Abstract
Ozone exposure in the lab and environment causes airway hyperreactivity lasting at least 3 days in humans and animals. In guinea pigs 1 day after ozone exposure, airway hyperreactivity is mediated by eosinophils that block neuronal M2 muscarinic receptor function, thus increasing acetylcholine release from airway parasympathetic nerves. However, mechanisms of ozone-induced airway hyperreactivity change over time, so that depleting eosinophils 3 days after ozone makes airway hyperreactivity worse rather than better. Ozone exposure increases IL-1β in bone marrow, which may contribute to acute and chronic airway hyperreactivity. To test whether IL-1β mediates ozone-induced airway hyperreactivity 1 and 3 days after ozone exposure, guinea pigs were pretreated with an IL-1 receptor antagonist (anakinra, 30 mg/kg, intraperitoneally) 30 minutes before exposure to filtered air or to ozone (2 ppm, 4 h). One or three days after exposure, airway reactivity was measured in anesthetized guinea pigs. The IL-1 receptor antagonist prevented ozone-induced airway hyperreactivity 3 days, but not 1 day, after ozone exposure. Ozone-induced airway hyperreactivity was vagally mediated, since bronchoconstriction induced by intravenous acetylcholine was not changed by ozone. The IL-1 receptor antagonist selectively prevented ozone-induced reduction of eosinophils around nerves and prevented ozone-induced deposition of extracellular eosinophil major basic protein in airways. These data demonstrate that IL-1 mediates ozone-induced airway hyperreactivity at 3 days, but not 1 day, after ozone exposure. Furthermore, preventing hyperreactivity was accompanied by decreased eosinophil major basic protein deposition within the lung, suggesting that IL-1 affects eosinophil activation 3 days after ozone exposure.
Keywords: asthma, eosinophils, cytokines, parasympathetic nerves, lungs
CLINICAL RELEVANCE
It is well established that ozone causes airway hyperreactivity both acutely after exposure and after a lag phase, but it remains to be determined what mechanisms mediate hyperreactivity at both time points.
Ozone exposure induces airway hyperreactivity in both humans and animals (1–3). Hospitalizations for both asthma and for cardiovascular complaints increase immediately after environmental ozone exposure, followed by a second increase in hospitalizations after a 3-day lag (4, 5). In guinea pigs, ozone exposure also has an acute and lag phase, which we have shown are associated with changes in eosinophil populations in lungs (3).
Ozone significantly increases eosinophils in bronchoalveolar and nasal lavage of humans (6–8). Furthermore, these eosinophils are activated, as evidenced by increased eosinophil cationic protein (6). Eosinophils are also significantly increased in the lungs of mice, rats, dogs, and primates after ozone exposure (9–12).
Acutely, ozone-induced airway hyperreactivity in guinea pigs is mediated by recruitment and activation of eosinophils at airway nerves (3). Activated eosinophils release preformed proteins, including eosinophil major basic protein (MBP), which is an endogenous antagonist for M2 muscarinic receptors (13, 14). In the lungs, acetylcholine release from parasympathetic nerves is normally limited by M2 muscarinic receptors (15). Eosinophils, via release of MBP, inhibit neuronal M2 function, resulting in increased acetylcholine release, increased bronchoconstriction, and vagally mediated airway hyperreactivity (14, 16). One day after ozone exposure, neuronal M2 receptor dysfunction and airway hyperreactivity are prevented or reversed by depleting eosinophils, preventing eosinophil migration into the lungs, and by blocking eosinophil MBP (17). Therefore, airway hyperreactivity 1 day after ozone exposure is mediated by eosinophils, neuronal M2 muscarinic receptor blockade, and subsequent increased acetylcholine release from parasympathetic nerves.
In contrast, 3 days after ozone exposure, airway hyperreactivity is mediated by different mechanisms. Although neuronal M2 muscarinic receptors are dysfunctional at this time point, protecting or restoring M2 function does not prevent airway hyperreactivity (3). The role of eosinophils in hyperreactivity 3 days after ozone exposure has changed from causative to protective, in that eosinophils re-enter the lungs at this time point and that depleting them worsens airway hyperreactivity (3).
Interleukin 1β (IL-1β) is a central mediator of inflammation that increases granulocyte hematopoiesis (18), induces chemokines and adhesion molecules (19–21), and activates eosinophils (22, 23). Inhibiting IL-1β or blocking IL-1 receptors prevents virus-induced, toluene diisocyanate–induced, and antigen challenge–induced hyperreactivity in animals (24–27). In humans with asthma, IL-1β is present in lavage, epithelial cells, and alveolar macrophages (28–31). Ozone interacts with airway epithelium and alveolar macrophages to produce inflammation via increasing inflammatory cytokines, including IL-1β (29, 32–35). The role of IL-1β in ozone-induced airway hyperreactivity has been suggested, since an IL-1 receptor antagonist, anakinra, prevents airway hyperreactivity and inflammation in ozone-exposed mice (36, 37). Anakinra, a recombinant human form of the IL-1 receptor antagonist, is a very potent, highly selective inhibitor of IL-1 type 1 receptors with no biological activity of its own (38). These experiments were designed to test in guinea pigs whether IL-1β affects eosinophil activation and mediates airway hyperreactivity 1 day after ozone exposure, a point at which the role of eosinophils has been firmly established (3, 17), and during the lag phase 3 days after a single ozone exposure, a point at which eosinophils and M2 receptors do not cause hyperreactivity.
MATERIALS AND METHODS
Specific pathogen–free female Dunkin-Hartley guinea pigs were used (350–550 g; Elm Hill Breeding Labs, Chelmsford, MA). Guinea pigs were shipped in filtered crates, housed in high-efficiency particulate filtered air, and fed a normal diet. Three to seven animals were used in each treatment group. Animals were handled in accordance with National Institutes of Health guidelines. All protocols were approved by Oregon Health and Science University Animal Care and Use Committee.
Guinea pigs were exposed to 2 ppm ozone or filtered air for 4 hours as described previously (17). The IL-1 receptor antagonist, anakinra, is both potent and selective for the type I IL-1 receptor, and has been extensively used to demonstrate the role of IL-1 in airway hyperreactivity (24, 36, 38). A quantity of 10 to 30 mg/kg recombinant human IL-1 receptor antagonist (anakinra) (36, 39) or vehicle (6.5 mM sodium citrate, 140 mM NaCl, 48 mM EDTA, 1 mg/ml polysorbate 80) was diluted in phosphate-buffered saline (PBS) and given intraperitoneally 30 minutes before ozone or air exposure, and once daily thereafter. After ozone exposure, animals were housed in the animal care facility with access to food and water ad libitum.
One or three days after exposure to ozone or filtered air, animals were anesthetized with urethane (1.9 g/kg, intraperitoneally). Both jugular veins were cannulated for intravenous administration of drugs, and the right carotid artery was cannulated and connected to a transducer to measure heart rate and blood pressure. Both vagus nerves were cut and the distal ends placed on platinum electrodes submerged in liquid paraffin. Animals were tracheostomized, ventilated with positive pressure and constant volume (1 ml/100 g body weight, 100 breaths/min), paralyzed with a constant infusion of succinylcholine (10 μg/kg/min, intravenously) and chemically sympathectomized with guanethidine (5 mg/kg, intravenously). The pressure required to inflate the lungs (pulmonary inflation pressure) was measured at the trachea. Bronchoconstriction and bradycardia were induced either by electrical stimulation of both vagi (10 V, 0.2 ms, 2–25 Hz), or by intravenous injection of acetylcholine (1–10 μg/kg; animals were all vagotomized to prevent the reflex bronchoconstriction to acetylcholine (40, 41)) and were measured as previously described (42). At the end of each experiment, bronchoalveolar lavage fluid (BALF), blood, and bone marrow (from the left femur) were collected.
The lungs were lavaged with 5 × 10 ml aliquots of PBS containing 100 μg isoproterenol. Cells were washed and resuspended in PBS and counted using a hemocytometer, and cytospun cells were stained with Hemacolor (Harleco, Gibbstown, NJ). Whole blood was taken from the carotid cannula and lysed with 0.1 N HCl. Total peripheral white blood cells were counted with a hemocytometer and cell differentials obtained from a blood smear.
Lungs were also removed, fixed, and embedded in paraffin. Nerves were labeled with rabbit polyclonal antibody to protein gene product (PGP)9.5 (1:1,000; Biogenesis, Kingston, NH), eosinophils were stained with 1% chromotrope 2R (Sigma, St. Louis, MO), and eosinophil activation assessed by measuring extracellular MBP with a rabbit monoclonal antibody to guinea pig MBP (1:1,000) as previously described (42–44).
Eosinophils were quantified in six to eight different airways per guinea pig using four to six animals per group. Airways were photographed and airway area measured using MetaMorph software (version 6.2; Universal Imaging, Downington, PA). Total area of the conducting airways between basement membrane and alveoli, including airway smooth muscle but excluding cartilage and blood vessels, was measured, and eosinophils within this area were counted and expressed as eosinophils per mm2. Eosinophils within 8 μm of any airway nerve (as identified with PGP9.5 staining) were also counted.
Slides immunostained for MBP were coded, and analysis was performed in a blinded fashion. Airways were photographed under identical conditions. Photographs were analyzed using MetaMorph software (Universal Imaging). Deposition of extracellular MBP (intact eosinophils, as identified by solid spheres of MBP staining in excess of 8 μm in diameter, were excluded) was quantified using a technique adapted from Tuder and coworkers (45) by Verbout and colleagues (44).
IL-1β was measured in supernatant from the first 10-ml aliquot of BALF and from bone marrow supernatant. Protein was measured using the bicinchoninic acid method (BCA protein assay kit; Pierce, Rockford, IL) and IL-1β was measured using a commercially available enzyme-linked immunosorbent assay kit with standard controls (Immunotech, Marseille, France) as described by Sato and coworkers (39). IL-1β concentrations were normalized to total protein. IL-1α was not measured, although it is also an agonist for IL-1 receptors, because it typically only plays an autocrine role in disease and is not commonly found in body fluids (20, 46, 47).
Acetylcholine, guanethidine, atropine, succinylcholine, isoproterenol, and urethane were purchased from Sigma. All drugs were dissolved and diluted in PBS. Recombinant human IL-1Ra (anakinra) was obtained from Amgen (Thousand Oaks, CA).
All data are expressed as means ± SE. In vivo, frequency and dose–response curves were compared using two-way ANOVA for repeated measures. Lavage and MBP deposition data were analyzed by a multiple one-way ANOVA with Fisher's post hoc test. Baseline and histology data were analyzed by multiple one-way ANOVAs with Bonferroni correction. A P value of less than 0.05 was considered significant. Analyses were made with Kaleidagraph (version 4.01; Synergy Software, Reading, PA) or StatView 4.5 (Abacus Concepts, Berkeley, CA).
RESULTS
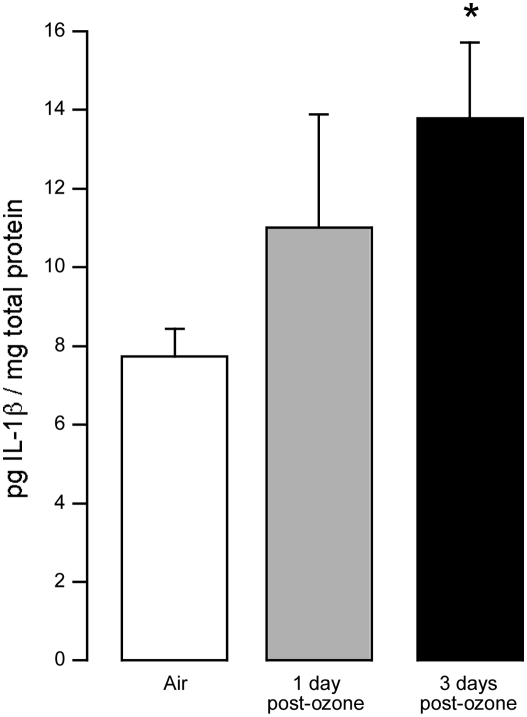
Three days after ozone exposure, IL-1β concentration in bone marrow was almost doubled (Figure 1), while 1 day after ozone exposure IL-1β was increased slightly. In contrast, IL-1β was below the limit of detection (2 pg/ml) in BALF from both ozone- and air-exposed guinea pigs (data not shown).
Figure 1.
IL-1β was present in bone marrow of control guinea pigs (open bar) and was slightly increased 1 day after ozone exposure (shaded bar). At 3 days after ozone exposure (solid bar), IL-1β levels in bone marrow were almost twice as high as in controls. IL-1β concentrations were normalized to total protein. *P = 0.057 from control. Data are means ± SE, n = 5.
Ozone exposure significantly increased baseline pulmonary inflation pressure 1 day after ozone exposure (Table 1). Pretreatment with the IL-1 receptor antagonist did not affect baseline pulmonary inflation pressure 1 day after ozone exposure. Neither ozone nor the IL-1 receptor antagonist affected resting heart rate 1 or 3 days after ozone exposure. Resting blood pressure was not affected by ozone or by the IL-1 receptor antagonist. Vehicle treatment did not affect baseline parameters compared with controls.
TABLE 1.
BASELINE CARDIOVASCULAR AND PULMONARY PARAMETERS
| Blood Pressure (mm Hg)
|
||||||
|---|---|---|---|---|---|---|
| Treatment | Group | n | Heart Rate (beats/min) | Systolic | Diastolic | Pulmonary Inflation Pressure (mm H2O) |
| Control | Air | 5 | 311 ± 24 | 40 ± 2 | 18 ± 2 | 104 ± 12 |
| 1 d after ozone exposure | Ozone | 6 | 295 ± 13 | 43 ± 4 | 21 ± 1 | 253 ± 30* |
| Ozone + IL-1Ra | 4 | 301 ± 6 | 38 ± 3 | 17 ± 2 | 240 ± 20* | |
| Control | Air | 7 | 298 ± 14 | 43 ± 5 | 21 ± 4 | 104 ± 4 |
| Air + IL-1Ra | 6 | 283 ± 7 | 40 ± 4 | 22 ± 2 | 98 ± 4 | |
| Air + Vehicle | 4 | 300 ± 8 | 38 ± 4 | 18 ± 2 | 105 ± 3 | |
| 3 d after ozone exposure | Ozone | 5 | 321 ± 7 | 49 ± 3 | 26 ± 3 | 121 ± 10 |
| Ozone + IL-1Ra | 4 | 283 ± 8 | 40 ± 3 | 24 ± 4 | 95 ± 5 | |
| Ozone + Vehicle | 3 | 297 ± 20 | 54 ± 7 | 31 ± 1 | 103 ± 3 | |
Values are means ± SE. Baseline pulmonary inflation pressure significantly increased 1 d after ozone exposure.
Significantly different from air-exposed controls.
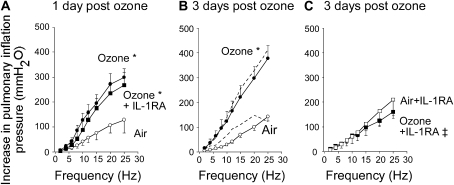
Bronchoconstriction in response to electrical stimulation of vagus nerves was significantly potentiated above control 1 and 3 days after ozone exposure (Figures 2A and 2B). The IL-1 receptor antagonist (30 mg/kg, intraperitoneally) had no effect on ozone-induced airway hyperreactivity at Day one (Figure 2A). In contrast, the IL-1 receptor antagonist completely blocked ozone-induced hyperreactivity to electrical stimulation of the vagus nerves 3 days after ozone exposure (Figure 2C). The effect was dose related, since 10 mg/kg intraperitoneally partially prevented ozone-induced airway hyperreactivity at 3 days (280 ± 73 mm H2O at 25 Hz). Vehicle administration did not inhibit ozone-induced airway hyperreactivity (Figure 2B).
Figure 2.
Blocking IL-1 receptors does not prevent airway hyperreactivity 1 day after ozone exposure (A) but does prevent airway hyperreactivity 3 days after exposure (C). In anesthetized guinea pigs, electrical stimulation of both vagus nerves causes frequency-dependent bronchoconstriction (measured as an increase in pulmonary inflation pressure) in air-exposed guinea pigs (open circles) that was significantly potentiated (A) 1 and (B) 3 days after ozone exposure (solid circles). Pretreatment with the IL-1 receptor antagonist prevented airway hyperreactivity 3 days after ozone exposure (C, solid squares), but not 1 day after exposure (A, solid squares). The IL-1 receptor antagonist did not affect air-exposed controls (C, open squares). Vehicle treatment did not change vagally mediated bronchoconstriction in either ozone- or air-exposed animals (B, dashed lines next to respective controls). *The entire frequency response after ozone exposure is significantly different from that of air-exposed guinea pigs. ‡The entire frequency response in the presence of IL-1 receptor antagonist is significantly different from ozone-exposed guinea pigs. Data are expressed as means ± SE, n = 3–7.
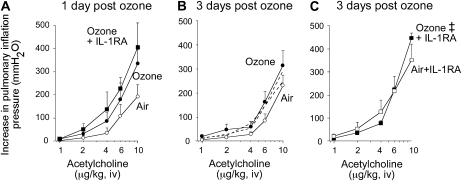
Ozone slightly, but not significantly, increased bronchoconstriction in response to intravenous acetylcholine at 1 and 3 days (Figures 3A and 3B). This increase probably does not contribute to ozone-induced airway hyperreactivity, since it was small compared with ozone's potentiation of vagally induced bronchoconstriction. The IL-1 receptor antagonist increased smooth muscle responsiveness to acetylcholine, but the effect was not significant and occurred regardless of whether animals were exposed to air or ozone (Figure 3C). Vehicle treatment did not affect acetylcholine-induced bronchoconstriction in either air- or ozone-exposed animals (Figure 3B).
Figure 3.
Blocking IL-1 receptors increases airway smooth muscle contraction to intravenous acetylcholine. Acetylcholine-induced bronchoconstriction was measured in vagotomized guinea pigs as an increase in pulmonary inflation pressure. (A) One and (B) three days after ozone exposure, smooth muscle contraction slightly increased (solid circles) compared with air-exposed animals (open circles). (C) The IL-1 receptor antagonist slightly increased smooth reactivity in all groups. Vehicle treatment did not change acetylcholine-induced bronchoconstriction in either air- or ozone-exposed animals (B, dashed lines next to respective controls). ‡The entire frequency response in the presence of IL-1 receptor antagonist is significantly different from that of ozone-exposed guinea pigs. Data are expressed as means ± SE, n = 4–7.
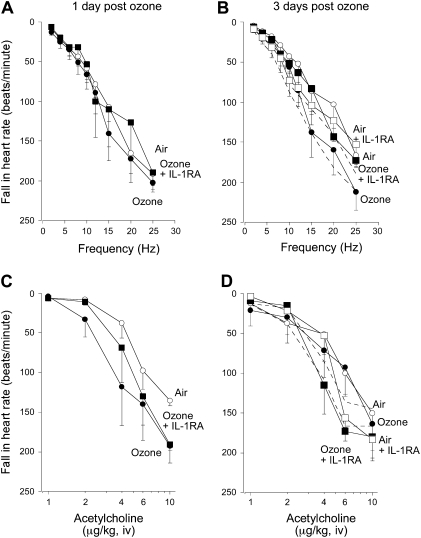
In the heart, ozone exposure increased acetylcholine-induced bradycardia, although not significantly, compared with air-exposed controls 1 day after ozone exposure (Figure 4C). Ozone did not affect either vagally induced or intravenous acetylcholine-induced bradycardia after 3 days (Figures 4B and 4D). Pretreatment with the IL-1 receptor antagonist also did not affect bradycardia in air- or ozone-exposed animals. Vehicle treatment did not affect vagally or acetylcholine-induced bradycardia in either air- or ozone-exposed guinea pigs (Figures 4B and 4D).
Figure 4.
Bradycardia in response to (A and B) electrical stimulation of both vagus nerves, or to (C and D) intravenous acetylcholine in vagotomized guinea pigs, was measured as a fall in heart rate in beats per minute. Neither ozone (A–D, solid circles) nor the IL-1 receptor antagonist (A–D, solid squares) affected vagally or acetylcholine induced bradycardia. Vehicle treatment did not change bradycardia in response to vagal stimulation or acetylcholine in either air- or ozone-exposed animals (B and D, dashed lines next to respective controls). Data are expressed as means ± SE, n = 4–6.
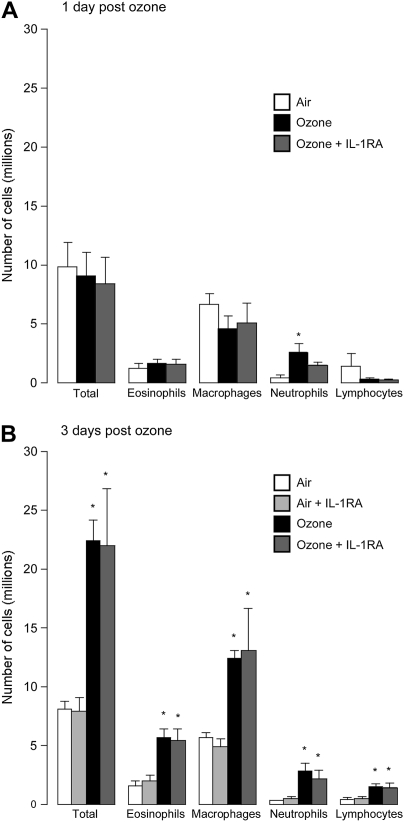
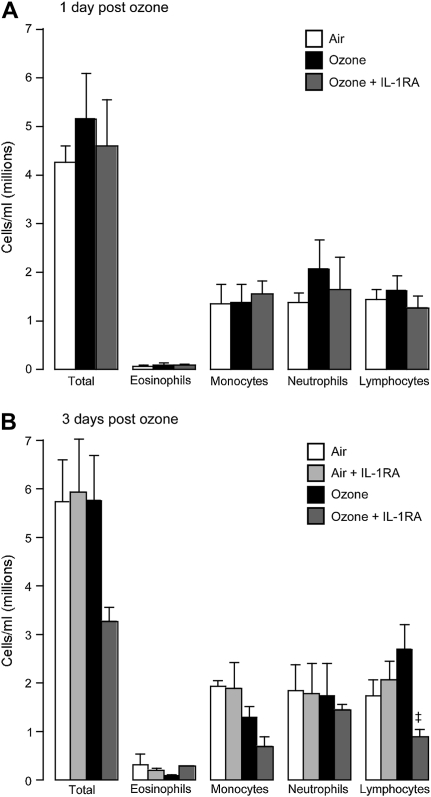
Only neutrophils were increased in bronchoalveolar lavage 1 day after ozone exposure (Figure 5A). In contrast, all inflammatory cells were increased 3 days after ozone exposure (Figure 5B). The protective effect of the IL-1 receptor antagonist 3 days after ozone exposure (Figure 2B) was not associated with a change in inflammatory cells in the bronchoalveolar lavage at this time point (Figure 5B). Neither was airway hyperreactivity, nor the protective effect of the antagonist due to changing inflammatory cell numbers in the blood, since circulating numbers of white cells were not changed by ozone, although the IL-1 receptor antagonist decreased circulating lymphocytes 3 days after ozone exposure (Figure 6). Vehicle treatment did not change inflammatory cell numbers in bronchoalveolar lavage or blood in either air- or ozone-exposed guinea pigs (data not shown).
Figure 5.
(A) Inflammatory cells in bronchoalveolar lavage were not increased 1 day after ozone exposure, with the exception of neutrophils. (B) However, 3 days after ozone exposure, all inflammatory cells were significantly increased in bronchoalveolar lavage. Treatment with IL-1 receptor antagonist did not change inflammatory cells in bronchoalveolar lavage in controls or at either time point after ozone exposure. *Significantly different from air-exposed controls. Data are expressed as means ± SE, n = 4–7.
Figure 6.
Circulating inflammatory cells in peripheral blood were not changed by ozone or by the IL-1 receptor antagonist at (A) 1 or (B) 3 days after ozone exposure, with the exception of lymphocytes, which were significantly inhibited by the IL-1 receptor antagonist 3 days after ozone exposure (B). Data are expressed as means ± SE, n = 3–6.
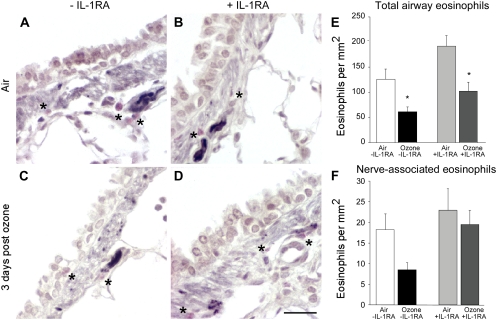
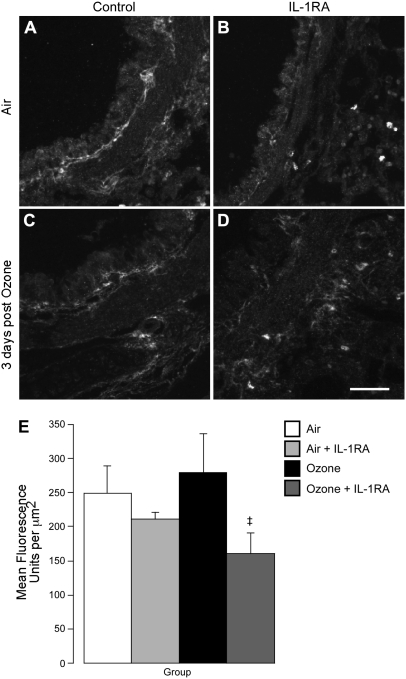
Since the IL-1 receptor antagonist only blocked airway hyperreactivity 3 days after ozone exposure, we counted eosinophils around the nerves only at this time point. Ozone alone decreased the number of eosinophils in the lungs and around the nerves (Figure 7). While the antagonist did not inhibit ozone-induced depletion of eosinophils in the whole lung, it did prevent loss of eosinophils around airway nerves (Figures 7E and 7F). The selective protection of eosinophils around nerves by the IL-1 receptor antagonist was accompanied by decreased degranulation as assessed by MBP deposition (Figure 8). Air-exposed animals have a basal level of eosinophil degranulation that was not enhanced by ozone exposure (Figures 8A and 8C).
Figure 7.
Eosinophils are present in airways and around nerves in control and ozone-exposed animals. Nerves in guinea pig lungs were stained black with antibody to PGP9.5, and eosinophils were stained red with chromotrope 2R (asterisks) (A–D) and quantified in six to eight different airways per guinea pig (E and F). Even in air-exposed control animals there are some eosinophils around airway nerves (A; open bars in E and F), which are decreased by ozone (C; solid bars in E and F). The ozone-induced decrease in total lung eosinophils was not affected by the IL-1 receptor antagonist (E), but the IL-1 receptor antagonist did prevent ozone induced eosinophil loss around nerves (F). Data in E and F are expressed as means ± SE, n = 4–6. *Significantly different from air-exposed animals treated with the IL-1 receptor antagonist.
Figure 8.
Blocking IL-1 receptors prevented extracellular deposition of eosinophil major basic protein (MBP) (labeled with an antibody to MBP) within airways 3 days after ozone exposure. After staining, slides were coded and analysis performed blinded, and intact eosinophils (as identified by solid spheres of MBP staining in excess of 8 μm in diameter) were excluded. Airways of air-exposed control guinea pigs do contain some extracellular MBP (A, and open bar in E), which was not significantly changed 3 days after ozone exposure (C, and solid bar in E). IL-1 receptor antagonist significantly decreased deposition of extracellular MBP (D, and dark gray bar in E). (E) Data are expressed as mean fluorescence intensity per area of the airway in μm2. Data are means ± SE, n = 4–6. ‡Significantly different from ozone-exposed animals. Scale bar for A–D, 50 μm.
DISCUSSION
Ozone transiently increases IL-1β in airway epithelium (48) and lungs (36, 37), and this declines over 24 hours. Our data at 24 hours are in agreement with this, since we did not see measurable levels of IL-1β in BALF at that time point. Since we previously demonstrated eosinophils increase in lungs 1 and 3 days after ozone (3), we checked bone marrow for cytokines involved in eosinophil hematopoiesis. IL-1β, a known inducer of granulocyte hematopoiesis (18), increases in bone marrow 3 days after ozone exposure and may be the systemic link between the lungs and increased eosinophil production.
In guinea pigs, ozone induces airway hyperreactivity that lasts at least 3 days, confirming previous findings (3). This is similar to what is seen in human populations after environmental exposure to ozone (4, 5). In contrast to our original hypothesis, IL-1β had no effect on ozone-induced airway hyperreactivity 1 day after ozone exposure. However, it completely prevented hyperreactivity 3 days after ozone exposure, demonstrating that the mechanisms of hyperreactivity have changed from IL-1 independent to IL-1 mediated over these 3 days. Thus, an IL-1 receptor antagonist given at the time of ozone exposure has no effect on airway hyperreactivity 1 day after exposure, but completely prevents hyperreactivity 3 days after exposure. Similarly, ozone-induced inflammation was not prevented 1 day after ozone exposure in IL-1 receptor type I knockout mice, but was prevented 3 days after exposure (37). These data show that hyperreactivity and inflammation after ozone change between 1 and 3 days after exposure. Protection against ozone-induced airway hyperreactivity occurs at the level of the nerves and not airway smooth muscle since the IL-1 receptor antagonist does not block acetylcholine-induced bronchoconstriction in air- or ozone-exposed animals.
Ozone-induced airway hyperreactivity is linked to inflammation (3, 49). Therefore, if mechanisms of hyperreactivity change, it might be expected that inflammatory cell populations also change over time. Although ozone exposure is traditionally associated with increased neutrophils, neutrophil populations in the lung do not change between 1 and 3 days after ozone exposure (3) (Figure 5). Ozone also induces eosinophilia in animals (9–12) and humans (6–8), and eosinophil populations in the lung do change between 1 and 3 days after ozone exposure (3) (Figure 5). Depleting eosinophils before ozone exposure completely prevents airway hyperreactivity 1 day later, demonstrating a role for eosinophils in ozone-induced hyperreactivity in guinea pigs (3). Preventing eosinophil migration into the lungs is not protective at this time point, suggesting that ozone is activating eosinophils that are resident in the lungs (3). Conversely, depleting eosinophils or preventing eosinophils from entering the lungs 3 days after ozone exposure makes hyperreactivity significantly worse (3), suggesting a beneficial role for late eosinophils that enter the lung 3 days after ozone exposure.
In humans with asthma, and in antigen-challenged, virus-infected, and ozone- or organophosphate-exposed animals, airway hyperreactivity is mediated by loss of neuronal M2 receptor function on the vagus nerves, leading to increased acetylcholine release and increased bronchoconstriction (14, 17, 50–52). This mechanism is established in mouse, rat, guinea pig, and horse models of airway hyperreactivity and in some humans with asthma (14, 17, 40, 50, 53–55). In antigen-challenged guinea pigs, loss of neuronal M2 muscarinic receptor function is mediated by degranulation of eosinophils at the nerves in the lungs, resulting in release of the M2 antagonist MBP (42, 53). A similar mechanism of hyperreactivity has been established 1 day after ozone exposure (17). However, mechanisms of hyperreactivity 3 days after ozone exposure are not understood. Although IL-1β decreases M2 receptor expression (56), this is unlikely to explain the ability of the IL-1 receptor antagonist to prevent airway hyperreactivity 3 days after ozone exposure, since we have previously shown that restoring M2 receptor function at this time point does not prevent airway hyperreactivity (3).
Airway hyperreactivity does not correlate with eosinophils in bronchoalveolar lavage or in airway tissues (3), but does correlate with the presence of activated eosinophils, as measured by major basic protein deposition in airways and especially around airway nerves (44, 57). Similarly, it is the presence of eosinophils in airway tissues, but not bronchoalveolar lavage or blood, that accompanies airway hyperreactivity in human asthma (58) and that may be important after ozone exposure. However, there are more eosinophils in bronchoalveolar lavage 3 days after ozone exposure than 1 day after, suggesting that eosinophils are moving through the lungs differently at these time points. A new, beneficial population of eosinophils has been suggested, since eosinophil depletion makes airway hyperreactivity significantly worse (3), and these beneficial eosinophils may arrive at the later time point. Here we show that eosinophils within the lungs are significantly decreased 3 days after ozone exposure while they are increased in bronchoalveolar lavage. Thus, ozone is either moving eosinophils from the lungs to the bronchoalveolar lavage, or they are disappearing via activation and degranulation (44).
IL-1β is known to activate eosinophils directly to stimulate cytokine release (23). IL-1 may also affect eosinophil reactivity, since IL-1 receptor antagonists decrease hypodense eosinophils in bronchoalveolar lavage of antigen-challenged animals (22), suggesting that IL-1 contributes to eosinophil activation. The same mechanism is suggested here, since the IL-1 receptor antagonist prevented eosinophil degranulation in ozone-exposed animals, measured as a decrease in extracellular MBP. In addition, the IL-1 receptor antagonist also prevented ozone-induced loss of eosinophils around airway nerves. Since hyperreactivity depends upon an interaction between eosinophils and nerves (42), it may be significant that only eosinophils around nerves were protected by the antagonist. Thus, the IL-1 receptor antagonist protects eosinophil populations around nerves and prevents eosinophil activation in ozone-exposed animals.
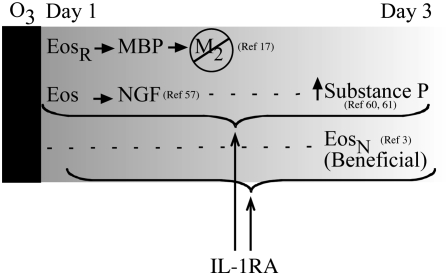
Depletion of eosinophils does not prevent hyperreactivity 3 days after ozone exposure, but actually makes airway hyperreactivity significantly worse, suggesting that eosinophils 3 days after ozone exposure are beneficial (3). The IL-1 receptor antagonist may inhibit hyperreactivity 3 days after ozone exposure not by preventing eosinophil degranulation, but by protecting beneficial eosinophils (3). Eosinophils are known to release neurotrophins, including nerve growth factor (59), leukemia inhibitory factor (60), and bone-derived neurotrophic factor (59), which may all be involved in neural repair mechanisms. It has been suggested that there are multiple populations of eosinophils (61); thus, while the IL-1 receptor antagonist may have no effect on resident eosinophils that mediate hyperreactivity 1 day after ozone exposure, it may preserve the integrity of secondary, beneficial eosinophils, preventing airway hyperreactivity 3 days after exposure (Figure 9).
Figure 9.
Airway hyperreactivity 1 day after ozone exposure is mediated by resident eosinophils (EosR) that release MBP and inhibit M2 muscarinic receptor function on parasympathetic nerves. The mechanisms of hyperreactivity 3 days after ozone exposure have not been determined; however, it is possible that hyperreactivity is mediated by substance P induced by nerve growth factor (NGF) released from eosinophils and that the IL-1 receptor antagonist (IL-1RA) is blocking this pathway. Alternatively, the IL-1 receptor antagonist may protect a new, beneficial population of eosinophils (EosN).
Substance P–containing airway nerves are increased in individuals with asthma compared with those without asthma (62), and ozone-induced airway hyperreactivity is associated with increased substance P in bronchoalveolar lavage in humans and in ferret lung (63, 64). Substance P is a peptide neurotransmitter usually expressed by sensory nerves. Although not usually present in efferent parasympathetic nerves of humans or guinea pigs (65, 66), ozone induces substance P expression in guinea pig parasympathetic nerves (67) and increases substance P in ferret parasympathetic nerves (64). Ozone-induced airway hyperreactivity is blocked by a neurokinin receptor (NK1) antagonist in guinea pigs 3 days after ozone exposure (67) and in ferret tracheas in vitro (64), demonstrating a role for tachykinins in ozone-induced hyperractivity. IL-1β can stimulate substance P expression directly (68, 69), and also indirectly via induction of nerve growth factor (70), which also increases substance P (71). One potential source of nerve growth factor is eosinophils (59). Therefore, the IL-1 receptor antagonist may prevent hyperreactivity by decreasing eosinophil activation, resulting in decreased nerve growth factor–mediated induction of substance P (Figure 9).
Asthma exacerbations increase not only at the time environmental ozone levels are high, but also up to 3 days later (2, 4, 5). This ozone-induced airway hyperreactivity also persists over 3 days in guinea pigs. The early phase is mediated by eosinophils (3), and here we show that the lag phase is mediated by IL-1β. The IL-1 receptor antagonist may protect against ozone-induced hyperreactivity during the lag phase by blocking degranulation of beneficial eosinophils, or by inhibiting eosinophil activation and subsequent release of a nerve growth factor, thus preventing induction of substance P (Figure 9). Environmental ozone exposure is associated with significant morbidity and mortality (72) that may be underestimated (73). These data suggest that different strategies will be required to treat ozone-induced airway hyperreactivity at early and late time points. Blocking eosinophils may be effective within the first 24 hours after ozone exposure, while blocking IL-1 receptors may be beneficial over the long term.
Acknowledgments
The authors thank Gerald Gleich (University of Utah) for generously providing the antibody to guinea pig MBP.
This work was supported by National Institutes of Health Grants HL-55543 (A.D.F.), ES-014601 (A.D.F.), HL-54659 (D.B.J.), HL-071795 (D.B.J.), RR-023424 (D.B.J.), the M.E. Steinberg Fellowship (K.C.V.), and AHA 0810148Z (K.C.V).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0045OC on July 10, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Shore SA, Schwartzman IN, Le Blanc B, Murthy GG, Doerschuk CM. Tumor necrosis factor receptor 2 contributes to ozone-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med 2001;164:602–607. [DOI] [PubMed] [Google Scholar]
- 2.Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987–2000. JAMA 2004;292:2372–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yost BL, Gleich GJ, Jacoby DB, Fryer AD. The changing role of eosinophils in long-term hyperreactivity following a single ozone exposure. Am J Physiol Lung Cell Mol Physiol 2005;289:L627–L635. [DOI] [PubMed] [Google Scholar]
- 4.Lewis TC, Robins TG, Dvonch JT, Keeler GJ, Yip FY, Mentz GB, Lin X, Parker EA, Israel BA, Gonzalez L, et al. Air pollution-associated changes in lung function among asthmatic children in Detroit. Environ Health Perspect 2005;113:1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko FW, Tam WW, Chan DP, Wong T, Tung AH, Lai CK, Hui DS. The temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax 2007;62:780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiltermann TJ, de Bruijne CR, Stolk J, Zwinderman AH, Spieksma FT, Roemer W, Steerenberg PA, Fischer PH, van Bree L, Hiemstra PS. Effects of photochemical air pollution and allergen exposure on upper respiratory tract inflammation in asthmatics. Am J Respir Crit Care Med 1997;156:1765–1772. [DOI] [PubMed] [Google Scholar]
- 7.Peden DB, Boehlecke B, Horstman D, Devlin R. Prolonged acute exposure to 0.16 ppm ozone induces eosinophilic airway inflammation in asthmatic subjects with allergies. J Allergy Clin Immunol 1997;100:802–808. [DOI] [PubMed] [Google Scholar]
- 8.Peden DB, Setzer RW Jr, Devlin RB. Ozone exposure has both a priming effect on allergen-induced responses and an intrinsic inflammatory action in the nasal airways of perennially allergic asthmatics. Am J Respir Crit Care Med 1995;151:1336–1345. [DOI] [PubMed] [Google Scholar]
- 9.Fabbri LM, Aizawa H, Alpert SE, Walters EH, O'Byrne PM, Gold BD, Nadel JA, Holtzman MJ. Airway hyperresponsiveness and changes in cell counts in bronchoalveolar lavage after ozone exposure in dogs. Am Rev Respir Dis 1984;129:288–291. [PubMed] [Google Scholar]
- 10.Hyde DM, Hubbard WC, Wong V, Wu R, Pinkerton K, Plopper CG. Ozone-induced acute tracheobronchial epithelial injury: relationship to granulocyte emigration in the lung. Am J Respir Cell Mol Biol 1992;6:481–497. [DOI] [PubMed] [Google Scholar]
- 11.Ishii Y, Hashimoto K, Nomura A, Sakamoto T, Uchida Y, Ohtsuka M, Hasegawa S, Sagai M. Elimination of neutrophils by apoptosis during the resolution of acute pulmonary inflammation in rats. Lung 1998;176:89–98. [DOI] [PubMed] [Google Scholar]
- 12.Park JW, Taube C, Joetham A, Takeda K, Kodama T, Dakhama A, McConville G, Allen CB, Sfyroera G, Shultz LD, et al. Complement activation is critical to airway hyperresponsiveness after acute ozone exposure. Am J Respir Crit Care Med 2004;169:726–732. [DOI] [PubMed] [Google Scholar]
- 13.Jacoby DB, Gleich GJ, Fryer AD. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J Clin Invest 1993;91:1314–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans CM, Fryer AD, Jacoby DB, Gleich GJ, Costello RW. Pretreatment with antibody to eosinophil major basic protein prevents hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. J Clin Invest 1997;100:2254–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fryer AD, Maclagan J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol 1984;83:973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fryer AD, Jacoby DB. Function of pulmonary M2 muscarinic receptors in antigen-challenged guinea pigs is restored by heparin and poly-L-glutamate. J Clin Invest 1992;90:2292–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yost BL, Gleich GJ, Fryer AD. Ozone-induced hyperresponsiveness and blockade of M2 muscarinic receptors by eosinophil major basic protein. J Appl Physiol 1999;87:1272–1278. [DOI] [PubMed] [Google Scholar]
- 18.Hestdal K, Ruscetti FW, Chizzonite R, Ortiz M, Gooya JM, Longo DL, Keller JR. Interleukin-1 (IL-1) directly and indirectly promotes hematopoietic cell growth through type I IL-1 receptor. Blood 1994;84:125–132. [PubMed] [Google Scholar]
- 19.Birdsall HH, Lane C, Ramser MN, Anderson DC. Induction of VCAM-1 and ICAM-1 on human neural cells and mechanisms of mononuclear leukocyte adherence. J Immunol 1992;148:2717–2723. [PubMed] [Google Scholar]
- 20.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood 1996;87:2095–2147. [PubMed] [Google Scholar]
- 21.Jedrzkiewicz S, Nakamura H, Silverman ES, Luster AD, Mansharamani N, In KH, Tamura G, Lilly CM. IL-1beta induces eotaxin gene transcription in A549 airway epithelial cells through NF-kappaB. Am J Physiol Lung Cell Mol Physiol 2000;279:L1058–L1065. [DOI] [PubMed] [Google Scholar]
- 22.Okada S, Inoue H, Yamauchi K, Iijima H, Ohkawara Y, Takishima T, Shirato K. Potential role of interleukin-1 in allergen-induced late asthmatic reactions in guinea pigs: suppressive effect of interleukin-1 receptor antagonist on late asthmatic reaction. J Allergy Clin Immunol 1995;95:1236–1245. [DOI] [PubMed] [Google Scholar]
- 23.Gounni AS, Nutku E, Koussih L, Aris F, Louahed J, Levitt RC, Nicolaides NC, Hamid Q. IL-9 expression by human eosinophils: regulation by IL-1beta and TNF-alpha. J Allergy Clin Immunol 2000;106:460–466. [DOI] [PubMed] [Google Scholar]
- 24.Selig W, Tocker J. Effect of interleukin-1 receptor antagonist on antigen-induced pulmonary responses in guinea pigs. Eur J Pharmacol 1992;213:331–336. [DOI] [PubMed] [Google Scholar]
- 25.Watson ML, Smith D, Bourne AD, Thompson RC, Westwick J. Cytokines contribute to airway dysfunction in antigen-challenged guinea pigs: inhibition of airway hyperreactivity, pulmonary eosinophil accumulation, and tumor necrosis factor generation by pretreatment with an interleukin-1 receptor antagonist. Am J Respir Cell Mol Biol 1993;8:365–369. [DOI] [PubMed] [Google Scholar]
- 26.Hakonarson H, Carter C, Maskeri N, Hodinka R, Grunstein MM. Rhinovirus-mediated changes in airway smooth muscle responsiveness: induced autocrine role of interleukin-1beta. Am J Physiol 1999;277:L13–L21. [DOI] [PubMed] [Google Scholar]
- 27.Johnson VJ, Yucesoy B, Luster MI. Prevention of IL-1 signaling attenuates airway hyperresponsiveness and inflammation in a murine model of toluene diisocyanate-induced asthma. J Allergy Clin Immunol 2005;116:851–858. [DOI] [PubMed] [Google Scholar]
- 28.Borish L, Mascali JJ, Dishuck J, Beam WR, Martin RJ, Rosenwasser LJ. Detection of alveolar macrophage-derived IL-1 beta in asthma: inhibition with corticosteroids. J Immunol 1992;149:3078–3082. [PubMed] [Google Scholar]
- 29.Arsalane K, Gosset P, Vanhee D, Voisin C, Hamid Q, Tonnel AB, Wallaert B. Ozone stimulates synthesis of inflammatory cytokines by alveolar macrophages in vitro. Am J Respir Cell Mol Biol 1995;13:60–68. [DOI] [PubMed] [Google Scholar]
- 30.Jarjour NN, Busse WW. Cytokines in bronchoalveolar lavage fluid of patients with nocturnal asthma. Am J Respir Crit Care Med 1995;152:1474–1477. [DOI] [PubMed] [Google Scholar]
- 31.Sousa AR, Lane SJ, Nakhosteen JA, Lee TH, Poston RN. Expression of interleukin-1 beta (IL-1beta) and interleukin-1 receptor antagonist (IL-1ra) on asthmatic bronchial epithelium. Am J Respir Crit Care Med 1996;154:1061–1066. [DOI] [PubMed] [Google Scholar]
- 32.Pendino KJ, Shuler RL, Laskin JD, Laskin DL. Enhanced production of interleukin-1, tumor necrosis factor-alpha, and fibronectin by rat lung phagocytes following inhalation of a pulmonary irritant. Am J Respir Cell Mol Biol 1994;11:279–286. [DOI] [PubMed] [Google Scholar]
- 33.Cohen MD, Sisco M, Li Y, Zelikoff JT, Schlesinger RB. Ozone-induced modulation of cell-mediated immune responses in the lungs. Toxicol Appl Pharmacol 2001;171:71–84. [DOI] [PubMed] [Google Scholar]
- 34.Fakhrzadeh L, Laskin JD, Laskin DL. Ozone-induced production of nitric oxide and TNF-alpha and tissue injury are dependent on NF-kappaB p50. Am J Physiol Lung Cell Mol Physiol 2004;287:L279–L285. [DOI] [PubMed] [Google Scholar]
- 35.Polosa R, Sapsford RJ, Dokic D, Cacciola RR, Prosperini G, Devalia JL, Holgate ST, Howarth PH, Davies DE. Induction of the epidermal growth factor receptor and its ligands in nasal epithelium by ozone. J Allergy Clin Immunol 2004;113:120–126. [DOI] [PubMed] [Google Scholar]
- 36.Park JW, Taube C, Swasey C, Kodama T, Joetham A, Balhorn A, Takeda K, Miyahara N, Allen CB, Dakhama A, et al. Interleukin-1 receptor antagonist attenuates airway hyperresponsiveness following exposure to ozone. Am J Respir Cell Mol Biol 2004;30:830–836. [DOI] [PubMed] [Google Scholar]
- 37.Johnston RA, Mizgerd JP, Flynt L, Quinton LJ, Williams ES, Shore SA. Type I interleukin-1 receptor is required for pulmonary responses to subacute ozone exposure in mice. Am J Respir Cell Mol Biol 2007;37:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dripps DJ, Brandhuber BJ, Thompson RC, Eisenberg SP. Interleukin-1 (IL-1) receptor antagonist binds to the 80-kDa IL-1 receptor but does not initiate IL-1 signal transduction. J Biol Chem 1991;266:10331–10336. [PubMed] [Google Scholar]
- 39.Sato K, Kawana M, Nonomura N, Nakano Y. Course of IL-1beta, IL-6, IL-8, and TNF-alpha in the middle ear fluid of the guinea pig otitis media model induced by nonviable Haemophilus influenzae. Ann Otol Rhinol Laryngol 1999;108:559–563. [DOI] [PubMed] [Google Scholar]
- 40.Belmonte KE, Fryer AD, Costello RW. Role of insulin in antigen-induced airway eosinophilia and neuronal M2 muscarinic receptor dysfunction. J Appl Physiol 1998;85:1708–1718. [DOI] [PubMed] [Google Scholar]
- 41.Wagner EM, Jacoby DB. Methacholine causes reflex bronchoconstriction. J Appl Physiol 1999;86:294–297. [DOI] [PubMed] [Google Scholar]
- 42.Fryer AD, Stein LH, Nie Z, Curtis DE, Evans CM, Hodgson ST, Jose PJ, Belmonte KE, Fitch E, Jacoby DB. Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction. J Clin Invest 2006;116:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis DM, Loegering DA, Gleich GJ. Antiserum to the major basic protein of guinea pig eosinophil granules. Immunochemistry 1976;13:743–746. [DOI] [PubMed] [Google Scholar]
- 44.Verbout NG, Lorton JK, Jacoby DB, Fryer AD. Atropine pretreatment enhances airway hyperreactivity in antigen-challenged guinea pigs through an eosinophil-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 2007;292:L1126–L1135. [DOI] [PubMed] [Google Scholar]
- 45.Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 2003;29:88–97. [DOI] [PubMed] [Google Scholar]
- 46.Kurt-Jones EA, Beller DI, Mizel SB, Unanue ER. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci USA 1985;82:1204–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe N, Kobayashi Y. Selective release of a processed form of interleukin 1 alpha. Cytokine 1994;6:597–601. [DOI] [PubMed] [Google Scholar]
- 48.Dokic D, Howarth HP. Effects of ozone on the nasal mucosa (epithelial cells). Prilozi 2006;27:115–125. [PubMed] [Google Scholar]
- 49.Gambone LM, Elbon CL, Fryer AD. Ozone-induced loss of neuronal M2 muscarinic receptor function is prevented by cyclophosphamide. J Appl Physiol 1994;77:1492–1499. [DOI] [PubMed] [Google Scholar]
- 50.Minette PA, Lammers JW, Dixon CM, McCusker MT, Barnes PJ. A muscarinic agonist inhibits reflex bronchoconstriction in normal but not in asthmatic subjects. J Appl Physiol 1989;67:2461–2465. [DOI] [PubMed] [Google Scholar]
- 51.Fryer AD, Jacoby DB. Parainfluenza virus infection damages inhibitory M2 muscarinic receptors on pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol 1991;102:267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lein PJ, Fryer AD. Organophosphorus insecticides induce airway hyperreactivity by decreasing neuronal M2 muscarinic receptor function independent of acetylcholinesterase inhibition. Toxicol Sci 2005;83:166–176. [DOI] [PubMed] [Google Scholar]
- 53.Elbon CL, Jacoby DB, Fryer AD. Pretreatment with an antibody to interleukin-5 prevents loss of pulmonary M2 muscarinic receptor function in antigen-challenged guinea pigs. Am J Respir Cell Mol Biol 1995;12:320–328. [DOI] [PubMed] [Google Scholar]
- 54.Zhang XY, Robinson NE, Zhu FX. Modulation of ACh release from airway cholinergic nerves in horses with recurrent airway obstruction. Am J Physiol 1999;276:L769–L775. [DOI] [PubMed] [Google Scholar]
- 55.Larsen GL, White CW, Takeda K, Loader JE, Nguyen DD, Joetham A, Groner Y, Gelfand EW. Mice that overexpress Cu/Zn superoxide dismutase are resistant to allergen-induced changes in airway control. Am J Physiol Lung Cell Mol Physiol 2000;279:L350–L359. [DOI] [PubMed] [Google Scholar]
- 56.Haddad EB, Rousell J, Lindsay MA, Barnes PJ. Synergy between tumor necrosis factor alpha and interleukin 1beta in inducing transcriptional down-regulation of muscarinic M2 receptor gene expression. Involvement of protein kinase A and ceramide pathways. J Biol Chem 1996;271:32586–32592. [DOI] [PubMed] [Google Scholar]
- 57.Costello RW, Schofield BH, Kephart GM, Gleich GJ, Jacoby DB, Fryer AD. Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am J Physiol 1997;273:L93–L103. [DOI] [PubMed] [Google Scholar]
- 58.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med 2003;167:199–204. [DOI] [PubMed] [Google Scholar]
- 59.Noga O, Englmann C, Hanf G, Grutzkau A, Seybold J, Kunkel G. The production, storage and release of the neurotrophins nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 by human peripheral eosinophils in allergics and non-allergics. Clin Exp Allergy 2003;33:649–654. [DOI] [PubMed] [Google Scholar]
- 60.Zheng X, Knight DA, Zhou D, Weir T, Peacock C, Schellenberg RR, Bai TR. Leukemia inhibitory factor is synthesized and released by human eosinophils and modulates activation state and chemotaxis. J Allergy Clin Immunol 1999;104:136–144. [DOI] [PubMed] [Google Scholar]
- 61.Giembycz MA, Lindsay MA. Pharmacology of the eosinophil. Pharmacol Rev 1999;51:213–340. [PubMed] [Google Scholar]
- 62.Ollerenshaw SL, Jarvis D, Sullivan CE, Woolcock AJ. Substance P immunoreactive nerves in airways from asthmatics and nonasthmatics. Eur Respir J 1991;4:673–682. [PubMed] [Google Scholar]
- 63.Hazbun ME, Hamilton R, Holian A, Eschenbacher WL. Ozone-induced increases in substance P and 8-epi-prostaglandin F2 alpha in the airways of human subjects. Am J Respir Cell Mol Biol 1993;9:568–572. [DOI] [PubMed] [Google Scholar]
- 64.Wu ZX, Satterfield BE, Dey RD. Substance P released from intrinsic airway neurons contributes to ozone-enhanced airway hyperresponsiveness in ferret trachea. J Appl Physiol 2003;95:742–750. [DOI] [PubMed] [Google Scholar]
- 65.Canning BJ, Reynolds SM, Anukwu LU, Kajekar R, Myers AC. Endogenous neurokinins facilitate synaptic transmission in guinea pig airway parasympathetic ganglia. Am J Physiol Regul Integr Comp Physiol 2002;283:R320–R330. [DOI] [PubMed] [Google Scholar]
- 66.Lundberg JM, Hokfelt T, Martling CR, Saria A, Cuello C. Substance P-immunoreactive sensory nerves in the lower respiratory tract of various mammals including man. Cell Tissue Res 1984;235:251–261. [DOI] [PubMed] [Google Scholar]
- 67.Hazari MS, Jacoby DB, Fryer AD. Neurokinin NK1 receptor antagonists prevent vagally-induced hyperresponsiveness in ozone exposed guinea pigs [abstract]. Am J Respir Crit Care Med 2003;167.
- 68.Hurst SM, Stanisz AM, Sharkey KA, Collins SM. Interleukin 1 beta-induced increase in substance P in rat myenteric plexus. Gastroenterology 1993;105:1754–1760. [DOI] [PubMed] [Google Scholar]
- 69.Wu ZX, Satterfield BE, Fedan JS, Dey RD. Interleukin-1beta-induced airway hyperresponsiveness enhances substance P in intrinsic neurons of ferret airway. Am J Physiol Lung Cell Mol Physiol 2002;283:L909–L917. [DOI] [PubMed] [Google Scholar]
- 70.Frossard N, Naline E, Olgart Hoglund C, Georges O, Advenier C. Nerve growth factor is released by IL-1beta and induces hyperresponsiveness of the human isolated bronchus. Eur Respir J 2005;26:15–20. [DOI] [PubMed] [Google Scholar]
- 71.de Vries A, Engels F, Henricks PA, Leusink-Muis T, McGregor GP, Braun A, Groneberg DA, Dessing MC, Nijkamp FP, Fischer A. Airway hyper-responsiveness in allergic asthma in guinea-pigs is mediated by nerve growth factor via the induction of substance P: a potential role for trkA. Clin Exp Allergy 2006;36:1192–1200. [DOI] [PubMed] [Google Scholar]
- 72.Gryparis A, Forsberg B, Katsouyanni K, Analitis A, Touloumi G, Schwartz J, Samoli E, Medina S, Anderson HR, Niciu EM, et al. Acute effects of ozone on mortality from the “air pollution and health: a European approach” project. Am J Respir Crit Care Med 2004;170:1080–1087. [DOI] [PubMed] [Google Scholar]
- 73.Zanobetti A, Schwartz J. Mortality displacement in the association of ozone with mortality: an analysis of 48 U.S. cities. Am J Respir Crit Care Med 2008;177:184–189. [DOI] [PubMed] [Google Scholar]