Abstract
Following repeated exposure to foster pups, virgin female rats acquire and eventually express a full spectrum of maternal caretaking behaviors directed toward pups. Though these behaviors are vigorous, these females are reportedly less motivated to seek out and interact with pups (i.e. maternally motivated) than parturient females during early postpartum. The present study systematically assesses how the length of pup-exposure and nature of interactions between the female-pup dyad affect maternal motivation in the virgin female rat. Virgin females were exposed to young pups consistently (24 h/day) across a prolonged period (21 days), briefly (1 h/day) across a relatively brief period (7 days), or distally (pups inaccessible in mesh bag). During final pup-exposure days, females were conditioned and tested for their preference for a pup-associated chamber (e.g. maternal motivation) using conditioned place preference. Early postpartum females provided a comparison group. Fully maternal behavior only emerged in females given prolonged pup-exposure; this behavior improved significantly over time and was maximally expressed for a duration equivalent to early postpartum. Females given brief pup-exposure expressed only emergent maternal behaviors initiated by pups; distal pup-exposure evoked pup-avoidance. Virgin females given prolonged or brief pup-exposure expressed substantial pup-associated chamber preference, with more females preferring the pup-associated chamber following longer pup-exposures in a subtle stepwise relationship. Maternal motivation was strikingly similar in prolonged pup-exposure virgin and early postpartum females. Females given distal pup-exposure completely lacked maternal motivation. Maternal behavior did not predict chamber preference. Results suggest that pup-exposure, regardless of length, is sufficient to support strong maternal motivation, whereas parity is not required.
Keywords: Conditioned place preference, Maternal, Motivation, Rat, Female, Lactating, Maternal behavior, Offspring
1. Introduction
Offspring (pups) elicit strongly motivated, rigorous caregiving behaviors from the maternally responsive female rat [1]. In the postpartum female, the performance of these maternal behaviors coincides with a strong drive to seek out and interact with pups, or maternal motivation. Early postpartum females voluntarily spend the vast majority of their time with their pups [2–6], bar-press insatiably for access to pups [7,8], prefer a pup-associated context over a cocaine-associated context [9,10], and readily retrieve pups from anxiety-provoking areas [11–13]. The complex motivational state of the early postpartum female not only promotes the expression of maternal behaviors critical to the development and survival of her offspring, but also promotes vigorous pup-seeking behavior when her pups are absent or unavailable. This maternal motivation is highly adaptive during the early postpartum period and thus may only emerge in females that have recently given birth.
Maternal caregiving behaviors, however, can be induced in virgin (nulliparous) female and male rats following continuous exposure to pups [14–16]. Constant (24 h/day) exposure to young pups typically induces stable maternal behavior in the adult, gonadally intact virgin female after 2–9 days [14–21]; these females do not need to experience gestation, parturition, or lactation (i.e. parity) to behave maternally [1,14–16]. Importantly, the pup-induced maternal behavior expressed by these maternally responsive virgin females includes most components of the naturally occurring maternal behavior displayed by postpartum females [21,22] and can remain in place over extended periods of time [23]. The present study uses the pup-exposed virgin female as a unique model to explore whether pup-exposure also promotes maternal motivation outside of the influences of parity.
Limited evidence suggests that maternal motivation is substantially weaker in the maternally responsive virgin female than in the postpartum female. Maternally responsive virgins retrieve pups more slowly in both familiar and anxiety-provoking environments [11–13,22] and lick pups less frequently than do early postpartum females [22]. In the absence of pups, maternally responsive virgin females express less pup-seeking behavior than do early postpartum females, only preferring a pup-associated chamber after extensive deprivation from pups [17]; such extensive pup-deprivation periods bolster maternal motivation even in females in late postpartum that spend little voluntary time around pups [3–5,24].
To assess the virgin females’ motivation to seek out and interact with pups in their absence, we use a well-established conditioned place preference (CPP) procedure to compare virgin and postpartum females’ conditioned preference for a pup-associated chamber, considered to reflect the incentive-motivational value of pups and thus a female’s maternal motivation [9,10,24]. Work by Fleming and colleagues suggests that both virgin and postpartum females must be exposed to pups in their homecage and express fully maternal behavior in order to prefer the pup-associated chamber [17]; further, females must be allowed to interact physically with pups during CPP conditioning in order to prefer the pup-associated chamber [25]. However, the length of pup-exposure and nature of the direct physical interactions between the female-pup dyad have not been systematically assessed with respect to maternal motivation. We posit that extending the length of time that virgin females are exposed to pups will enhance the incentive-motivational value of pups [26–28], resulting in strong preference for the pup-associated chamber in females given prolonged pup-exposure and little chamber preference in females given limited or no pup-exposure.
Virgin females were exposed to young pups using one of three pup-exposure regimens: either consistently across a prolonged period (24 h/day for 21 days), briefly across a relatively brief period (1 h/day for 7 days), or distally, with pups remaining inaccessible in a mesh bag (1 h/day for 4 days). The novel prolonged pup-exposure regimen closely replicates the consistent homecage exposure to pups and the intensive time naturally spent with pups during the highly motivated early postpartum period [2]; accordingly, prolonged-exposure females were only deprived of pups for minimal, behaviorally relevant lengths of time prior to CPP. The brief pup-exposure regimen represents an important, early behavioral transition from pup-avoidance to tolerance but prior to the emergence of full maternal behavior, addressing for the first time whether a motivational transition occurs concurrently during this early exposure period. Distal pup-exposure evokes neither maternal behavior nor motivation, replicating previous work [25]. All females were scored daily on pup retrieval, nest-building, and crouching/hovering over pups, to characterize the expression of maternal behaviors across pup-exposure regimens and during the CPP procedure.
To our knowledge, this is the first study to systematically manipulate the length of pup-exposure in virgin females, relative to conditioned preference for a pup-associated chamber, and is only the second [17] study to assess maternal motivation in virgin females using CPP.
2. Methods
2.1. Animals
Subjects (n=56) were 90–120 day old female Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA) raised in an animal colony maintained at the Laboratory Animal Facility at Rutgers University, accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC). All procedures comply the “National Institutes of Health Guide for Care and Use of Laboratory Animals” [29] and are approved by the Rutgers University Animal Care and Facilities Committee. Females were moved into a quiet testing suite 2–5 days prior to the initial pup-exposure (virgins females, n=32) or parturition (primiparous females, n=24). Each female was housed individually in opaque shoebox homecages (25.5 cm W×47 cm L×23 cm H) with woodchip bedding and food and water ad libitum. Virgin and parturient females’ homecages were adjacent so that virgin females were exposed to olfactory and auditory stimuli associated with pups as their parturient neighbors experienced parturition and initiated maternal caregiving. A 12 h:12 h light:dark cycle was maintained (lights on 0700). Females remained healthy and pups gained weight and developed normally across the experiment [details in 10,30]. Females were not tested for estrous cycle stage, as vaginal lavage can elicit CPP [31].
2.2. Pup-exposure procedures
Virgin females were assigned to receive either prolonged (n=12), brief (n=10), or distal (n=8) exposure to pups prior to the conditioned place preference (CPP) procedure. Pups were provided by parturient, lactating females (n=10) and, by carefully controlling the precisely timed births of foster litters, always ranged from 1–8 days old to elicit maximal maternal responsivity from females [2,32]. Pup age did not affect onset or quality of any behavior. To limit females’ neophobic response and prevent pup attacking/killing, only two freshly-nourished pups were given to females on each pup-exposure day. Females that attacked/killed pups were removed from the study (n=2).
2.2.1. Prolonged pup-exposure
Two 1-day old pups were placed in each virgin female’s homecage, opposite her nest. Pups remained with virgin females for 12 h before being returned to lactating females for nursing and care; two fresh pups were immediately given to each virgin female for the subsequent 12 h. These 12 h rotations allowed pups to be nursed and cared for by lactating females more frequently than typical 24 h rotations [see 33], with vocalization rate, corporal cooling, and hunger reaching a relatively steady-state between 12 and 24 h [34,35]. Continuous (24 h/day) pup-exposure continued across CPP for a total of 21 days.
2.2.2. Brief pup-exposure
Two 1-day old pups were presented for 1 h daily for two days prior to the CPP procedure. During CPP, virgin females received 1 h daily exposures during each CPP pup-conditioning session. The brief (1 h/day) pup-exposures occurred over a total of 7 days.
2.2.3. Distal pup-exposure
Virgin females were exposed to distal pup stimuli during each 1 h CPP pup-conditioning session. Two pups were enclosed in a mesh bag that allowed females to smell, hear, and see pups but not to interact physically with them; females were unable to mouth, carry, or lick the pups. The bag was promptly removed from the chamber if the female attacked the bag; one female attacked on the first conditioning day and no attacks were observed on any other day.
2.2.4. Early postpartum pup-exposure
Parturient, lactating females that did not donate pups to virgin females (n=14) remained with culled litters of eight pups. Every day, pups were collected from all females, commingled, and redistributed to ensure all lactating and virgin females were similarly exposed to “foster” pups.
2.3. Maternal behavior observations and scoring
All subjects were observed and scored daily on three maternal behaviors: retrieval of pups, nest-building, and crouching/hovering over pups. A female met the criterion for full maternal behavior if she achieved a retrieval score of 1 or higher on two consecutive days and a crouch/hover score of 1 or higher on one of those days.
For all subjects exposed to pups in their homecage, maternal behaviors were scored between 0700 and 0900 h; pups were first removed from homecages (if necessary) and placed into small boxes adjacent to each homecage for 1 h prior to testing. Homecage nest-building and crouching/hovering scoring began eight days into the prolonged pup-exposure regimen, at their first emergence. All subjects were also observed during each 1 h CPP pup-conditioning session.
2.3.1. Retrieval
Two freshly-nourished pups were placed in the corner of the cage opposite each female’s nest site and preferred corner. Criterion for pup retrieval required a female to pick up the pup with her mouth and transport the pup to her nest. Scores ranged from 0–2, corresponding to the number (0–2) of pups retrieved to the nest; scores of 0.5 and 1.5 reflected retrieval of one or both pups to a non-nest location, respectively. Females were observed for 10 min.
2.3.2. Crouching/hovering
After retrieval was scored, females were scored on crouching/hovering postures on a scale ranging from 0–3: 0=lack of definitive hovering; 1=active hovering over pup(s) or lying/resting on top of pup (s); 2=low crouch over pup(s) or prone nursing posture; 3=active/high rigid crouch (kyphosis) over pup(s).
2.3.3. Nest building
Nests were scored immediately after crouching/hovering, on a scale ranging from 0–4: 0=no nest, nest material scattered across cage; 1=poor, a flat nest without walls built without using all nest material; 2=fair, a flat nest without walls built using all nest material; 3=good, nest with low/medium walls constructed using all material; 4=excellent, nest with high walls built using all material. Scores of 2 or higher were considered maternal. After scoring, nests were destroyed. Nest material (shredded paper towels) was changed every 3–4 days.
2.4. Conditioned place preference procedure
2.4.1. Apparatus
The custom-designed conditioned place preference (CPP) apparatus consisted of three equal-sized clear Plexiglas chambers (27.5 cm W×21 cm L×20.5 cm H) each decorated with unique contextual cues [details in 10,30] and containing infrared beams that traversed the floor of each chamber. Beam breaks recorded time spent and locomotion within each chamber.
2.4.2. Pre-conditioning baseline session
Each female was placed into the center chamber and allowed access to all three chambers for 1 h. This session occurred on pup-exposure day 16 (prolonged), day 3 (brief), prior to pup-exposure (distal), or on postpartum day 1 (postpartum group). The day of this session, particularly with respect to postpartum, does not influence CPP (Wansaw and Morrell, unpublished observations).
2.4.3. Conditioning phase
Females received a separate conditioning session, one with young pups and one with nothing (empty), once a day for four consecutive days. Pups were 4–7 days old on conditioning days 1–4, respectively, and thus age-matched to the postpartum day of the early postpartum females. At 0800, pups were removed from each female’s homecage (if necessary) and freshly-nourished pups were placed into small, adjacent cages so that subjects could not see or physically interact with pups but were exposed to pups’ auditory and olfactory stimuli. At 1000, females were confined to one cue-decorated side chamber containing pups (pup-associated chamber) that had been deprived of female contact for 2 h and were thus hungry and demanding of maternal care [36]. Two pups were given to each virgin female given brief or prolonged pup-exposure, to mimic homecage exposures and minimize pup attacks; similarly, two pups were confined in each mesh bag given to each virgin female given distal pup-exposure. Five pups were given to each postpartum female [2,10]. Females and pups remained in the conditioning chamber for 1 h [17] to allow sufficient time for female-pup interactions; females’ behavior was recorded every 5 min. Females were then returned to homecages for 4 h, and pups were returned to early postpartum and prolonged pup-exposure females. At 1500, females were confined to the opposite cue-decorated side chamber, which remained empty (empty chamber), for 1 h.
2.4.4. Post-conditioning test session
Females were tested for conditioned chamber preference on the day after the final conditioning session. Females were allowed access to all chambers for 1 h. Pups were not present. Virgin females were tested on pup-exposure day 21 (prolonged), day 8 (brief), or after 4 days of exposure to the mesh bag containing pups (distal). Postpartum females were tested on postpartum day 8.
2.5. Analyses and statistics
Statistical analyses were performed as before [10,30], with P<0.05 as significance level. Preferences are presented as the percentages of individual subjects within each population that met criteria for each of the four preference categories. Chamber times (averaged across all subjects) and behavior scores are presented as means and standard errors of the mean (sem).
2.5.1. Conditioned place preference
The time spent in each chamber (chamber time) during pre- and post-conditioning sessions was used to identify each subject’s chamber preference [9,10,24,30]. Subjects spending at least 30 min in one chamber and 25% more time in that chamber than either other chamber were categorized as preferring that chamber; those failing to meet criteria were categorized as having no preference. Thus, each individual subject was categorized into one of four preference categories: preference for the pup-associated, center, or empty chamber, or no preference. Within-groups. In each group, pre- and post-conditioning chamber preferences were compared using a chi-square goodness-of-fit test for specified proportions. Pre- and postconditioning chamber times were compared with a two-way ANOVA (chamber and session as repeated measures). Between-groups. Chamber preferences within a session were compared using Fisher’s exact tests or one-tailed tests for significance of difference between two proportions, and chamber times within a session were compared using a two-way ANOVA (chamber as repeated).
2.5.2. Maternal behavior scores
The mean latency to express fully maternal behavior was calculated as a percentage of the total number of pup-exposure days, in order to standardize data across pup-exposure groups, and compared using a one-way independent ANOVA followed by Tukey’s posthoc tests. After CPP testing, prolonged and brief pup-exposure females were separated by their preference for the pup-associated (prolonged n=5; brief n=3) or empty chamber (prolonged n=4; brief n=4) and mean scores within each preference group were analyzed using two-way ANOVAs (day as repeated) and Tukey’s posthocs. Preference groups did not differ on any behavioral measure, so scores were pooled and compared across days using a one-way ANOVA (day as repeated) and Tukey’s posthocs.
2.5.3. Locomotion during conditioning
Infrared beam breaks in each CPP chamber provided a measure of subjects’ locomotion during each conditioning session. Data from prolonged and brief pup-exposure females that eventually preferred the pup-associated chamber or empty chamber were compared to identify any relationships between conditioning session locomotion and conditioned chamber preference. Locomotion did not differ by chamber preference category and was pooled within each pup-exposure group. Locomotion in the empty chamber was similar across pup-exposure groups and pooled for presentation. Locomotion in the pup-associated and empty chambers was compared across pup-exposure groups using a two-way ANOVA (day as repeated) and within each pup-exposure group across chambers using paired t-tests.
3. Results
3.1. Expression of full maternal behavior
All postpartum females expressed full maternal behavior immediately after parturition (Fig. 1). No virgin female expressed full maternal behavior upon initial exposure to pups. Virgin females only expressed full maternal behavior after 8–15 days of prolonged pup-exposure, whereas females never met criterion for full maternal behavior after brief or distal pup-exposure (Fig. 1). The latency to express full maternal behavior (Fig. 2) was substantially shorter in postpartum females than any virgin female group; amongst virgin females, prolonged pup-exposure resulted in a shorter latency to fully maternal behavior than seen in brief or distal pup-exposure, during which fully maternal behavior was never expressed (F(3,38)=446.19, P<0.0001; Tukey’s: P<0.05).
Fig. 1.
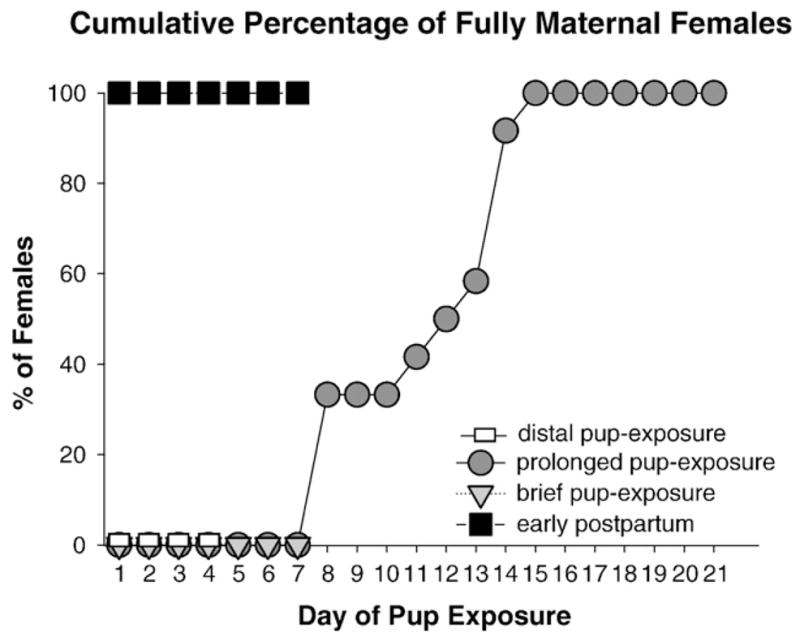
Cumulative percentage of fully maternal virgin females given brief, prolonged, or distal exposure to young pups and percentage of fully maternal postpartum females beginning immediately after parturition.
Fig. 2.
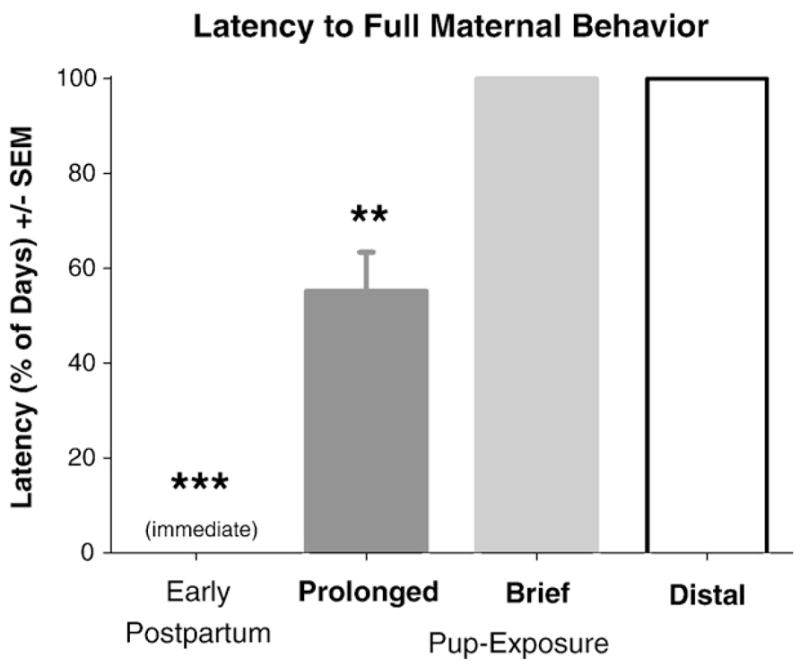
Latency, presented as the percentage of total days of pup-exposure, to express full maternal behavior in virgin females given brief, prolonged, or distal exposure to pups and in early postpartum females toward their own pups beginning immediately after parturition. The latency to express full maternal behavior was significantly shorter in postpartum females than all three groups of virgin females (***). Prolonged pup-exposure decreased the latency to full maternal behavior compared to females given brief or distal exposure to pups, which never expressed full maternal behavior (**). All P<0.05.
3.2. Conditioned place preference
3.2.1. Pre-conditioning session
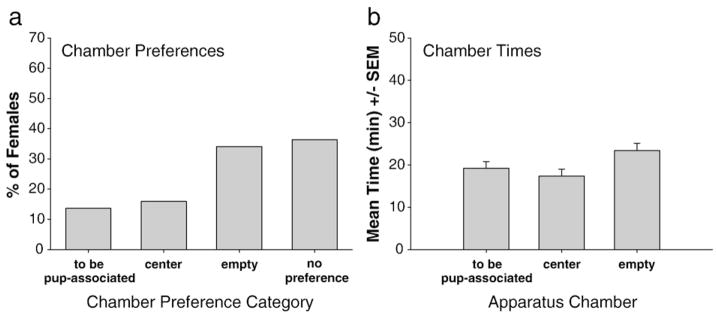
Chamber preferences and times were similar across all females and pooled. Females were equally distributed across chamber preference categories (P>0.05), with the majority of females lacking a chamber preference (36%) or preferred the empty side chamber (34%) (Fig. 3a). Females spent equal amounts of time in each chamber (P>0.05) (Fig. 3b).
Fig. 3.
Pre-conditioning chamber preferences (a) and mean time spent in each chamber (b) during the pre-conditioning (baseline) session. Data did not differ across groups of females and were pooled for presentation.
3.2.2. Confirmation of conditioning
Conditioning was confirmed in each group of females if the distribution of chamber preferences and/or the mean time spent in each chamber changed significantly between pre- and post-conditioning sessions. In all females, chamber preferences changed between sessions [distal exposure: χ2 (3, N=8)=20.10; brief: χ2 (3, N=10)=96.92; prolonged: χ2 (3, N=12)=13.44; early postpartum: χ2 (3, N=14)=18.32; all P<0.01]. In prolonged pup-exposure females, chamber times also changed [chamber×session: F(2,22)=3.73, P<0.05].
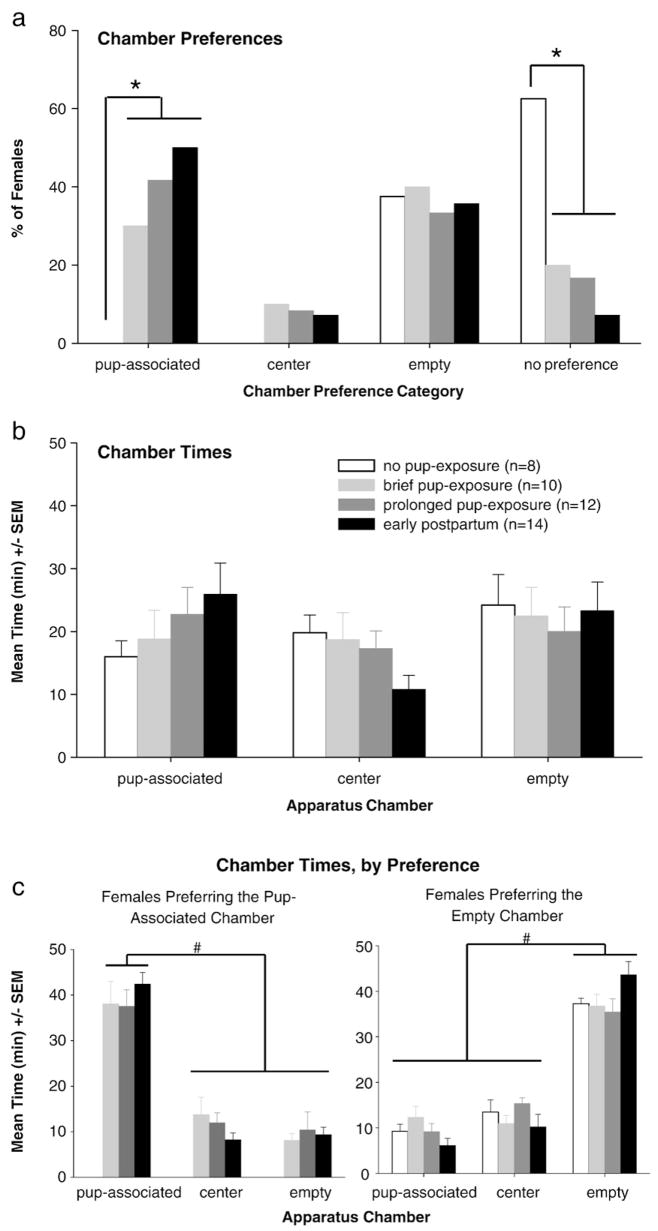
3.2.3. Post-conditioning session
After conditioning, chamber preferences were strikingly similar between early postpartum females and virgin females given prolonged pup-exposure. Specifically, substantial preference for the pup-associated chamber emerged in early postpartum females (50%) and in virgin females given prolonged pup-exposure (42%) (Fig. 4a). Pup-associated chamber preference was slightly reduced in virgin females given only brief pup-exposure (33%). No (0%) virgin females preferred the pup-associated chamber following distal pup-exposure, which was significantly less than any other group (Fisher’s: P<0.05; prolonged: z=2.12; brief: z=1.70; postpartum: z=2.40; all P<0.05); instead, most (64%) of these females lacked chamber preference, more than in any other group (pooled groups, as did not differ: z=−2.96; P<0.01). No differences emerged between chamber times in any group (Fig. 4b).
Fig. 4.
Post-conditioning chamber preferences (a), mean time spent in each chamber by all females (b), and mean time spent in each chamber by only females preferring the pup-associated or empty chambers (c) during the post-conditioning (test) session. Between-group differences within each chamber (*) and within-group differences between chambers (#) are identified; all P<0.05.
Data from all females were pooled and separated by preference for additional analyses. Females preferring the pup-associated chamber (n=15) spent substantially more time in that chamber than the empty chamber [F(2,28)=80.36, P<0.0001], whereas females preferring the empty chamber (n=16) spent more time in that chamber than the pup-associated chamber [F(2,30)=113.30, P<0.0001] (Fig. 4c).
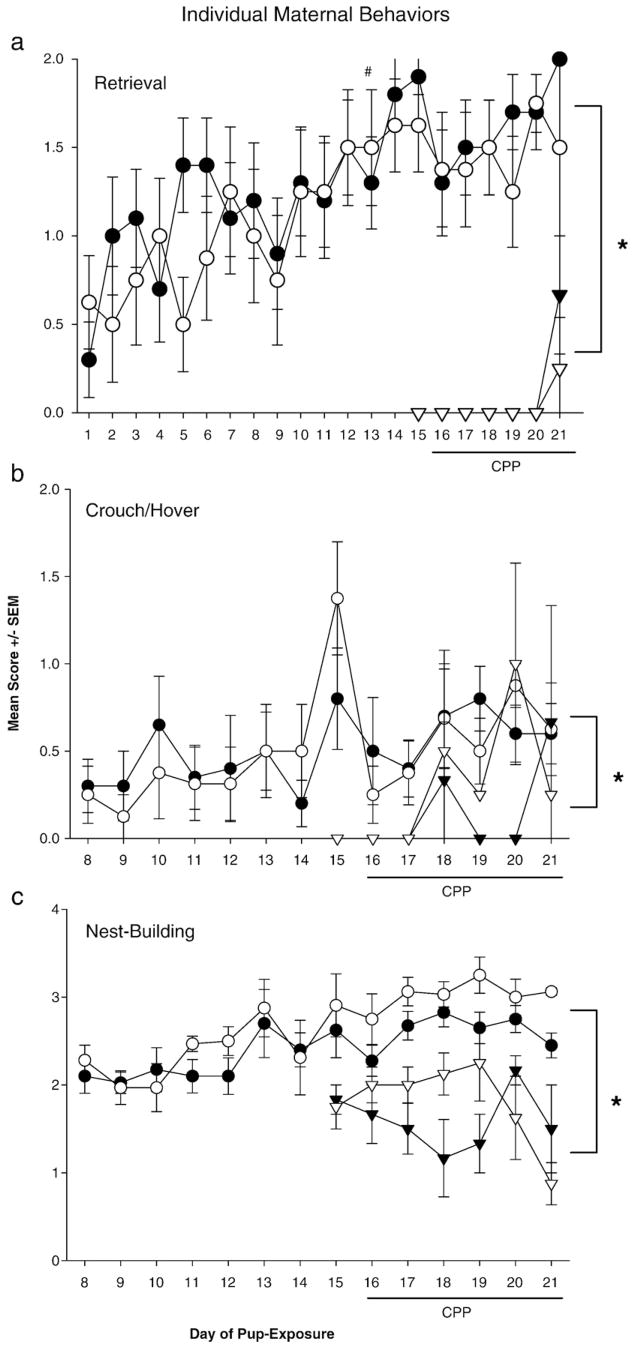
3.3. Emergence and strength of individual maternal behaviors
Virgin females given prolonged or brief pup-exposure expressed a range of individual maternal behaviors toward pups (Fig. 5). Retrieval scores of females given prolonged pup-exposure improved significantly across the pup-exposure period [retrieval on days 1–13; F (12,96)=2.22, P<0.05; Tukey’s: P <0.05] and retrieval and nest-building scores were consistently maternal across the final 11–13 days of pup-exposure. Females given brief pup-exposure never attained consistently maternal scores on any behavior, though inconsistent retrieval, nest-building, and crouching/hovering behaviors were observed on various days. All daily scores were reliably and substantially higher in females given prolonged pup-exposure than those given brief pup-exposure [main group effect: retrieval, F(1,17)= 163.94; crouch/hover, F(1,17)=10.37; nest, F(1,17)=50.98; all P<0.05; Tukey’s: all P<0.05] (Fig. 5a–c).
Fig. 5.
Mean daily scores on retrieval (a), crouching/hovering (b) and nest-building (c) behaviors of virgin females given prolonged pup-exposure (circles) or brief pup-exposure (triangles). Scores are separated by females’ post-conditioning preference for the pup-associated (filled shapes) or empty chamber (open shapes); within each pup-exposure group, scores were similar across preferences. Retrieval scores increased significantly between days 1–13 in the prolonged pup-exposure group (#). Scores of females given prolonged and brief pup-exposure differed every day (*). All P<0.05.
To examine the relationship between the expression of individual behaviors and the subjects’ post-conditioning chamber preference, daily maternal behavior scores were separated by each female’s preference for the pup-associated or empty chamber (Fig. 5a–c). Both preference groups achieved similar scores on each behavior and did not differ in onset (i.e. first day of expression) of behavior, regardless of pup-exposure length.
3.4. Locomotion during conditioning
As expression of maternal behaviors differed dramatically in females given prolonged and brief pup-exposure across CPP conditioning, locomotion within the pup-associated chamber during the first and last conditioning sessions was analyzed in these groups. On the first conditioning day, locomotion in the pup-associated chamber was higher in females given prolonged pup-exposure than those given brief pup-exposure [F(1,12)=119.37, P<0.0001] and was higher than in the empty chamber only in the prolonged pup-exposure group [t(9)=4.96, P<0.01] (Fig. 6). On the last conditioning day, locomotion in the pup-associated chamber was similar, regardless of pup-exposure length, and was higher than in the empty chamber [t(16)=3.07, P<0.01]. Between the first and last conditioning days, locomotion in the empty chamber decreased in both pup-exposure groups [t(16)=3.17, P<0.01] whereas locomotion in the pup-associated chamber decreased only in females given prolonged pup-exposure [t(9)=2.88, P<0.05].
Fig. 6.
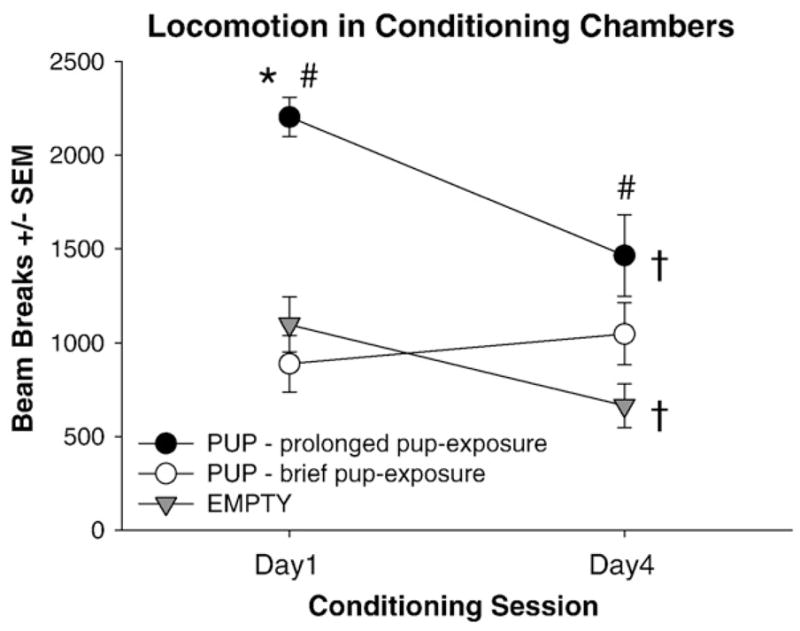
Mean locomotion within the pup-associated and empty chamber during the first and last conditioning sessions of virgin females given prolonged or brief exposure to pups. Locomotion within the pup-associated chamber (PUP) is separated into females given prolonged (filled circles) or brief (open circles) pup-exposure. Locomotion within the empty chamber (EMPTY) did not differ across pup-exposure and was pooled (grey triangles). Between-group differences within chambers (*) and within-group differences between chambers (#) and within each chamber across days (†) are identified; all P<0.05.
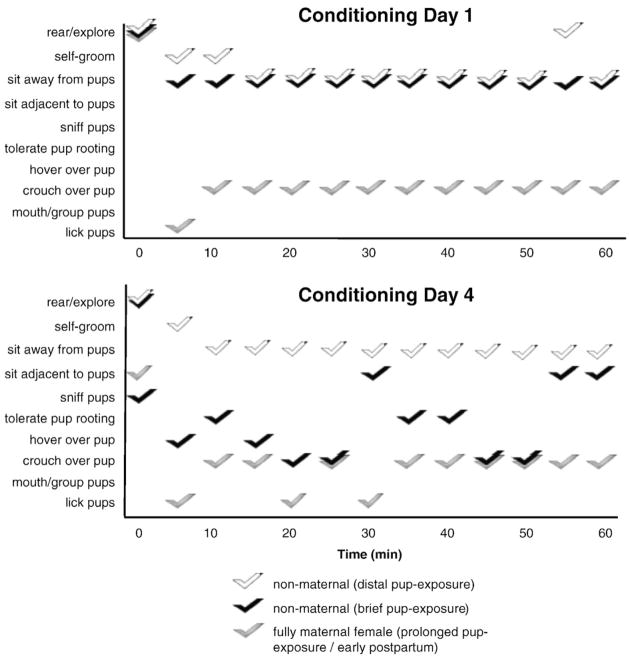
3.5. Maternal behavior during conditioning
Select females were observed and their behaviors recorded every 5 min throughout the first and last pup-conditioning sessions. Behavior raster plots were compiled for three representative females, typifying a fully maternal individual (prolonged and early postpartum), a non-maternal individual with limited pup-exposure (brief), and a non-maternal individual lacking full exposure to pups (distal) (Fig. 7). Across both conditioning sessions, maternally responsive females (prolonged and early postpartum) expressed mostly pup-directed, maternal behaviors, whereas non-maternal females (distal pup-exposure) avoided the mesh bag containing pups. Non-maternal females given brief pup-exposure shifted notably from avoidant to pup-tolerant behaviors between the first and last conditioning days. In all pup-exposure groups, behavioral patterns during conditioning did not correspond with female’s eventual chamber preference.
Fig. 7.
Behavior raster plot for three representative females during the first and last conditioning sessions in the pup-associated chamber. Observed behaviors were typical for all maternally responsive (prolonged pup-exposed and early postpartum) females (light grey), non-maternal females with brief pup-exposure (black) and females with only distal pup-exposure (white).
4. Discussion
Virgin females will strongly prefer a pup-associated chamber after being exposed to pups for prolonged periods of time (24 h/day for 21 days), rivaling the robust pup-associated chamber preference seen in parturient females during the early postpartum period, when maternal motivation is strongest [9,10,24,37]. Fewer virgin females (33%) exposed to pups for relatively brief periods of time (1 h/day for 7 days) preferred the pup-associated chamber, compared to virgin females given prolonged pup-exposure (42%) and, particularly, early postpartum females (50%), revealing a subtle stepwise relationship between length of pup-exposure and strength of maternal motivation. No females lacking full exposure to pups and receiving only distal (auditory and olfactory) pup-cues preferred the pup-associated chamber. This work reveals, for the first time, that even brief exposure to pups is sufficient to promote maternal motivation in a strong subpopulation of virgin females and that this maternal motivation can be enhanced to postpartum levels by increasing the length of exposure to pups.
Importantly, our prolonged pup-exposure regimen is the closest published replication of the natural behavioral patterns of the parturient female during the first eight days of the postpartum period, when the strength of her maternal motivation peaks [9,10,24,37]. First, the maternal female expresses vigorous, consistent maternal behaviors toward her pups during the early postpartum period [1]. In the present study, virgin females given prolonged pup-exposure expressed maternal behavior that improved significantly over time and was maximally established by CPP testing. That nearly half of these virgin females preferred the pup-associated chamber at CPP test suggests that maximizing females’ maternal responsivity may increase her attribution of incentive value to pups.
Second, the parturient female spends the majority of her time with her pups during the early postpartum period [2–5,24]. In the present study, females were only deprived of pups for relatively brief, biologically relevant periods (2 h) prior to pup-conditioning [10,24]. Our data demonstrates, for the first time, that dramatic pup-deprivation is not necessary for virgin females to strongly prefer a pup-associated chamber [17]. As even parturient females will not prefer a pup-associated chamber following brief pup-deprivations in late postpartum, when increasing amounts of time are spent away from pups and maternal care declines [24], maternal motivation in virgin females can actually surpass that of the naturally parturient female in late postpartum.
Together, our data demonstrate that, by mimicking the natural behavioral patterns seen in early postpartum, virgin females can express strong maternal motivation that rivals that of the parturient female. We conclude that maternal motivation is not established exclusively by the complex neuroendocrine states of gestation, parturition, and postpartum, or by the physiological, behavioral, and polysensory experiences of parity.
Additionally, many virgin females given brief pup-exposure, which never expressed fully maternal behavior, expressed strong preference for the pup-associated chamber. These non-maternal females expressed a markedly reduced locomotor response during the first pup-conditioning session compared to the fully maternal females given prolonged pup-exposure and expressed mostly freezing and/or avoidant behavior toward pups. By the last conditioning session, brief pup-exposure females were increasingly tolerant of pup-initiated physical interaction and no longer expressed freezing/avoidant behaviors, and locomotor response to pups matched that of prolonged pup-exposure females. We posit that the emergence of these inconsistent, maternal-like behaviors represents a transitional state of maternal responsivity during which pups shift from an aversive to a neutral or slightly attractive stimulus to the female. Females given brief pup-exposure did not express locomotor habituation across pup-conditioning sessions, as did prolonged pup-exposure females, indicating that the females’ perception of the pup stimulus differed dramatically across these four exposures [38]. We posit that the initial tolerance for pup-initiated contact (e.g. rooting) represents an emerging transition in the motivational state of the female that is potentiated by the changing incentive-motivational status of pups and ultimately expressed as a drive to initiate contact and interact with pups.
Notably, the motivational transition observed in virgin females given brief pup-exposure was not observed in females given only distal exposure to a limited range of pup-related stimuli. While both groups of females were completely non-maternal, the olfactory and auditory cues provided by distal pup-exposure were insufficient to initiate a similar shift in the incentive-motivational value of pups to the distally exposed female. We posit that the limited time spent in the pup-associated chamber by these distally exposed females reflects their sub-threshold attraction to the novel odors and vocalizations of pups enclosed within the mesh bag [39].
Together, these findings suggest that pup-associated chamber preference in the virgin female requires a basal level of physical interaction between the female and pups, which distal pup-exposure prevents. Pups confined inside a mesh bag were prevented from initiating physical contact with these non-maternal virgin females, e.g. rooting at females’ ventrum and nipples, whereas pup-initiated contact occurred in all other groups of females, even those given brief pup-exposure, with contact reciprocated and supplemented by maternally responsive females. Prior work by Fleming and colleagues [7,25] suggests that maternal motivation is contingent upon direct female-pup interactions: bar-pressing for access to pups declines dramatically if pups cannot be retrieved upon delivery [7] and postpartum females will not prefer a pup-associated chamber if prevented from interacting with pups in that chamber [25]. Our data importantly extend these findings by revealing that physical interactions can be initiated by the maternally responsive female (i.e. during prolonged pup-exposure) or by pups (i.e. during brief pup-exposure) and must include a complete range of polysensory input in order to promote maternal motivation in both virgin and parturient females.
It is also important to note that the expression maternal behavior, an important correlate to our appetitive-motivational CPP measures, remained remarkably consistent between virgin females preferring the pup-associated or empty chamber. At no point did any female’s maternal behavior in the homecage or behavioral patterns recorded during pup-conditioning predict or correlate with her preference for the pup-associated or empty chamber; this dissociation was evident across our novel, prolonged pup-exposure regimen, including the 15 days prior to CPP, the six days of CPP (Fig. 5), and during each pup-conditioning session (data not shown; see Fig. 7 for exemplar). Strongly motivated, ‘active’ maternal behaviors (retrieval and nest-building) [12,13,22,37], which were expressed consistently after prolonged pup-exposure and were emerging after brief pup-exposure, did not predict any females’ preference for the pup-associated chamber. Thus, even well-established, motivated caretaking behaviors do not predict a virgin females’ maternal motivation and that behavior and motivation remain distinguishable components of the maternal state, in accord with others’ work [7,17,40].
As females are the primary providers of parental care in rodents and most other small mammals, many factors may mediate the shift of the incentive-motivational value of pups from aversive to attractive, initiate the emergence of a drive to interact with pups in the non-maternal female, and promote the expression of a range of maternal behaviors that further strengthen maternal motivation in the female [1,2]. These factors include neurobiological, environmental, and experiential factors, which act in concert to regulate maternal responsivity in the female rat. The present work uniquely addresses how two of these critical factors, postpartum status and exposure to pups, affect maternal motivation in the female rat, a model that is uniquely evolutionarily primed to respond to changes in the incentive-motivational value of pups.
It has been proposed that females’ motivated behavior may be influenced by dynamic differences in levels of gonadal hormones and lactogenic peptides in cycling and postpartum females. While preference for a pup-associated chamber is strongest in gonadally intact (versus ovariectomized) females [17], this preference is likely attributable to hormonally facilitated female-pup interactions [1,15,17,23]. In the present study, pup-exposed virgin and postpartum females expressed comparable preference for the pup-associated chamber, despite dramatic neuroendocrine differences [41–45]. Furthermore, CPP is relatively insensitive to differences across the estrus cycle [30], suggesting that any perseverative changes in gonadal hormone levels induced by pups [41] do not influence CPP, either. Mere exposure to pups can up-regulate long-form prolactin receptor mRNA in both virgin and postpartum females [46]; circulating prolactin levels are substantially higher in females expressing pup-induced maternal behavior than in non-maternal females exposed briefly to pups [41,47]. Prolactin has been proposed as a likely factor promoting maternal motivation [2], and it is possible that pup-exposed virgin females that prefer the pup-associated chamber express higher levels of centrally acting prolactin.
The mesolimbic dopamine (DA) system also contributes importantly to the expression of highly motivated maternal behaviors [6,33,48–56] and, as emerging evidence suggests, the attribution of incentive-motivational value to pups in the parturient female [6,17]. Dopamine release into the nucleus accumbens increases markedly during bouts of licking/grooming [57,58], while blocking dopamine transmission impairs both licking and motivated pup retrieval [2,48–52]. Recent work indicates that dopamine antagonism blocks the acquisition of pup-associated chamber preference in postpartum females [17], whereas stimulation of D1 receptors can promote it [2,6]. Thus, we posit that individual differences in D1-receptor responsivity may modulate the incentive value attributed to pups by the maternally responsive female. This hypothesis was supported by a recent study [33] revealing that accumbal dopamine is chronically elevated in maternally responsive virgin females; we posit that this persistent increase in mesolimbic dopamine may contribute to strong maternal motivation in our virgin females given prolonged pup-exposure. Also, as reunion with pups transiently increases accumbal dopamine in the maternal female [57,58], we further posit that such increases in dopamine may also enhance the incentive-motivational value of pups during each pup-conditioning session, facilitating the shift in incentive-motivational value of pups in our virgin females given brief pup-exposure.
It is possible that pup-induced elevations in dopaminergic transmission may have enhanced associative learning in all pup-exposed females [59], thereby preventing most females given only distal pup-exposure from strongly preferring a conditioning chamber. However, pup-associated stimuli (e.g. odors, vocalizations) and novel stimuli, such as the mesh bag containing pups, elicit arousal in females and depends upon mesolimbic dopamine activity [60,61]. Thus, whether in the form of vigorous caretaking behaviors (maternal females) or as mild anxiety (non-maternal females) [1], we believe that attention and learning of conditioned associations (e.g. CPP) was similarly enhanced in all females in this study [62].
Ultimately, the present study reveals, for the first time, striking levels of maternal motivation in the virgin female rat. This motivation is contingent upon physical interactions between the female and pups, including full exposure to a broad spectrum of polysensory stimuli associated with pups. Furthermore, maternal motivation can be enhanced by systematically extending the length of time that females are exposed to pups. Early work revealed that consistent, repeated exposures to a stimulus can increase the attractiveness of that stimulus [63]; we posit that the systematic increases in maternal motivation seen in the present study may be attributable to the systematic increases in pup-exposure length and, as a consequence, the quality of maternal behavior. This critical shift in incentive-motivational value of pups from aversive to attractive may be attributable to endocrinological, neurochemical and behavioral changes emerging during initial pup-exposures. Importantly, this motivational shift contributes critically to pups’ survival and health by promoting motivated caregiving and, as revealed in the present study, can occur equally in both virgin and parturient female rats.
Acknowledgments
This research was supported by NIH DA014025 and March of Dimes #12FY02-05-103 and #12FY05-06-134 awarded to J.I.M. The authors thank Jonathan Perez for assistance with data collection and the Laboratory Animal Facility staff of Rutgers University, Newark NJ, for animal breeding and care. All experiments described within this manuscript comply with the current laws of the country in which they were performed. The authors have no conflicts of interest, financial or otherwise, pertaining to any aspect of the work reported in this manuscript.
References
- 1.Numan M, Insel T. The neurobiology of parental behavior. New York: Springer-Verlag; 2003. [Google Scholar]
- 2.Pereira M, Seip KM, Morrell JI. The neurobiology of the parental mind. Amsterdam: Elsevier; 2008. Maternal motivation and its neural substrate across the postpartum period. [Google Scholar]
- 3.Grota LJ, Ader R. Continuous recording of maternal behaviour in Rattus norvegicus. Anim Behav. 1969;17:722–9. doi: 10.1016/0003-3472(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 4.Grota LJ, Ader R. Behavior of lactating rats in a dual-chambered maternity cage. Horm Behav. 1974;5:275–82. doi: 10.1016/0018-506x(74)90014-2. [DOI] [PubMed] [Google Scholar]
- 5.Reisbick S, Rosenblatt JS, Mayer AD. Decline of maternal behavior in the virgin and lactating rat. J Comp Physiol Psychol. 1975;89:722–32. doi: 10.1037/h0077059. [DOI] [PubMed] [Google Scholar]
- 6.Pereira M, Dziopa E, Morrell JI. Expression of maternal behavior during the late postpartum period: effect of D1 dopamine receptor stimulation. Parent Brain Conf. 2007:P2. [Google Scholar]
- 7.Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 2000;108:215–31. doi: 10.1016/s0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- 8.Wilsoncroft WE. Babies by bar-press: maternal behavior in the rat. Behav Res Meth Instrum. 1969;1:229–30. [Google Scholar]
- 9.Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–94. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- 10.Seip KM, Morrell JI. Increasing the incentive salience of cocaine challenges preference for pup- over cocaine-associated stimuli during early postpartum: place preference and locomotor analyses in the lactating female rat. Psychopharmacology. 2007;194:309–19. doi: 10.1007/s00213-007-0841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bridges R, Zarrow MX, Gandelman R, Denenberg VH. Differences in maternal responsiveness between lactating and sensitized rats. Dev Psychobiol. 1972;5:123–7. doi: 10.1002/dev.420050205. [DOI] [PubMed] [Google Scholar]
- 12.Stern JM, Mackinnon DA. Postpartum, hormonal, and nonhormonal induction of maternal behavior in rats: effects on T-maze retrieval of pups. Horm Behav. 1976;7:305–16. doi: 10.1016/0018-506x(76)90036-2. [DOI] [PubMed] [Google Scholar]
- 13.Pereira M, Uriarte N, Agrati D, Zuluaga MJ, Ferreira A. Motivational aspects of maternal anxiolysis in lactating rats. Psychopharmacology. 2005;180:241–8. doi: 10.1007/s00213-005-2157-y. [DOI] [PubMed] [Google Scholar]
- 14.Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–4. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- 15.Fleming AS, Rosenblatt JS. Maternal behavior in the virgin and lactating rat. J Comp Physiol Psychol. 1974;86:957–72. doi: 10.1037/h0036414. [DOI] [PubMed] [Google Scholar]
- 16.Terkel J, Rosenblatt JS. Aspects of nonhormonal maternal behavior in the rat. Horm Behav. 1971;2:161–71. [Google Scholar]
- 17.Fleming AS, Korsmit M, Deller M. Rat pups are potent reinforcers to the maternal animal: effects of experience, parity, hormones and dopamine function. Psychobiology. 1994;22:44–53. [Google Scholar]
- 18.Mayer AD, Rosenblatt JS. Hormonal influences during the ontogeny of maternal behavior in female rats. J Comp Physiol Psychol. 1979;93:879–98. [Google Scholar]
- 19.Mayer AD, Freeman NC, Rosenblatt JS. Ontogeny of maternal behavior in the laboratory rat: factors underlying changes in responsiveness from 30 to 90 days. Dev Psychobiol. 1979;12:425–39. doi: 10.1002/dev.420120503. [DOI] [PubMed] [Google Scholar]
- 20.Olazabal DE, Kalinichev M, Morrell JI, Rosenblatt JS. MPOA cytotoxic lesions and maternal behavior in the rat: effects of midpubertal lesions on maternal behavior and the role of ovarian hormones in maturation of MPOA control of maternal behavior. Horm Behav. 2002;41:126–38. doi: 10.1006/hbeh.2001.1753. [DOI] [PubMed] [Google Scholar]
- 21.Bridges R, Zarrow MX, Gandelman R, Denenberg VH. Differences in maternal responsiveness between lactating and sensitized rats. Dev Psychobiol. 1972;5:123–7. doi: 10.1002/dev.420050205. [DOI] [PubMed] [Google Scholar]
- 22.Lonstein JS, Wagner CK, De Vries GJ. Comparison of the “nursing” and other parental behaviors of nulliparous and lactating female rats. Horm Behav. 1999;36:242–51. doi: 10.1006/hbeh.1999.1544. [DOI] [PubMed] [Google Scholar]
- 23.Fleming AS, Sarker J. Experience–hormone interactions and maternal behavior in rats. Physiol Behav. 1990;47:1165–73. doi: 10.1016/0031-9384(90)90368-e. [DOI] [PubMed] [Google Scholar]
- 24.Wansaw MP, Pereira M, Morrell JI. Characterization of maternal motivation in the lactating rat: contrasts between early and late postpartum responses. Horm Behav. 2008;54(2):294–301. doi: 10.1016/j.yhbeh.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnusson JE, Fleming AS. Rat pups are reinforcing to the maternal rat: role of sensory cues. Psychobiology. 1995;23:69–75. [Google Scholar]
- 26.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 28.Salamone JD, Correa M. Motivational view of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:2–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- 29.National Institutes of Health Guide for Care and Use of Laboratory Animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- 30.Seip KM, Pereira M, Wansaw MP, Reiss JI, Dziopa EI, Morrell JI. Incentive salience of cocaine remains remarkably stable across the postpartum period of the female rat, yet differs from virgin female and male responses. Psychopharmacology. 2008;199:119–30. doi: 10.1007/s00213-008-1140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker QD, Nelson CJ, Smith D, Kuhn C. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–52. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- 32.Stern JM, Mackinnon DA. Sensory regulation of maternal behavior in rats: effects of pup age. Dev Psychobiol. 1978;11:579–86. doi: 10.1002/dev.420110607. [DOI] [PubMed] [Google Scholar]
- 33.Afonso VM, Grella SL, Chatterjee D, Fleming AS. Previous maternal experience affects accumbal dopaminergic responses to pup-stimuli. Brain Res. 2008;1198:115–23. doi: 10.1016/j.brainres.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 34.Blumberg MS, Alberts JR. Both hypoxia and milk deprivation diminish metabolic heat production and ultrasound emission by rat pups during cold exposure. Behav Neurosci. 1991;105:1030–7. doi: 10.1037//0735-7044.105.6.1030. [DOI] [PubMed] [Google Scholar]
- 35.Hofer MA, Shair H. Ultrasonic vocalization during social interaction and isolation in 2-weeek-old rats. Dev Psychobiol. 1978;11:495–504. doi: 10.1002/dev.420110513. [DOI] [PubMed] [Google Scholar]
- 36.Pereira M, Ferreira A. Demanding pups improve maternal behavioral impairments in sensitized and haloperidol-treated lactating female rats. Behav Brain Res. 2006;175:139–48. doi: 10.1016/j.bbr.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- 38.Mazur JE. Learning and behavior. 6. Upper Saddle River, NJ: Prentice-Hall; 2005. [Google Scholar]
- 39.Bardo MT, Neisewander JL, Pierce RC. Novelty-induced place preference behavior in rats: effects of opiate and dopaminergic drugs. Pharmacol Biochem Behav. 1989;32:683–9. doi: 10.1016/0091-3057(89)90018-x. [DOI] [PubMed] [Google Scholar]
- 40.Mattson BJ, Williams SE, Rosenblatt JS, Morrell JI. Preference for cocaine- or pup-associated chambers differentiates otherwise behaviorally identical postpartum maternal rats. Psychopharmacology. 2003;167:1–8. doi: 10.1007/s00213-002-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marinari KT, Moltz H. Serum prolactin levels and vaginal cyclicity in concaveated and lactating remale rats. Physiol Behav. 1978;21:525–8. doi: 10.1016/0031-9384(78)90124-5. [DOI] [PubMed] [Google Scholar]
- 42.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–26. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 43.Smith MS, Neill JD. Inhibition of gonadotropin secretion during lactation in the rat: relative contribution of suckling and ovarian steroids. Biol Reprod. 1977;17:255–61. doi: 10.1095/biolreprod17.2.255. [DOI] [PubMed] [Google Scholar]
- 44.Taya K, Greenwald GS. Peripheral blood and ovarian levels of sex steroids in the lactating rat. Endocrinol Jpn. 1982;29:453–9. doi: 10.1507/endocrj1954.29.453. [DOI] [PubMed] [Google Scholar]
- 45.Grota LJ, Eik-Nes KB. Plasma progesterone concentrations during pregnancy and lactation in the rat. J Reprod Fertil. 1967;13:83–91. doi: 10.1530/jrf.0.0130083. [DOI] [PubMed] [Google Scholar]
- 46.Sugiyama T, Minoura H, Toyoda N, Sakaguchi K, Tanaka M, Sudo S, et al. Pup contact induces the expression of long form prolactin receptor mRNA in the brain of female rats: effects of ovariectomy and hypophysectomy on receptor gene expression. J Endocrinol. 1996;149:335–40. doi: 10.1677/joe.0.1490335. [DOI] [PubMed] [Google Scholar]
- 47.Bridges RS, Goldman BD, Bryant LP. Serum prolactin concentrations and the initiation of maternal behavior in the rat. Horm Behav. 1974;5:219–26. doi: 10.1016/0018-506x(74)90030-0. [DOI] [PubMed] [Google Scholar]
- 48.Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, et al. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in the rat. Behav Neurosci. 2005;119:1588–604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- 49.Byrnes EM, Rigero BA, Bridges RS. Dopamine antagonists during parturition disrupt maternal care and the retention of maternal behavior in rats. Pharmacol Biochem Behav. 2002;73:869–75. doi: 10.1016/s0091-3057(02)00941-3. [DOI] [PubMed] [Google Scholar]
- 50.Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67:659–69. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- 51.Silva MR, Bernardi MM, Felicio LF. Effects of dopamine receptor antagonists on ongoing maternal behavior in rats. Pharmacol Biochem Behav. 2001;68:461–8. doi: 10.1016/s0091-3057(01)00471-3. [DOI] [PubMed] [Google Scholar]
- 52.Pereira M, Ferreira A. Demanding pups improve maternal behavioral impairments in sensitized and haloperidol-treated lactating female rats. Behav Brain Res. 2006;175:139–48. doi: 10.1016/j.bbr.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 53.Gaffori O, Le Moal M. Disruption of maternal behavior and appearance of cannibalism after ventral mesencephalic tegmentum lesions. Physiol Behav. 1979;23:317–23. doi: 10.1016/0031-9384(79)90373-1. [DOI] [PubMed] [Google Scholar]
- 54.Giordano AL, Johnson AE, Rosenblatt JS. Haloperidol-induced disruption of retrieval behavior and reversal with apomorphine in lactating rats. Physiol Behav. 1990;48:211–4. doi: 10.1016/0031-9384(90)90288-f. [DOI] [PubMed] [Google Scholar]
- 55.Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci. 1991;105:588–98. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- 56.Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. The effects of 6-OHDA-induced dopamine depletions in the ventral or dorsal striatum on maternal and sexual behavior in the female rat. Pharmacol Biochem Behav. 1991;39:71–7. doi: 10.1016/0091-3057(91)90399-m. [DOI] [PubMed] [Google Scholar]
- 57.Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45:673–6. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- 58.Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–23. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alcaro A, Huber R, Panksepp J. Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev. 2007;56:283–321. doi: 10.1016/j.brainresrev.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776:61–7. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- 61.Saigusa T, Tuinstra T, Koshikawa N, Cools AR. High and low responders to novelty: effects of a catecholamine synthesis inhibitor on novelty-induced changes in behaviour and release of accumbal dopamine. Neuroscience. 1999;88:1153–63. doi: 10.1016/s0306-4522(98)00275-9. [DOI] [PubMed] [Google Scholar]
- 62.Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol. 1908;18:459–82. [Google Scholar]
- 63.Zajonc RB. Attitudinal effects of mere exposure. J Pers Soc Psychol. 1968;8:1–29. [Google Scholar]