Abstract
Objective
To evaluate the effects of whole-body vibration on fat, bone, leptin and muscle mass.
Methods/Design
Thirty 7-month-old female 344 Fischer rats were randomized by weight into three groups (baseline, vibration or control; n=7–10 per group). Rats in the vibration group were placed inside individual compartments attached to a Pneu-Vibe vibration platform (Pneumex, Sandpoint, ID, USA) and vibrated at 30–50 Hz (6mm peak to peak) for 30 min per day, 5 days per week, for 12 weeks. The vibration intervention consisted of six 5-min cycles with a 1-min break between cycles.
Results
There were significant body composition differences between the whole-body vibration and the control groups. The whole-body vibration group weighed approximately 10% less (mean ± s.d.; 207 ± 10 vs 222 ± 15 g, P<0.03) and had less body fat (20.8 ± 3.8 vs 26.8 ± 5.9 g, P<0.05), a lower percentage of body fat (10.2 ± 1.7 vs 12 ± 2.0%, P<0.05), and lower serum leptin levels (1.06 ± 0.45 vs 2.27 ± 0.57 ng ml−1, P<0.01) than the age-matched controls. No differences were observed for total lean mass, bone mineral content (BMC), bone mineral density (BMD), insulin-like growth factor-I (IGF-I) or soleus (SOL) and extensor digitorum longus (EDL) mass or function. Regional high-resolution dual-energy X-ray absoptiometry scans of the lumbar spine (L1-4) revealed that the whole-body vibration group had significantly greater BMC (0.33 ± 0.05 vs 0.26 ± 0.03 g, P<0.01) and BMD (0.21 ± 0.01 vs 0.19 ± 0.01 gcm−2, P<0.01) than the control group. No differences between the groups were observed in the amount of food consumed.
Conclusion
These findings show that whole-body vibration reduced body fat accumulation and serum leptin without affecting whole body BMC, BMD or lean mass. However, the increase in vertebral BMC and BMD suggests that vibration may have resulted in local increases in bone mass and density. Also, whole-body vibration did not affect muscle function or food consumption.
Keywords: weight gain, fat mass, leptin, lean mass, bone
Introduction
Nearly 66% of Americans are overweight or obese.1,2 Obesity is an important co-morbidity factor in many common diseases such as heart disease, diabetics and cancer.3–5 Although weight control is a commonly reported behavior, the prevalence of obesity is increasing in the United States5 in part, because traditional approaches for treating overweight and obese adults, which have focused on individual weight loss have had limited success.6,7
Prevention has been suggested as the most important approach to decreasing obesity.8 Physical activity increases energy metabolism, contributes to a healthy energy balance and can be used to increase lean mass and bone mass while decreasing fat mass.9 Physical activity is also beneficial in attenuating or improving chronic disease conditions.10 However, despite the proven benefits of physical activity, more than 50% of US adults do not get enough physical activity to provide health benefits; and 24% are not active at all in their leisure time.2
Recently, whole-body vibration has been proposed as a potential alternative, or adjuvant, to exercise. Whole-body vibration has been reported to increase energy metabolism through an increased oxygen uptake to values comparable to moderate walking.11,12 Additionally, standing on a vibration platform at a frequency of 26 Hz and amplitude of 6mm increased heart rate response, Borg rating of perceived exertion and blood lactate concentrations to levels found when performing moderate exercise.11,12 These data suggest that the increase in energy expenditure with whole-body vibration is of sufficient magnitude to impact body composition. In support of this finding, Rubin et al.13 report that short bursts of low-intensity vibration in young mice reduce the differentiation of precursor cells to adipocytes, suggesting a plausible mechanism by which mechanical vibration may prevent fat accumulation.
Although obesity is associated with negative health outcomes, weight loss is also not without risks.14 Weight loss is associated with bone and muscle loss, potentially increasing the risk for sarcopenia and osteoporosis.15 Thus, weight reduction may accelerate bone and muscle loss associated with menopause.16
Whole-body vibration training has been promoted as a method of improving bone mass17–24 and muscle strength.24,25 However, to our knowledge, the long-term effect of vibration on body composition has not been investigated. Thus, the purpose of this study was to determine whether whole-body vibration could promote weight loss while maintaining or improving bone and muscle mass in mature rats.
Methods
This study was approved by the Institutional Animal Care and Use Committee at the Oregon State University.
Animals
Seven-month-old virgin female Fisher 344 rats (Harlan Laboratories, Indianapolis, IN, USA) were used. Each animal was housed separately in a translucent polycarbonate cage with enclosed filters on the sides and tops of the cages. The colony room was maintained on a 14/10 h light–dark cycle, with temperature maintained at 21–23 °C. The attending veterinarian inspected the animals once a day for overall health. The author (GFM) also inspected the animals once a day and noted their health and any signs of distress; including changes in daily cleaning behavior, food and water consumption, activity level, shedding and porphyrin secretion around the eyes and nose.
Food and diet
The animals had free access to water and a standard commercial diet (Teklad Rodent Chow, no. 8604 Harlan Teklad, Madison, WI, USA) containing 1.46% calcium, 0.99% phosphorus and 4.96 IU per gram of vitamin D3. Food intake was determined over weeks 3–4, 7–8 and 11–12.
Experimental design
The animals were randomized by weight into one of three groups (8–10 per group): (a) baseline, (b) vibration or (c) control. The baseline group was sacrificed at initiation of the study for analysis of body composition, bone mass and muscle function. For vibration treatment, animals in the vibration group were placed in individual 13 × 28 × 15cm compartments attached to a Pneu-Vibe vibration platform (Pneumex, Sandpoint, ID, USA). This group received 30 min of whole-body vibration each day, 5 days per week, for 12 weeks.
The vibration intervention consisted of six 5-min cycles at an amplitude of 6mm. Each minute of vibration consisted of 35 s at 30 Hz, followed by 20 s at 40 Hz, followed by 5 s at 50 Hz. The cycle was repeated. After 5 min of vibration, the animals were given a 1-min rest period as recent data suggest that high-frequency loading regimes in combination with the insertion of rest periods may augment osteogenesis.26 The non-vibration animals remained in their cages, which were placed approximately 4m from the vibration platform.
Whole-body scans
Whole-body bone mineral content (BMC), whole-body bone mineral density (BMD), lean and fat mass, percentage of body fat and femoral and lumbar spine (L1-L4) BMC and BMD were determined by dual-energy X-ray absorptiometry using small animal software (Model 4500-A, Hologic Inc., Waltham, MA, USA). The rats were anesthetized with 2–3% isoflurane delivered in 100% oxygen (1.5 l min−1) via a nose cone during the scanning procedure. The least significant changes for whole body and spine BMD for rats in our laboratory are 0.003 and 0.006 gcm−2 and less than 1% for whole body composition (lean muscle mass and fat mass) at 95% confidence level, respectively.
Muscle mass and function
Following the scans, soleus (SOL) and extensor digitorum longus (EDL) muscles were excised from the anesthetized animals. The muscles were mounted between an isometric force transducer and an immovable post via the tendons, and suspended in a temperature-controlled (22 °C) bicarbonate buffer that was continuously bubbled with 95% O2 and 5% CO2.27 Supramaximal stimuli (200 μs pulses at 60 Hz for SOL and 120 Hz for EDL) were delivered to platinum electrodes flanking the muscle. Data were collected with muscles at their optimal length for tetanic tension. At the conclusion of the experiment, muscle mass was determined and the muscle’s physiological cross-sectional area was calculated as described previously.27
Micro-computed tomography
After the removal of the muscles, the animals were euthanized by exsanguination. The right tibia, femur and lumbar vertebrae (L1-4) were removed, stored in 70% alcohol at 4 °C. Micro-computed tomography was used for non-destructive three-dimensional evaluation of vertebral cancellous bone architecture.28 The lumbar vertebrae were scanned using a Scanco μCT40 scanner (Scanco Medical AG, Basserdorf, Switzerland) at a voxel size of 20 × 20 × 20 μm. Analysis of the lumbar vertebra included the entire region of secondary spongiosa between the cranial and caudal growth plates at a threshold of 265. Direct cancellous bone measurements included (1) cancellous bone volume/tissue volume (volume of total tissue occupied by cancellous bone,), (2) trabecular thickness (mean thickness of individual trabeculae, μm), (3) trabecular number (number of trabeculae within the sampling site, 1mm−1) and (4) trabecular separation (the distance between trabeculae, μm).
Serum Leptin and IGF-I
Serum leptin was measured using a DSL-10-23100 ACTIVE Human Leptin Enzyme-Linked Immunosorbent (ELISA) kit according to the manufacturer’s instructions (Diagnostic Systems Laboratories, INC. Webster, TX, USA). Serum insulin-like growth factor-I (IGF-I) was measured using a rat IGF-I radioimmunoassay kit according to the manufacturer’s instructions (Diagnostics Systems Laboratories, Inc., Webster, TX, USA).
Statistical analyses
Data are expressed as mean ± s.d. One-way analysis of variance (ANOVA) was used to evaluate the effects of treatment (baseline, control, vibration) on total body mass, lean and fat mass, percentage of body fat, BMC, BMD, muscle mass and function, leptin and IGF-1 levels. When the ANOVA was significant, a Bonferroni post-hoc test was used to evaluate differences among the three treatment groups. A t-test was used to evaluate the effects of treatment (control, vibration) on food consumption, lumbar BMC and BMD, vertebral cancellous bone architecture and serum leptin levels. A P-value of less than0.05 was considered significant for all effects.
Results
The rats adjusted quickly to the vibration, were healthy, and tolerated the vibration well. Subsequent to the first two vibration sessions the animals exhibited no signs of distress as evidenced by normal condition of their tail and fur, minimal urine and feces excretion during vibration and the absence of porphyrin secretion around the animal’s eyes. Furthermore, no difference was observed in the amount of food and water consumed between the control and vibration groups (Table 1).
Table 1.
Food consumption, total body mass, total body lean mass and serum IGF-I levels
| Variables | Baseline n = 10 | Control n = 10 | Vibration n = 8 | ANOVA (P-value =) |
|---|---|---|---|---|
| B | C | V | ||
| Food consumption (g week−1) | 77±5 | 78±2 | 0.79 | |
| Total body mass (g) | 198.9±5.2a | 221.8±14.8b | 207.3±10.1a | 0.03 |
| Total body lean mass (g) | 170.9±8.2a | 187.0±10.0b | 182.4±9.2b | 0.01 |
| IGF-I (ng ml−1) | 426.0±52.0 a | 354.0±44.0b | 354.0±47.0b | 0.01 |
Abbreviation: IGF-I, insulin-like growth factor-I. Groups sharing a common superscript do not differ significantly from one another (P>0.05). Values are mean ± s.d.
Whole-body composition
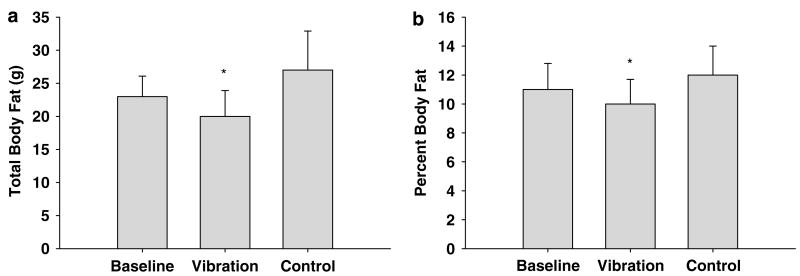
The control and vibrated rats weighted more than the baseline group at the end of the study. (Table 1). However, at termination of the study, the control group weighed more than the vibration group. The control and vibration groups had greater lean mass than the baseline group. Differences in lean mass were not detected between the control and vibration groups (Table 1). The vibration group had less body fat and a lower percentage of body fat than the age-matched control and baseline groups (Figure 1).
Figure 1.
Whole-body vibration reduced total body fat (a) and percent body fat (b). Values are mean ± s.d. for total body fat (a) and percent body fat (b), the vibration group had less fat mass and percent body fat than the baseline and control group. *P<0.05.
Bone mass and architecture
Dual-energy X-ray absorptiometry analysis revealed that the vibration and control groups had greater BMC and BMD than the baseline group. However, no differences were observed between the control and vibration groups for total body BMC or BMD (Table 2). Regional high-resolution dual-energy X-ray absorptiometry scans of the lumbar spine (L1-4) revealed that the vibration group had greater BMC and BMD than the control group.
Table 2.
Total body and lumbar spine (L1-4) bone mineral content (BMC) and bone mineral density (BMD)
| Variables | Baseline n = 10 | Control n = 10 | Vibration n = 8 | ANOVA (P-value =) |
|---|---|---|---|---|
| B | C | V | ||
| Total body BMC (g) | 7.20±0.33a | 7.99±0.44b | 7.91±0.31b | 0.01 |
| Total body BMD (g cm−2) | 0.160±0.007a | 0.168±0.005b | 0.170±0.006b | 0.01 |
| Spine BMC (g) | NA | 0.258±0.030a | 0.331±0.050b | 0.001 |
| Spine BMD (g cm−2) | NA | 0.192±0.010a | 0.214±0.010b | 0.005 |
Abbreviations: BMC, bone mineral content; BMD, bone mineral density. Groups sharing a common superscript do not differ significantly from one another (P>0.05). Values are mean ± s.d.
Micro-computed tomography analysis of cancellous bone in the first lumbar vertebra revealed no differences between the whole-body vibration and control groups in cancellous bone volume/tissue volume, trabecular number, trabecular thickness or trabecular separation (Table 3).
Table 3.
Cancellous bone volume and architecture in the first lumbar vertebra as determined by microcomputed tomography
| Variables | Control n = 10 | Vibration n = 8 | ANOVA (P-value =) |
|---|---|---|---|
| Bone volume/tissue volume | 0.44±0.05 | 0.47±0.04 | 0.28 |
| Trabecular number (1 mm−1) | 5.80±0.40 | 6.00±0.20 | 0.26 |
| Trabecular thickness (mm) | 0.076±0.01 | 0.077±0.01 | 0.71 |
| Trabecular separation (mm) | 0.149±0.01 | 0.143±0.01 | 0.17 |
Muscle mass
The mass of the SOL and the EDL was similar for both vibration and control groups (Tables 3 and 4). In addition, no differences were observed in twitch force or kinetics (twitch contraction time and relaxation time) or in absolute or specific (per unit muscle cross-sectional area) tetanic force.
Table 4.
Muscle weight and isometric contractile properties
| Variables | Non-vibration control | Vibration | ANOVA (P-value =) |
|---|---|---|---|
| Soleus | n = 10 | n = 8 | |
| Muscle mass (mg) | 75.4±2.7 | 76.3±5.1 | 0.63 |
| Twitch force (mN) | 199±29 | 201±26 | 0.85 |
| Twitch contraction time (ms) | 124±7 | 121±4 | 0.25 |
| Twitch relaxation time (ms) | 171±32 | 166±16 | 0.66 |
| Tetanic force (mN) | 1104±116 | 1105±64 | 0.98 |
| Tetanic force/muscle CSA (kN m−2) | 219±27 | 215±12 | 0.74 |
| Extensor digitorum longus | n = 10 | n = 7 | |
| Muscle mass (mg) | 90.7±4.9 | 86.1±4.4 | 0.07 |
| Twitch force (mN) | 342±64 | 316±24 | 0.33 |
| Twitch contraction time (ms) | 35±3 | 38±5 | 0.15 |
| Twitch relaxation time (ms) | 43±4 | 46±6 | 0.14 |
| Tetanic force (mN) | 1783±134 | 1668±108 | 0.08 |
| Tetanic force/muscle CSA (kN m−2) | 264±20 | 253±13 | 0.22 |
Abbreviation: CSA cross-sectional area. Values are mean ± s.d.
IGF-I
No differences in serum levels of IGF-I were observed between the vibration and non-vibration groups. However, serum levels of IGF-I were lower in both the vibration and non-vibration groups compared with the baseline group (Table 1).
Leptin
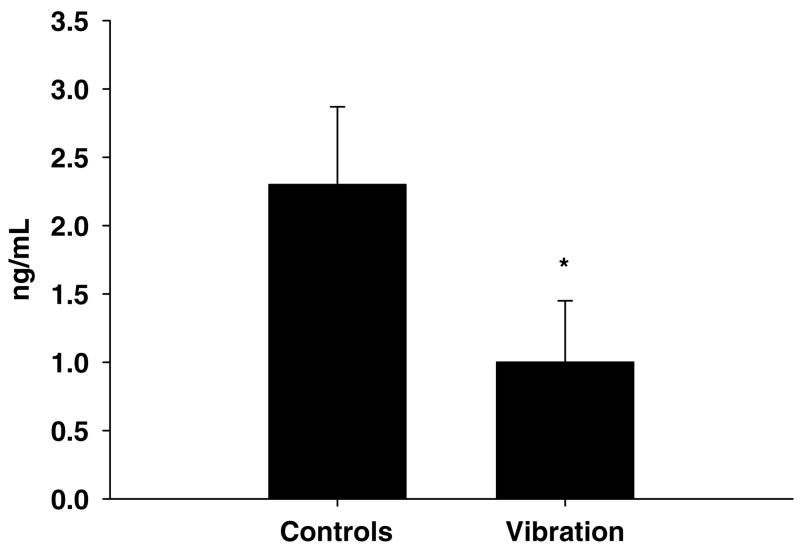
Serum leptin levels were lower in the vibration group compared with the age-matched control group (Figure 2). Serum leptin levels in the baseline group were not evaluated.
Figure 2.
Effects of whole-body vibration on serum leptin. Values are mean ± s.d. The vibration group had lower serum leptin levels than the control group. *P ≤ 0.01.
Discussion
Over the course of the study the vibrated rats were healthy, tolerated the vibration well and exhibited no obvious signs of distress following adaptation to vibration. Twelve weeks of whole-body vibration resulted in significant body composition differences between the vibration and control groups. The whole-body vibration group weighed less, had less body fat and had a lower overall percentage of body fat than the age-matched non-vibration group. In addition, the vibrated rats had lower serum leptin levels than the control animals. However, differences in food intake were not observed between the two groups.
Serum leptin concentrations are strongly correlated with body fat and body mass index (BMI).29 Leptin acts on peripheral tissue and increases the inflammatory response by stimulating the production of tumor necrosis factor-α, interleukin-6, and interleukin-12. As a consequence, elevated leptin levels are considered to be an independent risk factor for increased cardiovascular disease, prostate cancer and breast cancer.30,31 Thus, the observed large reduction in serum leptin levels suggests that whole-body vibration may have beneficial effects on disease risk that are disproportionately large compared with the relatively small reduction in body weight.
Epidemiologic, cross-sectional and prospective correlation studies suggest an essential role for physical activity in weight-loss maintenance, and post-hoc analysis of prospective trials shows a clear dose–response relationship between physical activity and weight maintenance.5 The mechanism responsible for the reduced fat accumulation in the vibration group is not known. Whole-body vibration has been proposed to increase energy expenditure via repetitive muscle contractions.5 Whole-body vibration has been reported to upregulate energy metabolism through an increased oxygen uptake comparable to moderate walking11,12 and corresponding increases in heart rate response, rating of perceived exertion and blood lactate levels expected when performing moderate exercise.11,12 Thus, increased energy expenditure without a concomitant increase in food consumption could explain the reduced weight gain in vibrated rats.
Previous whole-body vibration studies showing effects on bone mass and muscle strength have used frequencies ranging from 20 to 90 Hz.25,32–34 Recently, Rubin et al.13 demonstrated that growing mice receiving 15 weeks of high-frequency, low-magnitude mechanical vibration at 90 Hz, 0.2-g peak acceleration developed far fewer fat cells than controls not receiving the treatment. Similar to our study, Rubin et al. reported no difference in food intake between the two groups of animals. This suggests that vibration does not affect appetite in spite of decreased leptin levels and that an increase in energy expenditure may attenuate or decrease fat cell accumulation. Moreover, at the termination of the Rubin et al. study,13 the vibrated mice had nearly 28% less fat in the torso than control animals. In addition, levels of fatty compounds linked to type 2 diabetes, such as triglycerides and free fatty acids, were reduced by 43 and 39%, respectively, in the livers of the vibrated mice.13 The present study extends this work by showing that vibration also reduces fat accumulation in mature rats fed a normal diet.
Increased body weight is associated with higher BMD and lower incidence of hip fractures in older men and women,35 whereas the converse is true for low body weight. Weight loss is a risk factor for osteoporosis.14 The mechanisms for the osteo-protective effect of body weight are not completely understood. Data suggest that this may be a result of increased skeletal loading and/or increased plasma levels of bone-active hormones (for example, estradiol),35 as body weight and estradiol levels are considered to be major factors determining BMD in both women and men.35
Whole-body-vibration has been reported to increase bone mass in disabled children,34 young women with low bone mass32 and postmenopausal women.33 Thus, whole-body-vibration may potentially increase bone mass similar to increases associated with weight gain by mimicking the increased skeletal loading associated with increased weight. We did not detect an increase in total body bone mass in this study. However, the increase in vertebral BMC and BMD suggests that vibration may have resulted in local increases in bone mass and density. Importantly, our findings indicate that prevention of weight gain by vibration had no negative impact on muscle or bone mass.
Vibration reduced fat mass but not lean mass. Even though the whole-body vibration group weighed less, had less fat mass and a lower percentage of body fat, no differences were observed for lean mass and bone mass between the control and whole-body vibration group. Some studies have suggested that up to 30% of weight loss because of energy restriction may be due to a reduction of lean mass.36 Thus, it is possible that a weight reduction program supplemented with whole-body vibration may positively affect body composition by increasing the rate of fat loss while preserving lean mass. Further research is needed to test this possibility.
No differences in lean mass between the whole-body vibration and control groups are consistent with our finding that vibration had no effect on the mass or function of the oxidative SOL and glycolytic EDL muscles. There was a tendency for EDL mass and tetanic force to be reduced by 5–6% in the vibration group. Although our statistical power for this comparison was low (0.42–0.45), these findings deserve further investigation because whole-body vibration has been proposed to augment strength gains during human resistance training exercise programs.24,25
Previous whole-body vibration studies37,38 have reported acute increases in plasma concentrations of growth hormone and serum IGF-I. In our study, whole-body vibration had no effect on IGF-I levels. However, both the whole-body vibration and control groups had lower IGF-I levels than the baseline group. This is consistent with previous research showing serum IGF-I levels to decline with age in a predictable manner in healthy individuals. After reaching a peak during the pubertal growth spurt, mean circulating IGF-I levels decrease twofold by the third decade and continue to steadily decline with age.39 As our treatment groups were 12 weeks older than the baseline group, we attribute the lower IGF-I levels in the older rats to the natural effects of aging.
In conclusion, these studies show that whole-body vibration reduced age-related increases in fat mass in mature rats. This effect occurred without changes in lean body mass, bone mass or muscle mass and function and without alterations in food intake. These findings suggest that whole-body vibration exercise suppresses the accumulation of fat in young adult rats. Further studies are necessary to determine whether whole-body vibration can prevent fat accumulation in humans. If successful, this research could have implications in cases such as preventing childhood obesity and other metabolic disorders associated with weight gain.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. J Am Med Assoc. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. Centers for Disease Control and Prevention Web site. National Health and Nutrition Examination Survey Data. [October 17, 2007. Accessed October 17, 2007]; http://www.cdc.gov/nchs/nhanes.htm.
- 3.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. J Am Med Assoc. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 4.Slyper AH. The pediatric obesity epidemic: causes and controversies. J Clin Endocrinol Metab. 2004;89:2540–2547. doi: 10.1210/jc.2003-031449. [DOI] [PubMed] [Google Scholar]
- 5.Stein CJ, Colditz GA. The epidemic of obesity. J Clin Endocrinol Metab. 2004;89:2522–2525. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]
- 6.American Dietetic Association (ADA) Position of the American Dietetic Association: individual-, family-, school-, and community-based interventions for pediatric overweight. J Am Diet Assoc. 2006;106:925–945. doi: 10.1016/j.jada.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Stice E, Shaw H, Marti CN. A meta-analytic review of obesity prevention programs for children and adolescents: the skinny on interventions that work. Psychol Bull. 2006;132:667–691. doi: 10.1037/0033-2909.132.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Styne DM. A plea for prevention. Am J Clin Nutr. 2003;78:199–200. doi: 10.1093/ajcn/78.2.199. [DOI] [PubMed] [Google Scholar]
- 9.Jakicic JM, Otto AD. Physical activity considerations for the treatment and prevention of obesity. Am J Clin Nutr. 2005;82(1 Suppl):226S–229S. doi: 10.1093/ajcn/82.1.226S. [DOI] [PubMed] [Google Scholar]
- 10.Catenacci VA, Wyatt HR. The role of physical activity in producing and maintaining weight loss. Nat Clin Pract Endocrinol Metab. 2007;3:518–529. doi: 10.1038/ncpendmet0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rittweger J, Ehrig J, Just K, Mutschelknauss M, Kirsch KA, Felsenberg D. Oxygen uptake in whole-body vibration exercise: influence of vibration frequency, amplitude, and external load. Int J Sports Med. 2002;23:428–432. doi: 10.1055/s-2002-33739. [DOI] [PubMed] [Google Scholar]
- 12.Rittweger J, Schiessl H, Felsenberg D. Oxygen uptake during whole-body vibration exercise: comparison with squatting as a slow voluntary movement. Eur J Appl Physiol. 2001;86:169–173. doi: 10.1007/s004210100511. [DOI] [PubMed] [Google Scholar]
- 13.Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci USA. 2007;104:17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapses SA, Riedt CS. Bone, body weight, and weight reduction: what are the concerns? J Nutr. 2006;136:1453–1456. doi: 10.1093/jn/136.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 16.Pouilles JM, Tremollieres F, Saint-Martin F, Ribot C. [Postmenopausal bone loss: results of a topographic study by X-ray absorptiometry] Rev Rhum Ed Fr. 1993;60:891–896. [PubMed] [Google Scholar]
- 17.Flieger J, Karachalios T, Khaldi L, Raptou P, Lyritis G. Mechanical stimulation in the form of vibration prevents postmenopausal bone loss in ovariectomized rats. Calcif Tissue Int. 1998;63:510–514. doi: 10.1007/s002239900566. [DOI] [PubMed] [Google Scholar]
- 18.Fritton JC, Rubin CT, Qin YX, McLeod KJ. Whole-body vibration in the skeleton: development of a resonance-based testing device. Ann Biomed Eng. 1997;25:831–839. doi: 10.1007/BF02684167. [DOI] [PubMed] [Google Scholar]
- 19.Rubin C, Pope M, Fritton JC, Magnusson M, Hansson T, McLeod K. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine. 2003;28:2621–2627. doi: 10.1097/01.BRS.0000102682.61791.C9. [DOI] [PubMed] [Google Scholar]
- 20.Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J. 2001;15:2225–2229. doi: 10.1096/fj.01-0166com. [DOI] [PubMed] [Google Scholar]
- 21.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66:397–402. [PubMed] [Google Scholar]
- 22.Rubin CT, McLeod KJ. Promotion of bony ingrowth by frequency-specific, low-amplitude mechanical strain. Clin Orthop Relat Res. 1994:165–174. [PubMed] [Google Scholar]
- 23.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 24.Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res. 2004;19:352–359. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 25.Roelants M, Delecluse C, Verschueren SM. Whole-body-vibration training increases knee-extension strength and speed of movement in older women. J Am Geriatr Soc. 2004;52:901–908. doi: 10.1111/j.1532-5415.2004.52256.x. [DOI] [PubMed] [Google Scholar]
- 26.LaMothe JM, Zernicke RF. Rest insertion combined with high-frequency loading enhances osteogenesis. J Appl Physiol. 2004;96:1788–1793. doi: 10.1152/japplphysiol.01145.2003. [DOI] [PubMed] [Google Scholar]
- 27.Widrick JJ, Fuchs R, Maddalozzo GF, Marley K, Snow C. Relative effects of exercise training and alendronate treatment on skeletal muscle function of ovariectomized rats. Menopause. 2007;14(3 Pt 1):528–534. doi: 10.1097/01.gme.0000227861.35226.fa. [DOI] [PubMed] [Google Scholar]
- 28.Thomsen JS, Laib A, Koller B, Prohaska S, Mosekilde L, Gowin W. Stereological measures of trabecular bone structure: comparison of 3D micro computed tomography with 2D histological sections in human proximal tibial bone biopsies. J Microsc. 2005;218(Pt 2):171–179. doi: 10.1111/j.1365-2818.2005.01469.x. [DOI] [PubMed] [Google Scholar]
- 29.Dedeoglu EN, Erenus M, Yoruk P. Effects of hormone therapy and tibolone on body composition and serum leptin levels in postmenopausal women. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 30.Scholze A, Tepel M. Role of leptin in reverse epidemiology in chronic kidney disease. Semin Dial. 2007;20:534–538. doi: 10.1111/j.1525-139X.2007.00334.x. [DOI] [PubMed] [Google Scholar]
- 31.Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8:395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 32.Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–1474. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 33.Gusi N, Raimundo A, Leal A. Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: a randomized controlled trial. BMC Musculoskelet Disord. 2006;7:92. doi: 10.1186/1471-2474-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 35.Zarrabeitia MT, Hernandez JL, Valero C, Zarrabeitia A, Amado JA, Gonzalez-Macias J, et al. Adiposity, estradiol, and genetic variants of steroid-metabolizing enzymes as determinants of bone mineral density. Eur J Endocrinol. 2007;156:117–122. doi: 10.1530/eje.1.02318. [DOI] [PubMed] [Google Scholar]
- 36.Ballor DL, Poehlman ET. Exercise-training enhances fat-free mass preservation during diet-induced weight loss: a meta-analytical finding. Int J Obes Relat Metab Disord. 1994;18:35–40. [PubMed] [Google Scholar]
- 37.Bosco C, Iacovelli M, Tsarpela O, Cardinale M, Bonifazi M, Tihanyi J, et al. Hormonal responses to whole-body vibration in men. Eur J Appl Physiol. 2000;81:449–454. doi: 10.1007/s004210050067. [DOI] [PubMed] [Google Scholar]
- 38.Kvorning T, Bagger M, Caserotti P, Madsen K. Effects of vibration and resistance training on neuromuscular and hormonal measures. Eur J Appl Physiol. 2006;96:615–625. doi: 10.1007/s00421-006-0139-3. [DOI] [PubMed] [Google Scholar]
- 39.Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jørgensen K, et al. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab. 1994;78:744–752. doi: 10.1210/jcem.78.3.8126152. [DOI] [PubMed] [Google Scholar]