Abstract
IL-11 and IL-11 receptor (R)α are induced by Th2 cytokines. However, the role(s) of endogenous IL-11 in antigen-induced Th2 inflammation has not been fully defined. We hypothesized that IL-11, signaling via IL-11Rα, plays an important role in aeroallergen-induced Th2 inflammation and mucus metaplasia. To test this hypothesis, we compared the responses induced by the aeroallergen ovalbumin (OVA) in wild-type (WT) and IL-11Rα–null mutant mice. We also generated and defined the effects of an antagonistic IL-11 mutein on pulmonary Th2 responses. Increased levels of IgE, eosinophilic tissue and bronchoalveolar lavage (BAL) inflammation, IL-13 production, and increased mucus production and secretion were noted in OVA-sensitized and -challenged WT mice. These responses were at least partially IL-11 dependent because each was decreased in mice with null mutations of IL-11Rα. Importantly, the administration of the IL-11 mutein to OVA-sensitized mice before aerosol antigen challenge also caused a significant decrease in OVA-induced inflammation, mucus responses, and IL-13 production. Intraperitoneal administration of the mutein to lung-specific IL-13–overexpressing transgenic mice also reduced BAL inflammation and airway mucus elaboration. These studies demonstrate that endogenous IL-11R signaling plays an important role in antigen-induced sensitization, eosinophilic inflammation, and airway mucus production. They also demonstrate that Th2 and IL-13 responses can be regulated by interventions that manipulate IL-11 signaling in the murine lung.
Keywords: IL-11, mutein, airway inflammation, mucus, IL-13
CLINICAL RELEVANCE
This study is the first to demonstrate that endogenous IL-11 signaling is critical to Th2 aeroallergen- or IL-13–induced inflammation and airway mucus production.
Asthma is a chronic disorder of the airway in which a Th2-dominated inflammatory response and subsequent airway remodeling are felt to play mechanistically important roles (1–4). In keeping with their importance, the cytokines that mediate these responses have been subject to significant investigation. These studies demonstrated that IL-4 plays a key role in Th2 cell differentiation (5). In contrast, IL-13 is now believed to be the major effector of Th2 inflammation and tissue remodeling (4, 6). Studies from our laboratory and others have also defined many of the pathways that are used to induce these alterations. As a result, it is now known that chemokines, matrix metalloproteinases (MMPs), TGF-β, and chitinases contribute to the pathogenesis of pulmonary Th2 inflammation and remodeling (4, 7, 8). However, the importance of IL-6–type cytokines in the generation of OVA- or IL-13–induced inflammation or tissue responses has not been fully defined.
IL-11 is a multifunctional IL-6–type cytokine that stimulates hematopoiesis, thrombopoiesis, and megakaryocytopoiesis; enhances bone marrow resorption; regulates macrophage differentiation; and confers mucosal protection after chemotherapy and radiation therapy (9–12). Previous studies from our laboratory demonstrated that epithelial cells, fibroblasts, and airway smooth muscle cells produce IL-11 in response to TGF-β1, IL-1, histamine, eosinophil major basic protein, and specific respiratory viruses (10, 13–18), and that the transgenic overexpression of IL-11 causes asthma-like airway fibrosis, myocyte hyperplasia, and airways hyperresponsiveness on methacholine challenge (19, 20). Our studies also demonstrated that IL-11 can be detected in exaggerated quantities in the nasal secretions of children with viral upper respiratory tract infections (16) and the airways of patients with asthma (21, 22). However, the role of endogenous IL-11 and IL-11 signaling in the pathogenesis of Th2 inflammation has not been adequately defined.
To define the role(s) of endogenous IL-11 in antigen-induced Th2 inflammation, we compared the acute responses induced by the aeroallergen ovalbumin (OVA) in wild-type (WT) and IL-11Rα–null mutant mice. We also generated and evaluated the effects of an antagonistic IL-11 mutein, on OVA-induced Th2 inflammation and IL-13–induced tissue responses. These studies demonstrate that the IL-11 pathway plays an important role in antigen sensitization and the eosinophilic inflammation, mucus metaplasia, and IL-13 elaboration that are characteristic of pulmonary Th2 responses. They also demonstrate that IL-11 signaling contributes to the effector functions of IL-13 in the murine lung.
MATERIALS AND METHODS
Genetically Modified Mice
IL-11Rα −/− mice were provided by Drs. Lorraine Robb and C. Glenn Begley (The Walter and Eliza Hall Institute, Victoria, Australia) (23). These mice were bred for more than 10 generations onto a C57BL/6 genetic background. C57BL/6 WT controls were obtained from the Jackson Labs (Bar Harbor, ME). CC10-rtTA-IL-13 transgenic mice were generated in our laboratory, bred onto a C57BL/6 background, and used in these studies. These mice use the Clara cell 10-kD protein (CC10) promoter and the reverse tetracycline transactivator (rtTA) to target IL-13 to the lung in a doxycycline-inducible manner. The methods that were used to generate and characterize these mice were described previously (24).
OVA Sensitization and Challenge
OVA sensitization and challenge were accomplished using a modification of the protocols previously described by our laboratory (23). In brief, 6- to 8-wk-old IL-11Rα–null mutant mice and control littermates received injections containing 20 μg of chicken OVA (Sigma, St. Louis, MO) complexed to alum (Resorptar; Indergen, New York, NY), or alum alone. This process was repeated 5 days later. After an additional 7 days, the animals received three aerosol challenges (40 min/d, 3 d) with 1% OVA (wt/vol) in endotoxin-free PBS or PBS alone. The aerosol was generated in an NE-U07 ultrasonic nebulizer (Omron Health Care, Vernon Hills, IL). The mice were killed 24, 48, or 72 hours after aerosol exposure.
Bronchoalveolar Lavage and Lung Inflammation
Lung inflammation was assessed by bronchoalveolar lavage (BAL) as described previously (23). In brief, animals were anesthetized, a median sternotomy was performed, the trachea was dissected free from the underlying soft tissues, and BAL was performed by perfusing the lungs in situ with 0.8 ml of PBS and gently aspirating the fluid back. This was repeated twice. The samples were then pooled and centrifuged, and cell numbers and differentials were assessed. The cell-free BAL fluid was stored at –70°C until used.
Histologic Analysis
The lungs were removed en bloc as described above, inflated at 25 cm pressure with PBS containing 0.5% low-melting-point agarose gel, fixed in Streck solution (Streck Laboratories, La Vista, NE), embedded in paraffin, sectioned, and stained. Hematoxylin and eosin, and diastase-periodic acid–Schiff (D-PAS) or modified Congo Red stains, were performed in the Research Histology Laboratory of the Department of Pathology at the Yale University School of Medicine. Histologic mucus index was estimated by the D-PAS stains as previously described (25), and tissue infiltration of the eosinophils was evaluated by Congo Red stains.
Fluorescence-Activated Cell Sorter Analysis
Cells from BAL fluids and lungs cell digests were subjected to fluorescence-activated cell sorter (FACS) analysis. BAL was performed as described previously. Whole lung cell suspensions were obtained by a method modified from that of Rice and coworkers (26). In brief, lung tissue was digested using dispase (5 mg/ml; Stem Cell Technologies, Vancouver, BC, Canada), collagenase (0.04%; Sigma-Aldrich, St. Louis, MO), and 100 U/ml DNase I (Sigma-Aldrich). After several centrifugations (10 min, 300–1,000 × g) and hemolysis (precooled hemolysis solution containing 11 mM KHCO3, 152 mM NH4Cl; washing 5 min, 400 × g at 4°C), cells were strained through progressively smaller cell strainers (100–20 μm) and nylon gauze and were finally resuspended in FACS buffer (PBS, 2% BSA, 2% FCS) supplemented with 10 U/ml DNase I. Adherent cells were removed and nonadherent cells were then incubated for 30 minutes at room temperature with purified rat anti-mouse CD16/CD32 mAb (1 μg per 105 cells; BD Biosciences, San Diego, CA) to prevent nonspecific binding of antibodies to Fc receptors. For intracellular cytokine staining, cells were stimulated with PMA (50 ng/ml)/ionomycin (500 ng/ml) in RPMI 1640 in the presence of Brefeldin A (2 μM; Sigma-Aldrich) for 6 hours before extracellular staining. Afterward, the cells were incubated for 30 minutes at 4°C with specific antibodies for surface marker staining CD4, CD16, CCR3, CD11c, MHCII, CD80, CD86, CD40, CD8, CD54, CD11b, and B220 to characterize specific cell types. For intracellular staining, cells were fixed with 0.5 ml of ice-cold 2% paraformaldehyde, were permeabilized using 0.5% saponin (Sigma-Aldrich), and were stained with anti–IL-4 or the appropriate isotype control to assess nonspecific staining. Cells were analyzed immediately by flow cytometry (FACSCalibur; Becton-Dickinson, Heidelberg, Germany). All antibodies and FACS reagents were from BD Biosciences. Saturating concentrations of the antibodies as determined by titration experiments before the study. Ten thousand cells per sample were analyzed. Isotype controls were subtracted from the respective specific antibody expression, and the results are reported as mean fluorescence intensity (MFI). Calculations were performed with Cell Quest analysis software (Becton-Dickinson). All experiments were performed in triplicate.
Analysis of Mucus and Mucin Gene Expression
To quantitate the levels of Muc5ac, the major airway mucus in the BAL fluids, 0.1 ml of BAL fluid was slot blotted onto nitrocellulose membranes using a Minifold II slot blot apparatus (Schleicher and Schuell, Keene, NH) according to the protocol provided by the manufacturer. After air-drying, the membrane was blocked with 5% skim milk in TTBS (0.1% Tween 20, 20 mM Tris-Cl, 500 mM NaCl) for 2 hours and washed three times with TTBS. The membranes were then incubated overnight at 4°C with a monoclonal antibody against Mucin-5AC (45M1; NeoMarkers, Union City, CA). After washing with TTBS, the membranes were incubated for 1 hour at room temperature with horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin (Ig)-G (Pierce, Rockford, IL). Immunoreactive mucins were detected using a chemiluminescent procedure (ECL Plus Western blotting detection system; Amersham Biosciences, Piscataway, NJ) according to instructions from the manufacturer. The relative density was quantitated using an AlphaImager 2000 Image analyzing system (Alpha Innotech, San Leandro, CA). The levels of Muc5ac mRNA were evaluated via real-time RT-PCR using Muc5ac specific primers (25) and a 7500 real-time PCR machine (Applied Biosystems Inc., Foster City, CA).
Generation of IL-11 Specific Antagonist Mutein
To further demonstrate the specific role of IL-11 signaling in our assay system, we generated IL-11 antagonist mutein, which can specifically inhibit the binding of IL-11 to IL-11Rα. The proposed binding sites of the antagonist to inhibit the interaction between IL-11 and IL11-Rα are schematically illustrated in Figure E1A in the online supplement. After screening a number of antagonistic variants from multiple phage libraries, several clones demonstrating the highest affinities were selected through evaluation of binding affinity and dose-dependent kinetics of the antagonist using competition enzyme-linked immunosorbent assay (ELISA) and Ba/F3 cell proliferation assay (Figures E1B–E1D). A clone 1.21 was found to bind with 20-fold higher affinity relative to the established reference of W147A IL-11 (27). After site-specific polyethyleneglycosylation (PEGylation) of this clone to increase its in vivo half life, it was produced in a large quantities, shown to be endotoxin free in the Limulus assay, and used to inhibit IL-11 signaling in these studies.
In Vivo Mutein Treatment
To inhibit IL-11 signaling in the OVA aeroallergen model, mice received OVA plus alum as noted above. The mutein (500 μg/mouse/d) was administrated via intraperitoneal injection at the time of the aerosol OVA challenge. In the experiments with the CC10-rtTA-IL-13 transgenic mice, mutein (500 μg/mouse/d) was administered starting 1 day before transgene induction with doxycycline (Dox).
Serum Total IgE and OVA-Specific IgE Measurements
The levels of total and OVA-specific IgE in the serum were measured by ELISA using BD OptEIA kit (BD Biosciences) according to the manufacturer's protocol with modification (28). In brief, 96-well plates were coated with purified anti-mouse IgE mAb (clone R35-72; BD Biosciences) and a purified mouse IgE isotype (27-25; BD Biosciences) was used as a standard. HRP-conjugated anti-mouse IgE (23G3; Southern Biotechnology, Birmingham, AL) (for total IgE) and HRP-labeled anti-biotin (Vector Laboratories, Barlingame, CA) following biotin-labeled OVA (for OVA-specific IgE) (kindly provided by Dr. Lauren Cohn, Pulmonary and Critical Care Medicine, Yale University) were added to the plates as detection enzymes. Pooled sera with known concentrations of IgE (5,000 pg/ml) were also obtained from Dr. Cohn and used as OVA-specific IgE standards. After adding HRP substrate chromogen 3,3′,5,5′-tetramethylbenzidine, the reaction was terminated and detected with an ultraviolet spectrometer.
Cytokine Measurements
The levels of IL-13 and other Th2 cytokines in BAL fluids were evaluated by ELISA using commercial assays (R&D Systems Inc., Minneapolis, MN) as described by the manufacturer.
Statistics
Normally distributed data are expressed as the mean ± SEM and assessed for significance by Student's t test or ANOVA as appropriate.
RESULTS
Role of IL-11α in OVA-Induced Inflammation
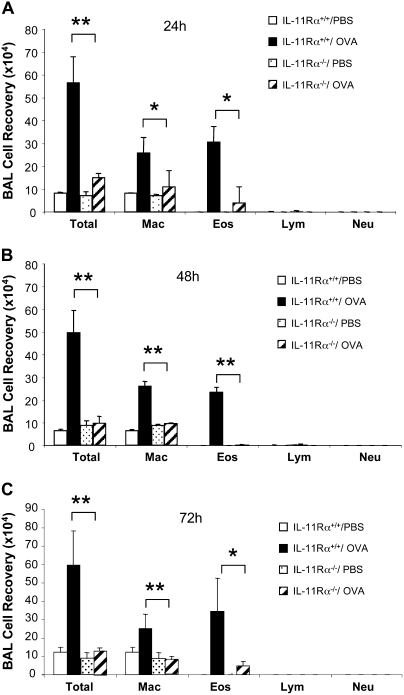
To address the importance of IL-11 signaling in Th2 inflammation, we compared the OVA-induced tissue and BAL inflammatory responses in WT and IL-11Rα–null mutant (IL-11Rα−/−) mice. In the WT mice, OVA sensitization and challenge caused an increase in BAL cellularity and a comparable increase in tissue inflammatory cell accumulation (Figure 1A and data not shown). In both compartments, increases in eosinophils and macrophages were readily appreciated (Figure 1A and data not shown). Comparable levels of BAL and tissue inflammation were not seen in animals that were not sensitized before aeroallergen challenge or that received systemic sensitization and nebulized saline (data not shown). When similar experiments were undertaken in IL-11Rα −/− mice, an impressive decrease in BAL and tissue cellularity was noted (Figure 1 and data not shown). In these experiments, the recovery of eosinophils and macrophages was reduced at all time points (24, 48, and 72 h) after aerosol Ag exposure (Figure 1 and data not shown). The difference between the OVA-induced inflammation in WT and IL-11−/− mice was particularly impressive 48 hours and 72 hours after Ag exposure. At these time points, total cell, eosinophil, and macrophage recoveries were reduced to levels that were comparable to those in control mice that did not receive OVA challenge (Figures 1B and 1C). When viewed in combination, these studies demonstrate that endogenous IL-11 signaling is essential for BAL and tissue inflammation and eosinophila after aeroallergen challenge.
Figure 1.
Differential counts of bronchoalveolar lavage (BAL) cells from wild-type (WT) and IL-11Rα–null mutant mice (IL-11Rα−/−) after ovalbumin (OVA) sensitization and challenge. BAL was performed at (A) 24-hour, (B) 48-hour, and (C) 72-hour intervals after the last OVA challenge. Values represent the mean ± SEM of a minimum of five animals at each time point. Total, total cell number; Mac, macrophages; Eos, eosinophilis; Lym, lymphocytes; Neu, neutrophils. *P < 0.05; **P < 0.01.
Role of IL-11α in OVA-Induced Airway Mucus and Mucin Gene Epression
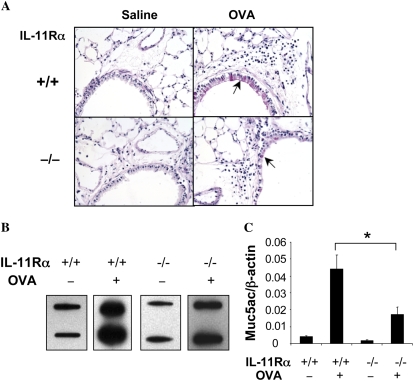
Mucus metaplasia is a characteristic feature of asthma that is also seen at sites of Th2 inflammation in the murine airway. To define the role(s) of IL-11 signaling in this response, we compared the D-PAS staining, levels of Muc5ac mRNA, and levels of Muc5ac in the airways of appropriately sensitized WT and IL-11Rα−/− mice. PAS+ cells were not found or were extremely rare in the airways of WT mice that received a saline aerosol challenge. In contrast, PAS+ cells were readily appreciated after aeroallergen challenge of sensitized WT mice (Figure 2A). The goblet cell hyperplasia in these sensitized and challenged WT mice was also associated with an increase in the levels of Muc5ac in the BAL fluid and the levels of mRNA encoding Muc5ac (Figures 2B and 2C). Each of these responses appeared to involve the IL-11 pathway because, in the absence of IL-11Rα, the frequency of PAS+ cells in the airway was significantly decreased and the levels of BAL Muc5ac and Muc5ac mRNA were impressively decreased (Figure 2).
Figure 2.
Role of IL-11Rα in the regulation of OVA-induced airway mucus, mucin gene expression. WT (IL-11Rα+/+) and IL-11Rα−/− mice were OVA sensitized, challenged, and killed 24 hours after challenge. (A) D-PAS stains (×20 original magnification). (B) Slot and immunoblotting with Muc5ac antibody. (C) Real-time RT-PCR on muc5ac gene. The solid arrows in A highlight the pink-stained airway mucus. A and B are illustrative of a minimum of three similar experiments. The values in C represent the mean ± SEM of evaluations in a minimum of five animals (*P < 0.05).
Role of IL-11α in Aeroallergen Sensitization
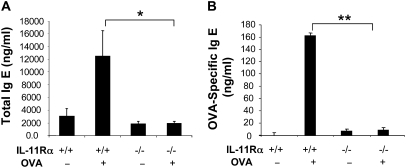
Because IL-11 signaling played a critical role in OVA-induced inflammation and mucus metaplasia, studies were next undertaken to determine if it also contributed to antigen sensitization. This was accomplished by comparing the levels of total and antigen-specific IgE in WT and IL-11Rα−/− mice after treatment with OVA plus alum. In these experiments significant increases in the levels of total and antigen-specific IgE were seen in WT mice (Figure 3). In contrast, both of these responses were significantly decreased in mice that were deficient in IL-11Rα−/− (Figure 3). These studies demonstrate that endogenous IL-11 signaling is essential for the optimal aeroallergen-induced sensitization.
Figure 3.
Role of IL-11Rα in the regulation of OVA-induced IgE production. WT (IL-11Rα+/+) and IL-11Rα−/− mice were sensitized, challenged, and killed 24 hours after challenge. (A) Total and (B) OVA-specific IgE levels in sera were evaluated using enzyme-linked immunosorbent assay (ELISA). Values represent the mean ± SEM of minimum of five animals at each time point (*P < 0.05; **P < 0.01).
Role of IL-11Rα on OVA-Induced Th2 Cytokine Expression
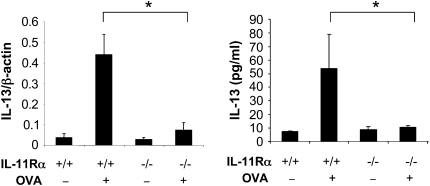
Studies were next undertaken to define the role(s) of IL-11Rα signaling in OVA-induced Th2 cytokine production. IL-4, IL-5, and IL-13 protein and mRNA were not readily detected in the BAL fluids and lung mRNA preparations, respectively, from WT mice that were not challenged with OVA. In contrast, IL-4, IL-5, and IL-13 mRNA and protein were readily detected in the BAL fluids and lungs from WT mice that were sensitized and challenged with OVA (Figure 4 and data not shown). Importantly, in the absence of IL-11Rα, the levels of BAL IL-13 and IL-13 mRNA were significantly decreased (Figure 4 and data not shown). Similar decreases in IL-4 and IL-5 mRNA and protein were also appreciated in sensitized and challenged IL-11Rα–deficient animals (data not shown). These data demonstrate that IL-11Rα plays a critical role in OVA-induced Th2 cytokine induction.
Figure 4.
Role of IL-11Rα in the regulation of OVA-induced IL-13. WT (IL-11Rα+/+) and IL-11Rα−/− mice were OVA sensitized, challenged, and killed 24 hours after challenge. The expression levels of BAL IL-13 were measured by ELISA. The values represent the mean ± SEM of evaluations in a minimum of five animals (*P < 0.05).
Effects of Mutein on OVA-Induced Th2 Inflammation
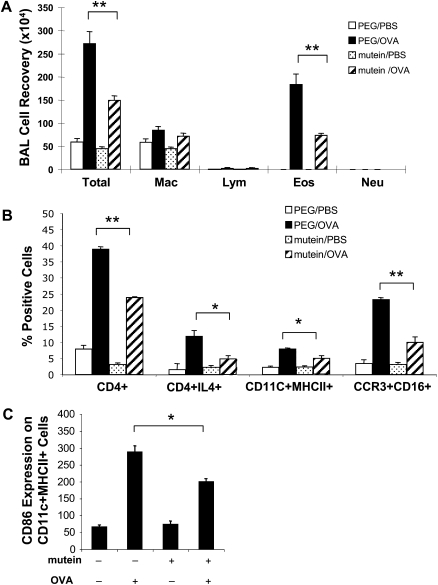
The studies noted above demonstrate that IL-11 signaling plays a critical role in antigen sensitization, Th2 inflammation, mucus metaplasia, and Th2 cytokine elaboration. The alterations that are seen in the absence of IL-11Rα could be entirely due to the defect in sensitization. Alternatively, they could be due to defects in sensitization and defects in Th2 effector responses. To differentiate among these options, we characterized the ability of the IL-11 mutein to alter Th2 responses in OVA-sensitized and -challenged WT mice. This was done by administering the mutein or its polyethylene glycol (PEG) control at the time of OVA aerosol challenge. In these experiments, OVA sensitization and challenge increased total BAL cell recovery by 5- to 6-fold in mice that received the PEG control. In contrast, BAL cell recovery was significantly decreased in mice that received the IL-11 mutein (Figure 5A). In accord with our studies with the IL-11 Rα−/− mice, eosinophil recovery was significantly decreased in the mutein-treated animals (Figure 5A). FACS analysis of the BAL cells further demonstrated that OVA-induced Th2 cell (CD4+IL-4+), dendritic cell (CD11C+ MHCII+), and eosinophil (CCR3+CD16+) numbers were significantly reduced in the mutein treated versus the PEG control–treated animals (Figure 5B). Interestingly, OVA-induced dendritic cell activation (CD86 expression) was also significantly reduced in the mutein-treated mice compared with vehicle-treated mice (Figure 5C).
Figure 5.
Effect of mutein in the regulation of OVA-induced BAL inflammation. C57 BL/6 mice were OVA sensitized, challenged, and killed 24 hours after challenge. At the time of OVA challenge, mutein (500 μg/mouse/d) or vehicle (PEG) were administrated via intraperitoneal injection. Differential count on BAL cells was illustrated (A) and further cellular characterization on BAL cells were performed by FACS analysis (B and C). The values represent the mean ± SEM of evaluations in a minimum of five animals (*P < 0.05; **P < 0.01).
Effects of Mutein on OVA-Induced IL-13 and Mucin Gene Expression
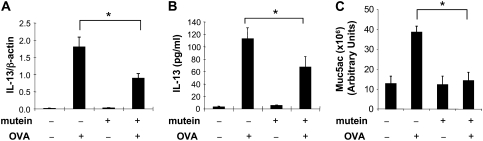
We next compared the IL-13 production and mucin gene expression in OVA-sensitized WT mice treated with PEG vehicle control or the IL-11 mutein. As noted above, OVA sensitization and challenge increased BAL IL-13 mRNA accumulation, the levels of BAL IL-13, and the levels of Muc5ac mRNA accumulation in the PEG control–treated animals (Figure 6). In all cases, these responses were significantly decreased in mice treated with the IL-11 mutein (Figure 6).
Figure 6.
Effect of mutein in the regulation of OVA-induced IL-13 and muc5ac expression. C57 BL/6 mice were OVA sensitized, challenged, and killed 24 hours after challenge. At the time of OVA challenge, mutein (500 μg/mouse/d) or vehicle (PEG) were administrated via intraperitoneal injection. The expression of IL-13 mRNA and protein was evaluated by real-time RT-PCR and ELISA, respectively (A and B). (C) The Muc5ac levels in BAL were quantitated by densitometry after slot and immunoblotting with Muc5ac antibody. The values represent the mean ± SEM of evaluations in a minimum of five animals (*P < 0.05).
Effects of Mutein on IL-13–Induced Tissue Phenotypes
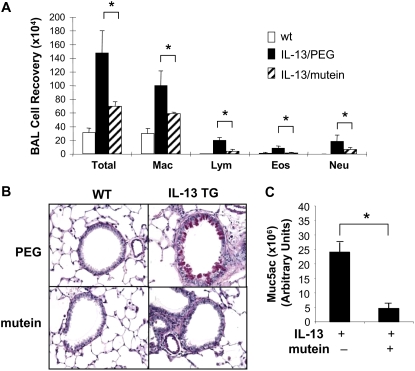
To gain insight into the mechanism(s) by which the IL-11 mutein could alter OVA-induced Th2 responses, we tested the hypothesis that IL-11 signaling contributes to the pathogenesis of IL-13–induced tissue alterations. This was done by comparing the tissue responses in CC10-rtTA-IL-13 transgenic mice treated with the PEG vehicle control or the IL-11 mutein. In keeping with our prior studies, transgenic IL-13 caused an eosinophilic inflammatory response, goblet cell hyperplasia, and enhanced mucin gene expression (4) (Figure 7). Importantly, comparisons of transgenic mice treated with the PEG vehicle control and the IL-11 mutein demonstrated that each of these responses was decreased in the mutein-treated animals (Figure 7). These studies demonstrate that IL-11 signaling plays an important role in the pathogenesis of IL-13 effector responses in the murine lung.
Figure 7.
Effect of mutein in the regulation of IL-13–induced BAL inflammation and mucus elaboration. Mutein (500 μg/mouse/d, intraperitoneally) or vehicle (PEG) were daily administrated to the inducible IL-13 transgenic mice (CC10-rtTA-IL-13) from a day before transgene induction by Doxycycline (Dox). After 2 weeks of Dox induction, mice were killed and (A) differential count on BAL cells, (B) airway mucus evaluation by D-PAS stains (×20 original magnification), and (C) Muc5ac protein expression quantitated by densitometry after slot and immunoblotting with Muc5ac antibody were illustrated. Values in A and C represent the mean ± SEM of evaluations in a minimum of five animals. B is a representative of a minimum of three similar evaluations (*P < 0.05).
DISCUSSION
IL-11 was originally discovered as an IL-6–like molecule that stimulated the proliferation of IL-6–dependent plasmacytoid cells (29). Subsequent studies have focused largely on the effects of exogenously administered rIL-11 and its role as a potential therapeutic agent. These studies demonstrated that IL-11 is an important regulator of platelet accumulation (30, 31) and that it confers cytoprotection, inhibits inflammation, and diminishes cell death (32, 33) at sites of mucosal and tissue injury. As a result of these investigations, rIL-11 has been approved as a therapeutic agent to treat thrombocytopenia (34, 35). It has also received significant attention as an agent that can be a prophylaxis against toxin-induced tissue injury (12, 36) and that can control pathologic inflammatory disorders (9, 37). In contrast, the roles of endogenous IL-11 have not been as extensively investigated. Because IL-11 is known to be overexpressed at sites of Th2 inflammation (22, 38), we used genetically modified mice and developed a soluble mutein that antagonizes IL-11-IL-11Rα binding to characterize the roles of physiologic IL-11R signaling in the pathogenesis of these responses. These studies demonstrate that IL-11 signaling plays a critical role in allergen-induced sensitization, dendritic cell accumulation, and activation and IgE production. They also demonstrated that this IL-11 pathway plays a critical role in the pathogenesis of Th2 inflammation, Th2 cytokine elaboration, and mucus metaplasia. These studies suggest that interventions that control IL-11 signaling can play a useful role in the regulation of disorders characterized by excessive Th2 inflammation and tissue remodeling responses.
Airway inflammation and remodeling are characteristic features of the exaggerated Th2 responses seen in asthma and mechanistically related disorders (3, 39). A number of lines of investigation have demonstrated that IL-11 expression is characteristic of these responses. This includes studies demonstrating that exaggerated levels of IL-11 mRNA can be found in biopsies from individuals with asthma, where they correlate with disease severity and reductions in FEV1 (21). It also includes murine investigations which demonstrated that IL-11 is induced during antigen-stimulated Th2 responses via a leukotriene-dependent mechanism (38); that IL-11 signaling plays an important role in the pathogenesis of IL-13–induced tissue inflammation, fibrotic, and mucus responses (22); and that the transgenic overexpression of IL-11 induces asthma-like T and B cell–rich inflammation, airway fibrosis, myofibroblast accumulation, and physiologic dysregulation (20). Our present studies add to our understanding of the biology of IL-11 and the role that it plays in Th2 responses by demonstrating, for the first time, that endogenous IL-11 contributes to antigen-induced Th2 sensitization, inflammation, and tissue remodeling and that administration of an IL-11 antagonist can ameliorate these effects. These studies suggest that IL-11 plays a key role in the pathogenic Th2 responses that are seen in diseases like asthma. However, it is important to point out that this may actually be only part of the effector profile of IL-11 in this setting. A number of studies have demonstrated that IL-11 also has potent anti-inflammatory effects, including the ability to inhibit nuclear factor-κB activation (36) and induce the production of the TNF-α antagonist, soluble TNF-α Receptor 1 (40). In keeping with these inhibitory properties, previous studies from our laboratory demonstrated that exogenous IL-11 can also inhibit antigen-induced Th2 inflammation and endothelial VCAM-1 expression (23). When these bodies of investigation are viewed in combination, it is clear that IL-11 has complex, context-specific effects on Th2 inflammation with the endogenous product mediating proinflammatory and remodeling effects, while the exogenous administration of high concentrations of this cytokine activates compensatory pathways that are designed to provide feedback to and control the responses. In many ways, these properties of IL-11 are analogous to findings with transforming growth factor (TGF)-β1 and IL-6, which have similar bi-directional modulatory effects (41–44) This is an intriguing analogy because IL-6 and IL-11 are closely related members of the IL-6–type cytokine gene family (44), and studies from our laboratory and others have demonstrated that IL-11 is potently induced by TGF-β1 in a variety of cells and tissues (10, 17, 45, 46).
Patients with asthma can experience bronchorrhea, and their biopsies are characterized by goblet cell hyperplasia and mucus accumulation (47). This mucus response is nicely modeled in the antigen-sensitized and -challenged murine lung, and has been the topic of significant investigation. These studies have demonstrated that a number of pathways contribute to airway mucus metaplasia, including the elaboration of Th2 cytokines such as IL-13 and the activation of the epidermal growth factor receptor (EGFR) (48–50). Our studies demonstrate that IL-11 signaling also contributes to this important airway response because OVA- and IL-13–induced mucus metaplasia were significantly decreased in IL-11Rα–null mice and mice treated with the IL-11 mutein. This inhibition could be due to the important role that IL-11 signaling plays in the production of IL-13. It is important to point out, however, that direct IL-11–induced regulatory effects cannot be ruled out because the related cytokine IL-6 has been shown to have strong mucus-inducing capacity in primary tracheobronchial epithelial cells (51). Additional investigation will be required to differentiate among these mechanistic options.
IL-13 is a product of a gene on chromosome 5 at q31. Although it was initially felt to be an IL-4–like molecule, it is now known to be a powerful stimulator of eosinophilic inflammation, tissue fibrosis, mucus metaplasia, alveolar remodeling, and airways hyperresponsiveness. It is also known to be dysregulated in, and to potentially contribute to, the pathogenesis of a variety of disorders, including asthma, idiopathic pulmonary fibrosis, scleroderma, viral pneumonia, hepatic fibrosis, nodular sclerosing Hodgkin's disease, and chronic obstructive pulmonary disease (3, 7, 52–56). Previous studies from our laboratory demonstrated that IL-13–induced inflammatory and mucus responses are diminished in IL-11Rα–null mice (22). The present studies add to our understanding of the role of IL-11 in this setting by demonstrating the decreased tissue effects of IL-13 in mice with a normal IL-11 receptor complex by treatment with the IL-11 mutein. This observation demonstrates that interventions which abrogate IL-11-IL-11Rα binding can be used to control the tissue effects of IL-13 in the lung and potentially other organs. However, the mechanism underlying mutein regulation of IL-13–induced inflammation and mucus response still remains to be determined. No significant changes in the STAT (STAT1, STAT3, STAT6) phosphoryaltion or acidic mammalian chitinase (AMCase) expression between the IL-13 transgenic mice with and without mutein treatment were detected in the additional mechanistic studies (N.-Y. Chen and C. G. Lee, unpublished observation). STAT6 is well known to mediate IL-13 signaling, and AMCase plays an critical role in Th2- or IL-13–induced inflammation (8). IL-11 was also known to confer protective effects on epithelial and inflammatory cells via induction of antiapoptotic molecules such as A1(bfl1) or survivin (57, 58), and apoptosis is an important mechanism in the resolution of inflammation and maintenance of homeostasis of airway epithelial cells (50, 59). Thus, it is intriguing to speculate that the inhibition of IL-11 signaling by mutein enhances apoptosis of these cells, resulting in fewer inflammatory cells and mucus-producing epithelial cells. To define the exact underlying regulatory mechanism of endogenous IL-11 signaling in allergic inflammation and mucus response, further comprehensive in vitro and in vivo mechanistic studies are warranted.
In summary, these studies demonstrate that endogenous IL-11 signaling plays an important role in the pathogenesis of Th2 antigen sensitization and the pathogenesis of Th2-induced and IL-13–induced inflammatory and remodeling responses. This establishes the IL-11-IL-11Rα pathway as a worthwhile site for investigations designed to identify therapeutic agents that can be used to treat Th2- and IL-13–mediated disorders.
Supplementary Material
Acknowledgments
The authors thank Kathleen Bertier for excellent secretarial and administrative assistance.
These studies were funded by NIH Grants HL-56389 (J.A.E.) and HL-084225 (C.G.L.), and by CSL Limited (Australia) (J.A.E.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2008-0053OC on July 10, 2008
Conflict of Interest Statement: A.D.N. is an employee of CSL limited. F.M.D. is an employee of CSL limited. P.D.S. is an employee of CSL limited. L.J.F. is an employee of CSL limited. M.B. was an employee of Zenyth Therapeutics Ltd before its acquisition by CSL. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000;161:1720–1745. [DOI] [PubMed] [Google Scholar]
- 2.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 1992;326:298–304. [DOI] [PubMed] [Google Scholar]
- 3.Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest 2003;111:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 1999;103:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinke JW, Borish L. Th2 cytokines and asthma. Interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir Res 2001;2:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev 2004;202:175–190. [DOI] [PubMed] [Google Scholar]
- 7.Corry DB, Kheradmand F. Biology and therapeutic potential of the interleukin-4/interleukin-13 signaling pathway in asthma. Am J Respir Med 2002;1:185–193. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 2004;304:1678–1682. [DOI] [PubMed] [Google Scholar]
- 9.Trepicchio WL, Bozza M, Pedneault G, Dorner AJ. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J Immunol 1996;157:3627–3634. [PubMed] [Google Scholar]
- 10.Zheng T, Zhu Z, Wang J, Homer RJ, Elias JA. IL-11: insights in asthma from overexpression transgenic modeling. J Allergy Clin Immunol 2001;108:489–496. [DOI] [PubMed] [Google Scholar]
- 11.Du XX, Williams DA. Interleukin-11: a multifunctional growth factor derived from the hematopoietic microenvironment. Blood 1994;83:2023–2030. [PubMed] [Google Scholar]
- 12.Du X, Williams DA. Interleukin-11: review of molecular, cell biology, and clinical use. Blood 1997;89:3897–3908. [PubMed] [Google Scholar]
- 13.Waxman AB, Einarsson O, Seres T, Knickelbein RG, Warshaw JB, Johnston R, Homer RJ, Elias JA. Targeted lung expression of interleukin-11 enhances murine tolerance of 100% oxygen and diminishes hyperoxia-induced DNA fragmentation. J Clin Invest 1998;101:1970–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Meeren A, Mouthon MA, Vandamme M, Squiban C, Aigueperse J. Combinations of cytokines promote survival of mice and limit acute radiation damage in concert with amelioration of vascular damage. Radiat Res 2004;161:549–559. [DOI] [PubMed] [Google Scholar]
- 15.Orazi A, Du X, Yang Z, Kashai M, Williams DA. Interleukin-11 prevents apoptosis and accelerates recovery of small intestinal mucosa in mice treated with combined chemotherapy and radiation. Lab Invest 1996;75:33–42. [PubMed] [Google Scholar]
- 16.Einarsson O, Geba GP, Zhu Z, Landry M, Elias JA. Interleukin-11: stimulation in vivo and in vitro by respiratory viruses and induction of airways hyperresponsiveness. J Clin Invest 1996;97:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elias JA, Zheng T, Whiting NL, Trow TK, Merrill WW, Zitnik R, Ray P, Alderman EM. IL-1 and transforming growth factor-beta regulation of fibroblast-derived IL-11. J Immunol 1994;152:2421–2429. [PubMed] [Google Scholar]
- 18.Zheng T, Nathanson MH, Elias JA. Histamine augments cytokine-stimulated IL-11 production by human lung fibroblasts. J Immunol 1994;153:4742–4752. [PubMed] [Google Scholar]
- 19.Ray P, Tang W, Wang P, Homer R, Kuhn C III, Flavell RA, Elias JA. Regulated overexpression of interleukin 11 in the lung: use to dissociate development-dependent and -independent phenotypes. J Clin Invest 1997;100:2501–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang W, Geba GP, Zheng T, Ray P, Homer RJ, Kuhn C III, Flavell RA, Elias JA. Targeted expression of IL-11 in the murine airway causes lymphocytic inflammation, bronchial remodeling, and airways obstruction. J Clin Invest 1996;98:2845–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minshall E, Chakir J, Laviolette M, Molet S, Zhu Z, Olivenstein R, Elias JA, Hamid Q. IL-11 expression is increased in severe asthma: association with epithelial cells and eosinophils. J Allergy Clin Immunol 2000;105:232–238. [DOI] [PubMed] [Google Scholar]
- 22.Chen Q, Rabach L, Noble P, Zheng T, Lee CG, Homer RJ, Elias JA. IL-11 receptor alpha in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol 2005;174:2305–2313. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Homer RJ, Hong L, Cohn L, Lee CG, Jung S, Elias JA. IL-11 selectively inhibits aeroallergen-induced pulmonary eosinophilia and Th2 cytokine production. J Immunol 2000;165:2222–2231. [DOI] [PubMed] [Google Scholar]
- 24.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ Jr, Chapman HA Jr, Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest 2000;106:1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CG, Homer RJ, Cohn L, Link H, Jung S, Craft JE, Graham BS, Johnson TR, Elias JA. Transgenic overexpression of interleukin (IL)-10 in the lung causes mucus metaplasia, tissue inflammation, and airway remodeling via IL-13-dependent and -independent pathways. J Biol Chem 2002;277:35466–35474. [DOI] [PubMed] [Google Scholar]
- 26.Rice WR, Conkright JJ, Na CL, Ikegami M, Shannon JM, Weaver TE. Maintenance of the mouse type II cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol 2002;283:L256–L264. [DOI] [PubMed] [Google Scholar]
- 27.Underhill-Day N, McGovern LA, Karpovich N, Mardon HJ, Barton VA, Heath JK. Functional characterization of W147A: a high-affinity interleukin-11 antagonist. Endocrinology 2003;144:3406–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kweon MN, Yamamoto M, Kajiki M, Takahashi I, Kiyono H. Systemically derived large intestinal CD4(+) Th2 cells play a central role in STAT6-mediated allergic diarrhea. J Clin Invest 2000;106:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul SR, Bennett F, Calvetti JA, Kelleher K, Wood CR, O'Hara RM Jr, Leary AC, Sibley B, Clark SC, Williams DA, et al. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci USA 1990;87:7512–7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaye JA. FDA licensure of NEUMEGA to prevent severe chemotherapy-induced thrombocytopenia. Stem Cells 1998;16:207–223. [DOI] [PubMed] [Google Scholar]
- 31.Valent P. Use of interleukin-11 to stimulate platelet production in myelodysplastic syndromes. Leuk Lymphoma 2006;47:1999–2001. [DOI] [PubMed] [Google Scholar]
- 32.Uemura T, Nakayama T, Kusaba T, Yakata Y, Yamazumi K, Matsuu-Matsuyama M, Shichijo K, Sekine I. The protective effect of interleukin-11 on the cell death induced by X-ray irradiation in cultured intestinal epithelial cell. J Radiat Res (Tokyo) 2007;48:171–177. [DOI] [PubMed] [Google Scholar]
- 33.Kimura R, Maeda M, Arita A, Oshima Y, Obana M, Ito T, Yamamoto Y, Mohri T, Kishimoto T, Kawase I, et al. Identification of cardiac myocytes as the target of interleukin 11, a cardioprotective cytokine. Cytokine 2007;38:107–115. [DOI] [PubMed] [Google Scholar]
- 34.Ciurea SO, Hoffman R. Cytokines for the treatment of thrombocytopenia. Semin Hematol 2007;44:166–182. [DOI] [PubMed] [Google Scholar]
- 35.Bhatia M, Davenport V, Cairo MS. The role of interleukin-11 to prevent chemotherapy-induced thrombocytopenia in patients with solid tumors, lymphoma, acute myeloid leukemia and bone marrow failure syndromes. Leuk Lymphoma 2007;48:9–15. [DOI] [PubMed] [Google Scholar]
- 36.Kawakami T, Takahashi T, Shimizu H, Nakahira K, Takeuchi M, Katayama H, Yokoyama M, Morita K, Akagi R, Sassa S. Highly liver-specific heme oxygenase-1 induction by interleukin-11 prevents carbon tetrachloride-induced hepatotoxicity. Int J Mol Med 2006;18:537–546. [PubMed] [Google Scholar]
- 37.Opal SM, Keith JC Jr, Jhung J, Palardy JE, Parejo N, Marchese E, Maganti V. Orally administered recombinant human interleukin-11 is protective in experimental neutropenic sepsis. J Infect Dis 2003;187:70–76. [DOI] [PubMed] [Google Scholar]
- 38.Lee KS, Kim SR, Park HS, Park SJ, Min KH, Lee KY, Jin SM, Lee YC. Cysteinyl leukotriene upregulates IL-11 expression in allergic airway disease of mice. J Allergy Clin Immunol 2007;119:141–149. [DOI] [PubMed] [Google Scholar]
- 39.Jeffery PK. Comparative morphology of the airways in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994;150:S6–S13. [DOI] [PubMed] [Google Scholar]
- 40.Ellis M, Hedstrom U, Frampton C, Alizadeh H, Kristensen J, Shammas FV, al-Ramadi BK. Modulation of the systemic inflammatory response by recombinant human interleukin-11: a prospective randomized placebo controlled clinical study in patients with hematological malignancy. Clin Immunol 2006;120:129–137. [DOI] [PubMed] [Google Scholar]
- 41.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med 2004;200:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol 1998;16:137–161. [DOI] [PubMed] [Google Scholar]
- 43.Wahl SM, Swisher J, McCartney-Francis N, Chen W. TGF-beta: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J Leukoc Biol 2004;76:15–24. [DOI] [PubMed] [Google Scholar]
- 44.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 2003;374:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elias JA, Zheng T, Einarsson O, Landry M, Trow T, Rebert N, Panuska J. Epithelial interleukin-11. Regulation by cytokines, respiratory syncytial virus, and retinoic acid. J Biol Chem 1994;269:22261–22268. [PubMed] [Google Scholar]
- 46.Elias JA, Wu Y, Zheng T, Panettieri R. Cytokine- and virus-stimulated airway smooth muscle cells produce IL-11 and other IL-6-type cytokines. Am J Physiol 1997;273:L648–L655. [DOI] [PubMed] [Google Scholar]
- 47.Rubin BK. The pharmacologic approach to airway clearance: mucoactive agents. Respir Care 2002;47:818–822. [PubMed] [Google Scholar]
- 48.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 2006;86:245–278. [DOI] [PubMed] [Google Scholar]
- 49.Voynow JA, Gendler SJ, Rose MC. Regulation of mucin genes in chronic inflammatory airway diseases. Am J Respir Cell Mol Biol 2006;34:661–665. [DOI] [PubMed] [Google Scholar]
- 50.Cohn L. Mucus in chronic airway diseases: sorting out the sticky details. J Clin Invest 2006;116:306–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem 2003;278:17036–17043. [DOI] [PubMed] [Google Scholar]
- 52.Belperio JA, Dy M, Burdick MD, Xue YY, Li K, Elias JA, Keane MP. Interaction of IL-13 and C10 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 2002;27:419–427. [DOI] [PubMed] [Google Scholar]
- 53.Hancock A, Armstrong L, Gama R, Millar A. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol 1998;18:60–65. [DOI] [PubMed] [Google Scholar]
- 54.Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol 1997;24:328–332. [PubMed] [Google Scholar]
- 55.van der Pouw Kraan TC, Kucukaycan M, Bakker AM, Baggen JM, van der Zee JS, Dentener MA, Wouters EF, Verweij CL. Chronic obstructive pulmonary disease is associated with the -1055 IL-13 promoter polymorphism. Genes Immun 2002;3:436–439. [DOI] [PubMed] [Google Scholar]
- 56.Ohshima K, Akaiwa M, Umeshita R, Suzumiya J, Izuhara K, Kikuchi M. Interleukin-13 and interleukin-13 receptor in Hodgkin's disease: possible autocrine mechanism and involvement in fibrosis. Histopathology 2001;38:368–375. [DOI] [PubMed] [Google Scholar]
- 57.He CH, Waxman AB, Lee CG, Link H, Rabach ME, Ma B, Chen Q, Zhu Z, Zhong M, Nakayama K, et al. Bcl-2-related protein A1 is an endogenous and cytokine-stimulated mediator of cytoprotection in hyperoxic acute lung injury. J Clin Invest 2005;115:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahboubi K, Li F, Plescia J, Kirkiles-Smith NC, Mesri M, Du Y, Carroll JM, Elias JA, Altieri DC, Pober JS. Interleukin-11 up-regulates survivin expression in endothelial cells through a signal transducer and activator of transcription-3 pathway. Lab Invest 2001;81:327–334. [DOI] [PubMed] [Google Scholar]
- 59.Henson PM, Tuder RM. Apoptosis in the lung: induction, clearance and detection. Am J Physiol Lung Cell Mol Physiol 2008;294:L601–L611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.