Abstract
Enteric nervous system (ENS) precursors undergo a complex process of cell migration, proliferation, and differentiation to form an integrated network of neurons and glia within the bowel wall. Although retinoids regulate ENS development, molecular and cellular mechanisms of retinoid effects on the ENS are not well understood. We hypothesized that retinoids might directly affect ENS precursor differentiation and proliferation, and tested that hypothesis using immunoselected fetal ENS precursors in primary culture. We now demonstrate that all retinoid receptors and many retinoid biosynthetic enzymes are present in the fetal bowel at about the time that migrating ENS precursors reach the distal bowel. We further demonstrate that retinoic acid (RA) enhances proliferation of subsets of ENS precursors in a time dependent fashion and increases neuronal differentiation. Surprisingly, however, enteric neurons that develop in retinoid deficient media have dramatically longer neurites than those exposed to RA. This difference in neurite growth correlates with increased RhoA protein at the neurite tip, decreased Smurf1 (a protein that targets RhoA for degradation), and dramatically decreased Smurf1 mRNA in response to RA. Collectively these data demonstrate diverse effects of RA on ENS precursor development and suggest that altered fetal retinoid availability or metabolism could contribute to intestinal motility disorders.
Keywords: Enteric nervous system, vitamin A, neurite growth, retinoids, ubiquitin ligase
Introduction
The enteric nervous system (ENS) controls most aspects of intestinal function including intestinal motility, blood flow, and epithelial secretion. The ENS also responds to sensory stimuli from the bowel wall and gut lumen. To perform these functions there are estimated to be 500 million enteric neurons in 20 functional classes in humans (Furness, 2006). All of these neurons, and their supporting glia are derived from a small group of neural crest cells that originate in the vagal, sacral and upper thoracic neural tube (Gariepy, 2001; Heanue and Pachnis, 2007; Newgreen and Young, 2002a; Newgreen and Young, 2002b). These ENS precursors migrate through the fetal bowel wall, undergo sequential lineage restriction and then differentiate, but mechanisms controlling ENS precursor migration, proliferation, and differentiation remain incompletely understood. When these crest-derived precursors fail to reach the distal bowel, the aganglionic bowel tonically contracts resulting in life threatening intestinal obstruction that in humans is called Hirschsprung disease, an illness affecting 1:5000 infants (Skinner, 1996). A variety of less well understood human motility disorders called intestinal pseudo-obstruction syndromes may also result from both damage to the ENS and abnormal ENS development. For this reason, defining the molecular mechanisms of ENS precursor differentiation may provide novel methods to prevent intestinal motility disorders.
Two recent studies suggested that the active vitamin A metabolite retinoic acid may have important roles in ENS development. The first study demonstrated that excess retinoic acid during fetal development results in delayed colonization of the distal bowel by ENS precursors (Pitera et al., 2001). Retinoic acid toxicity also caused abnormal gut mesenchyme differentiation, and other abnormalities of gut looping, rotation and morphogenesis. The second study demonstrated aganglionosis of the bowel in retinaldehyde dehydrogenase 2 (Raldh2/Aldh1a2) deficient mice that had been partially “rescued” by treatment of the pregnant mothers with all-trans-retinoic acid (RA) (Niederreither et al., 2003). Because Raldh2 protein is critical for local production of RA in many regions of the developing embryo, Raldh2−/− mice remain quite abnormal even after RA supplementation including serious defects in gut mesenchyme and in other neural crest derivatives. Thus, these studies suggest that RA influences ENS precursor development, but do not demonstrate direct effects of RA on ENS precursors or define how RA signaling influences the proliferation, survival or differentiation of these cells. In particular, it is possible that the observed effects in vivo result from RA induced changes in the microenvironment through which ENS precursors migrate. In Raldh2−/− mice, it is also possible that the distal bowel aganglionosis results from defects in neural crest development that occur long before ENS precursors reach the bowel.
To determine if RA has any direct effects on ENS precursors, we have used immunoselection to separate crest-derived cells in the fetal gut from other cells within the gut wall and then maintained these ENS precursors in low density culture with or without added RA. These studies demonstrate significant effects on ENS precursor proliferation, neuronal differentiation, and neurite extension in vitro that suggest that RA influences many aspects of ENS development via direct effects on ENS precursors that are independent of retinoid induced changes in gut mesenchyme differentiation or RA effects on earlier stages of neural crest development. Furthermore, our studies demonstrated unexpectedly that while RA increases neuronal differentiation, it also reduces neurite growth from ENS precursors. This reduction in neurite growth correlates with a RA induced reduction in Smurf1 protein and mRNA abundance and an increase in RhoA protein levels in developing enteric neurons. Collectively these data demonstrate complex effects on many aspects of ENS development suggesting that vitamin A deficiency or excess could cause defective ENS development and contribute to human motility disorders.
Methods
Primary culture of non-selected and immunoselected ENS precursors
E12.5 CF-1 mouse stomach, small bowel, and colon were dissociated with collagenase (0.5 mg/mL) and dispase (0.5 mg/mL) to yield a single cell suspension. Cells were filtered through a 40 μm cell strainer (BD Falcon). Non-selected dissociated gut cells were plated at 10,000 cells/well in poly-D-lysine/laminin coated 8-well chamber slides (Biocoat, Fisher) and grown in Neurobasal medium plus vitamin A deficient B27 supplement (Invitrogen), glial cell line-derived neurotrophic factor (GDNF, 50ng/mL) and 2 mM L-glutamine (Invitrogen). To immunoselect enteric neural crest-derived cells, dissociated gut cells were exposed to p75NTR antibody (Chemicon, 1:1000) in vitamin A deficient B27 supplemented Neurobasal medium followed by incubation with goat anti-rabbit coupled paramagnetic beads (Miltenyi Biotec GmbH, 1:50, 1 hour, 4°C) before separating neural crest-derived cells from unselected cells with a positive selection column (MACS Separation columns, Miltenyi Biotec GmbH). Immunoselected cells were plated at 1000 cells/well using the same conditions as unselected cells. This low cell density was used to minimize cell-cell interaction and to maintain approximately the same number of crest-derived cells as in the non-selected cultures. In all the experiments, factors tested were added to the medium at the time of plating. Experimental sets were as follows: (a) defined RA-free medium plus 10−6 M all-trans retinoic acid (RA) (Sigma), (b) defined RA-free medium only, or (c) defined RA-free medium plus 10−5 M pan-retinoic acid receptor inhibitor (BMS493, generously provided by Dr. Chris Zusi at Bristol-Myers Squibb). RA and BMS493 were dissolved in ethanol to prepare stock solutions. One μl of each stock solution was added to 10 mL of medium. Control cultures also had 1 μL of ethanol per 10 mL of medium. Cells were maintained in a humidified environment with 5% CO2 at 37°C for 2 days or 7 days. For prolonged culture, medium was changed every 2 days by withdrawing half of the medium and adding new medium. For proliferation analysis, bromodeoxyuridine (BrdU, 10 μmol/L final concentration) was added to cells 5 h before fixation. Cells were washed with phosphate buffered saline (PBS) and fixed in 4% paraformaldehyde (room temperature, 25 min) for immunohistochemical analysis. All experiments were performed in triplicate.
Immunohistochemistry and image analysis
After fixation, cells were washed with PBS, blocked with 4% normal donkey serum in TBST (Tris-buffered saline plus 0.1% Triton X-100) (1 h, 37°C) and then incubated in primary antibody (4°C overnight). Primary antibodies include: Goat anti-RET (1:100; Neuromics), rabbit TuJ1 (1:500) and mouse TuJ1 (1:100; Covance), rabbit anti-S100β(1:500; Dako), anti-RhoA (sc-179, 1:50), anti-Smurf1 (sc-25510, 1:50), anti-PI 3-kinase p85α (B-9) (sc-1637, 1:50), anti-Cdc42 (P1) (sc-87, 1:50; Santa Cruz Biotechnology), rabbit anti-phospho-PKCζ (p-PKCζ), anti-phospho-GSK3β (p-GSK3β) (1:100; Cell Signaling Technology) and rabbit anti-Raldh2 antibody (kind gift of Dr. Peter McCaffery (University of Aberdeen) (Berggren et al., 1999; Moss et al., 1998). Antibody binding was visualized with Alexa Fluor 350-, 488-, 546- and 594-conjugated anti-mouse, anti-goat and anti-rabbit secondary antibodies (1:250; 25°C, 1 h, Invitrogen). For BrdU labeling, cells were treated with 4 N HCl (25°C, 10 min) either before primary antibody (Ret/TuJ1/BrdU labeling) or after secondary antibody (S100β/BrdU labeling). BrdU labeling was then visualized after PBS washing, by incubation with Alexa Fluor 488-conjugated anti-BrdU antibody (1 : 20, 37°C , 45 min, Invitrogen). Apoptotic cells were analyzed using an In Situ Cell Death Detection kit (Fluorescein) (Roche) following the manufacturer's protocol. Images were obtained with an Olympus BX60 microscope, an Axiocam digital camera and AxioVision imaging software (Zeiss) using identical exposure times. Neurite length was measured by using NIH ImageJ 1.36. Cell counts were obtained manually by using a counting grid and a 20X objective.
In situ hybridization
Wild type CF-1 mice were perfused with cold 4 % paraformaldehyde (PFA), post-fixed overnight at 4°C and then frozen in OCT before sectioning at 14 μm thickness. Slides were warmed to 25°C, baked 15 minutes at 50°C and then fixed again in 4 % PFA for 20 min at 25°C. After washing twice in diethylpyrocarbonate treated phosphate buffered saline (PBS-DEPC, 10 mM) for 5 minutes, tissues were digested in Proteinase K (25 μg/mL for E14 samples and 10 μg/mL for E12 samples) for 8−13 minutes in (50 mM Tris (pH 7.5), 5 mM EDTA, DEPC treated water). Slides were then washed again in PBS-DEPC (2 × 5 minutes), incubated in 4 % PFA for (15 minutes, 25°C), and rinsed in DEPC treated water. Tissues were then blocked with 0.2 % acetic anhydride/0.1 M triethanolamine (10 minutes, 25°C), washed in PBS-DEPC (5 minutes, 25°C), and pre-hybridized for 1hr at 65°C in pre-hybridization solution (50 % formamide, 5× SSC, 1 mg/ml Yeast tRNA, 100 μg/ml Heparin, 1× Denhardt's Solution, 0.1 % Tween 20 (Sigma P-1379), 0.1 % CHAPS (Sigma C-3023), 5 mM EDTA pH 8.0). Riboprobes (2 ng/mL final concentration, prepared using a MAXIscript kit from Ambion) were then added to fresh pre-hybridization solution, slides were covered with coverslips, and tissues were hybridized overnight at 65°C in humidified chamber. Following hybridization, tissues were washed in 1× SSC/50% formamide at 65°C (3 × 30minutes), then twice in PBT (10 mM PBS with 0.1% Triton X-100 and 2mg/mL BSA) for 20 min at 25°C, and then blocked with PBT/20 % NSS (normal sheep serum) for 1hr at 25°C. Hybridized probe was detected after incubation (overnight, 4°C) with an anti-digoxigenin antibody conjugated to alkaline phosphatase (Roche, 1:2000) in fresh blocking solution (PBT/20 % NSS). During the detection step for some E12 samples, we also used the TSA Plus DNP System (Perkin Elmer) according to the manufacturer's instructions. Slides were then washed in PBT (3 × 30min, 25°C) followed by washing once in alkaline phosphatase (AP) buffer (100 mM Tris pH 9.5, 50 mM MgCl2, 100 mM NaCl, 0.1 % TWEEN 20) with levamisole (5 mM, DakoCytomation) for 5 min and once in AP buffer without levamisole. Finally slides were incubated in AP buffer with 3.5 μL/mL BCIP (0.35 % final concentration) and 1.5 μL/mL NBT (0.15 % final concentration) for 1−3 days in dark at 4°C, or until desired stain is attained. Plasmids used to generate probes for Rarb (exons 3−9), Rxrb (exons 1−10), Rxrg (exons 1−10) and Cyp26c1 (exons 4−5) were generously provided by Dr. Pierre Chambon (IGBMC (Institut de Génétique et de Biologie Moléculaire et Cellulaire), INSERM (National Institute of Health and Medical Research), CNRS (Centre National de la Recherche Scientifique)). Plasmids for Raldh2 and Raldh3 were generously provided by Dr. Ursula Drager (University of Massachusetts). For the other genes, cDNA was cloned via PCR and used as a template for riboprobe preparation. Primers used to isolate these cDNA are listed as follows. Rara (exons 7−9), 5’ – TCTCCCTGGACATTGACCTC and 5’ – ATGCTCCGAAGGTCTGTGAT; Rarg (exons 4−9), 5’ – CTCGGGTCTATAAGCCATGC and 5’ – CATAGCCAGCATTGTGCATC; Rxra (exons 4−9), 5’ -GCTCACCAAATGACCCTGTT and 5’ - GAAGAACCAGGTTGCTCCAG; Raldh1(exons 1−2), 5’ – CATGCAAGGGTGCCTTTATT and 5’ – CTGTCCCTCAGTGACTCCTTG; Cyp26a1(exons 1−4), 5’ – CTGGGCGGCCTTATAAAGAG and 5’ – CGAAATGTTCTCCTCGATGC; Cyp26b1(exons 4−5), 5’ – TCAATGTGCCCAAGATCCTA and 5’ – AGCCTTCTGTAGGCCCTTCT. Sense and anti-sense riboprobes were prepared for all in situ hybridization studies and no signal was detected for any of the sense controls (Supplemental Figure 1).
β-Galactosidase histochemistry
For whole mount staining, tissues were fixed for 30 min on ice with 0.2 % glutaraldehyde in 100 mM phosphate buffer (pH 7.4) (PBS) plus 2 mM MgCl2, before washing twice (5 min each) in PBS plus 2 mM MgCl2. The blue precipitate was generated by incubation in the dark at 37°C in X-gal buffer (100 mM phosphate buffer (pH 7.4), 2 mM MgCl2, 0.01 % sodium deoxycholate, 0.02 % Nonidet P40, 5 mM potassium-ferricyanide, 5 mM potassium-ferrocyanide, and 1 mg/mL of X-Gal (5-bromo-4-chloro-3-indolyl- beta-D-galactopyranoside)). For sections, stained whole gut samples were frozen in OCT and 14 μm sections were cut with a cryostat.
Polymerase chain reaction (PCR) based analysis of gene expression
The following primers were designed to generate short amplicons (50−100 bp, Tm about 60°C) and were synthesized by Integrated DNA Technologies Inc.:
RhoA: 5’-GAATGACGAGCACACGAGAC-3’ and 5’-GTACCCAAAAGCGCCAATCC-3’;
Smurf1: 5’-CACTGGCTACCAGCGTTTG3’ and 5’-CCTATTCTGTCTCGGGTCTGTAA-3’.
Rara: 5’-TTCTTTCCCCCTATGCTGGGT-3’ and 5’-GGGAGGGCTGGGTACTATCTC-3’.
Rarb: 5’-TCCTGGGAGTTGGTGATGTC-3’ and 5’-TCGGAGCAGCTCACTTCCTA-3’.
Rarg: 5’-ATGTACGACTGCATGGAATCG-3’ and 5’-CCAGTGGCTCTGCGTAGTAA-3’.
Rxra: 5’-ATGGACACCAAACATTTCCTGC-3’ and 5’-CCAGTGGAGAGCCGATTCC-3’.
Rxrb: 5’-GCAGCCCAAATGACCCAGT-3’ and 5’-GGAGAGGGACCGATCAAAGAT-3’.
Rxrg: 5’-CATGAGCCCTTCAGTAGCCTT-3’ and 5’-CGGAGAGCCAAGAGCATTGAG-3’.
Raldh1: 5’-ATACTTGTCGGATTTAGGAGGCT-3’ and 5’-GGGCCTATCTTCCAAATGAACA-3’.
Raldh2: 5’-CAGAGAGTGGGAGAGTGTTCC-3’ and 5’-CACACAGAACCAAGAGAGAAGG-3’.
Raldh3: 5’-GGGTCACACTGGAGCTAGGA-3’ and 5’-CTGGCCTCTTCTTGGCGAA-3’.
Cyp26a1: 5’-AAGCTCTGGGACCTGTACTGT-3’ and 5’-CTCCGCTGAAGCACCATCT-3’.
Cyp26b1: 5’-TCATCGGAGAGACTGGTCACT-3’ and 5’-GGTGCTCACTAGCTGGTGTTC-3’.
Cyp26c1: 5’-GCGTAGTCAAGGAGGTGCTG-3’ and 5’-AGCCTTTGGGGATCTGGTAA-3’.
For each gene, analysis was performed in triplicate and control reactions were performed omitting reverse transcriptase from the cDNA synthesis. For RhoA and Smurf1, quantitative real time reverse transcriptase PCR (qRT-PCR) studies were performed with SYBR green PCR Master mix (Applied Biosystems) and the iCycler iQ (Bio-Rad). The mRNA template for reverse transcription was obtained from E12.5 CF-1 p75NTR+ immunoselected gut cells that had been maintained for 48 h in culture with or without RA as described above (n = 3 samples for each condition). The RNA content of samples was normalized based on Gapdh (glyceraldehyde-3-phosphate dehydrogenase) amplification. Gapdh primers: 5’-AACTTTGGCATTGTGGAAGG-3’ and 5’-GGATGCAGGGATGATGTTCT-3’. The threshold cycle (CT value) at which a significant increase in PCR product is first detected, was recorded. ΔCT=CT of gene of interest minus CT of GAPDH. Fold change was estimated based on the assumption that each cycle difference corresponds to a two fold change in mRNA abundance, an assumption we recently validated for a number of genes (Vohra et al., 2006). For the other genes, semi-quantitative PCR was performed using cDNA templates prepared from freshly isolated p75NTR expressing immunoselected ENS precursors and non-selected cells.
Quantitative analyses
All of our studies were performed at least in triplicate. For absolute cell counts, every cell in the culture well was counted after immunohistochemical staining. For data presented as percentages, and for neurite length studies, the number of cells analyzed is indicated in the figure legend. For studies of Smurf1 and RhoA, estimates of protein abundance were performed by digital image analysis of pixel intensity measurements because we needed to evaluate protein abundance in a subset of cultured cells or in specific regions of the cell. Images were obtained as described above and care was taken to avoid fluorescence photobleaching and to ensure that each image was obtained using identical exposure and illumination conditions. Average pixel intensity measurements were obtained using Photoshop 7.0 to measure the intensity of a four pixel area in the cell body, neurite shaft and neurite tip (n=75 neurites for each condition). Background pixel intensity was subtracted from the measured values.
Statistical analyses
Statistical analysis was performed using SigmaStat software (Systat Software). Either t-test or Mann–Whitney rank sum test were employed. Data are presented as mean ± SE. p < 0.05 was considered significant.
Results
RA increases the number of Ret immunoreactive cells in culture
To determine the cellular mechanisms through which RA influences ENS development, we dissociated E12.5 CF-1 mouse gut and maintained cells in culture. We selected this time point for analysis because enteric neural crest-derived cells (NCC) at this time are a heterogeneous population that includes proliferating and differentiating precursors for both neurons and glia. In addition, to determine if RA affects ENS precursors directly or indirectly, we used immunoselection with an antibody to p75NTR to separate NCC from other cells in the gut (Wu et al., 1999) or grew NCC in mixed gut cultures without immunoselection. The immunoselection procedure isolated 12.0 +/− 0.8 % of the total cell population from the bowel consistent with the work of other investigators, and 89.0 +/− 1.3 % of these immunoselected cells were p75NTR+ as evaluated by immunohistochemistry two hours after plating. Antibody staining also demonstrated that 50.8 +/− 3.5 % of the immunoselected cells were Ret+ two hours after plating consistent with prior data demonstrating that some p75NTR expressing cells in the bowel at this age are Ret negative (Young et al., 1999). Thus, the immunoselection procedure generates a highly enriched, but not pure population of crest-derived cells.
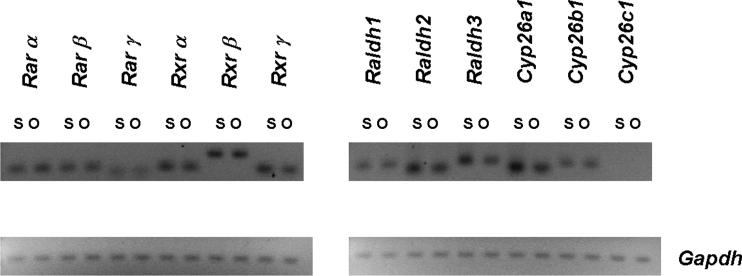
Retinoid responsiveness requires the expression of Rar and Rxr receptors (Blomhoff and Blomhoff, 2006) and the presence of RA. In vivo, RA is produced locally in tissues from retinaldehyde via the activity of retinaldehyde dehydrogenases (Raldh1−3). Local degradation of RA via Cyp26a1-c1 enzymes is also thought to be important for limiting retinoid action. As an initial experiment to determine if ENS precursors are likely to respond directly to RA, we performed PCR for retinoid receptors and retinoic acid metabolizing enzymes using cDNA derived from our immunoselected cells and the cells that were not selected with p75NTR antibody as template. These studies suggested that many of the genes required to produce, degrade and respond to RA were expressed in our immunoselected cells (Figure 1) including all of the Rar and Rxr receptors, Raldh1−3, Cyp26a1 and Cyp26b1. Interestingly these genes were also expressed in the non-selected cell pool suggesting that RA might have both direct and indirect effects on ENS precursor development.
Figure 1.
Many of the genes required to respond to RA and to synthesize or degrade RA are expressed in p75NTR antibody immunoselected cells from the E12.5 CF-1 mouse bowel. They are also expressed in the residual cells that were not selected with p75NTR antibody. Reverse transcriptase reactions were performed on freshly isolated cells to generate the cDNA templates for these studies. PCR amplification of Gapdh was used as a control to demonstrate similar levels of template abundance in the analyzed samples. With the exception of Cyp26c1, all of the genes investigated were expressed in the selected and non-selected cell populations. S = selected cells. O = “other cells” from the fetal bowel that were not immunoselected.
To evaluate the effect of RA on ENS precursors, we established cultures of E12.5 mouse gut cells obtained via p75NTR antibody immunoselection. In parallel we also analyzed total gut dissociated cell cultures from the same time point. Immunoselected cells were plated at low density so that most cells were not in contact at the time of plating. This strategy reduces cell-cell interaction and facilitates evaluation of RA effects on neurite growth. Mixed cell cultures were plated at 10-fold higher density so that both sets of cultured cells had the approximately the same number of ENS precursors at the time of plating. Cells were maintained in culture for 48 hr or for seven days in media containing RA or in RA-free media. We were also concerned about the possibility that our cultures might contain endogenous RA even after dissociation and dilution in media given the expression of Raldh1−3 genes in our cultured cells. To address this possibility, we included as a control, additional cultures with RA-free media and added BMS493, a pan-retinoic acid receptor antagonist that completely blocks all RAR isoforms (α, β, γ) and has been used by other investigators in both cell and organ culture experiments to evaluate RAR function (Chazaud et al., 2003; Hochgreb et al., 2003). As detailed below, results obtained in cultures containing BMS493 and in RA-free media without BMS493 were identical for all experiments (Figures 2-6) consistent with the idea that RA abundance is very low in these dissociated cell cultures in the absence of added RA. Control experiments confirmed that BMS493 reduced the effect of added RA in these cultures (data not shown). In addition, all cultures contained glial cell line-derived neurotrophic factor (GDNF) a potent promoter of ENS precursor survival, proliferation and neurite growth (Chalazonitis et al., 1998; Hearn et al., 1998; Heuckeroth et al., 1998).
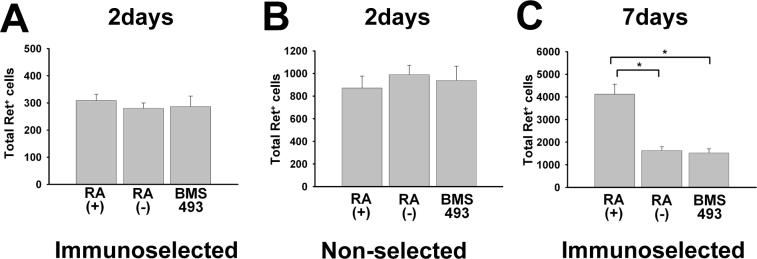
Figure 2.
Retinoic acid (RA) increases Ret+ cells in culture after seven days, but this is not evident after 48 hours. E12.5 CF-1 mouse ENS precursors were maintained in culture either after immunoselection with an antibody to p75NTR (A, C) or without prior immunoselection (i.e., non-selected total gut culture) (B). ENS precursors were grown for 48 hr (A, B) or 7 days (C) in culture in the presence of GDNF plus RA, GDNF without RA, or GDNF plus BMS493. (A, B) There are no significant differences between any of these conditions after 48 hours in culture. (C) Quantitative analysis of total Ret+ cells after seven days demonstrated more Ret+ cells in RA treated cultures. N = three experiments/six separate wells. All Ret+ cells in each culture well were counted. *P < 0.01.
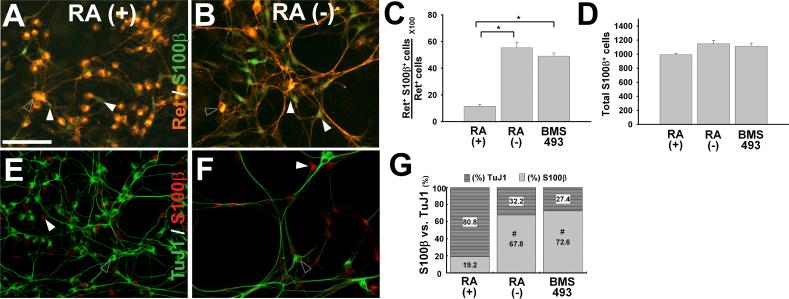
Figure 6.
RA enhances neuronal lineage commitment but does not reduce glial lineage marker expression. E12.5 CF-1 mouse immunoselected (p75NTR expressing) ENS precursors were grown for seven days (A-G) in the presence of GDNF plus RA, GDNF without RA (−), or GDNF plus BMS493. Representative images of Ret and S100β (A, B), or TuJ1 and S100β (E,F) immunohistochemistry are shown. (C, D) Quantitative analysis of S100β expression in Ret+ cells was performed by counting all Ret+ and S100β+ cells in each well. These analyses demonstrated that RA reduces the percentage of Ret+ S100β+ cells (C), but does not reduce the total number of S100β+ cells per well. For these studies, all S100β+ cells in each well were counted (D). (G) Quantitative analysis of the ratio of S100β+ to TuJ1 immunoreactive cells was also performed by counting all TuJ1+ and S100β+ cells in each well. (A, B) Open arrowheads point to Ret+ cells. (E, F) Open arrowheads point to TuJ1+ cells. In all images, white arrowheads point to S100β cells. At least 1800 S100β+ cells per condition were analyzed from a total 3 separate experiments. *P < 0.01. Scale bars, 100 μ m.
To begin to investigate the effect of RA on ENS precursor development, we first determined the number of Ret immunoreactive cells in the cultures at each time point. Recognizing that ENS precursors become more intensely Ret+ as they differentiate into neurons and lose Ret expression as they differentiate into glia (Heuckeroth et al., 1998; Young et al., 1999), we counted both strongly and weakly Ret antibody immunoreactive cells together as Ret+ cells. Many other cells in the culture dish were clearly Ret negative. These studies demonstrated the same number of Ret+ cells/well with or without added RA after 48 hours. Similar results were obtained with and without immunoselection after 48 hours (Figure 2A, B), but more Ret+ cells were present in the total gut cell cultures (i.e., non-selected cells) than in the immunoselected cell cultures. The etiology of this difference is not known, but given the data below, may reflect a difference in initial cell viability or adhesion to the plates after immunoselection. After 7 days of culture, however, there was a significant increase in the number of Ret+ cells in immunoselected cultures maintained in the presence of RA compared to cells cultured without RA (P = 0.002) or in the presence of BMS493 (P < 0.001) (Figure 2C). Unfortunately, because of high cell densities and cell aggregation, we could not obtain reliable quantitative data for total Ret+ cell counts from non-selected cells after 7 days in culture, so those data are not presented. Collectively these data demonstrate that culture of ENS precursors in the presence of RA leads to an increase in the number of Ret immunoreactive cells per well, but the mechanism for this effect needed additional investigation.
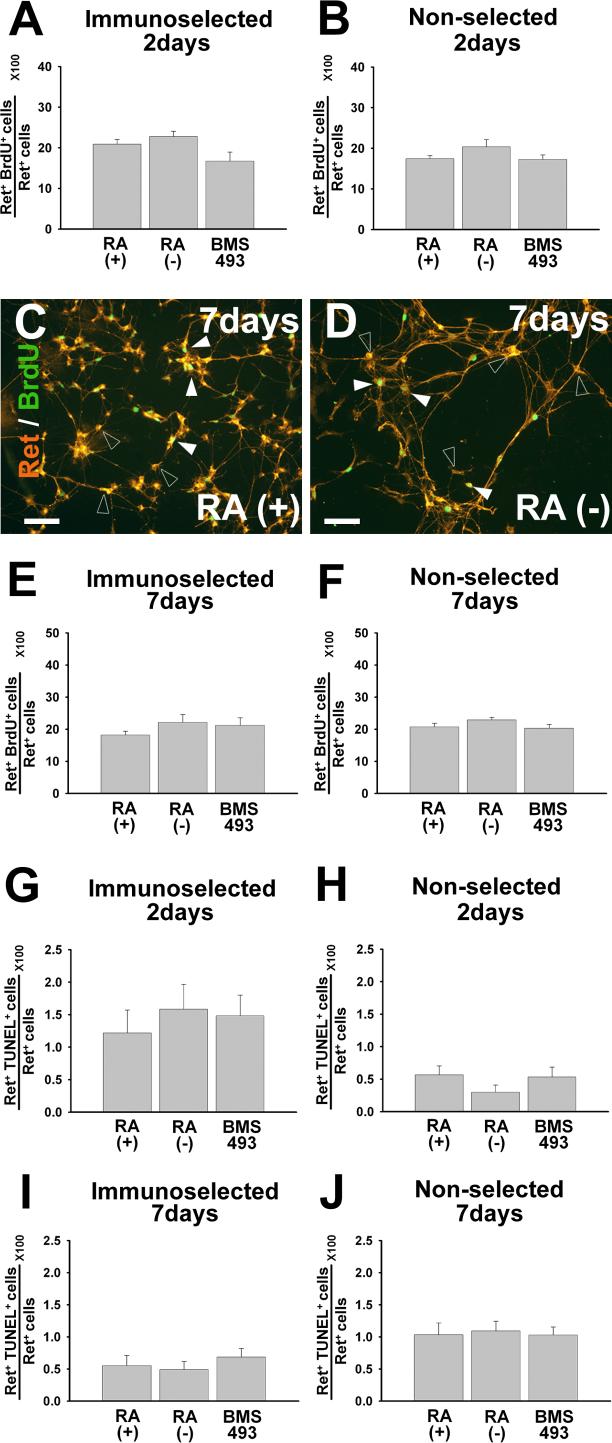
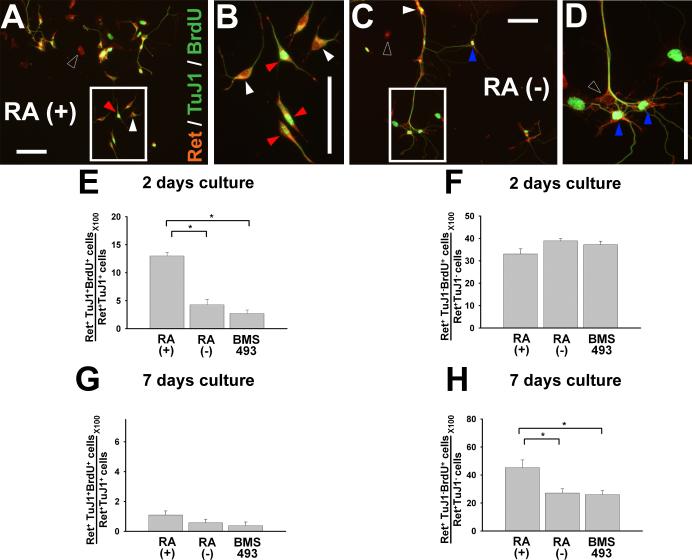
We initially hypothesized that the increased number of Ret+ cells per well after RA treatment reflected increased proliferation of Ret+ cells. We therefore evaluated cell proliferation after 48 hours or seven days in culture using BrdU incorporation and Ret immunohistochemistry. Cells were grown in the presence of: 1) GDNF plus RA, 2) GDNF without RA, or 3) GDNF plus BMS493. We evaluated the proliferation of Ret+ cells using both immunoselected and non-selected culture conditions. These studies failed to demonstrate any significant difference in the rate of Ret+ cell proliferation under any of the conditions tested (Figure 3A-F). We therefore determined if RA deficiency increased death of Ret+ cells in culture using Ret/TUNEL double label immunohistochemistry. Under all conditions tested, the rate of cell death was low (< 2 %) and there was no difference in the percentage of Ret+/TUNEL+ cells under any of the conditions tested (e.g. see Figure 3G-J). Collectively these data suggest the possibility that culture in RA deficient media results in a reduction in Ret+ cells/well via loss of Ret expression, but does not exclude the possibility that RA influences the proliferation of subsets of Ret+ cells.
Figure 3.
RA does not dramatically affect proliferation or death rates of Ret+ cells in culture when all Ret expressing cells are analyzed together. E12.5 CF-1 mouse p75NTR+ immunoselected (A, C, E, G, I) or non-selected (B, D, F, H, J) ENS precursors were maintained in culture for 48 hours or seven days before analysis. Cells were grown in the presence of GDNF plus RA, GDNF without RA, or GDNF plus BMS493. (C, D) Cultured cells were stained with antibodies to Ret and BrdU. Closed arrowheads point to Ret and BrdU double positive cells. Open arrowheads point to Ret expressing/BrdU negative cells. (A, B, E, F) Quantitative analysis of total Ret+ BrdU+ cells did not demonstrate any effect of RA on cell proliferation. (G-J) Quantitative analysis of total Ret+ TUNEL+ cells did not demonstrate any difference in cell death in response to RA. N = three experiments/six separate wells. At least 600 cells for each experiment were analyzed. *P < 0.01. Scale bars, 100 μm.
RA promotes proliferation of Ret+TuJ1+ cells after 48 hours in culture and increases proliferation of Ret+TuJ1− cells after seven days in culture
Because NCC isolated from the gut are not a homogeneous cell population, we hypothesized that RA might selectively affect the proliferation of only a subset of ENS precursors in culture, but that this effect might not be detected when all Ret+ cells are evaluated simultaneously. Specifically, we hypothesized that RA might differentially affect the proliferation of Ret+TuJ1+ and Ret+TuJ1-cells. TuJ1 antibody recognizes neuron specific β-III tubulin and has been used as a marker for committed neuronal precursors in the ENS. To test this hypothesis, E12.5 CF-1 mouse immunoselected ENS precursors were grown in culture for 48 hr in the presence of GDNF plus RA, GDNF without RA, or GDNF plus BMS493. Cultures were stained with Ret, TuJ1 and BrdU antibodies and analyzed for the proliferation of Ret+TuJ1+ and Ret+TuJ1− cells (Fig. 4A-F). These studies demonstrated that after 48 hours in culture, Ret+TuJ1+ cells proliferate more vigorously when maintained in RA containing media, than in RA deficient media or in media with BMS493 (P<0.001) (Fig. 4E). In contrast, the rate of proliferation of Ret+TuJ1− cells is not significantly affected by RA after 48 hours in culture (Figure 4F). Also note that with or without RA, TuJ1+ cells (Figure 4E) proliferate at a lower rate than TuJ1− cells (Figure 4F) consistent with the observation that cells tend to exit the cell cycle as they differentiate. Moreover, after seven days in culture, even in the presence of RA, only 1 +/− 0.3% of Ret+TuJ1+ cells continue to incorporate BrdU (Figure 4G). In contrast, in our seven day cultures, 45.4 +/− 5.4% of Ret+TuJ1− cells grown in the presence of RA were BrdU+, while only 27.1 +/− 3.0% of Ret+TuJ1− cells incorporated BrdU after growth in media without RA (P < 0.01). In combination, these data suggest that RA supports proliferation of committed neuronal precursors (i.e., TuJ1+ cells) at early time points, and encourages the proliferation of Ret+TuJ1− cells at later time points. Although these data may initially appear to contradict data concerning RA effects on the proliferation of all Ret+ cells in culture (Figure 3), they must be interpreted in the context of the cell culture composition at each time point under each condition. Specifically we hypothesized that proliferating subsets of Ret+ cells were too small a component of the total, or proliferated at too low an absolute rate, to be detected when all Ret+ cells were counted simultaneously.
Figure 4.
RA markedly enhances proliferation of immunoselected Ret+ TuJ1+ double positive cells after 48 hours in culture and increases proliferation of Ret+ TuJ1− cells after seven days in culture. E12.5 CF-1 mouse immunoselected (p75NTR expressing) ENS precursors were grown for 48 hours (A-F) or 7 days (G, H) in the presence of GDNF plus RA, GDNF without RA, or GDNF plus BMS493. (A-D) Representative images are shown. B and D are magnified views of the square regions in A and C respectively. Open arrowheads point to Ret single positive cells. White arrowheads point to RET and TuJ1 double positive cells. Blue arrowheads point to Ret and BrdU double positive cells. Red arrowheads point to Ret, TuJ1 and BrdU triple positive cells. Although both BrdU and TuJ1 staining employ green fluorescence, these are easily distinguished since BrdU is a nuclear antigen and TuJ1 antibody binds a cytoplasmic antigen. Quantitative analysis of cell proliferation for Ret+TuJ1+ (E) and Ret+TuJ1− (F) cells demonstrates that RA promotes proliferation of Ret and TuJ1 double positive cells after 2 days culture, however, this effect is not maintained after 7 days culture (G). (F) In contrast, RA has no effect on proliferation in the Ret+ TuJ1− population after 2 days culture, but increases proliferation of Ret+ TuJ1− cells after seven days in culture (H). All Ret+ cells in 2 days cultures were counted. At least 1800 cells per condition were analyzed for all figures from a total of 3 separate experiments. *P < 0.01. Scale bars, 100 μm.
RA induces neuronal differentiation of Ret expressing cells and reduces neurite outgrowth
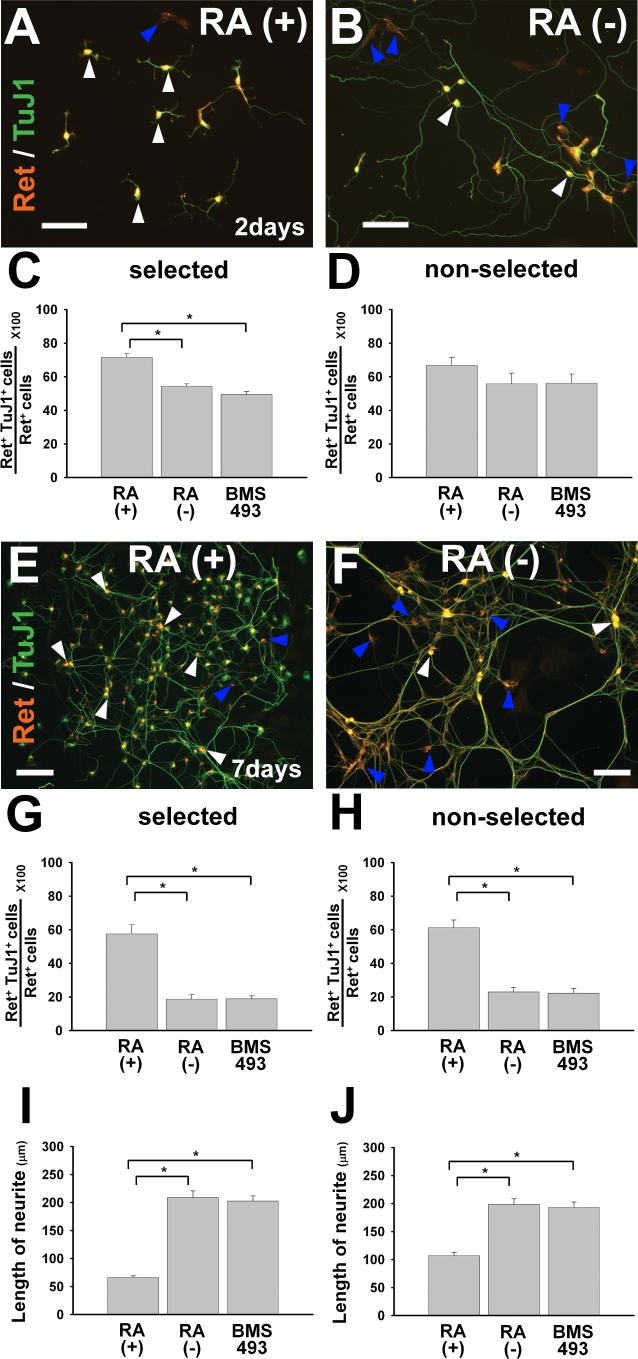
Ret is expressed in both uncommitted ENS precursors and in NCC that differentiate into enteric neurons (Young et al., 1999). Because RA increases neuronal differentiation in other neuronal lineages (Baldassarre et al., 2000; Elmi et al., 2007; Kumar et al., 2007; Scheibe et al., 1991), we hypothesized that RA might enhance neuronal differentiation in the developing ENS. To test this hypothesis, E12.5 CF-1 mouse immunoselected (p75NTR expressing) ENS precursors were grown in culture for 48 hr in the presence of GDNF plus RA, GDNF without RA, or GDNF plus BMS493 (Figure 5A-C). After immunohistochemistry, all Ret and TuJ1 immunoreactive cells were counted. These analyses demonstrated that Ret+ cells cultured in the presence of RA had a small but significantly increased likelihood of expressing neuron specific β-III tubulin (i.e., TuJ1 antibody reactivity) than cells grown without RA or with BMS493 (P<0.001) (Figure 5C). These data support the hypothesis that RA enhances neuronal differentiation of ENS precursors. To determine if other cells within the gut wall might influence RA induced neuronal differentiation, we performed non-selected NCC culture for 48 hours followed by Ret and TuJ1 immunohistochemistry (Figure 5D). In this case, the difference in the percentage of Ret+TuJ1+ cells after culture with or without RA did not reach statistical significance, suggesting the possibility that other cells within the gut might delay RA induced NCC differentiation. To test this hypothesis, we evaluated Ret and TuJ1 immunoreactivity in cultures maintained for seven days (Figure 5E-H). In this case, roughly 60% of Ret+ cells were TuJ1 immunoreactive after culture with RA independent of immunoselection. In contrast, culture without RA or with BMS493 resulted in many Ret+TuJ1− cells (approximately 80%), demonstrating that RA dramatically enhances neuronal differentiation of ENS precursors (based on TuJ1 immunoreactivity) and suggests that this effect becomes more significant with extended RA exposure.
Figure 5.
RA induces neuronal differentiation of Ret+ cells and reduces neurite outgrowth. E12.5 CF-1 mouse ENS precursors were maintained in culture after immunoselecting p75NTR expressing cells (A-C, E-G, I) or without prior immunoselection (i.e., in mixed cell culture) (D, H, J). Cultures were analyzed after 48 hours (A-D, I, J) or 7 days (E-H) in the presence of GDNF plus RA, GDNF without RA, or GDNF plus BMS493. Representative images of cells after 2 days culture (A, B) or seven days in culture are shown (E, F). White arrowheads point to Ret and TuJ1 double positive cells. Blue arrowheads point to Ret positive/TuJ1 negative cells. (C, D) Quantitative analysis of neuronal differentiation as manifest by TuJ1+ immunoreactivity in Ret+ cells demonstrated that RA increased neuronal differentiation of immunoselected cells after 2 days in culture, but did not significantly increase neuronal differentiation in mixed (non-selected) cell cultures. However, (E-H) the percentage of Ret+ cells that were TuJ1 immunoreactive was significantly increased by RA after seven days in culture with or without immunoselection. (I, J) Quantitative analysis of neurite length in selected or non-selected cells. For Ret+ TuJ1 double labeling studies, > 1800 cells were evaluated for each condition from a total of three experiments. For neurite length studies, 600 cells for each condition were analyzed from a total of 3 experiments. *P < 0.01. Scale bars, 100 μm.
Neuronal differentiation is characterized not only by TuJ1 immunoreactivity, but also by many other changes including neurite outgrowth. We had anticipated that RA, by enhancing neuronal differentiation, would also induce neurite outgrowth in cultured ENS precursors as it does in many other model neuronal systems (Clagett-Dame et al., 2006; Corcoran et al., 2000; So et al., 2006). Remarkably however, neurites from ENS precursors maintained in culture with RA appeared significantly shorter than neurites observed in cells grown without RA (Figure 5A, B). Quantitative analysis of neurite outgrowth confirmed this impression (P<0.001) (Figure 5I, J). Similar effects of RA on neurite growth were observed in both selected and non-selected NCC cultures (P<0.001), but the ability of RA to reduce neurite growth was less dramatic in mixed cell cultures than in immunoselected cells. Collectively these data suggest that there are complex effects of RA on both NCC and other cells within the bowel wall and unusual effects of RA on neurite outgrowth in these cells.
RA enhances ENS precursor neuronal lineage commitment but does not block glial lineage differentiation
Ret+ ENS precursors are known to differentiate into both enteric neurons and glia (Bixby et al., 2002; Chalazonitis et al., 1998). The preceding data suggest that RA enhances ENS precursor differentiation into TuJ1+ neurons. To determine the effect of RA on glial lineage differentiation, we performed immunohistochemistry with the glial lineage marker S100β. Cells were maintained in GDNF plus RA, GDNF without RA, or GDNF plus BMS493 before immunohistochemistry with Ret and S100β antibodies (Figure 6A, B) or using TuJ1 and S100β antibodies (Figure 6E, F). S100β was not detected after 48 hour culture of E12.5 CF-1 mouse immunoselected ENS precursors, but could be readily detected after seven days in culture. At this stage in development all of the S100β expressing cells and all TuJ1 immunoreactive cells express Ret, but Ret immunoreactivity was significantly more intense in TuJ1+ cells than in S100β+ cells where Ret immunoreactivity was weak, but present above background. Quantitative analysis demonstrated that RA treatment resulted in a dramatic reduction in the percentage of Ret+ cells that express S100β compared to when cells are cultured without RA or with BMS493 (P<0.001) (Fig. 6C). To determine if the reduced percentage of S100β+ cells reflected a retinoid induced block in S100β expression or an increase in Ret+ cells that are S100β negative, we counted all S100β+ cells in the wells of our seven day cultures (Figure 6D). Remarkably, the total number of S100β+ cells in these cultures was unaffected by retinoid signaling suggesting that while retinoids enhance neuronal differentiation, they do not block glial differentiation under these conditions. The change in proportion of TuJ1+ to S100β+ cells in response to RA signaling was also verified more directly by double label immunohistochemistry with TuJ1 and S100β antibodies (P<0.001) (Fig. 6E-G). Together these data demonstrate complex effects of retinoid signaling on ENS precursor differentiation, proliferation and neurite growth. They also provide an explanation for the apparent paradox in BrdU incorporation data outlined above for each condition.
Because RA influences both enteric neuron differentiation and proliferation, the relative abundance of Ret+TuJ1+ and Ret+TuJ1− cells under each condition and the proliferation rates for these subsets of cells must be considered together in the calculation for the proliferation rate of all Ret+ cells. More specifically, the proliferation rate of RET+ cells is equal to the sum of the proliferation rate for Ret+TuJ1+ and Ret+TuJ1− cells, adjusted for the percentage of these cells in the culture (i.e., (Ret+BrdU+/Ret+ cells) = (Ret+TuJ1+Brdu+/Ret+TuJ1+ cells)(Ret+TuJ1+/Ret+ cells) + (Ret+TuJ1-BrdU+/Ret+TuJ1− cells)(Ret+TuJ1−/Ret+ cells)). Thus, even when there is an apparently dramatic effect of RA on the rate of proliferation of a subset of Ret+ cells, this effect may not be noticeable when all Ret+ cells are analyzed simultaneously if the absolute rate of proliferation in this cell population is low, or the cells analyzed are of low enough abundance. For example, using data obtained after culturing p75NTR immunoselected cells for 48 hours with or without RA we find that although the proliferation rate of Ret+TuJ1+ cells increased dramatically with added RA (from 4.3 +/− 0.8% to 13.0 +/− 0.6%), and a sizeable percentage of the cells in RA containing cultures were Ret+TuJ1+ (71.5 +/− 2.1 %), the significantly higher rate of proliferation of Ret+TuJ1− cells (33.0 +/− 2.2% in the presence of RA) and the slight increase in the proliferation of Ret+TuJ1− cells in the absence of RA (38.9 +/− 0.9%) masks the increase proliferation of Ret+TuJ1+ cells when all Ret+ cells are analyzed together. To demonstrate consistency in all of our results, we used the data from Figures 4 and 5 for the proliferation and abundance of TuJ1+ and TuJ1− cell subpopulations to calculate the percentage of total Ret+ cells proliferating in these studies. These results compare very closely to the independently measured rates of total RET+ cell proliferation shown in Figure 3A and 3C. All of these data and calculations are tabulated in Table 1 and the results of these independent measurements agree within statistical error. Sample calculations are detailed in Supplemental Figure 1.
Table 1. Summary of proliferation results.
In this table, proliferation data generated from separate experiments are summarize to facilitate comparison of proliferation and differentiation data presented in Figure 3-5. These data demonstrate that our independent measurements of proliferation rates for total Ret+ cells and the proliferation rates for Ret+TuJ1+ and Ret+TuJ1− cell subpopulations are mutually consistent when the composition of Ret+TuJ1+ and Ret+TuJ1− cells in the culture is considered. Column E gives data for directly measured Ret+ cell proliferation rates. The calculated proliferation rate is equal to the proliferation rate for Ret+TuJ1+ cells x %Ret+TuJ1+ cells plus proliferation rate for Ret+TuJ1− × %Ret+TuJ1− cells. Note that calculated Ret+ cell proliferation rates are very similar to the measured rates even though the data are from separate experiments. Large errors for some of the calculated rates reflect propagation of the errors when very few cells are proliferating. Sample calculations are in Supplemental Figure 1.
| Ret+TuJ1+Brdu+/Ret+TuJ1+ | Ret+TuJ1+/Ret+ | Ret+TuJ1−Brdu+/Ret+TuJ1− | Ret+TuJ1−/Ret+ | Calculated Ret+BrdU+ percentage | Measured Ret+Brdu+/Ret+ | ||
|---|---|---|---|---|---|---|---|
| A | B | C | D | ((A x B) + (C x D))/100 | E | ||
| 2 days | RA + | 13.0 +/− 0.6 | 71.5 +/− 2.1 | 33.0 +/− 2.2 | 28.5 +/− 2.1 | 18.7 +/− 2.1 | 20.9 +/− 1.1 |
| RA - | 4.3 +/− 0.8 | 54.4 +/− 1.2 | 38.9 +/− 0.9 | 45.6 +/− 1.2 | 20.0 +/− 4.8 | 22.7 +−/ 1.2 | |
| 7 days | RA + | 1.0 +/− 0.3 | 57.5 +/− 5.5 | 45.4 +/− 5.4 | 42.5 +/− 5.5 | 19.9 +/− 7.2 | 18.3 +/− 1.1 |
| RA - | 0.6 +/− 0.2 | 18.6 +/− 2.5 | 27.1 +/− 3.0 | 81.4 +/− 2.5 | 22.2 +/− 8.3 | 22.3 +/− 2.2 |
RA alters the expression of RhoA and Smurf1 in developing murine enteric neurons
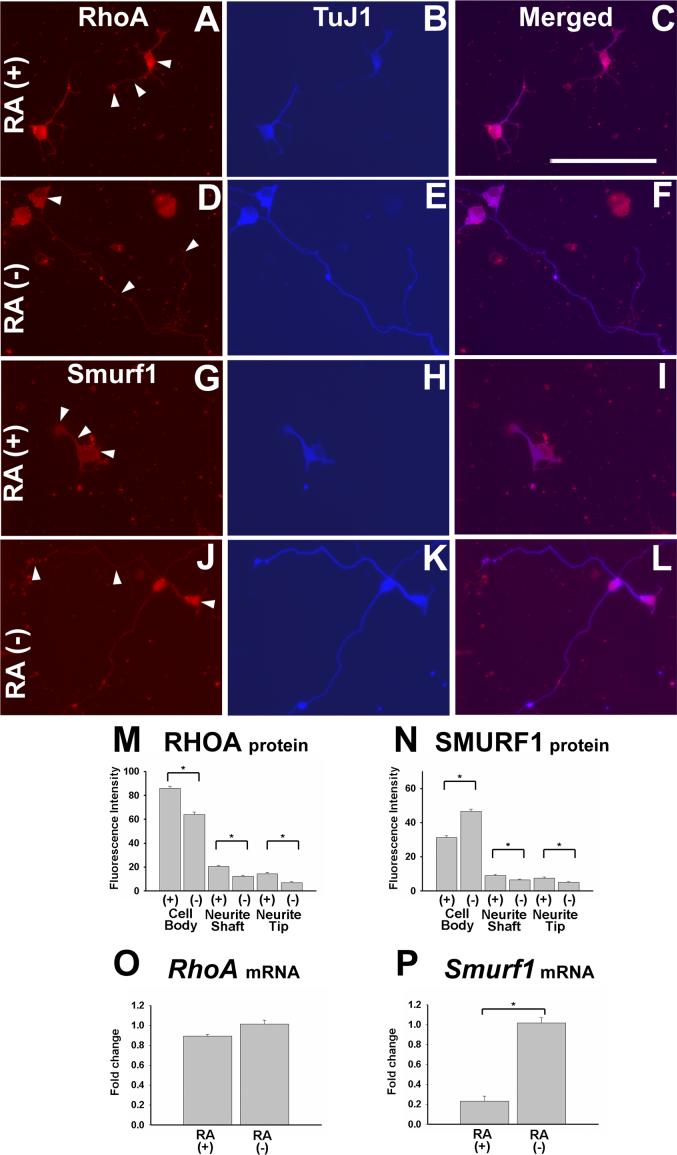
While the ability of RA to increase neuronal differentiation of ENS precursors mirrors findings in other regions of the nervous system, the observation that RA caused reduced neurite length in cultured ENS precursors was surprising since in most other model systems RA increases neurite outgrowth. Recent work has defined many of the molecular mediators required for neurite outgrowth in cultured ENS precursors (Vohra et al., 2007). Because of the unanticipated effect of RA on neurite outgrowth in these cells, we decided to explore in more detail the molecular mechanism of retinoid induced reductions in neurite length. Analysis by immunohistochemistry did not reveal any obvious difference in the abundance or distribution of Ret, phosphatidylinositol 3-kinase (PI-3K), phospho-glycogen synthase kinase 3 beta (p-GSK3β), phospho-protein kinase C zeta (p-PKCζ) or cell division cycle 42 (Cdc42) proteins in TuJ1+ ENS precursors maintained in culture with or without RA (data not shown). We therefore focused our analysis on RhoA, a small GTPase that regulates the actin cytoskeleton. For efficient neurite growth, RhoA protein levels must be reduced at the tip of the growing neurite (Bryan et al., 2005; Vohra et al., 2007). Because we needed to be able to evaluate to the subcellular distribution of RhoA protein in developing enteric neurons, we initially used quantitative immunohistochemical techniques to evaluate the abundance of RhoA in the neuronal cell body, neurite shaft and neurite tip of developing E12.5 CF-1 immunoselected ENS precursors after 48 hour in culture with GDNF plus RA or GDNF without RA (Figure 7A-F). Quantitative analysis of pixel intensity demonstrated reduced levels of RhoA protein in all regions of TuJ1+ cells grown under RA deficient conditions compared to those maintained in media with RA (P<0.001) (Figure 7M). The reduction in RhoA protein was even more prominent in the neurite tip (62% reduction) than in the cell body (23% reduction) and provides a plausible explanation for the enhanced neurite growth in enteric neurons deprived of RA.
Figure 7.
Cells maintained in RA containing media have elevated levels of RhoA protein and reduced levels of Smurf1 compared to cells grown without RA. E12.5 CF1 mouse immunoselected (p75NTR expressing) ENS precursors were grown for 2 days in the presence of GDNF plus RA (A-C, G-I), or in GDNF without RA (D-F, J-L). Cultures were stained with antibodies to RhoA (A, D), Smurf1 (G, J) or TuJ1 (B, E, H, K). (C, F, I, L) Merged images. (M) RhoA or (N) Smurf1 immunofluorescence intensity in the cell body, axon shaft and axon tip was determined by quantitative image analysis. Arrowheads point to the location of fluorescence intensity measurements. 75 cells for each condition were analyzed from a total of 3 separate experiments. (O) RhoA and (P) Smurf1 mRNA levels were determined by qRT-PCR. Relative expression levels are shown. *P < 0.01. Scale bar, 100 μm.
We initially hypothesized that the increased RhoA protein in RA treated developing enteric neurons resulted from a direct effect of RA on RhoA transcription. We therefore measured steady state mRNA levels for RhoA using quantitative real time polymerase chain reaction (qRT-PCR). For this experiment immunoselected p75NTR+ E12.5 CF-1 ENS precursors were maintained in culture with GDNF plus RA or GDNF minus RA for 48 hours before mRNA was prepared. Under these conditions, there was a dramatic effect on neurite length in TuJ1+ cells (Figure 5A, B, I). The qRT-PCR analyses, however, demonstrated comparable RhoA mRNA levels in RA treated and RA deficient cultures (Figure 7O). While this could be due to the relatively modest reduction in RhoA protein abundance in the cell body of RA deprived enteric neurons, or reflect the heterogeneous cell population analyzed at the mRNA level (i.e., effects on RhoA mRNA abundance may occur, but in only a subset of the cultured cells), an alternative explanation for reduced RhoA protein and unchanged RhoA mRNA levels in RA deprived cells would be that RhoA protein is degraded at a higher rate in RA deficient than in RA treated ENS precursors.
We recently demonstrated that RhoA protein abundance in ENS precursors is dramatically dependent on Smurf1 (SMAD specific E3 ubiquitin protein ligase 1), a protein that promotes RhoA degradation at the tip of growing neurites (Vohra et al., 2007). We therefore hypothesized that RA deficiency might reduce RhoA levels in developing enteric neurons by increasing Smurf1 abundance. To test this hypothesis we used quantitative pixel intensity analysis of cultured ENS precursors prepared in the same way as the cells used for RhoA analyses. These studies demonstrated that Smurf1 protein abundance was significantly increased in the cell body of RA deficient TuJ1+ cells, a finding that could account for the reduced RhoA protein levels in these neurons because of Smurf1 mediated RhoA degradation in the cell cytoplasm. Interestingly, based on pixel intensity, Smurf1 protein was actually marginally lower in abundance in the neurite shaft and tip in RA depleted cells than in RA treated cells, suggesting that increased Smurf1 in the cell body of RA deprived cells adequately enhances RhoA degradation to promote neurite elongation by reducing RhoA throughout the cell including in the neurite tip. To determine if RA affected Smurf1 mRNA levels we used qRT-PCR to analyze Smurf1 abundance in immunoselected ENS precursors maintained in culture with GDNF plus RA or GDNF without RA for 48 hours. These studies demonstrated a 5-fold reduction in Smurf1 mRNA in cells cultured with RA compared to cells cultured without RA (figure 7P), supporting the hypothesis that RA induced reductions in Smurf1 abundance account for the reduced Smurf1 protein levels, elevated RhoA protein levels and reduced neurite growth in RA treated cells.
Genes required for RA synthesis, degradation and responsiveness are expressed in the region of the developing ENS
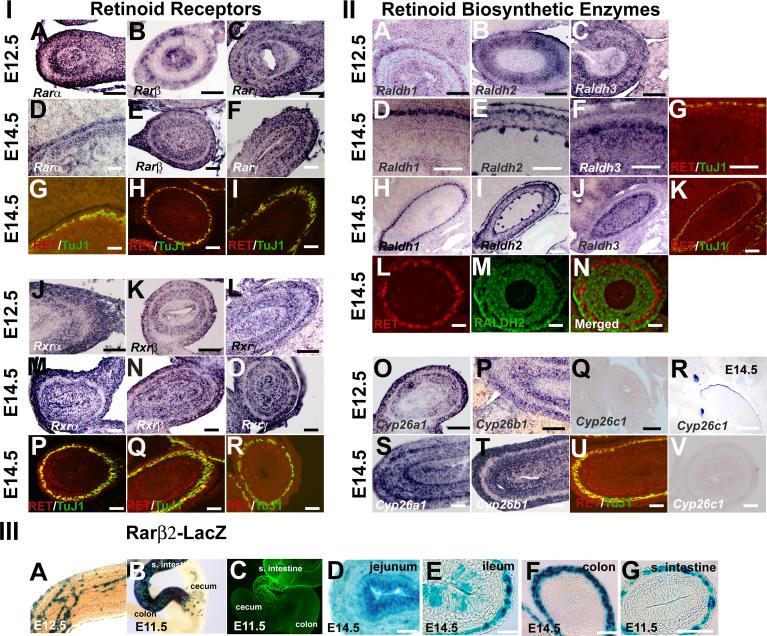
Collectively the studies above describe complex effects of RA on isolated ENS precursors maintained in low density cultures. In most cases, the effect of RA in mixed cell cultures was similar to the effect in p75NTR+ immunoselected cells, but there were some subtle differences in results obtained with or without immunoselection (e.g. absolute neurite length in the presence of RA) that suggested that even in these dissociated cultures, RA might have effects on other cells in the bowel wall that influence ENS development. More importantly, RA is known to affect gut looping, rotation and morphogenesis. For this reason, we decided to pursue a more detailed evaluation of the expression patterns for RA receptors and metabolizing enzymes in the bowel during the developmental period studied in culture. We therefore evaluated gene expression patterns in the developing small bowel for retinoid biosynthetic genes and receptors (Figure 8) at E12.5 and E14.5 by in situ hybridization. When reagents were available we also performed immunohistochemistry. Consistent with our PCR results we found abundant mRNA for Rara, Rarb, Rarg, Rxra, Rxrb, Rxrg, Raldh1, Raldh2, Raldh3, Cyp26a1 and Cyp26b1 in the bowel wall at both ages, but the expression pattern for each gene was distinct. Cyp26c1 expression was not detected in the bowel, but control studies readily detected Cyp26c1 expression in developing teeth and at the edge of the tongue (Figure 8R). For each probe, sense controls were also performed, but no signal was detected from any of the sense control probes (Supplemental Figure 2).
Figure 8.
Expression patterns for retinoid receptors and biosynthetic enzymes in the bowel wall at E12.5 and E14.5. Retinoid receptor (Section I: A-F, J-O) and retinoid biosynthetic enzyme (Section II: A-F, H, I,J, O-Q, S,T, V) gene expression was evaluated by in situ hybridization in the small bowel. At E14.5, adjacent sections of the bowel were also stained by immunohistochemistry with Ret and TuJ1 antibodies to demonstrate the position of the developing ENS (Section I: G-I, P-R; Section II: G, K, U). For Raldh2, protein expression was evaluated by double label immunohistochemistry with Ret (Section II: L, N) and Raldh2 (Section 2 M, N) antibodies. The immunohistochemical staining pattern for Raldh2 is similar to the in situ hybridization pattern for the same gene (Section II: E, I) and shows little overlap with Ret expression. Cyp26c1 was not detected in the bowel at E12.5 (Section II: Q) or E14.5 (Section II: V), but could be detected in developing teeth and the tongue surface at E14.5 in agreement with prior reports (Tahayato et al., 2003) demonstrating the probe specificity and sensitivity (Section II: R). Section III shows gene expression patterns for β-galactosidase in Rarβ2-LacZ transgenic mice that produce β-galactosidase from the Rarb promoter. (Section III: A, B) Images show whole mount staining patterns in the mid-colon at E12.5 (A) and at the ileocecal junction at E11.5 (B). These patterns are typical of the pattern of ENS precursors within the bowel at these ages. For comparison, TuJ1 immunoreactive cells at E11.5 are shown (C) at the ileocecal junction. (Section III: D-G) Sections of the bowel were obtained in the jejunum (D), ileum (E), and colon (F) at E14.5 demonstrating that the pattern of β-galactosidase expression varies along the length of the bowel at this age. (Section III: G) shows strong β-galactosidase expression in the small bowel in the region of the ENS at E11.5 consistent with the whole mount staining patterns. Scale bars, 50 μm. Because of common usage, we used Greek symbols for retinoid receptor gene names in this figure. Rarα = Rara, Rarβ = Rarb, Rarγ = Rarg, Rxrα = Rxrg, Rxrβ = Rxrb, and Rxrγ = Rxrg
The expression data for this large number of genes are complex. We have summarized our interpretation of these data in Table 2, but a few additional comments are required. Rara, Rarb, Rarg, Rxra, Rxrb, and Rxrg all appear to be expressed in the region of the developing ENS at both E12.5 and E14.5 (Figure 8, section I). All of these receptors are also expressed in other cells within the bowel wall including gut epithelium and gut mesenchyme. Some of the receptors (e.g., Rarg, Rxra, Rxrg) appear quite broadly expressed in all layers of the bowel, but others appear to have more restricted patterns of expression (e.g. Rarb at E12.5). Patterns of expression of retinoid biosynthetic enzymes (RALDH) and degrading enzymes (CYP26) are also complex (Figure 8, section II). Raldh1 is prominently expressed in the area of the ENS at E12.5 and E14.5. Raldh2 is expressed throughout the gut mesenchyme at E12.5 and E14.5, and prominently expressed adjacent to the region of the ENS. Raldh3 is broadly expressed in the gut wall at E12.5 and at E14.5, but with no epithelial expression detected at E12.5. Cyp26a1 and Cyp26b1 are both expressed in the region of the ENS and also more diffusely in gut mesenchyme at E12.5. At E14.5, Cyp26a1 and Cyp26b1 are expressed in the same areas, but are also expressed in gut epithelium. In contrast, Cyp26c1 was not expressed in the bowel at these ages.
Table 2. Summary of gene expression data.
The table outlines in an easily accessible format our interpretation of the gene expression studies. Many aspects of the gene expression data however, cannot be tabulated, so this table is meant to complement Figures 8.
| Gene | ENS | Gut epithelium | Gut mesenchyme | Comments | |||
|---|---|---|---|---|---|---|---|
| E12.5 | E14.5 | E12.5 | E14.5 | E12.5 | E14.5 | ||
| Rara | + | + | + | Weak | + | + | |
| Rarb | + | + | + | + | Absent | + | ENS expression confirmed in Rarb-LacZ mice |
| Rarg | + | + | + | + | + | + | |
| Rxra | + | + | Absent | + | + | + | |
| Rxrb | + | + | Absent | + | + | + | |
| Rxrg | + | + | + | + | + | + | |
| Raldh1 | + | + | + | Absent | Absent | Absent | |
| Raldh2 | Absent | Absent | Absent | Absent | + | + | Immunohistochemistry also performed |
| Raldh3 | + | + | Absent | + | + | + | |
| Cyp26a1 | + | + | Weak | + | + | + | |
| Cyp26b1 | + | + | Weak | + | + | + | |
| Cyp26c1 | Absent | Absent | Absent | Absent | Absent | Absent | |
To enhance our ability to evaluate these gene expression patterns relative to ENS precursors, we performed Ret and TuJ1 immunohistochemistry on adjacent sections for all of these genes (Figure 8, section I: G-H, P-R; section II: G, K, U). Despite this effort, the lack of available antibody reagents for immunohistochemistry makes it difficult in some cases to be absolutely certain about the expression of genes within ENS precursors or in closely adjacent cells. Because there was a well characterized antibody to Raldh2, we performed double label immunohistochemistry with Ret and Raldh2 antibodies (Figure 8, section II: L-N). These studies support our interpretation as outlined above, and also demonstrate essentially no overlap between Raldh2 and Ret expressing cells at E14.5.
Given the diverse effects of RA on ENS precursor development in vitro, and the dynamic nature of ENS development, we also wanted to pursue in more detail the hypothesis that the pattern or retinoid responsive genes might change over time and differ along the length of the bowel to facilitate ENS development. While it is impractical to pursue this hypothesis for all these genes in this manuscript, we did perform additional follow up experiments using a Rarb reporter mouse line to investigate this possibility. We focus on Rarb because Rarb is prominently expressed in the region of the ENS at E12.5 and because of the availability of β-galactosidase reporter mice to facilitate our studies. Rarβ2-Lacz mice expresses β-galactosidase from the Rarb promoter and the expression pattern appears to accurately recapitulate Rarb gene expression patterns in vivo (Mendelsohn et al., 1991). Because whole mount X-gal staining gives readily interpretable signal with minimal background staining, we analyzed β-galactosidase staining patterns in the gut of Rarβ2-Lacz mice as a surrogate for evaluating Rarb expression. These studies demonstrated a pattern of gene expression within the gut wall that is typical of the ENS, with a wavefront of β-galactosidase expressing cells in the mid-colon at E12.5 and at the ileocecal junction at E11.5 (Figure 8, section III: A, B). For comparison, whole mount TuJ1 immunohistochemistry at E11.5 shows a similar extent of intestinal colonization by cells of the developing ENS (Figure 8, section III: C) providing strong evidence of Rarb expression within ENS precursors in conjunction with our in situ hybridization data. These whole mount staining methods also supported our hypothesis that there might be variation in the expression pattern for β-galactosidase along the length of the bowel. To investigate this more carefully, we sectioned the bowel after X-gal staining in Rarβ2-Lacz mice. This analysis demonstrated that in the jejunum all cells in the bowel wall express β-galactosidase at E14.5 (Figure 8, section III: D), but in the ileum gene expression is restricted to the area of the ENS and a subset of epithelial cells (Figure 8, section III: E). In contrast, in the colon, β-galactosidase expression is restricted to the region of the ENS (Figure 8, section III: F) suggesting that there are dynamic changes in β-galactosidase expression during development in these mice. Further support for this hypothesis is provided by the observation that in sections of the mid-small bowel at E11.5 β-galactosidase expression is restricted to the region of the developing ENS with no epithelial or mesenchymal expression (Figure 8, section III: G). Collectively these data demonstrate complex expression patterns for retinoid receptors and biosynthetic enzymes within the ENS and other cells of the bowel wall. The data are consistent with the hypothesis that RA directly affects ENS precursor development since all of the retinoid receptors appear to be expressed within ENS precursors, but these data also suggest that in vivo there may be indirect effects of RA on ENS development mediated by retinoid signaling in other cells within the bowel wall.
Discussion
Retinoic acid is a morphogen that influences many aspects of development by regulating the transcriptional activity of Rar and Rxr receptors (Blomhoff and Blomhoff, 2006; Maden, 2007). Recent studies have shown profound effects of RA on both gut and ENS development (Niederreither et al., 2003; Pitera et al., 2001), but did not address whether retinoid effects on the ENS were direct or indirect, and did not define the cellular mechanisms of ENS precursor development affected by retinoid signaling. Our new data now demonstrate significant effects of RA on many aspects of ENS precursor development in vitro. These data suggest that RA directly affects ENS precursors and that maternal vitamin A deficiency or excess could increase the likelihood of intestinal motility disorders via altered ENS precursor development.
Because we were primarily interested in RA effects on cell proliferation and differentiation, our gene expression and cell culture studies focus on E12.5 and E14.5 small bowel, the source of most of the cells evaluated in culture. This is a period of rapid proliferation of ENS precursors in vivo (Pham et al., 1991) and a time when enteric neurons are differentiating, extending neurites and forming a complex interacting network (Gariepy, 2001; Gershon, 1997; Heanue and Pachnis, 2007). These studies demonstrated that all of the retinoid receptors are expressed in the region of the developing ENS and in other cells within the murine bowel wall at these developmental stages. In addition to retinoid receptors, we demonstrated expression of retinoic acid biosynthetic enzymes Raldh1−3 and the RA degrading enzymes Cyp26a1 and Cyp26b1 in or near ENS precursors. Furthermore, studies with Rarβ2-LacZ reporter mice, showed the intense LacZ expression at E11.5, E12.5 and E14.5 within the ENS supporting the hypothesis that RA directly affects ENS precursor cells. These studies, however, also suggest that the patterns of retinoid receptor and biosynthetic enzyme expression change over time and differ along the longitudinal axis of the bowel to facilitate ENS and intestinal development. Although additional studies are needed to fully define the temporal and spatial expression patterns of each of these genes, the gene expression data presented and the effects of RA on gut morphogenesis suggest that RA could directly affect ENS development or indirectly influence the ENS via modulation of gene expression in the gut epithelium or mesenchyme.
By using low density culture and immunoselected ENS precursor cells, our vitro studies are designed primarily to provide insight into direct effects of RA on ENS precursors by removing from the culture the majority of non-crest derived cells and reducing cell-cell interaction. Although not perfect, in the immunoselected cell pool 89% of the cells are crest-derived at the time of plating. Since retinoid receptors are expressed within ENS progenitors, it seems highly likely that the effects observed in our selected cells are independent of residual non-crest-derived cells in the culture. Furthermore, even the addition of roughly 9-fold more non-crest-derived cells to these cultures had only subtle effects on ENS precursor development in response to RA. These studies led to several important conclusions. 1) RA dramatically increased the number of TuJ1 immunoreactive ENS precursors that developed in vitro consistent with the ability of RA to enhance neuronal differentiation in other neuronal precursor cells. This occurred using immunoselected cells and non-selected (i.e., whole gut) cells in culture, although the percentage of TuJ1 immunoreactive Ret+ cells differed slightly under the two culture conditions. 2) RA increased the proliferation of Ret+ ENS precursors that had begun to differentiate into neurons as demonstrated by TuJ1 immunoreactivity early during the cell culture period (48 hrs) and enhanced proliferation of Ret+TuJ1− cells at the seven day time point. These data suggest complex effects of RA on proliferation of subsets of ENS precursors during development. 3) We did not detect any effect of RA or RA deficiency on cell death in our studies, but because apoptosis is rare in wild type ENS precursors in vivo (Gianino et al., 2003), we did not pursue these studies in detail. Collectively these analyses suggest that RA is important for enteric neuron differentiation and may influence the number of enteric neurons in the bowel, even after the wave front of migrating ENS precursors has reached the distal bowel. These effects contrast with the effect of endothelin-3 (Edn3) in cultured E11.5 ENS precursors, since Edn3 and GDNF synergistically enhance the proliferation of Ret+TuJ1− cells in the first 36 hours of culture, but Edn3 has minimal effect on the proliferation of Ret+TuJ1+ cells (Barlow et al., 2003). Edn3 also supports the proliferation a subset of Sox10+ ENS progenitors in vivo and prevents Ret expression and neuronal differentiation (Barlow et al., 2003; Bondurand et al., 2006). Thus, RA and Edn3 both support proliferation of ENS precursors, but differ in the subpopulation of cells supported and in their ability to enhance (RA) or suppress (EDN3) enteric neuron differentiation.
One of the most striking and surprising observations was that RA reduces neurite growth from committed ENS precursors in vitro. This is supported by the observed 3-fold decrease in neurite length in immunoselected cells maintained in RA compared to those grown without RA after 48 hours in culture. Similar effects of RA were also obvious after seven days in culture, but tracking the long neurites in our seven day cultures was not possible. This contrasts with the effect of RA in other model systems where RA enhances neurite growth (Clagett-Dame et al., 2006; Dmetrichuk et al., 2006; Maden, 2007). Similar, but less severe reductions in neurite length were also induced by RA in ENS precursors cultured in the presence of non-crest derived cells. These data are consistent with the hypothesis that non-crest-derived cells within the gut wall regulate ENS precursor neurite growth, but do not demonstrate convincingly if these effects of non-crest-derived cells are RA dependent. Together with previous observations, our new data emphasize that ENS precursors share some molecular strategies with cells in other regions of the nervous system, but that ENS progenitors have their own unique mechanisms that cannot be predicted from studies in other neuronal precursors. Additional differences between molecular mechanisms regulating ENS precursors and other neuronal subtypes include the absence of an effect of Mek inhibitors on neurite growth in cultured ENS precursors (Srinivasan et al., 2005) and the absence of significant cell death in the WT ENS during development (Gianino et al., 2003).
The molecular mechanisms that underlie all of these effects will be challenging to identify since it appears that there are complex stage-dependent and environment dependent effect of RA on ENS precursor development. Furthermore, it will be important to directly link RA induced transcriptional changes to the alterations in ENS precursor biology. Nonetheless, we have identified at least one RA induced molecular change within developing ENS precursors that could account for the RA induced reduction in neurite length. Specifically, we now demonstrate that ENS precursors cultured in RA have less Smurf1 protein in the cell body than cells maintained without RA. Retinoic acid also dramatically reduces Smurf1 mRNA levels in these cells. This is likely important since the E3 ubiquitin ligase Smurf1 is required in ENS precursors for efficient neurite growth (Vohra et al., 2007) because Smurf1 targets RhoA for degradation (Bryan et al., 2005; Wang et al., 2003) and RhoA reduces neurite growth under these conditions by altering actin cytoskeletal dynamics. Interestingly, although Smurf1 protein levels were significantly elevated in the cell body of RA deprived enteric neurons, Smurf1 protein levels were actually slightly lower along the axon shaft and tip in RA deprived cells. Because most of the Smurf1 protein is present in the cell body at this stage of development anyway, we now hypothesize that the increase in cell body Smurf1 level is adequate to account for the reduction in RhoA protein in the cell body, neurite shaft and neurite tip via increased degradation. This hypothesis is consistent with the observation that RA does not appear to affect RhoA mRNA levels, but significantly affects the level of RhoA protein. These findings do not exclude the possibility that there are other RA induced proteins that affect neurite growth in ENS precursors, but do provide a plausible molecular mechanism for the changes in neurite growth induced by RA.
A separate perplexing question is why ENS precursors should have developed mechanisms to reduce neurite growth in response to RA in vitro, while most other neurons have increased neurite growth in response to RA. This seems particularly striking in the context of RA induced neuronal differentiation in cultured ENS precursors. One potential explanation lies in the observation that RA has many distinct effects on the developing ENS that must be coordinated to generate an adequate number of enteric neurons, permit ENS precursors to migrate to the end of the bowel and then produce a network of interacting cells, some of which have long neurites. Furthermore, although Raldh and Cyp26 enzymes regulate RA availability in the gut wall, RA affects so many aspects of gut development outside the ENS, that individual cell populations may need to adopt novel strategies in response to RA signaling to permit normal development. Specifically in the context of ENS precursors, RA induces neuronal differentiation, but also supports precursor proliferation, arguably an unusual combination of effects that may be needed to produce enough neurons to populate the bowel. Similarly, extensive neurite growth too early in development may interfere with precursor migration (assuming TuJ1+ cell might still migrate) or could result in excessive neurite growth before other signals were available to provide axon guidance. Thus, each of the effects we observed in vitro, may be important to promote specific aspects of ENS development. Whether these apparently direct effects of RA on ENS precursors also occur in vivo, or are modulated by RA effects on other cells in the bowel wall is currently unknown and merits additional investigation. This work, however, may require the analysis of mice with conditional mutations in specific retinoid receptors or in the retinoid biosynthetic machinery.
While our new data provide important insight into the cellular and molecular mechanisms through which RA regulates ENS development, these data also raise many additional questions. For example, the effect of RA on Ret- p75NTR+ cells has not been investigated and the fate of these cells is not well understood. Furthermore, although RA increases the number of Ret+ cells in culture, it is not clear to what degree RA is required for the induction of Ret expression or for continued Ret expression in ENS precursors. It is clear, however, that some cells remain intensely Ret+ even after a week of RA deprivation, suggesting that in at least a subset of cells, Ret expression becomes RA independent. The effect of RA on developing enteric glia also deserves additional investigation. Our analyses demonstrate that RA enhances the number of neurons that developed in culture, but did not reduce the number of S100β+ cells. These S100β+ cells are presumed to differentiate into enteric glia, but the effect of RA on later stages of enteric glial differentiation has not been investigated. It remains possible, for example that continued exposure to RA could prevent other aspects of glial differentiation and that changes in RA abundance over time are required for normal differentiation of enteric glia. All of these questions will require additional investigation using a combination of primary culture and in vivo model systems.
These complex effects of RA on ENS precursors in vitro suggest that RA abundance will affect many aspects of ENS development. For this reason, polymorphisms that change the activity of Rar, Rxr, Raldh, and Cyp26 protein families could profoundly influence ENS morphogenesis and function. Even promoter or enhancer mutations that alter the temporal or regional expression patterns for these genes would be expected to influence the developing bowel wall and enteric nervous system. Furthermore, these data suggest the intriguing possibility that non-genetic factors, like vitamin A levels could impact the penetrance or expressivity of Hirschsprung disease. Finally, these data imply that retinoid deficiency could contribute to other human intestinal motility disorders by altering neuronal versus glial differentiation, ENS precursor proliferation, neurite growth or other aspects of intestinal development. These data therefore suggest that some cases of intestinal motility disorder or Hirschsprung disease might be preventable by ensuring adequate maternal nutrition (i.e. vitamin A levels) before and during early gestation.
Supplementary Material
Supplemental Figure 1. Sample calculations for proliferation rates in Table 1 are shown using data from cells maintained for 48 hours in culture in RA containing media.
Supplemental Figure 2. In situ hybridization results obtained using sense control probes failed to produce any signal from E14.5 gut sections. Scale bar, 50 μm.
Acknowledgements
We appreciate the guidance of Ming Fu and Bhupinder Vohra in pursuing these studies. We thank Dr. Pierre Chambon, Dr. Peter McCaffery, Dr. Ursula Drager, and Dr. Chris Zusi for sharing valuable reagents. We also thank Dr. Cathy Mendelsohn for sharing Rarβ2-lacZ transgenic mice, and Dr. Louis Muglia and Dr. Scott Saunders for recommendations on the manuscript. This work was supported by NIH/RO1 DK57038, DK6459201, the Digestive Disease Research Center Core (DDRCC) NIH/P30-DK52574, NIH Grant DK56341 (Clinical Nutrition Research Unit) and a grant from the March of Dimes FY02-182. Work cited in this publication was performed in a facility supported by the NCRR grant C06 RR015502.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldassarre G, Boccia A, Bruni P, Sandomenico C, Barone MV, Pepe S, Angrisano T, Belletti B, Motti ML, Fusco A, Voglietto G. Retinoic acid induces neuronal differentiation of embryonal carcinoma cells by reducing proteasome-dependent proteolysis of the cyclin-dependent inhibitor p27. Cell Growth and Differentiation. 2000;11:517–526. [PubMed] [Google Scholar]
- Barlow A, de Graaff E, Pachnis V. Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron. 2003;40:905–16. doi: 10.1016/s0896-6273(03)00730-x. [DOI] [PubMed] [Google Scholar]
- Berggren K, McCaffery P, Drager U, Forehand CJ. Differential distribution of retinoic acid synthesis in the chicken embryo as determined by immunolocalization of the retinoic acid synthetic enzyme, RALDH-2. Dev Biol. 1999;210:288–304. doi: 10.1006/dbio.1999.9286. [DOI] [PubMed] [Google Scholar]
- Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35:643–56. doi: 10.1016/s0896-6273(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–30. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- Bondurand N, Natarajan D, Barlow A, Thapar N, Pachnis V. Maintenance of mammalian enteric nervous system progenitors by SOX10 and endothelin 3 signalling. Development. 2006;133:2075–86. doi: 10.1242/dev.02375. [DOI] [PubMed] [Google Scholar]
- Bryan B, Cai Y, Wrighton K, Wu G, Feng XH, Liu M. Ubiquitination of RhoA by Smurf1 promotes neurite outgrowth. FEBS Lett. 2005;579:1015–9. doi: 10.1016/j.febslet.2004.12.074. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, Rothman TP, Chen J, Gershon MD. Age-dependent differences in the effects of GDNF and NT-3 on the development of neurons and glia from neural crest-derived precursors immunoselected from the fetal rat gut: expression of GFRalpha-1 in vitro and in vivo. Dev Biol. 1998;204:385–406. doi: 10.1006/dbio.1998.9090. [DOI] [PubMed] [Google Scholar]
- Chazaud C, Dolle P, Rossant J, Mollard R. Retinoic acid signaling regulates murine bronchial tubule formation. Mech Dev. 2003;120:691–700. doi: 10.1016/s0925-4773(03)00048-0. [DOI] [PubMed] [Google Scholar]
- Clagett-Dame M, McNeill EM, Muley PD. Role of all-trans retinoic acid in neurite outgrowth and axonal elongation. J Neurobiol. 2006;66:739–56. doi: 10.1002/neu.20241. [DOI] [PubMed] [Google Scholar]
- Corcoran J, Shroot B, Pizzey J, Maden M. The role of retinoic acid receptors in neurite outgrowth from different populations of embryonic mouse dorsal root ganglia. J Cell Sci. 2000;113(Pt 14):2567–74. doi: 10.1242/jcs.113.14.2567. [DOI] [PubMed] [Google Scholar]
- Dmetrichuk JM, Carlone RL, Spencer GE. Retinoic acid induces neurite outgrowth and growth cone turning in invertebrate neurons. Dev Biol. 2006;294:39–49. doi: 10.1016/j.ydbio.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Elmi M, Faigle R, Yang W, Matsumoto Y, Rosenqvist E, Funa K. Mechanism of MASH1 induction by ASK1 and ATRA in adult neural progenitors. Mol Cell Neurosci. 2007;36:248–59. doi: 10.1016/j.mcn.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Furness JB. The enteric nervous system. Blackwell Publishing; Malden, MA: 2006. [Google Scholar]
- Gariepy CE. Intestinal motility disorders and development of the enteric nervous system. Pediatr Res. 2001;49:605–13. doi: 10.1203/00006450-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Gershon M. Genes and lineages in the formation of the enteric nervous system. Current Opinion in Neurobiology. 1997;7:101–109. doi: 10.1016/s0959-4388(97)80127-4. [DOI] [PubMed] [Google Scholar]
- Gianino S, Grider JR, Cresswell J, Enomoto H, Heuckeroth RO. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development. 2003;130:2187–2198. doi: 10.1242/dev.00433. [DOI] [PubMed] [Google Scholar]
- Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–79. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- Hearn CJ, Murphy M, Newgreen D. GDNF and ET-3 differentially modulate the numbers of avian enteric neural crest cells and enteric neurons in vitro. Dev Biol. 1998;197:93–105. doi: 10.1006/dbio.1998.8876. [DOI] [PubMed] [Google Scholar]
- Heuckeroth RO, Lampe PA, Johnson EMJ, Milbrandt J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Developmental Biology. 1998;200:116–129. doi: 10.1006/dbio.1998.8955. [DOI] [PubMed] [Google Scholar]
- Hochgreb T, Linhares VL, Menezes DC, Sampaio AC, Yan CY, Cardoso WV, Rosenthal N, Xavier-Neto J. A caudorostral wave of RALDH2 conveys anteroposterior information to the cardiac field. Development. 2003;130:5363–74. doi: 10.1242/dev.00750. [DOI] [PubMed] [Google Scholar]
- Kumar M, Bagchi B, Gupta SK, Meena AS, Gressens P, Mani S. Neurospheres derived from human embryoid bodies treated with retinoic Acid show an increase in nestin and ngn2 expression that correlates with the proportion of tyrosine hydroxylase-positive cells. Stem Cells Dev. 2007;16:667–81. doi: 10.1089/scd.2006.0115. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–65. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Ruberte E, LeMeur M, Morriss-Kay G, Chambon P. Developmental analysis of the retinoic acid-inducible RAR-beta 2 promoter in transgenic animals. Development. 1991;113:723–34. doi: 10.1242/dev.113.3.723. [DOI] [PubMed] [Google Scholar]
- Moss JB, Xavier-Neto J, Shapiro MD, Nayeem SM, McCaffery P, Drager UC, Rosenthal N. Dynamic patterns of retinoic acid synthesis and response in the developing mammalian heart. Dev Biol. 1998;199:55–71. doi: 10.1006/dbio.1998.8911. [DOI] [PubMed] [Google Scholar]
- Newgreen D, Young HM. Enteric nervous system: development and developmental disturbances--part 1. Pediatr Dev Pathol. 2002a;5:224–47. doi: 10.1007/s10024-001-0142-y. [DOI] [PubMed] [Google Scholar]
- Newgreen D, Young HM. Enteric nervous system: development and developmental disturbances--part 2. Pediatr Dev Pathol. 2002b;5:329–49. doi: 10.1007/s10024-002-0002-4. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Roux IL, Schuhbaur B, Chambon P, Dolle P. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development. 2003;130:2525–2534. doi: 10.1242/dev.00463. [DOI] [PubMed] [Google Scholar]
- Pham TD, Gershon MD, Rothman TP. Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. Journal of comparative neurology. 1991;314:789–798. doi: 10.1002/cne.903140411. [DOI] [PubMed] [Google Scholar]
- Pitera JE, Smith VV, Woolf AS, Milla PJ. Embryonic gut anomalies in a mouse model of retinoic Acid-induced caudal regression syndrome: delayed gut looping, rudimentary cecum, and anorectal anomalies. Am J Pathol. 2001;159:2321–9. doi: 10.1016/S0002-9440(10)63082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe RJ, Ginty DD, Wagner JA. Retinoic acid stimulates the differentiation of PC12 cells that are deficient in cAMP-dependent protein kinase. J Cell Biol. 1991;113:1173–82. doi: 10.1083/jcb.113.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. Hirschsprung's Disease. Curr. Probl. Surg. 1996;33:391–461. doi: 10.1016/s0011-3840(96)80009-8. [DOI] [PubMed] [Google Scholar]
- So PL, Yip PK, Bunting S, Wong LF, Mazarakis ND, Hall S, McMahon S, Maden M, Corcoran JP. Interactions between retinoic acid, nerve growth factor and sonic hedgehog signalling pathways in neurite outgrowth. Dev Biol. 2006;298:167–175. doi: 10.1016/j.ydbio.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Anitha M, Mwangi S, Heuckeroth RO. Enteric neuroblasts require the phosphatidylinositol 3-kinase/Akt/Forkhead pathway for GDNF-stimulated survival. Mol Cell Neurosci. 2005;29:107–19. doi: 10.1016/j.mcn.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Tahayato A, Dolle P, Petkovich M. Cyp26C1 encodes a novel retinoic acid-metabolizing enzyme expressed in the hindbrain, inner ear, first branchial arch and tooth buds during murine development. Gene Expr Patterns. 2003;3:449–54. doi: 10.1016/s1567-133x(03)00066-8. [DOI] [PubMed] [Google Scholar]
- Vohra BP, Fu M, Heuckeroth RO. Protein kinase Czeta and glycogen synthase kinase-3beta control neuronal polarity in developing rodent enteric neurons, whereas SMAD specific E3 ubiquitin protein ligase 1 promotes neurite growth but does not influence polarity. J Neurosci. 2007;27:9458–68. doi: 10.1523/JNEUROSCI.0870-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra BP, Tsuji K, Nagashimada M, Uesaka T, Wind D, Fu M, Armon J, Enomoto H, Heuckeroth RO. Differential gene expression and functional analysis implicate novel mechanisms in enteric nervous system precursor migration and neuritogenesis. Dev Biol. 2006;298:259–71. doi: 10.1016/j.ydbio.2006.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HR, Zhang Y, Ozdamar B, Ogunjimi AA, Alexandrova E, Thomsen GH, Wrana JL. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302:1775–9. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Chen J-X, Rothman TP, Gershon MD. Inhibition of in vitro enteric neuronal development by endothelin-3: mediation by endothelin B receptors. Development. 1999;126:1161–1173. doi: 10.1242/dev.126.6.1161. [DOI] [PubMed] [Google Scholar]
- Young HM, Ciampoli D, Hsuan J, Canty AJ. Expression of Ret-, p75NTR -, Phox2a-, Phox2b-, and tyrosine hydroxylase-immunoreactivity by undifferentiated neural crest-derived cells and different classes of enteric neurons in the embryonic mouse gut. Developmental Dynamics. 1999;216:137–152. doi: 10.1002/(SICI)1097-0177(199910)216:2<137::AID-DVDY5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Sample calculations for proliferation rates in Table 1 are shown using data from cells maintained for 48 hours in culture in RA containing media.
Supplemental Figure 2. In situ hybridization results obtained using sense control probes failed to produce any signal from E14.5 gut sections. Scale bar, 50 μm.