Abstract
This investigation used a non-randomized controlled design to evaluate the effect and feasibility of a mindfulness based stress reduction (MBSR) program on immune function, quality of life (QOL), and coping in women recently diagnosed with breast cancer. Early stage breast cancer patients, who did not receive chemotherapy, self-selected into an 8-week MBSR program or into an assessment only, control group. Outcomes were evaluated over time. The first assessment was at least 10 days after surgery and prior to adjuvant therapy, as well as before the MBSR start-up. Further assessments were mid-MBSR, at completion of MBSR, and at 4-weeks post MBSR completion. Women with breast cancer enrolled in the control group (Non-MBSR) were assessed at similar times. At the first assessment (i.e., before MBSR start), reductions in peripheral blood mononuclear cell NK cell activity (NKCA) and IFN gamma production with increases in IL-4, IL-6, and IL-10 production and plasma cortisol levels were observed for both the MBSR and Non-MBSR groups of breast cancer patients. Over time women in the MBSR group re-established their NKCA and cytokine production levels. In contrast, breast cancer patients in the Non-MBSR group exhibited continued reductions in NKCA and IFN gamma production with increased IL-4, IL-6, and IL-10 production. Moreover, women enrolled in the MBSR program had reduced cortisol levels, improved QOL, and increased coping effectiveness compared to the Non-MBSR group. In summary, MBSR is a program that is feasible for women recently diagnosed with early stage breast cancer and the results provide preliminary evidence of beneficial effects of MBSR on immune function, QOL, and coping effectiveness.
Keywords: Breast cancer, NK cell activity, Cytokines, Coping, Optimistic coping, Supportant coping, Quality of life, Psychoneuroimmunology
1. Introduction
Cancer diagnosis of any type evokes fear and dread, but for women a diagnosis of breast cancer is an especially devastating emotional experience (Shapiro, et al., 2001; Stark and House, 2000), as breast cancer is the second leading cause of deaths due to cancer in American women (ACS, 2007). Anxiety, fear, depression and uncertainty are prevalent at diagnosis (Northouse, 1992; Spiegel, 1996, 1997; Stark and House, 2000; Witek-Janusek, et al., 2007) and distress typically intensifies with treatment burden (Berger, 1998; Berger, et al., 2007; Maraste et al., 1992; Nail and Winningham, 1995; Schreier and Williams, 2004; Theobald, 2004). The emotional response to breast cancer is independent of disease stage, as women diagnosed with non-invasive breast cancers also experience powerful emotions (Witek-Janusek, et al., 2007; Northouse, 1992; Rakovitch, et al., 2003). The emotional impact of a cancer diagnosis and the rigors of cancer treatment adversely affect quality of life (QOL) and this may persist beyond treatment (Longman, et al., 1999). Evidence demonstrates that psychosocial variables during diagnosis and treatment are key predictors of both short-term and long-term quality of life (Carver, et al., 2005, 2006) and emphasize the importance of early psychosocial intervention for individuals diagnosed with cancer.
Emotional distress activates neuroendocrine stress response systems and increases stress hormone secretion (Chrousos and Gold, 1992; Chrousos, 2000). Stress hormones are well-known to alter immune function (Kiecolt-Glaser, et al., 2002; Sanders and Kavelaars, 2007; Schoneveld and Cidlowski, 2007). Breast cancer diagnosis and treatment leads to psychological and immunological disturbance. Women who report greater subjective stress after breast cancer surgery, but prior to adjuvant therapy, have lower basal and interferon (IFN) augmented NK cell activity (NKCA) and reduced T cell proliferative response to mitogens (Andersen, et al., 1998, 2004). We have previously shown that this stress-induced immune dysregulation begins early in the diagnostic phase, as women stressed by the experience of breast biopsy have lower production of IFN gamma but increased production of IL-4, IL-10 and IL-6, when compared to non-biopsied control women (Witek-Janusek et al., 2007). This increase in IL-4 and IL-10 is similar to results of other studies in non-cancer populations that demonstrated stress-associated shifts in Th1/Th2 cytokine balance toward a Th2 response (Maes, et al., 1999; Marshall, et al., 1998).
Integrative approaches to promote wellness and reduce the distress associated with cancer are increasingly considered as essential components of cancer care. Mindfulness based stress reduction (MBSR) is a program that shows promise as an approach to not only mange the emotional distress that accompanies disease, such as cancer, but to also produce biological benefits that may promote health and contribute to cancer control (Grossman, et al., 2004). MBSR, as developed and propagated by Jon Kabat-Zinn, stems from contemplative Eastern spiritual practices that use meditation to cultivate conscious awareness (i.e., mindfulness) of one’s experience in a non-judgmental or accepting manner (Kabat-Zinn, et al., 1990). In predominately non-controlled studies of individuals with a variety of medical conditions, MBSR has been shown to assist individuals to more skillfully manage emotions and somatic reactivity to life stressors (Bishop, 2002; Grossman et al., 2004). Speca and colleagues have demonstrated, using a randomized wait-list control design, that MBSR benefits individuals who continue to harbor emotional distress well-beyond their cancer diagnosis and treatment. In that study, MBSR was effective at reducing symptoms of stress and mood disturbance compared to the control group (Speca, et al., 2000). A follow-up of the combined group of MBSR participants (immediate intervention group and the wait-list MBSR participants) from the Speca et al. study showed that although the greatest psychological benefit was observed at completion of the program, effects persisted at 6 month (Carlson, et al., 2001) and at 1-year follow-up (Carlson, et al., 2007). A series of uncontrolled evaluations in cancer patients showed that MBSR improved QOL, increased sleep quality (Carlson, et al., 2003), and attenuated disturbed cortisol secretory patterns (Carlson, et al., 2004, 2007).
Given that MBSR reduces psychological distress, it is possible that it may also reverse stress-associated immune dysregulation in cancer patients. Optimal immune function is important for cancer control, especially at times when tumor burden is removed by surgery and immune mechanisms become more essential in defending against any nascent tumor cells (Avraham and Ben-Eliyahu, 2007; Lutgendorf, S., et al., 2007). The preponderance of evidence supports the importance of optimal immune function in individuals with cancer. Therefore, interventions that not only reduce psychological stress but also support immune function are advantageous to individuals with cancer.
Previously, we have shown that breast cancer diagnosis and treatment produce an increase in psychological distress (anxiety and mood disturbance) accompanied by immune dysregulation. The immune dysregulation included an increase in production of IL-4 and IL-6 and reduced NKCA and IFN gamma production (Nagabhushan, et al., 2001; Witek-Janusek et al., 2007). The dysregulation in immune function was dissociated from treatment effects and was likely due to the psychosocial distress evoked by a diagnosis of cancer and its treatment. We also conducted a study in HIV+ men to evaluate the immune effects of MBSR. We found that MBSR normalized aspects of immune function that were dysregulated in these men (Robinson, et al., 2003). Others have also reported evidence of immune benefits of MBSR. Well individuals experiencing job-related stress who participated in MBSR showed reductions in anxiety and negative affect and significantly greater antibody responses to the influenza vaccine when compared to a control group. In that study MBSR participants also exhibited increased brain electrical activity indicative of positive mood and the magnitude of change in brain activity predicted the magnitude of change in immune response (Davidson, et al., 2003). Evidence from uncontrolled studies of cancer patients, who were evaluated well-beyond diagnosis, also suggests that MBSR may effect the immune system (Carlson et al., 2003, 2007).
Given that few studies have evaluated the immune effects of MBSR in cancer patients, and to our knowledge no studies have evaluated the effects of MBSR for recently diagnosed breast cancer patients, we conducted this study. The purpose was to determine the feasibility of MBSR for women undergoing diagnosis and treatment for breast cancer and to initially assess the effect of MBSR on immune function, quality of life, and coping effectiveness in these women. We enrolled women who had completed surgery for early stage breast cancer and assessed outcomes over time, from pre to post MBSR program completion. Results are compared to women with early stage breast cancer who did not participate in MBSR but consented to an assessment only control condition.
2. Methods
2.1. Subjects
Women (35–75 years of age) diagnosed with early stage breast cancer and who did not receive systemic chemotherapy, participated in this study. The majority of these women had small lesions (< 1 cm) and uninvolved axillary nodes. Women with larger tumors or with positive axillary nodes were included if their treatment did not include chemotherapy. Eighty three percent of the women enrolled were treated with breast conserving surgery followed by radiation therapy, while the rest were treated with surgery only. Women were excluded if they had an immune-based disease, were incapable of reading and writing English, were diagnosed with psychoses, anxiety disorders or cognitive impairments, were substance abusers, were taking corticosteroids, anxiolytics or antidepressant drugs, or if they were already trained in MBSR. Inclusion and exclusion criteria for the Non-MBSR control group (usual care) were the same as those for the MBSR intervention group. In addition, a group of age-matched healthy women who did not have cancer or a history of cancer (i.e., Cancer Free) was included to provide normative data for immune variables as measured in our laboratory. These women were recruited from the local community and from the University by use of recruitment flyers and were assessed in the same manner as the groups of women with cancer. Participants were asked not to engage in other structured stress reduction programs during the course of the study. This study was approved by the Loyola University Medical Center Institutional Review Board for the Study of Human Subjects. All procedures were carried out with the adequate understanding and written consent of the subjects.
2.2. Procedures
2.2.1. Recruitment
Women with cancer were recruited from three cancer centers. Eligible women were identified (via physicians) after completion of their breast surgery and when their full surgical pathology report was available and diagnosis and treatment plans were complete. Women who expressed interest in the study were met by research staff at a scheduled clinic visit. The study was further described and those willing to participate were consented. At this time, women self-selected to either the MBSR or a Non-MBSR group that received assessments only.
2.2.2. Intervention
The MBSR program was modeled after that of Kabat-Zinn and has been previously described in detail (Kabat-Zinn et al., 1985, 1990). Briefly, the program consisted of 8-weekly (2.5 hr/wk) group sessions plus one full day session held after the 5th week. A clinical psychologist trained as an instructor in the Kabat-Zinn MBSR program, provided the instruction to all groups. Pre program interviews were conducted during which the expectations and commitments of MBSR were discussed. Specific weekly objectives and content were accomplished in the same manner for each cohort. As per the Kabat-Zinn program, mindfulness was taught through the use of breath awareness, sitting and walking meditation, and mindful yoga. Participants were provided with a standardized session-by-session program workbook containing weekly objectives and assignments, as well as two practice CDs or audiotapes and the Kabat-Zinn book, Full Castastrophe Living (Kabat-Zinn, 1990). The sessions were all provided at the University Cancer Center and were conducted in a large multipurpose room, which allowed group sitting arrangements as well as free floor space for yoga mats and meditation cushions.
2.2.3. Assessments
Immune, QOL, coping and cortisol were assessed at 4 time points. The first assessment (designated as T1; pre MBSR) was at least 10 days after surgery, as well as before the start of an 8-week MBSR training program (for the intervention group). With respect to the timing of blood sampling and radiation treatment, women were sampled within a small window of time around the start of radiation therapy. In a separate pilot study, we demonstrated radiation therapy of breast cancer patients within a similar window of time to have no observed effect on the immune system, as judged by PBMC assessment of both function and subset percentage and number as judged phenotypically. The second assessment was 4 weeks after the MBSR intervention began (T2; mid-MBSR). The third assessment occurred at the completion of MBSR training (T3). The fourth assessment took place 1 month after completion of MBSR training (T4). Assessments for breast cancer patients in the Non-MBSR group were matched in time with that of the breast cancer patients participating in MBSR. Attempts were made to collect all blood samples during the late afternoon/evening, but this was not possible in all cases. Health, medical history, cancer treatment, medication and supplement use, and demographic information were obtained at each assessment period.
2.2.4 Study Flow and Subject Retention
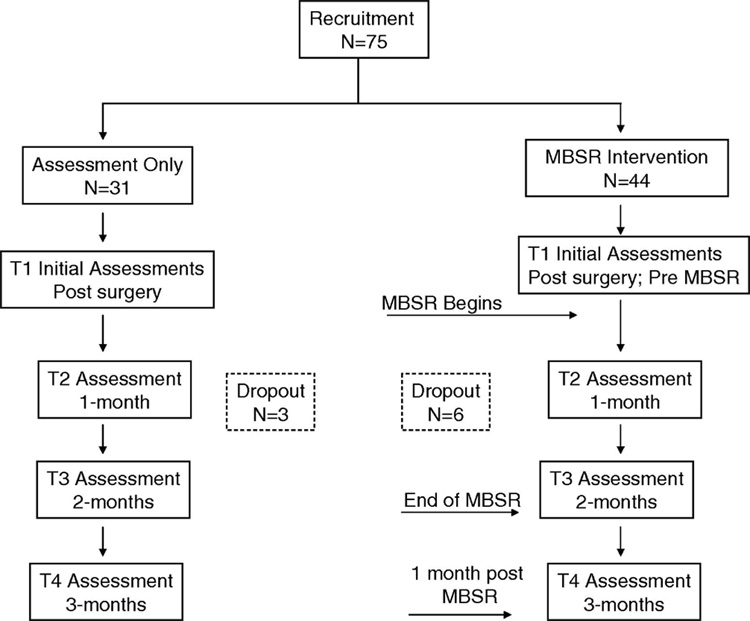
Figure 1 depicts the experimental design and study flow. Seventy-five women were enrolled (44 into the MBSR group and 31 into the control group). Of the 44 women enrolled into the MBSR group, 6 withdrew and the reasons were; 4 lacked interest in the program and 2 had transportation problems. All of these attritions occurred during the first weeks of the MBSR program and these women are not included in the analysis. Three women withdrew from the control condition for the following reasons: one did not want additional blood draws (after the first assessment), another started a different stress reduction program, and the third individual failed follow-up appointments. Age, tumor stage, and QOL scores for the women that withdrew from the study were similar to that of the women who remained in the study. Attendance of the 38 women in the 8-week MBSR program was good with 71% of the women attending 7–8 sessions, 26% attending 5–6 sessions, and 1 woman (3%) attending 4 sessions. In addition, 71% of the women in the MBSR group attended the all day session.
Figure 1.
The experimental design and study flow diagram is illustrated.
2.3 Instruments
2.3.1. Quality of Life Index Cancer Version III
The Quality of Life Index Cancer Version III evaluates quality of life in terms of an individual’s satisfaction or dissatisfaction with the areas of life that are important to him/her (Ferrans, 1990). The Quality of Life Index measures life satisfaction in four domains: health and functioning, socioeconomic, psychological/spiritual, and family. Two parts evaluate 34 items using a 6-point Likert scale. Part I measures satisfaction within each identified domain; Part II measures the perceived importance of each item. Satisfaction scores are weighted by importance. The Quality of Life Index has been used in several studies of breast cancer patients and content and construct validity is established (Ferrans and Powers, 1985). Internal consistency (Cronbach alpha) in breast cancer patients was 0.93–0.96 (Ferrans, 1990; Hughes, 1993). Criterion validity was 0.80 (Ferrans, 1990). Sensitivity to change was demonstrated in 27 studies. Test-retest reliability was 0.87 (Ferrans and Powers, 1985).
2.3.2. Jalowiec Coping Scale (JCS)
The JCS is a 60 item self report measure that assesses use and efficacy of coping behaviors. JCS allows for classification of dispositional coping style into the following categories: evasive, confrontive, fatalistic, palliative, self reliant, emotive and optimistic and has been used in cancer patients. The Cronbach alpha coefficient for the total score, based on 24 studies, was 0.86. Stability reliability was demonstrated by test-retest procedures with results ranging from 0.78 to 0.91 (Jalowiec, 1993).
2.3.3. Demographic, Health and Medical History Form
Demographic information was collected and included age, race, marital status, education, and employment status. Medical records were reviewed to obtain information regarding cancer pathology and cancer treatment. At each assessment a health history form was completed by participants and included questions related to presence of other medical conditions or diseases, current use of prescription and non-prescription medications and supplements, and occurrence of recent infectious illness.
2.3.4. Mindful Attention Awareness Scale (MAAS)
MAAS is a 15-item scale that measures the general tendency to be attentive to and aware of present moment experiences in daily life, using both general and situation specific statements. MAAS scores range from 1 to 6 and higher scores indicate greater mindfulness. Factor analyses of adult sample data show a single factor structure (Brown and Ryan, 2003) and in a cancer population, levels of internal consistency of >0.67 (Carlson and Brown, 2005).
2.4. Immune and Cortisol Measures
2.4.1. Isolation of peripheral blood mononuclear cells
Blood was collected in sterile heparinized tubes and processed immediately. Some blood samples had insufficient volume (e.g., tubes incompletely filled due to technical or venous access issues) that prevented analysis of all immune outcomes. In such cases, priority was always given to NKCA followed by cytokine analysis. Heparinized peripheral blood was overlaid onto Ficoll/Hypaque and centrifuged at 1000 × g for 20 min. The peripheral blood mononuclear cells (PBMC) at the interface were washed twice with Hank's Balanced Salt Solution prior to assessment of NKCA, phenotypic analysis, or cytokine production. Phenotypic analysis was as described previously (Nagabhushan et al., 2001). Briefly, isolated PBMCs were analyzed with specific fluorochrome conjugated antibodies in order to identify specific subsets of PBMCs including: CD3 for T lymphocytes, CD16 and/or CD56 for NK cells; CD4 for helper lymphocytes; CD8 for cytotoxic lymphocytes, and CD16 single positive cells were identified as circulating monocytes. Semi-quantitative analysis of the phenotypic expression of relevant leukocyte surface molecules was determined by immunofluorescence using a FACS Star Plus System. Antibodies reactive with surface markers were obtained as direct conjugates from BD Biosciences, San Jose, CA. Interassay variability ranges for laboratory PBMC values ranged from 1.5 – 7.9%.
2.4.2. Natural killer cell activity (NKCA)
K562 tumor cells, obtained from the American Type Culture Collection, Rockville, MD, were radioactively labeled with 100 uCi of [51Cr] (New England Nuclear, Boston, MA). Radiolabled K562 cells were incubated for 4 hr with PBMC. Following incubation, the supernatants were removed using a Skatron harvesting press (Skatron Inc., Sterling, VA) and the associated radioactivity was determined. Effector to target ratios for NKCA was 50, 25, 12 and 6:1.
Results are expressed as % cytotoxicity and calculated by the formula:
All experimental means were calculated from triplicate values. Lytic units (LU) were calculated by a program written by David Coggins, FCRC, Frederick, MD and represents the number of cells per 107 effectors required to achieve 20% lysis of the targets. *DPM=disintegrations per minute.
2.4.3. Evaluation of PBMC for cytokine production
Cytokines were measured under optimal conditions in bulk PBMC culture supernatant fluids as described previously (Witek-Janusek and Mathews, 1999). Briefly, PBMC (1 × 106 cells/ml) were cultured with and without PMA/PHA (PMA @ 20 ng/well; PHA @ 0.05%/well) in 24 well plates for 48 hr at 37 °C. Aliquots of the culture supernatants were stored at −80 °C for subsequent cytokine analysis.
2.4.4. Cytokine measurement (ELISA)
All cytokines were measured using quantitative sandwich enzyme immunoassay techniques (Quantikine kits, R & D Systems, Minneapolis, MN). Sensitivities for cytokines were; IL-2 < 7 pg/ml, IL-6 <0.7 pg/ml, IFN gamma <3 pg/ml, IL-10 < 2 pg/ml, and IL-4 < 4.1 pg/ml). The coefficient of variation ranged between 2.6 – 4.9% for the individually assessed cytokines.
2.4.5. Cortisol
To avoid confounds due to the diurnal pattern of cortisol secretion, cortisol (PM values) was measured only in those women whose blood was obtained in the late afternoon and/or evening (4–6PM). The number of women who had PM samples in the MBSR group was (T1=26, T2=25, T3=32, T4=16), while numbers for the control women were (T1=17, T2=15, T3=10, and T4=11). Cortisol was determined by radioimmunoassay using commercially available kits (Diagnostics Products Corporation, Los Angeles, CA). The sensitivity of the assay was 0.2ug/dl and the coefficient of interassay variability ranged from 3.0–4.8%.
2.5. Statistical methods
Data are expressed as means with the standard error of the mean (SEM) or standard deviation (SD) as noted. Main study variables were analyzed using repeated measures ANOVA to determine whether there were within group differences over time, between group differences (MBSR versus Non-MBSR), as well as if there were any interactions of treatment group with time. Significant outcomes were further analyzed using one-way ANOVA to determine post-MBSR program group differences at the T3 and T4 time points only. To accommodate these pre-planned comparisons, the Bonferroni correction was applied and the alpha was adjusted to 0.025. Analysis of other variables used an alpha of 0.05. The Statistical Package for Social Sciences (SPSS: version 13.0) was used for data analysis.
3. Results
3.1. Subjects
Table 1 summarizes the demographic and cancer-related variables of women in the three study groups. There was no difference in the age of women in the MBSR, Non-MBSR, or Cancer Free comparison group. Stage of cancer and surgical treatment for cancer was not statistically different for women in the MBSR group compared to the women in the Non MBSR group. Overall women with breast cancer had either Stage 0 (in situ), Stage 1, or Stage 2 breast cancer, with the majority of women in both groups (MBSR and Non-MBSR) having Stage 1. Women in both cancer groups were predominately treated with breast conservation surgery. The majority of women in all groups were married, employed, and Caucasian and no statistical difference in these demographics were found.
Table 1.
Characteristics of study groups.
| MBSR Group | Non-MBSR Group | Cancer Free Group | |
|---|---|---|---|
| N=38 | N=28 | N=30 | |
| Age (mean years +/− SD) | 55+/−10 | 54+/−8 | 55+/−9 |
| Stage of Cancer | |||
| • Stage 0 (in situ) | 37% | 25% | |
| • Stage 1 | 50% | 71% | |
| • Stage 2 | 13% | 4% | |
| Breast Cancer Surgery | |||
| • Breast Conservation | 87% | 96% | |
| • Mastectomy | 13% | 4% | |
| Education (mean years +/−SD) | 16+/−4 | 16+/−3 | 18+/−4 |
| Marital Status | |||
| • Married | 66% | 75% | 78% |
| • Single | 11% | 11% | 22% |
| • Divorced | 13% | 4% | 0 |
| • Widowed | 8% | 0 | 0 |
| • Unknown | 2% | 10% | 0 |
| Employment | |||
| • Employed | 61% | 68% | 84% |
| • Unemployed | 29% | 21% | 16% |
| • Unknown | 10% | 11% | 0 |
| Race | |||
| • Caucasian | 84% | 79% | 93% |
| • African American | 10% | 11% | 7% |
| • Hispanic | 3% | 0 | 0 |
| • PI/Asian | 3% | 7% | 0 |
| • Unknown | 0 | 0 | 0 |
SD = Standard Deviation; No statistical differences were observed among any of the groups.
3.2. Immunological Assessments
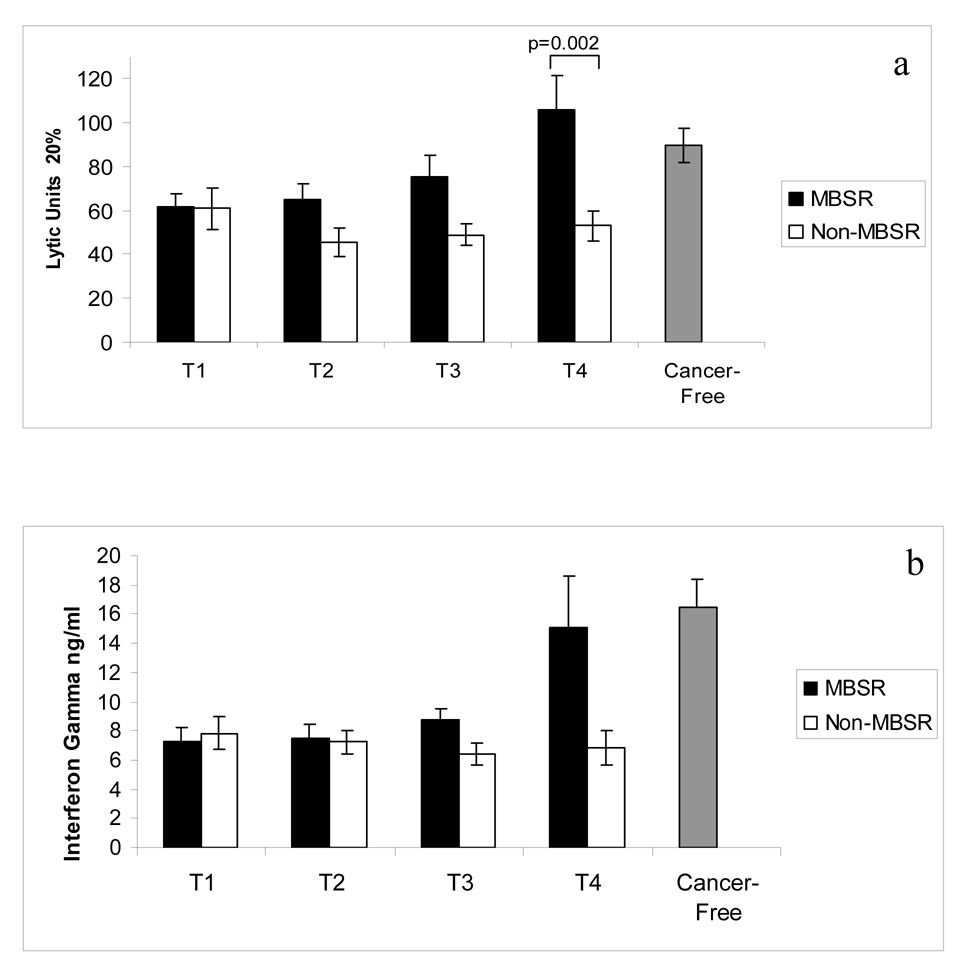
Figure 2a illustrates NKCA, expressed as lytic units, for women in the MBSR and Non-MBSR groups for the 4 assessment times (T1–T4). For normative purposes, NKCA is also shown for a group of age-matched women who were Cancer Free (assessed at one time point only). A significant main effect of time was revealed, indicating that NKCA changed within groups over the time periods of assessment [(F=2.941; df (3,141); p=0.035]. Also, a significant main effect of treatment [F=7.308; df (1,47); p=0.010] and a significant interaction between treatment and time [(F=3.480; df (3,141); p=0.018] was revealed. These analyses demonstrate that women in the MBSR group had an increase in their NKCA that emerged with time so that by T4 the NKCA of women in the MBSR group was significantly greater than that of the women in the Non-MBSR group (p=0.002). The confidence interval for the difference between the means at T4 is (98% CI = 43.6 to 62.2 LU). It is noteworthy that at T3 and T4 the NKCA for the MBSR group, but not the Non-MBSR group, returned to levels that did not differ (p>0.05) from that of Cancer Free women. These results show improvements in NKCA for the group of women enrolled in the MBSR program but not so for women in the Non MBSR group. Phenotypic analysis of circulating NK cells and other PBMC subsets was performed for CD56+ bright and dim NK cells, CD16+56+ NK cells, CD3+, CD4+, CD8+, CD16+ and CD19+ subsets. No difference between the MBSR and Non-MBSR groups of women (or the Cancer Free group of women) for any of these subsets was found at any time point. Data are not shown. These results imply that the difference in NKCA between these groups of women was not due to numerical changes in circulating NK cells but rather was due to changes in functional NKCA.
Figure 2.
a. NKCA, expressed as lytic units at 20%, is illustrated for the MBSR and Non-MBSR groups, and for women without cancer (Cancer-Free). Peripheral blood was collected and NKCA was measured using K562 tumor cells as the target. ANOVA: Time effect p=0.035, Treatment effect p=0.010, Interaction of Treatment × Time p=0.018. b. PBMC production of IFN gamma is depicted for MBSR and Non-MBSR groups and for women without cancer (Cancer-Free). Peripheral blood was collected, PBMC were activated with PMA/PHA and culture supernatants were collected at 48 hr. Cytokine concentration was determined by ELISA. Repeated measures ANOVA; Treatment effect, p=0.001 and Interaction of Treatment × Time p=0.043. Bars represent the mean values +/− SEM.
Production of cytokines by PBMC of these groups of women was analyzed statistically in a similar manner. Figure 2b illustrates the production of IFN gamma, expressed as ng/ml of cell culture media, for women in the MBSR and Non-MBSR groups for the 4 assessment times. The analysis revealed a significant main effect of treatment [F=8.193; df (2,59); p=0.001] and a significant interaction between treatment and time [(F=2.981; df (6,77; p=0.043] such that production of IFN gamma by PBMC of women in the MBSR group over time was greater than for women in the Non-MBSR group. The difference between the MBSR and Non-MBSR group approached significance (p=0.027; 98% CI = 6.2–14.7 ng/ml) at T4. It is noteworthy that at T4 the IFN gamma production by the MBSR group, but not the Non-MBSR group, attained levels that did not differ (p > 0.05) from that of Cancer Free women.
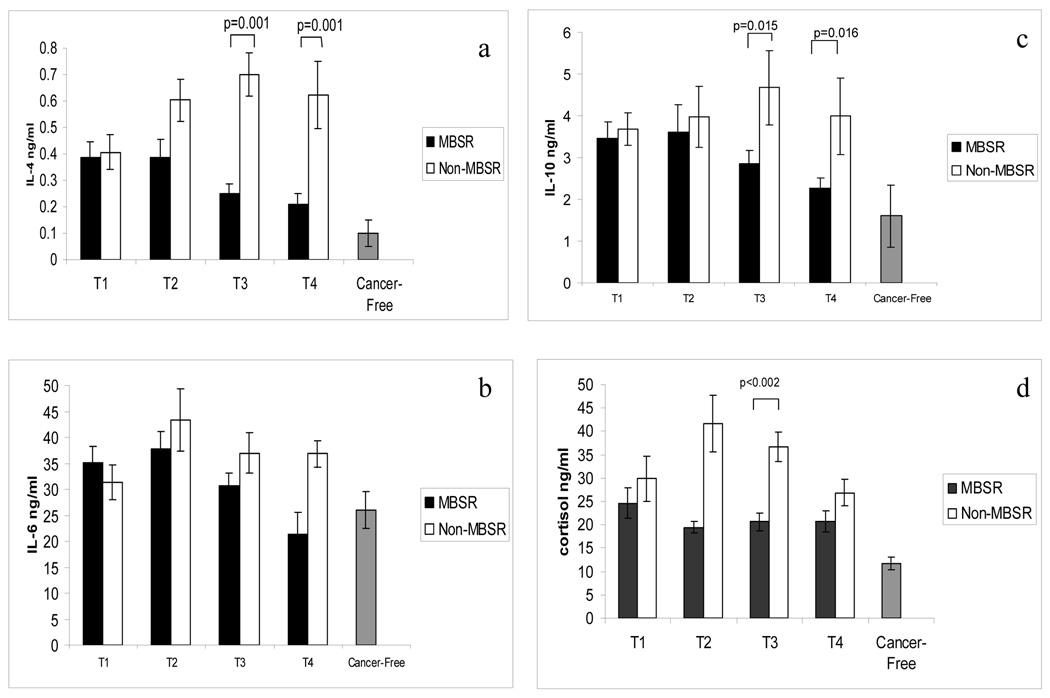
For IL-4 production, the analysis revealed a significant main effect of time, indicating that IL-4 production changed within groups over the time periods of assessment [(F=2.897; df (3,99); p=0.039]. Also, a significant main effect of treatment [(F=12.420; df (1,33); p=0.001] and a significant interaction between treatment and time [(F=3.298; df (3,99); p=0.024] were revealed, such that PBMC from the women in the MBSR group produced less IL-4 over time, while the PBMC of women in the Non-MBSR group produced more IL-4 over time. See Figure 3a. These differences were significant (p =0.001) at both post MBSR time points, T3 (98%CI = 0.033 to 0.057) and T4 (98% CI = 0.034 to 0.035). At T4, production of IL-4 by the PBMC of the MBSR group, but not that of the Non-MBSR group, was similar (p > 0.05) to that of the PBMC of the Cancer Free women.
Figure 3.
a. PBMC production of IL-4 is depicted for the MBSR and Non-MBSR groups, and for women without cancer (Cancer-Free). Repeated measures ANOVA: Time effect p=0.039, Treatment effect p=0.001, Interaction of Treatment × Time p=0.024. b. PBMC production of IL-6 is depicted for the MBSR and Non-MBSR groups, and for women without cancer (Cancer-Free). Repeated measures ANOVA: Treatment effect p=0.031. c. PBMC production of IL-10 is depicted for women in the MBSR and Non-MBSR groups, and in comparison to women without cancer (Cancer-Free). Repeated measures ANOVA: Treatment effect p=0.035. For a.-c. Peripheral blood was collected, PBMC were activated with PMA/PHA and culture supernatants were collected at 48 hr. Cytokine concentration was determined by ELISA. d. Circulating cortisol levels (PM values) are shown for women in the MBSR and Non-MBSR groups, and in comparison to women without cancer (Cancer-Free). Repeated measures ANOVA: Treatment effect p=0.024. Cortisol concentration was determined by ELISA. Bars represent the mean values +/− SEM.
For IL-6 production, repeated measures ANOVA revealed a significant main effect of treatment [(F=5.091; df (1,33); p=0.031] in that women in the MBSR group showed reduced production of IL-6 with respect to the Non-MBSR group, which emerged as significant (p=0.008) at T4 (98% CI = 14.37 to 15.09). See Figure 3b. At T3 and T4, IL-6 production observed in the MBSR group was similar (p > 0.05) to that of the Cancer Free group.
For IL-10 production by PBMC, the analysis revealed only a significant main effect of treatment (i.e., group) [(F=4.822; df (1,33); p=0.035] indicating that women in the Non-MBSR group showed increased PBMC production of IL-10 with respect to the MBSR group. See Figure 3c. The difference between the MBSR and Non-MBSR group was significant at T3 (p = 0.015; 98% CI = 2.12 to 2.28) and T4 (p = 0.016; 98% CI = 1.88 to 1.93). At T4, IL-10 production for the MBSR group of women was similar (p > 0.05) to that of Cancer Free women. Phenotypic analysis of circulating PBMC subsets showed no difference between the MBSR and Non-MBSR groups of women (or the Cancer Free group of women) at any time point. These results imply that differences in cytokine production between these groups of women were not due to numerical changes in the PBMC subsets.
3.2. Plasma Cortisol
Figure 3d illustrates cortisol levels for the two groups of women with breast cancer (MBSR and Non-MBSR) and for the Cancer-Free group of women. Repeated measures ANOVA revealed a significant group effect [F=5.115; df (1,42); p=0.001], such that women who were not enrolled in the MBSR program exhibited greater cortisol levels. At completion of MBSR (T3) cortisol levels were significantly less (p=0.002) in the MBSR group compared to the Non-MBSR group (98% CI = 10.7 to 30.59 ng/ml). With respect to the Cancer Free women, women in both the MBSR and the Non-MBSR groups had significant elevations (p<0.05) at all times.
3.3. Quality of Life and Coping Assessments
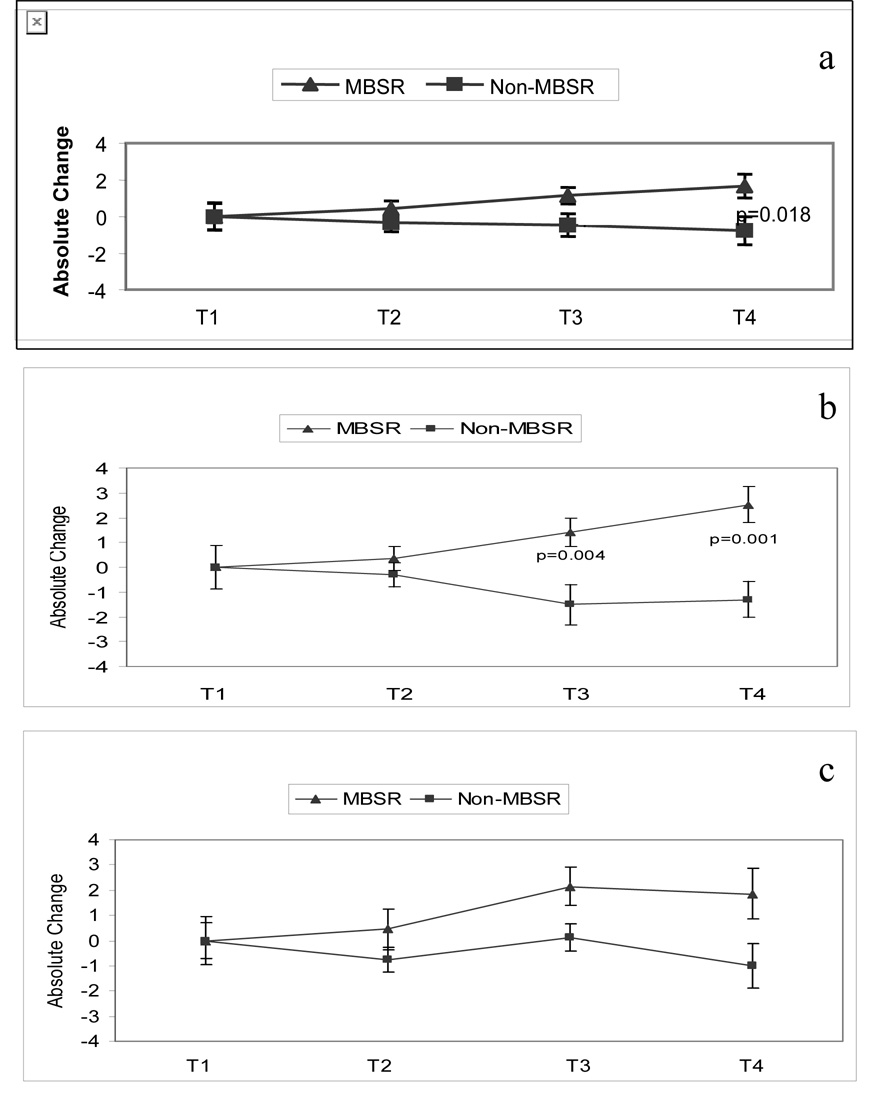
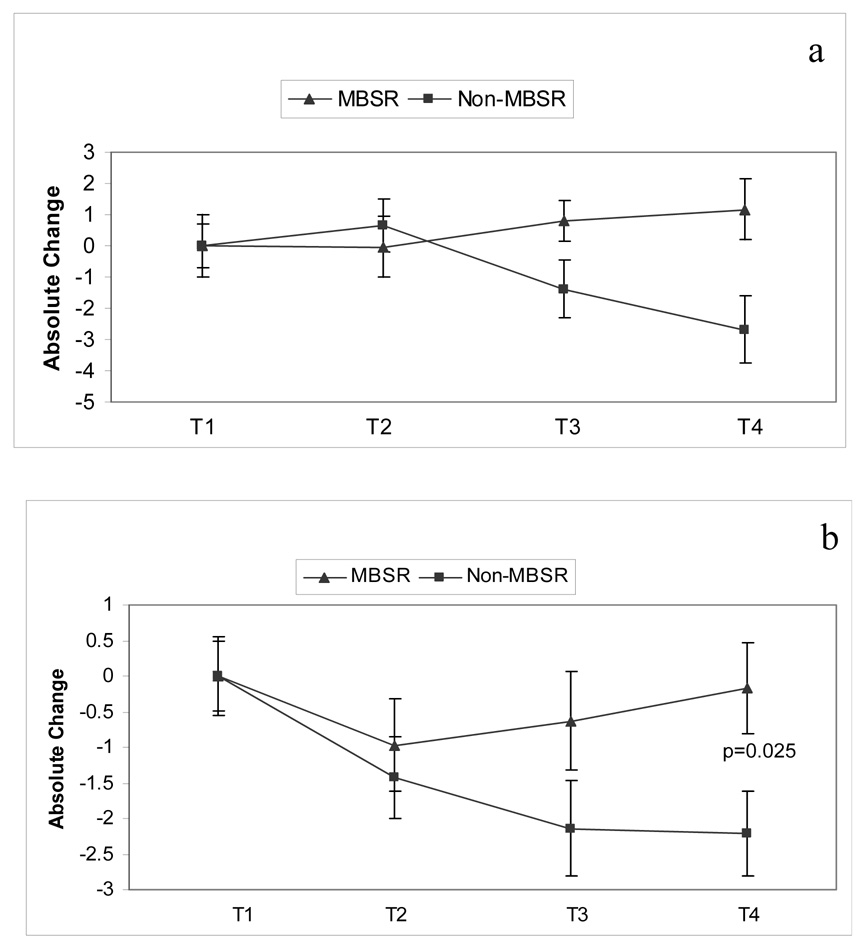
Figure 4a shows absolute changes in the total QOL scores from baseline (T1) for the MBSR group compared to the Non-MBSR group. Repeated measures ANOVA revealed a significant group effect [F=5.582; df (1,45); p=0.023], in that women who completed MBSR reported better total QOL with respect to women in the Non-MBSR group. One way ANOVA between the two groups approached significance at T3 (p = 0.033; 98%CI = 2.24 to 2.38) and attained significance at T4 (p = 0.018; 98%CI = 2.11 to 2.27). There was no interaction of treatment by time for the total QOL score.
Figure 4.
a. Total quality of life is depicted for women in the MBSR and Non-MBSR groups. Quality of life was measured using the Ferrans Quality of Life Index and absolute change from T1 is depicted. Repeated measures ANOVA; Treatment effect p=0.023. b. The Psychological/Spiritual subscale of the Ferrans Quality of Life Index is depicted for women in the MBSR and Non-MBSR groups. Quality of life was measured using the Ferrans Quality of Life Index and absolute change from T1 is depicted. Repeated measures ANOVA; Treatment effect p=0.001, Interaction of Treatment × Time p=0.009. c. The Family subscale of the Ferrans Quality of Life Index is depicted for women in the MBSR and Non-MBSR groups. Quality of life was measured using the Ferrans Quality of Life Index and absolute change from T1 is depicted. Repeated measures ANOVA: Treatment effect p=0.046.
An analysis of QOL subscales showed that changes in total QOL were predominately due to differences in the psychological-spiritual domain and the family domain. As shown in Figure 4b for the psychological-spiritual domain, there was a significant treatment effect [F=12.493; df (1,44); p=0.001] and a significant interaction of treatment by time [F=4.955; df (2,88); p=0.009] in that women in the MBSR group reported more satisfaction over time in psychological-spiritual QOL than Non-MBSR women. One way ANOVA showed that significant group differences occurred at T3 (p=0.004; 98% CI = 3.43 to 4.57) and T4 (p=0.001; 98% CI = 2.99 to 4.01). Regarding the family domain, a significant treatment effect [F=4.214; df (1.44); p=0.046] revealed that women in the MBSR group reported increased changes in life satisfaction with respect to family compared to the Non-MBSR group of women (Figure 4c). This effect was marginal at T3 (p=0.06; 98% CI = 2.75 – 3.21) and marginal at T4 (p=0.046; 98% CI = 1.72 – 3.80). No interaction of treatment by time was found for the family subscale. No significant differences were found in the remaining 2 domains (health/functioning and socioeconomic) of QOL.
An assessment of coping was made using the Jalowiec Coping Scale, which asks respondents to report the frequency of coping styles used, followed by an evaluation of the effectiveness of those coping styles. Eight coping styles were assessed: confrontive, evasive, optimistic, fatalistic, emotive, palliative, supportant, and self-reliant. Of these eight styles the effectiveness of two coping styles was found to differ between the MBSR group compared to the Non-MBSR group, these being Optimistic and Supportant Coping effectiveness. For optimistic coping there was a significant interaction of treatment over time [(F=10.188; df (2,94); p=0.001] and a significant main effect of treatment [(F=3.504; df (2,94); p=0.034]. See Figure 5a. One-way ANOVA revealed a marginal significance (p=0.04) at T4 only (98% CI = 3.44 to 4.54). With respect to the effectiveness of Supportant coping (Figure 5b), a significant treatment effect was found [F= 4.347; df (1, 50); p=0.04]. This effect was significant at T4 only (p=0.025; 98% CI = 2.19 to 2.86).
Figure 5.
a. The Optimistic Effectiveness subscale of the Jalowiec Coping Scale is depicted for women in the MBSR and Non-MBSR groups. Absolute change from T1 is depicted. Repeated measures ANOVA; Time effect p=0.08, Treatment effect p=0.001 and Treatment × Time effect p=0.034. b. The Supportant Effectiveness subscale of the Jalowiec Coping Scale is depicted for women in the MBSR and Non-MBSR groups. Absolute change from T1 is depicted. Repeated measures ANOVA: Treatment effect p=0.04.
Assessment of mindfulness using the MAAS showed no significant changes (p>0.05) from T1–T4, or differences in MAAS scores between the MBSR group versus the Non MBSR group. MAAS scores for the MBSR group from T1 to T4 were: 61±4, 59±4, 64±4, 62±4, respectively; while MAAS scores for the Non MBSR group from T1 to T4 were: 62±4, 63±4, 65±4, 61±4, respectively.
4. Discussion
The emotional impact and the rigors of cancer treatment can adversely affect QOL (Carver et al., 2005; Carver et al., 2006; Longman et al., 1999) and efforts to improve QOL for individuals with cancer is a priority of integrative cancer care (Boyd, 2007). Cancer survivors do not recover from the crisis of cancer as rapidly as those recovering from acute medical situations but journey through distinct phases of adaptation (Cella and Tross, 1986; Marcus, et al., 1998). Interventions provided at critical times during the adaptation to cancer can equip patients with skills that facilitate long-term adaptation and improve QOL. In this study women who enrolled in the MBSR program during their cancer treatment reported more improvement in their QOL compared to the women in the Non-MBSR control group. The emergence with time and the persistence of this effect at the 1 month follow-up likely reflect the progressive attainment of skills taught over the 8 week MBSR program. Others also showed that MBSR improves global QOL (using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire) in cancer patients enrolled into MBSR after completion of cancer treatment (range: 3 months to 20 years post treatment) (Carlson et al., 2003; Carlson et al., 2004). QOL was measured in the present study by administering the Ferrans and Powers Quality of Life Index. Ferrans defines QOL as “a person’s sense of well-being that stems from satisfaction or dissatisfaction with the areas of life that are important to him/her” (Ferrans, 1990). Measuring QOL as an individual’s satisfaction with the aspects of life that they value is unique compared to other measures of QOL. The results reported herein show that only two of the four domains of QOL evaluated, the psychological-spiritual and the family domains showed improvements; whereas, health and functioning and socioeconomic domains were not changed by MBSR. These findings suggest that MBSR allows individuals facing a potentially life-threatening situation to find more value or meaning and satisfaction in family and psychological-spiritual domains of QOL. An evolving literature supports the notion that some individuals with cancer undergo post-traumatic growth, which leads to benefit finding in the face of adversity (Bellizzi, 2004; Carver and Antoni, 2004; Lechner, et al., 2006) and MBSR may facilitate such growth (Garland, et al., 2007).
Certain coping mechanisms are useful to deal with the emotional trauma that cancer engenders and may facilitate adaptation and positive growth; whereas, other coping behaviors (pessimism, cognitive avoidance, substance abuse, hopelessness) are likely to be maladaptive and lead to poor adjustment (Hack and Degner, 2004; Low, et al., 2006; Schou, et al., 2004; Stanton, et al., 2002). The results of this study show that women in the midst of breast cancer treatment, who participated in the MBSR program, reported more improvements in coping effectiveness. Of the eight coping styles measured, coping effectiveness improved for supportant coping and for optimistic coping styles only. Supportant coping styles include the use of personal, professional, and spiritual support systems. Optimistic coping styles include use of positive thinking, maintaining a positive outlook, and making positive comparisons (Jalowiec, et al., 1984). Those coping styles not improved by MBSR were: confrontive, avoidant, fatalistic, emotive, palliative, and self-reliant coping. It is purported that MBSR increases an individual’s ability to cope, yet little, if any, data exists to substantiate this. These results suggest that MBSR does not have a wide-ranging impact on coping but rather has specific effects that promote better use of support systems and promote a more positive outlook regarding one’s cancer experience. Being optimistic and using positive reframing styles of coping have been found to be related to benefit finding in breast cancer patients and may facilitate adaptation to cancer (Uchino, 2006). Further, an evaluation of early-stage breast cancer patients found that trait optimism was a key predictor of long-term QOL (Carver et al., 2006). These results suggest that facilitation of psychological adjustment, conferred by MBSR, during the diagnostic and treatment phase may yield long-term benefits on QOL for cancer survivors. An increase in optimistic coping may promote a more positive affect. Others have shown that MBSR in well-individuals leads to increased electrical activity in areas of the brain that mediate positive emotions, while also increasing the antibody response to the influenza vaccine in those individuals (Davidson et al., 2003). Both trait optimism and positive affect have been shown to produce beneficial effects on immune function (Marsland, et al., 2006, 2007; Pressman and Cohen, 2005; Segerstrom, 2005; Stone, et al., 1987; Valdimarsdottir and Bovbjerg, 1997). Likewise, social support has been shown to have beneficial effects on immune function in cancer patients (Lutgendorf, S. K., et al., 2005; Marucha, et al., 2005; Spiegel and Sephton, 2001; Uchino, 2006). In the present study, participants in the MBSR program perceived greater effectiveness of their support systems, suggesting social support as another possible mediator of the immune effects observed.
Mindfulness is defined as paying attention to one’s inner experiences (i.e., emotions and cognitions) in a non-judgmental or accepting manner. The cultivation of mindfulness through training allows an individual to decrease habitual or automatic responses to stressful experiences. Over time one develops insight, and possibly acceptance, of life events (which one cannot change) so that activation of stress response systems is reduced (Brown and Ryan, 2003; Kabat-Zinn, 1990). Mindfulness was measured in this study using the Mindful Attention Awareness Scale (MAAS), which measures the general tendency to be attentive to and aware of present moment experiences in daily life (Brown and Ryan, 2003). No changes in MAAS scores was observed in the women enrolled in the MBSR program, nor were any differences observed between the MBSR group and the Non-MBSR group. A factor analysis of five mindfulness instruments found that collectively these instruments assess five distinct facets of mindfulness, supporting the notion that mindfulness is a multifaceted construct (Baer, et al., 2006). Our observation of no change in MAAS scores in women who completed the MBSR program may be because the MAAS measures only one facet of mindfulness and did not capture other facets of mindfulness that may have been affected by the MBSR program (Baer et al., 2006). Use of a multifaceted measure of mindfulness in future studies may discern how MBSR benefits ill populations.
Baseline assessments of immune function for both groups of women with cancer (MBSR and Non MBSR) revealed a general reduction in NKCA and in IFN gamma production, with an increase in IL-4, IL-6 and IL-10 production. Women who were enrolled in the MBSR program exhibited a restoration of NKCA and cytokine balance; whereas, women in the Non MBSR control group continued to exhibit immune dysregulation. By T4 and in some cases T3, women enrolled into the MBSR program obtained “cancer free” levels, immunologically. In contrast, the women in the Non-MBSR group did not return to cancer free levels even at 3 months (T4) following study entry. These results may have implications for cancer control, as there is now abundant evidence in experimental animals (most convincingly in genetically altered mice) that immune function (particularly components of the innate immune system such as NK cells and immune modulating cytokines like IFN gamma) controls tumor growth and progression, with the most dramatic effect on epithelial tumors like breast cancer (Dighe, et al., 1994; Kagi, et al., 1994; Kaplan, et al., 1998; Seki, et al., 2003; Smyth, et al., 1998, 1999, 2005a, 2005b; Street, et al., 2001; van den Broek, et al., 1996; Wallace and Smyth, 2005). In humans, not unexpectedly, this issue is not as clearly resolved. However, there is substantial evidence that human NK cells can recognize and destroy transformed tumor cells. This evidence is not simply the lysis of tumor cell lines in cell culture, but includes an understanding of the molecular display of DNA damaged tumor cells that express genotoxic, danger signals recognized by NK cells (Cosman, et al., 2001; Gasser, et al., 2005; Onda, et al., 2001; Oppenheim, et al., 2005; Radosavljevic, et al., 2002; Smyth et al., 2005a). The clinical significance of immune modulation during cancer is supported in several ways. High levels of NKCA in cancer patients correlate with a good prognosis (Gonzales, et al., 1998; Koda, et al., 1997; Liljefors, et al., 2003; Nakamura, et al., 2000; Seo and Tokura, 1999; Taketomi, et al., 1998). Impaired NKCA correlates with invasiveness of human malignancy (Levy, et al., 1984) and reduced NKCA is a significant prognostic indicator of lymphatic involvement, serosal invasion, lymph node metastatic disease and a poorer survival rate (Takeuchi, et al., 2001). NK cell infiltration into primary tumors is associated with fewer metastases to the lymph nodes and less lymphatic invasion (Koda et al., 1997). Notably, in a 13 year follow-up of cancer patients, NKCA was significantly related to overall survival, progression free survival, and response rate. Of particular importance, patients in that study were stratified into those with high versus low NKCA and overall survival for patients was 71 weeks versus 30 weeks, respectively. Further, NKCA was an independent prognostic factor for overall survival in that study (Liljefors et al., 2003). It is worth noting that breast cancer cells can express relatively little or no HLA (Redondo, et al., 2003; Zia, et al., 2001). This is a particularly important observation with relevance to breast cancer in that “recognition” of missing HLA is one means by which NK cells destroy tumor cells. This form of cancer control may be of importance for cancers that disseminate via the lymphatics and blood stream (e.g. breast cancer). For early stage breast cancer, surgery and/or adjuvant therapy, like radiation therapy, removes tumor burden and is also thought to eliminate any nascent tumor cells. Prognosis is good, but the disease can recur after apparent successful eradication of the tumor. Recurrence occurs in some women but not in others. The reasons for this are unclear and certainly are multiple, but it is possible that reduced NKCA may contribute to this recurrence. Whether or not reductions in this form of immune function can be related to recurrence incidence, remains unresolved. However, it is clear that the early survivorship period is a vulnerable period and is an important “window of opportunity” for such an intervention like MBSR (Lutgendorf, S. et al., 2007).
The effect of MBSR on NKCA in patients newly diagnosed with cancer has not been evaluated previously. However, a series of uncontrolled evaluations in cancer survivors (i.e., beyond treatment) have reported changes in PBMC intracellular cytokine production (Carlson et al., 2004, 2007). Those changes were best revealed at 6 and 12 months post MBSR training and were most pronounced for T lymphocyte intracellular production of IFN gamma and TNF. Those results are difficult to compare to the results reported herein in that follow-up of subjects evaluated in this study was at 1 month after the completion of MBSR training. Further, PBMC activation in this study was for 48 hours with a measurement of released cytokines, while PBMC activation was for 4 hours with assessment of intracellular cytokines in the Carlson et al. studies. Each approach has distinct advantages for the measurement of immune function but the assessment of released cytokines after 48 hours permits a measurement of the global cytokine response by PBMC within the context of other produced cytokines. Further the Carlson et al. studies evaluated subjects who had completed multiple immune-altering treatments for periods ranging from 3 months to 20 years prior to study enrollment; since a control group was not used, observed improvements, as noted by the authors, could be a consequence of recovery from the effects of treatment (Carlson et al., 2007). It is clear that the two study populations are quite dissimilar and that the methods of immune assessment differ as well. These differences may explain any inconsistencies among the studies.
Women in the MBSR group had reduced cortisol levels compared to women who received usual care. Albeit, the results obtained for cortisol are limited in that only a subgroup of women, who had blood drawn in the late afternoon could be evaluated. However, it is likely that reduction of circulating cortisol may contribute to the immune changes observed. By sequence association of neurochemical and immunological variables, it is apparent that differences between the groups of women with breast cancer were observed for cortisol concentration and for IL-4 production. By T3 such differences were observed for cortisol concentration and for IL-4 and IL-10 production. By T4, differences between the two groups of women with breast cancer were observed for NKCA, IL-4 and IL-10 production, as well as IFN gamma production. This temporal sequence suggests that changes in cortisol concentration and IL-4 production may influence NKCA and IFN gamma production and also influence IL-10 production. Cortisol, IL-4 and IL-10 have been separately shown to diminish NKCA and IFN gamma production. (Chrousos, 2000; Daynes, et al., 1990; Maes et al., 1999; Marshall et al., 1998; Rook, et al., 1994). Both NKCA and IFN gamma production have been demonstrated to be involved in immune surveillance, controlling not only tumor initiation, but also tumor metastasis and tumor growth (Dighe et al., 1994; Kagi et al., 1994; Kaplan et al., 1998; Seki et al., 2003, Smyth et al., 1998, 1999; Smyth et al., 2005a, 2005b; Street et al., 2001; van den Broek et al., 1996; Wallace and Smyth, 2005). Therefore, these data support the concept that women enrolled in MBSR would have in place ”normalized” cancer control measures that women in the Non-MBSR group would not have. Neuroendocrine activation (as evidenced by cortisol) can result in the production of proinflammatory (IL-6) and Th2 (IL-4, IL10) cytokines with reductions in Th1 (IFN gamma) cytokines and NKCA. The milieu of the immune system is influenced by neuroendocrine activation and also by the cytokine microenvironment of the immune cells. IL-4 and particularly IL-10 can negatively impact Th1 cytokines and NK cells. This possibility is reflected in the global cytokine data, wherein IL-4, IL-6 and IL-10 were initially increased for all women with breast cancer. Over time, these cytokine levels increase in the Non-MBSR group, while these cytokine levels decrease in the MBSR group. Reductions in these cytokines may allow for normalization of Th1 cytokines (e.g. IFN gamma) as well as NKCA. The net result is that women enrolled in MBSR return to immune homeostasis more rapidly than women not enrolled in MBSR.
The conclusions drawn from the results of this study are limited because women were not randomly assigned to the MBSR program and group membership may reflect selection bias. For example, women who selected MBSR may have had preconceived expectations of MBSR and this may have influenced their response to the quality of life and coping measures. Although this is a possibility, if this were true, one would expect that there would be a global improvement in all domains of QOL, as well as improvement in most of the coping styles assessed. Yet, only two of the four domains of QOL and two of the eight styles of coping showed improvement. Also, the efficacy of MBSR in providing psychological and quality of life benefits is consistent with what others have shown in previous studies (Speca et al., 2000; Carlson et al., 2001, 2003, 2004, 2007). Imbalance in treatment and demographic variables, as a result of lack of randomization, may also confound results. However, this study used restricted diagnostic and treatment inclusion criteria, which allowed enrollment of a relatively homogenous group of women with breast cancer. Overall, the cancer groups were well balanced with respect to disease, treatment, and demographic variables. Nevertheless, the lack of randomization remains a design limitation.
Despite such limitation, the findings of this study provide valuable and unique contributions to the study of MBSR and to psychoneuroimmunology. Regarding MBSR, the majority of published studies lack a control group and adaptation (i.e., “recovery”) of cancer patients is not accounted for in those previous studies (Carlson et al., 2001, 2003, 2004, 2007). In fact, only one randomized wait list control study has been published, which was limited to an evaluation of the psychological effects of MBSR in cancer patients who were enrolled well-beyond their cancer treatment (Speca et al., 2000). To our knowledge, no other study has evaluated benefits of MBSR in recently diagnosed cancer patients during treatment. Immune dysregulation may jeopardize cancer control, especially at critical times marked by risk of tumor dissemination, such as in the peri- treatment period. Immune cancer control mechanisms are thought to be more important at this time, when the bulk of the tumor burden is removed, and when there is risk that nascent tumor cells might disseminate (Lutgendorf et al., 2007). The early provision of interventions to restore immune function during this pivotal time is clearly important. Finally, interventions offered at vulnerable times during the adaptation to cancer, can equip patients with skills that will facilitate long-term adaptation and improved QOL. The results reported herein, support the need for future studies that evaluate these concepts in large scale randomized clinical trials of MBSR for individuals diagnosed with cancer.
Acknowledgements
The study was supported in part by the National Cancer Institute R21- CA – 9864 and the Niehoff School of Nursing Palmer Funds. Our heartfelt thanks are extended to all the women who so graciously agreed to participate in this study and to Margaret J. LaPlante, Ph.D., who provided her expertise in initiating the MBSR program. The authors gratefully acknowledge the expert assistance of Jonna Peterson, Sara Shanti and Kelly Loster in performing laboratory analyses. Special thanks are extended to Cheryl Peterson, R.N., who unfailingly supported study recruitment and to Linda Milbrandt R.N. who assisted with medical record reviews. We also recognize and thank James Sinacore Ph.D. for data analysis and especially, Kathy Albain, M.D., Ann McCall, M.D. and the clinic physicians and staff of the Loyola University Breast Care Center who facilitated the implementation of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ACS. Cancer Facts & Figures 2007. 2007 [Google Scholar]
- Andersen BL, Farrar WB, Golden-Kreutz D, Kutz LA, MacCallum R, Courtney ME, Glaser R. Stress and immune responses after surgical treatment for regional breast cancer. J. Natl. Cancer Inst. 1998;90:30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, Shapiro CL, Carson WE., 3rd Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J. Clin. Oncol. 2004;22:3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham R, Ben-Eliyahu S. Neuroendocrine regulation of cancer progression: II. Immunological mechanisms, clinical relevance, and prophylactic measures. In: Ader R, editor. Psychoneuroimmunology. Fourth ed. Amsterdam: Elsevier; 2007. pp. 251–265. 2007. [Google Scholar]
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Bellizzi KM. Expressions of generativity and posttraumatic growth in adult cancer survivors. Int. J. Aging Hum. Dev. 2004;58:267–287. doi: 10.2190/DC07-CPVW-4UVE-5GK0. [DOI] [PubMed] [Google Scholar]
- Berger AM. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol. Nurs. Forum. 1998;25:51–62. [PubMed] [Google Scholar]
- Bishop SR. What do we really know about mindfulness-based stress reduction? Psychosom. Med. 2002;64:71–83. doi: 10.1097/00006842-200201000-00010. [DOI] [PubMed] [Google Scholar]
- Boyd DB. Integrative oncology: the last ten years--a personal retrospectve. Altern. Ther. Health Med. 2007;13:56–64. [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J. Pers. Soc. Psychol. 2003;84:822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Ursuliak Z, Goodey E, Angen M, Speca M. The effects of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients: 6-month follow-up. Support. Care Cancer. 2001;9:112–123. doi: 10.1007/s005200000206. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom. Med. 2003;65:571–581. doi: 10.1097/01.psy.0000074003.35911.41. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology. 2004;29:448–474. doi: 10.1016/s0306-4530(03)00054-4. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Brown KW. Validation of the Mindful Attention Awareness Scale in a cancer population. J. Psychosom. Res. 2005;58:29–33. doi: 10.1016/j.jpsychores.2004.04.366. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Patel KD, Faris P. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav. Immun. 2007;21:1038–1049. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Carver CS, Antoni MH. Finding benefit in breast cancer during the year after diagnosis predicts better adjustment 5 to 8 years after diagnosis. Health Psychol. 2004;23:595–598. doi: 10.1037/0278-6133.23.6.595. [DOI] [PubMed] [Google Scholar]
- Carver CS, Smith RG, Antoni MH, Petronis VM, Weiss S, Derhagopian RP. Optimistic personality and psychosocial well-being during treatment predict psychosocial wellbeing among long-term survivors of breast cancer. Health Psychol. 2005;24:508–516. doi: 10.1037/0278-6133.24.5.508. [DOI] [PubMed] [Google Scholar]
- Carver CS, Smith RG, Petronis VM, Antoni MH. Quality of life among long-term survivors of breast cancer: Different types of antecedents predict different classes of outcomes. Psychooncology. 2006;15:749–758. doi: 10.1002/pon.1006. [DOI] [PubMed] [Google Scholar]
- Cella DF, Tross S. Psychological adjustment to survival from Hodgkin's disease. J. Consult. Clin. Psychol. 1986;54:616–622. doi: 10.1037//0022-006x.54.5.616. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Chrousos GP. The stress response and immune function: clinical implications. The 1999 Novera H. Spector Lecture. Ann. N. Y. Acad. Sci. 2000;917:38–67. doi: 10.1111/j.1749-6632.2000.tb05371.x. [DOI] [PubMed] [Google Scholar]
- Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, Urbanowski F, Harrington A, Bonus K, Sheridan JF. Alterations in brain and immune function produced by mindfulness meditation. Psychosom. Med. 2003;65:564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- Daynes RA, Araneo BA, Dowell TA, Huang K, Dudley D. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J. Exp. Med. 1990;171:979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Ferrans CE, Powers MJ. Quality of life index: development and psychometric properties. ANS Adv. Nurs. Sci. 1985;8:15–24. doi: 10.1097/00012272-198510000-00005. [DOI] [PubMed] [Google Scholar]
- Ferrans CE. Development of a quality of life index for patients with cancer. Oncol. Nurs. Forum. 1990;17:15–19. discussion 20-11. [PubMed] [Google Scholar]
- Garland SN, Carlson LE, Cook S, Lansdell L, Speca M. A non-randomized comparison of mindfulness-based stress reduction and healing arts programs for facilitating post-traumatic growth and spirituality in cancer outpatients. Support. Care Cancer. 2007;15:949–961. doi: 10.1007/s00520-007-0280-5. [DOI] [PubMed] [Google Scholar]
- Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales FM, Vargas JA, Lopez-Cortijo C, Castejon R, Forriz C, Ramirez-Camancho R, Millan F, Durantez A. Prognostic significance of natural killer cell activity in patients with laryngeal carcinoma. Arch. Otolaryngol. Head Neck Surg. 1998;124:852–856. doi: 10.1001/archotol.124.8.852. [DOI] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J. Psychosom. Res. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Hack TF, Degner LF. Coping responses following breast cancer diagnosis predict psychological adjustment three years later. Psychooncology. 2004;13:235–247. doi: 10.1002/pon.739. [DOI] [PubMed] [Google Scholar]
- Hughes KK. Psychosocial and functional status of breast cancer patients. The influence of diagnosis and treatment choice. Cancer Nurs. 1993;16:222–229. [PubMed] [Google Scholar]
- Jalowiec A, Murphy SP, Powers MJ. Psychometric assessment of the Jalowiec Coping Scale. Nurs. Res. 1984;33:157–161. [PubMed] [Google Scholar]
- Jalowiec A. Effectiveness of coping strategies in critical care nurses. Dimens Crit Care Nurs. 1993;12:204–205. [PubMed] [Google Scholar]
- Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J. Behav. Med. 1985;8:163–190. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full Catastrophe Living. New York: Delacorte; 1990. [Google Scholar]
- Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: New Perspectives from Psychoneuroimmunology. Annu. Rev. Psychol. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- Koda K, Saito N, Takiguchi N, Oda K, Nunomura M, Nakajima N. Preoperative natural killer cell activity: correlation with distant metastases in curatively research colorectal carcinomas. Int. Surg. 1997;82:190–193. [PubMed] [Google Scholar]
- Lechner SC, Carver CS, Antoni MH, Weaver KE, Phillips KM. Curvilinear associations between benefit finding and psychosocial adjustment to breast cancer. J. Consult. Clin. Psychol. 2006;74:828–840. doi: 10.1037/0022-006X.74.5.828. [DOI] [PubMed] [Google Scholar]
- Levy S, Tempe JL, Caussade P, Aleksijevic A, Grosshans E, Mayer S, Lang JM. Stage-related decrease in natural killer cell activity in untreated patients with mycosis fungoides. Cancer Immunol. Immunother. 1984;18:138–140. doi: 10.1007/BF00205749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljefors M, Nilsson B, Hjelm Skog AL, Ragnhammar P, Mellstedt H, Frodin JE. Natural killer (NK) cell function is a strong prognostic factor in colorectal carcinoma patients treated with the monoclonal antibody 17-1A. Int. J. Cancer. 2003;105:717–723. doi: 10.1002/ijc.11139. [DOI] [PubMed] [Google Scholar]
- Longman AJ, Braden CJ, Mishel MH. Side-effects burden, psychological adjustment, and life quality in women with breast cancer: pattern of association over time. Oncol. Nurs. Forum. 1999;26:909–915. [PubMed] [Google Scholar]
- Low CA, Stanton AL, Thompson N, Kwan L, Ganz PA. Contextual life stress and coping strategies as predictors of adjustment to breast cancer survivorship. Ann. Behav. Med. 2006;32:235–244. doi: 10.1207/s15324796abm3203_10. [DOI] [PubMed] [Google Scholar]
- Lutgendorf S, Costanzo E, Siegel S. Psychosocial influences in oncology: An expanded model of biobehavioral mechanisms. In: Ader R, editor. Psychoneuroimmunology. Burlington: Elsevier Academic Press; 2007. pp. 869–895. [Google Scholar]
- Lutgendorf SK, Sood AK, Anderson B, McGinn S, Maiseri H, Dao M, Sorosky JI, De Geest K, Ritchie J, Lubaroff DM. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J. Clin. Oncol. 2005;23:7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin AH, Delmeire L, Van Gastel A, Kenis G, De Jongh R, Bosmans E. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol. Psychiatry. 1999;45:833–839. doi: 10.1016/s0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- Marcus AC, Garrett KM, Cella D, Wenzel LB, Brady MJ, Crane LA, McClatchey MW, Kluhsman BC, Pate-Willig M. Telephone counseling of breast cancer patients after treatment: a description of a randomized clinical trial. Psychooncology. 1998;7:470–482. doi: 10.1002/(SICI)1099-1611(199811/12)7:6<470::AID-PON325>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Marshall GD, Jr, Agarwal SK, Lloyd C, Cohen L, Henninger EM, Morris GJ. Cytokine dysregulation associated with exam stress in healthy medical students. Brain Behav. Immun. 1998;12:297–307. doi: 10.1006/brbi.1998.0537. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Cohen S, Rabin BS, Manuck SB. Trait positive affect and antibody response to hepatitis B vaccination. Brain Behav. Immun. 2006;20:261–269. doi: 10.1016/j.bbi.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Pressman S, Cohen S. Positive affect and immune function. In: Ader R, editor. Psychoneuroimmunology. Burlington: Elsevier Academic Press; 2007. pp. 761–779. [Google Scholar]
- Marucha PT, Crespin TR, Shelby RA, Andersen BL. TNF-alpha levels in cancer patients relate to social variables. Brain Behav. Immun. 2005;19:521–525. doi: 10.1016/j.bbi.2005.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagabhushan M, Mathews HL, Witek-Janusek L. Aberrant nuclear expression of ap-1 and NFKB in lymphocytes of women stressed by the experience of breast biopsy. Brain Behav. Immun. 2001;15:78–84. doi: 10.1006/brbi.2000.0589. [DOI] [PubMed] [Google Scholar]
- Nail LM, Winningham ML. Fatigue and weakness in cancer patients: the symptoms experience. Semin. Oncol. Nurs. 1995;11:272–278. doi: 10.1016/s0749-2081(05)80008-7. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kawasaki N, Hagiwara M, Saito M, Konaka C, Kato H. Cellular immunologic parameters related to age, gender, and stage in lung cancer patients. Lung Cancer. 2000;28:139–145. doi: 10.1016/s0169-5002(99)00133-6. [DOI] [PubMed] [Google Scholar]
- Northouse LL. Psychological impact of the diagnosis of breast cancer on the patient and her family. J. Am. Med. Womens Assoc. 1992;47:161–164. [PubMed] [Google Scholar]
- Onda H, Ohkubo S, Shintani Y, Ogi K, Kikuchi K, Tanaka H, Yamamoto K, Tsuji I, Ishibashi Y, Yamada T, Kitada C, Suzuki N, Sawada H, Nishimura O, Fujino M. A novel secreted tumor antigen with a glycosylphosphatidylinositol-anchored structure ubiquitously expressed in human cancers. Biochem. Biophys. Res. Commun. 2001;285:235–243. doi: 10.1006/bbrc.2001.5149. [DOI] [PubMed] [Google Scholar]
- Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- Pressman S, Cohen S. The influence of positive affect on heatlh: A Review. Psychol. Bull. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Radosavljevic M, Cuillerier B, Wilson MJ, Clement O, Wicker S, Gilfillan S, Beck S, Trowsdale J, Bahram S. A cluster of ten novel MHC class I related genes on human chromosome 6q24.2-q25.3. Genomics. 2002;79:114–123. doi: 10.1006/geno.2001.6673. [DOI] [PubMed] [Google Scholar]
- Rakovitch E, Franssen E, Kim J, Ackerman I, Pignol JP, Paszat L, Pritchard KI, Ho C, Redelmeier DA. A comparison of risk perception and psychological morbidity in women with ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res. Treat. 2003;77:285–293. doi: 10.1023/a:1021853302033. [DOI] [PubMed] [Google Scholar]
- Redondo M, Garcia J, Villar E, Rodrigo I, Perea-Milla E, Serrano A, Morell M. Major histocompatibility complex status in breast carcinogenesis and relationship to apoptosis. Hum. Pathol. 2003;34:1283–1289. doi: 10.1016/j.humpath.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Robinson FP, Mathews HL, Witek-Janusek L. Psycho-endocrine-immune response to mindfulness-based stress reduction in individuals infected with the human immunodeficiency virus: a quasiexperimental study. J Altern Complement Med. 2003;9:683–694. doi: 10.1089/107555303322524535. [DOI] [PubMed] [Google Scholar]
- Rook GA, Hernandez-Pando R, Lightman SL. Hormones, peripherally activated prohormones and regulation of the Th1/Th2 balance. Immunol. Today. 1994;15:301–303. doi: 10.1016/0167-5699(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Sanders VM, Kavelaars A. Adrenergic regulation of immunity. In: Ader R, editor. Psychoneuroimmunology. Burlington: Elsevier Academic Press; 2007. pp. 63–83. [Google Scholar]
- Schoneveld O, Cidlowski JA. Glucocorticoids and immunity: mechanisms of regulation. In: Ader R, editor. Psychoneuroimmunology. Burlington: Elsevier Academic Press; 2007. pp. 45–61. [Google Scholar]
- Schou I, Ekeberg O, Ruland CM, Sandvik L, Karesen R. Pessimism as a predictor of emotional morbidity one year following breast cancer surgery. Psychooncology. 2004;13:309–320. doi: 10.1002/pon.747. [DOI] [PubMed] [Google Scholar]
- Schreier AM, Williams SA. Anxiety and quality of life of women who receive radiation or chemotherapy for breast cancer. Oncol. Nurs. Forum. 2004;31:127–130. doi: 10.1188/04.ONF.127-130. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC. Optimism and immunity: do positive thoughts always lead to positive effects? Brain Behav. Immun. 2005;19:195–200. doi: 10.1016/j.bbi.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki N, Hayakawa Y, Brooks AD, Wine J, Wiltrout RH, Yagita H, Tanner JE, Smyth MJ, Sayers TJ. Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis is an important endogenous mechanism for resistance to liver metastases in murine renal cancer. Cancer Res. 2003;63:207–213. [PubMed] [Google Scholar]
- Seo N, Tokura Y. Downregulation of innate and acquired antitumor immunity by bystander gammadelta and alphabeta T lymphocytes with Th2 or Tr1 cytokine profiles. J. Interferon Cytokine Res. 1999;19:555–561. doi: 10.1089/107999099313686. [DOI] [PubMed] [Google Scholar]
- Shapiro SL, Lopez AM, Schwartz GE, Bootzin R, Figueredo AJ, Braden CJ, Kurker SF. Quality of life and breast cancer: relationship to psychosocial variables. J. Clin. Psychol. 2001;57:501–519. doi: 10.1002/jclp.1026. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Kelly JM, Baxter AG, Korner H, Sedgwick JD. An essential role for tumor necrosis factor in natural killer cell-mediated tumor rejection in the peritoneum. J. Exp. Med. 1998;188:1611–1619. doi: 10.1084/jem.188.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Thia KY, Cretney E, Kelly JM, Snook MB, Forbes CA, Scalzo AA. Perforin is a major contributor to NK cell control of tumor metastasis. J. Immunol. 1999;162:6658–6662. [PubMed] [Google Scholar]
- Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J. Exp. Med. 2005a;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J. Exp. Med. 2005b;201:1973–1985. doi: 10.1084/jem.20042280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speca M, Carlson LE, Goodey E, Angen M. A randomized, wait-list controlled clinical trial: the effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosom. Med. 2000;62:613–622. doi: 10.1097/00006842-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Spiegel D. Psychological distress and disease course for women with breast cancer: one answer, many questions. J. Natl. Cancer Inst. 1996;88:629–631. doi: 10.1093/jnci/88.10.629. [DOI] [PubMed] [Google Scholar]
- Spiegel D. Psychosocial aspects of breast cancer treatment. Semin. Oncol. 1997;24:S1–S31. S36–S47. [PubMed] [Google Scholar]
- Spiegel D, Sephton SE. Psychoneuroimmune and endocrine pathways in cancer: effects of stress and support. Semin Clin Neuropsychiatry. 2001;6:252–265. doi: 10.1053/scnp.2001.26995. [DOI] [PubMed] [Google Scholar]
- Stanton AL, Danoff-Burg S, Huggins ME. The first year after breast cancer diagnosis: hope and coping strategies as predictors of adjustment. Psychooncology. 2002;11:93–102. doi: 10.1002/pon.574. [DOI] [PubMed] [Google Scholar]
- Stark DP, House A. Anxiety in cancer patients. Br. J. Cancer. 2000;83:1261–1267. doi: 10.1054/bjoc.2000.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, Cox DS, Valdimarsdottir H, Jandorf L, Neale JM. Evidence that secretory IgA antibody is associated with daily mood. J. Pers. Soc. Psychol. 1987;52:988–993. doi: 10.1037//0022-3514.52.5.988. [DOI] [PubMed] [Google Scholar]
- Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- Taketomi A, Shimada M, Shirabe K, Kajiyama K, Gion T, Sugimachi K. Natural killer cell activity in patients with hepatocellular carcinoma: a new prognostic indicator after hepatectomy. Cancer. 1998;83:58–63. doi: 10.1002/(sici)1097-0142(19980701)83:1<58::aid-cncr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Maehara Y, Tokunaga E, Koga T, Kakeji Y, Sugimachi K. Prognostic significance of natural killer cell activity in patients with gastric carcinoma: a multivariate analysis. Am. J. Gastroenterol. 2001;96:574–578. doi: 10.1111/j.1572-0241.2001.03535.x. [DOI] [PubMed] [Google Scholar]
- Theobald DE. Cancer pain, fatigue, distress, and insomnia in cancer patients. Clin Cornerstone. 2004;6 Suppl 1D:S15–S21. doi: 10.1016/s1098-3597(05)80003-1. [DOI] [PubMed] [Google Scholar]
- Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J. Behav. Med. 2006;29:377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Valdimarsdottir HB, Bovbjerg DH. Positive and negative mood: association with natural killer cell activity. Psychol. Health. 1997;12:319–327. [Google Scholar]
- van den Broek ME, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK, Melief CJ, Zinkernagel RM, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J. Exp. Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace ME, Smyth MJ. The role of natural killer cells in tumor control--effectors and regulators of adaptive immunity. Springer Semin. Immunopathol. 2005;27:49–64. doi: 10.1007/s00281-004-0195-x. [DOI] [PubMed] [Google Scholar]
- Witek-Janusek L, Mathews HL. Differential effects of glucocorticoids on colony stimulating factors produced by neonatal mononuclear cells. Pediatr. Res. 1999;45:224–229. doi: 10.1203/00006450-199902000-00011. [DOI] [PubMed] [Google Scholar]
- Witek-Janusek L, Gabram S, Mathews HL. Psychologic stress, reduced NK cell activity, and cytokine dysregulation in women experiencing diagnostic breast biopsy. Psychoneuroendocrinology. 2007;32:22–35. doi: 10.1016/j.psyneuen.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zia A, Schildberg FW, Funke I. MHC class I negative phenotype of disseminated tumor cells in bone marrow is associated with poor survival in R0M0 breast cancer patients. Int. J. Cancer. 2001;93:566–570. doi: 10.1002/ijc.1362. [DOI] [PubMed] [Google Scholar]