.Miyata M, Sakuma F, Yoshimura A, Ishikawa H, Nishimaki T, Kasukawa R. Pulmonary hypertension in rats: 2. Role of interleukin-6. Int Arch Allergy Immunol 1995;108:287–291. [DOI] [PubMed] [Google Scholar]
- 18.Golembeski SM, West J, Tada Y, Fagan KA. Interleukin-6 causes mild pulmonary hypertension and augments hypoxia-induced pulmonary hypertension in mice. Chest 2005;128:572S–573S. [DOI] [PubMed] [Google Scholar]
- 19.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995;151:1628–1631. [DOI] [PubMed] [Google Scholar]
- 20.Boudreau N, Weaver V. Forcing the third dimension. Cell 2006;125:429–431. [DOI] [PubMed] [Google Scholar]
- 21.Archer S, Rich S. Primary pulmonary hypertension: a vascular biology and translational research “work in progress”. Circulation 2000;102:2781–2791. [DOI] [PubMed] [Google Scholar]
- 22.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 2005;115:2811–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vieillard-Baron A, Frisdal E, Raffestin B, Baker AH, Eddahibi S, Adnot S, d'Ortho MP. Inhibition of matrix metalloproteinases by lung TIMP-1 gene transfer limits monocrotaline-induced pulmonary vascular remodeling in rats. Hum Gene Ther 2003;14:861–869. [DOI] [PubMed] [Google Scholar]
- 24.Vieillard-Baron A, Frisdal E, Eddahibi S, Deprez I, Baker AH, Newby AC, Berger P, Levame M, Raffestin B, Adnot S, et al. Inhibition of matrix metalloproteinases by lung TIMP-1 gene transfer or doxycycline aggravates pulmonary hypertension in rats. Circ Res 2000;87:418–425. [DOI] [PubMed] [Google Scholar]
- 25.Frisdal E, Gest V, Vieillard-Baron A, Levame M, Lepetit H, Eddahibi S, Lafuma C, Harf A, Adnot S, Dortho MP. Gelatinase expression in pulmonary arteries during experimental pulmonary hypertension. Eur Respir J 2001;18:838–845. [DOI] [PubMed] [Google Scholar]
- 26.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 2003;3:422–433. [DOI] [PubMed] [Google Scholar]
- 27.Lepetit H, Eddahibi S, Fadel E, Frisdal E, Munaut C, Noel A, Humbert M, Adnot S, D'Ortho MP, Lafuma C. Smooth muscle cell matrix metalloproteinases in idiopathic pulmonary arterial hypertension. Eur Respir J 2005;25:834–842. [DOI] [PubMed] [Google Scholar]
- 28.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 1994;266:1865–1869. [DOI] [PubMed] [Google Scholar]
- 29.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay MF, Clauvel JP, Raphael M, Degos L. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 1995;86:1276–1280. [PubMed] [Google Scholar]
- 30.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 1995;332:1186–1191. [DOI] [PubMed] [Google Scholar]
- 31.Bull TM, Cool CD, Serls AE, Rai PR, Parr J, Neid JM, Geraci MW, Campbell TB, Voelkel NF, Badesch DB. Primary pulmonary hypertension, Castleman's disease and human herpesvirus-8. Eur Respir J 2003;22:403–407. [DOI] [PubMed] [Google Scholar]
- 32.Cool CD, Rai PR, Yeager ME, Hernandez-Saavedra D, Serls AE, Bull TM, Geraci MW, Brown KK, Routes JM, Tuder RM, et al. Expression of human herpesvirus 8 in primary pulmonary hypertension. N Engl J Med 2003;349:1113–1122. [DOI] [PubMed] [Google Scholar]
- 33.Laney AS, De Marco T, Peters JS, Malloy M, Teehankee C, Moore PS, Chang Y. Kaposi sarcoma-associated herpesvirus and primary and secondary pulmonary hypertension. Chest 2005;127:762–767. [DOI] [PubMed] [Google Scholar]
- 34.Nicastri E. Human herpesvirus 8 and pulmonary hypertension. Emerg Infect Dis 2005;11:1480–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henke-Gendo C, Mengel M, Hoeper MM, Alkharsah K, Schulz TF. Absence of Kaposi's sarcoma-associated herpesvirus in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2005;172:1581–1585. [DOI] [PubMed] [Google Scholar]
- 36.Hammock L, Reisenauer A, Wang W, Cohen C, Birdsong G, Folpe AL. Latency-associated nuclear antigen expression and human herpesvirus-8 polymerase chain reaction in the evaluation of Kaposi sarcoma and other vascular tumors in HIV-positive patients. Mod Pathol 2005;18:463–468. [DOI] [PubMed] [Google Scholar]
- 37.Montani D, Achouh L, Marcelin AG, Viard JP, Hermine O, Canioni D, Sitbon O, Simonneau G, Humbert M. Reversibility of pulmonary arterial hypertension in HIV/HHV8-associated Castleman's disease. Eur Respir J 2005;26:969–972. [DOI] [PubMed] [Google Scholar]
- 38.Gutierrez F, Masia M, Padilla S, Ramos JM, Bernal E, Morales P, Pozo F, Andrada E, Martin-Hidalgo A. Occult lymphadenopathic Kaposi's sarcoma associated with severe pulmonary hypertension: a clinical hint about the potential role of HHV-8 in HIV-related pulmonary hypertension? J Clin Virol 2006;37:79–82. [DOI] [PubMed] [Google Scholar]
- 39.Shan B, Morris CA, Zhuo Y, Shelby BD, Levy DR, Lasky JA. Activation of proMMP-2 and Src by HHV8 vGPCR in human pulmonary arterial endothelial cells. J Mol Cell Cardiol 2007;42:517–525. [DOI] [PubMed] [Google Scholar]
- 40.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med 1996;2:342–346. [DOI] [PubMed] [Google Scholar]
- 41.Sakurada S, Katano H, Sata T, Ohkuni H, Watanabe T, Mori S. Effective human herpesvirus 8 infection of human umbilical vein endothelial cells by cell-mediated transmission. J Virol 2001;75:7717–7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geraci MW, Moore M, Gesell T, Yeager ME, Alger L, Golpon H, Gao B, Loyd JE, Tuder RM, Voelkel NF. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res 2001;88:555–562. [DOI] [PubMed] [Google Scholar]
- 43.Bull TM, Coldren CD, Moore M, Sotto-Santiago SM, Pham DV, Nana-Sinkam SP, Voelkel NF, Geraci MW. Gene microarray analysis of peripheral blood cells in pulmonary arterial hypertension. Am J Respir Crit Care Med 2004;170:911–919. [DOI] [PubMed] [Google Scholar]
- 44.Moses AV, Jarvis MA, Raggo C, Bell YC, Ruhl R, Luukkonen BG, Griffith DJ, Wait CL, Druker BJ, Heinrich MC, et al. Kaposi's sarcoma-associated herpesvirus-induced upregulation of the c-kit proto-oncogene, as identified by gene expression profiling, is essential for the transformation of endothelial cells. J Virol 2002;76:8383–8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moses AV, Jarvis MA, Raggo C, Bell YC, Ruhl R, Luukkonen BG, Griffith DJ, Wait CL, Druker BJ, Heinrich MC, et al. A functional genomics approach to Kaposi's sarcoma. Ann N Y Acad Sci 2002;975:180–191. [DOI] [PubMed] [Google Scholar]
- 46.Wang HW, Trotter MW, Lagos D, Bourboulia D, Henderson S, Makinen T, Elliman S, Flanagan AM, Alitalo K, Boshoff C. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat Genet 2004;36:687–693. [DOI] [PubMed] [Google Scholar]
- 47.Naranatt PP, Krishnan HH, Svojanovsky SR, Bloomer C, Mathur S, Chandran B. Host gene induction and transcriptional reprogramming in Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells: insights into modulation events early during infection. Cancer Res 2004;64:72–84. [DOI] [PubMed] [Google Scholar]
- 48.Vart RJ, Nikitenko LL, Lagos D, Trotter MW, Cannon M, Bourboulia D, Gratrix F, Takeuchi Y, Boshoff C. Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6 and G-protein-coupled receptor regulate angiopoietin-2 expression in lymphatic endothelial cells. Cancer Res 2007;67:4042–4051. [DOI] [PubMed] [Google Scholar]
- 49.Voelkel NF, Cool C, Taraceviene-Stewart L, Geraci MW, Yeager M, Bull T, Kasper, M, Tuder RM. Janus face of vascular endothelial growth factor: the obligatory survival factor for lung vascular endothelium controls precapillary artery remodeling in severe pulmonary hypertension. Crit Care Med 2002;30:S251–S256. [DOI] [PubMed] [Google Scholar]
- 50.Leger-Ravet MB, Peuchmaur M, Devergne O, Audouin J, Raphael M, Van Damme J, Galanaud P, Diebold J, Emilie D. Interleukin-6 gene expression in Castleman's disease. Blood 1991;78:2923–2930. [PubMed] [Google Scholar]
- 51.Pellicelli AM, Palmieri F, Cicalini S, Petrosillo N. Pathogenesis of HIV-related pulmonary hypertension. Ann N Y Acad Sci 2001;946:82–94. [DOI] [PubMed] [Google Scholar]
- 52.Poole LJ, Yu Y, Kim PS, Zheng QZ, Pevsner J, Hayward GS. Altered patterns of cellular gene expression in dermal microvascular endothelial cells infected with Kaposi's sarcoma-associated herpesvirus. J Virol 2002;76:3395–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrington WJ Jr, Bagasra O, Sosa CE, Bobroski LE, Baum M, Wen XL, Cabral L, Byrne GE, Pomerantz RJ, Wood C. Human herpesvirus type 8 DNA sequences in cell-free plasma and mononuclear cells of Kaposi's sarcoma patients. J Infect Dis 1996;174:1101–1105. [DOI] [PubMed] [Google Scholar]
- 54.Monini P, Colombini S, Sturzl M, Goletti D, Cafaro A, Sgadari C, Butto S, Franco M, Leone P, Fais S, et al. Reactivation and persistence of human herpesvirus-8 infection in B cells and monocytes by Th-1 cytokines increased in Kaposi's sarcoma. Blood 1999;93:4044–4058. [PubMed] [Google Scholar]
- 55.Aboulafia DM. The epidemiologic, pathologic, and clinical features of AIDS-associated pulmonary Kaposi's sarcoma. Chest 2000;117:1128–1145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.