Abstract
Bleomycin hydrolase is a multifaceted neutral cysteine protease with a suggested role in antigen presentation, homocysteine-thiolactone metabolism, and Alzheimer’s disease pathogenesis. Deletion of the protease in mice results in increased neonatal mortality and dermatopathology. Immunohistochemical and behavioral studies of BLMH knockout mice were undertaken to further evaluate the role of the protease in the brain. No gross abnormalities in the central nervous system were observed upon preliminary histological examination of B6.129Blmhtm1Geh/J null animals. However, glial fibrillary acid protein immunohistochemistry revealed a global reactive astrogliosis in the aged null animals, indicative of undefined brain pathology. The role of BLMH in the brain was further explored by characterizing the behavioral phenotype of hybrid [129S6-Blmhtm1Geh/J X B6.129 Blmhtm1Geh/J]F1 null and littermate controls using multiple behavioral paradigms. In the water maze, deletion of BLMH resulted in poorer performance during water maze probe trials without detectable effect of the mutation on sensorimotor function. In addition, no age-dependent decline in discriminative performance on probe trials was observed in null animals. These data suggest a physiological non-redundant function for BLMH in the central nervous system.
Keywords: knockout, hippocampus, learning, memory, aging, Alzheimer’s disease
Bleomycin hydrolase (BLMH) is a highly conserved and unusual aminopeptidase with suggested importance in antigen presentation, homocysteine-thiolactone metabolism, and Alzheimer’s disease (AD). A genetic polymorphism within the conserved carboxyterminal region of BLMH is a susceptibility locus for sporadic AD and has been reported by us (Montoya et al., 1998) and others (Papassotiropoulos et al., 2000) to be associated with an approximate two-fold increased risk for development of the disease, although this effect has not been replicated in all studies. Accumulation of β-amyloid peptide derived from proteolytic cleavage of the amyloid precursor protein is believed to be a critical event in the early pathogenesis of AD (Hardy and Allsop, 1991; Hardy and Higgins, 1992). In vitro studies indicate BLMH can process β-amyloid peptides (Kajiya et al., 2006). Surprisingly, ectopic overexpression of catalytically active human BLMH in HEK 293 or CHO cells results in increased secretion of β-amyloid (Lefterov et al., 2000; Lefterov et al., 2001). Despite these observations, the molecular substrates and physiological functions of the BLMH in the central nervous system (CNS) remain unknown.
BLMH is a broad-specificity, cytoplasmic papain superfamily cysteine protease (Sebti et al., 1989; Enenkel et al., 1993, Chapot-Charter et al., 1994; Bromme et al., 1996) expressed in many tissues, including brain (Bromme et al., 1996; Ferrando et al., 1996; Raina et al., 1999; Namba et al., 1999; Malherbe et al., 2000). The protease possesses an unusual ring barrel homohexameric structure that encloses the active sites and restricts substrate access (Joshua-Tor et al., 1995). Although no endogenous inhibitors have been identified, protease activity is regulated through the BLMH carboxyterminus which overlays and limits entry to each active site cleft (Joshua-Tor et al., 1995; Zheng et al., 1998). Curiously, the polymorphism associated with AD results in a conserved amino acid change within the regulatory domain of the BLMH carboxyterminus (Montoya et al., 1998).
The protease was initially identified through its ability to deaminate and inactivate the chemotherapeutic glycopeptide bleomycin (Umezawa, 1966; Umezawa, 1972; Lazo and Humphreys, 1983). Later studies with BLMH knockout mice revealed non-redundant functions in neonatal survival and epidermal integrity (Schawartz et al., 1999). BLMH has emerged as a candidate aminopeptidase for MHC I epitope production (Stolze et al., 2000) and consequently could play a putative role in neuronal development or synaptic plasticity as mediated by MHC derived peptides (Neumann et al., 1995; Corrveau et al., 1998; Huh et al., 2002). Most recently, BLMH has been identified as an intracellular homocysteine-thiolactonase, which protects cells against homocysteine toxicity (Zimny et al., 2006). Interestingly, mild hyperhomocysteinemia is a strong risk factor for dementia and AD (Seshadri et al., 2002).
Genetically modified mice have proven powerful tools for elucidating specific protein functions as well as providing animal models for disease (Saura et al., 2004; Duff and Rao, 2001). In this report, the effects of the targeted deletion of BLMH in a murine model were evaluated by CNS immunohistochemistry and behavioral phenotyping of both young and aged null animals and littermate controls. The studies revealed an increase in astrogliosis in the BLMH null mice as compared to appropriate controls. Furthermore, null animals exhibited poorer performance in the hippocampus-dependent paradigm of the Morris water maze compared to wild-type littermates. These results suggest non-redundant physiological functions for BLMH in the brain.
EXPERIMENTAL PROCEDURES
Animal maintenance
BLMH knockout mice were generated by targeted deletion of 129/Sv and 129/J hybrid embryonic stem cells injected into C57BL/6J blastocysts as previously described (Schwartz et al., 1999). Resulting offspring were backcrossed 7–9 generations to 129S6/SvEv (Taconic, Germantown, NY) or 9–11 generations to C57BL/6J (Jackson Labs, Bar Harbor, Maine). B6.129 Blmhtm1Geh/J null and wild-type littermate controls used for preliminary CNS immunohistochemistry were bred from male and female heterozygote B6.129 Blmhtm1Geh/J mice. Hybrid [129S6-Blmhtm1Geh/J X B6.129 Blmhtm1Geh/J]F1 BLMH null and littermate controls were bred from female 129S6-Blmhtm1Geh/J and male B6.129Blmhtm1Geh/J. Mice were housed four or five to a cage and maintained under controlled conditions with a 12 h light cycle and free access to food and water. The Animal Care and Use Committee of the University of Pittsburgh approved all experimental procedures.
Genotyping
Genomic DNA was isolated from tail clippings taken at weaning (Laird et al., 1991) and genotyped by using the BLMH intron 2 forward primer p1 (CACTGTAGCTGTACTCACAC), BLMH exon 3 reverse primer p2 (GCGACAGAGTACCATGTAGG) and neomycin cassette reverse primer p3 (ATTTGTCACGTCCTGCACGACG) (Schwartz et al., 1999). Briefly, the 25 μl PCR mixture contained 6 μl InstaGene (BioRad, Hercules, CA) purified DNA, 2 mM Mg2, 0.2 mM dNTP, 2 μM p1, 0.8 μM p2, 6.4 μM p3 and 0.5 units of Taq polymerase (Invitrogen, Carlsbad, CA). The thermal cycling reaction was run for 34 cycles of 92°C for 30s, 65°C for 40s and 72°C for 90 s. The 0.6 kb amplicon from the BLMH wild-type allele and the 0.95 kb amplicon from the null allele were distinguished on a 1% agarose gel stained with ethidium bromide.
Immunohistochemistry
Each animal was deeply anesthetized by intraperitoneal injection of 125 mg/kg sodium pentobarbitol prior to sequential transcardial perfusion of physiological saline and 4% paraformaldehyde fixative infused at controlled pressure using a peristaltic pump (Cole-Palmer, Vernon Hills, IL). The isolated brain was postfixed for 2 h and placed in phosphate buffered sucrose solution at 4° C overnight. Each brain was sectioned in the coronal plane using a freezing microtome, and 35 μm sections were sequentially collected in six bins of cryoprotectant and stored at −20° C. Bins of tissue from paired null and wild-type animals were stained with Nissl or processed for immunohistochemistry using the avidin-biotin modification of the immunoperoxidase procedure. Briefly, tissues were washed four times to remove cryoprotectant solution and then transferred to a mouse monoclonal primary antibody (Chemicon) diluted to a final concentration of 1:1000 in 10 mM sodium phosphate (NaPO4) buffer containing 0.3% Triton X-100 and 1% normal donkey serum. Following overnight incubation at 4° C, the tissues were washed three times and then incubated for 1 h in affinity purified biotinylated donkey secondary diluted to 1:200 in NaPO4 buffer (Jackson ImmunoResearch Laboratories, West Grove, PA). The sections were subsequently washed and incubated in ABC complex for 90 min using reagents from the Vector Elite Kit (Vector Laboratories, Burlingame, CA). Thereafter, the tissue was washed in buffer and incubated for 10 min in a 0.1 M Tris-saline buffer containing diaminobenzidine. The immunoperoxidase reaction product was visualized by adding H2O2. Sections were mounted on gelatin-coated slides, dried at room temperature, dehydrated in a graded ethanol series, cleared in xylene and coverslipped with Cytoseal60 (Richard Allan Scientific, Kalamazoo, MI). All sections were visualized and recorded with a Leica DMR microscope equipped with a DC100 digital camera (Leica Microsystems, Wetzlar, Germany). Cell counts for glial fibrillary acidic protein (GFAP) stained astroglia were performed blind to genotype in at least three 200 × 200 μm fields from hippocampal areas CA1 and CA3 for each animal evaluated. A minimum of three to five age and strain matched animals of each genotype were examined for immunohistochemical studies.
Behavioral tests
Young (<8 months) and aged (>14 months) BLMH null and wild-type littermate controls were tested in a series of established behavioral paradigms. Males and females were evaluated separately in groups consisting of a minimum of 5 mice of each genotype. Littermate null and wild type animals comprising each group were derived from 2 to 4 litters differing in age by no more than 2 months. Five groups of animals were tested on the following tasks in the following order: open-field test, cued and contextual fear conditioning, light-dark box exploration, and the spatial and visible-platform versions of the water maze paradigm. One group each of male and female mice also were evaluated on the rotarod apparatus prior to testing on the other behavioral paradigms.
Rotarod testing
Mice were tested for seven consecutive days on a 3- to 19-rpm accelerating Ugo Basile Model 7650 rotarod apparatus (Ugo Basile, Camerio, Italy) to evaluate motor coordination and balance. Each animal was scored for time on the rotarod, which was defined as latency to fall from the apparatus during the 180s trial.
Open field exploration
Locomotion and exploratory behavior were evaluated by observing each animal in an open field chamber (43 × 43 × 28 cm) (MED Associates, St. Albans, VT) for 10 min. Distance, location, and duration of travel bouts, duration of rearing, and occurrence of stereotypical behaviors were recorded. The inner 50% of the chamber surface was considered as the center of the test arena.
Contextual and cued fear conditioning
Associative learning and memory were evaluated by contextual fear conditioning (Paylor et al., 1994; Thiels et al., 2000). Mice were individually transferred to the conditioning chamber, which was housed in a sound-attenuating cubicle. After a 2-min acclimatization period, the animals were presented with 2 pairings of a 30-s tone (80 dB, 2000 Hz) immediately followed by a 2-s scrambled foot shock (0.75 mA). Pairings were separated by 2 min, and the mice were returned to their home cages 30 s after the second pairing. After 24 h, the mice were returned to the conditioning chamber for 5 min, and freezing behavior was scored to assess acquisition of the training context-foot shock association. Two h later, the mice were placed into a novel context and scored for freezing behavior for 3 min before and for 3 min during continuous presentation of the tone. Freezing was scored manually every 10 s by an experimenter blind to the animals’ genotype. Freezing was defined as the absence of movement except respiration.
Light-dark exploration
Exploration of a light-dark box was used to evaluate anxiety as reflected by time spent in the darkened portion of the test chamber. Each animal was placed for 10 min into a test arena (43 × 43 × 28 cm) (MED Associates) that was divided into two compartments (each 21.5 cm × 43 cm). One of the compartments was well illuminated, and the other one was dark due to a black plexiglass insert (walls and ceiling). Animals could move freely between the two compartments through a cut-out (4 × 4 cm) in the center of the wall separating the compartments. Time spent in each compartment was recorded.
Water maze testing
Spatial learning and memory were evaluated in the Morris water maze (Morris, 1984). The maze consisted of a tank (83 cm diameter) that was filled with water to a depth of 29 cm, with a circular, translucent platform (5 cm diameter) submerged 1 cm below the water surface. Extra-maze visual cues in the testing room and the platform location remained fixed for all trials. On five consecutive training days, each animal received four 120s training trials in which animals were released into the tank from each of the four cardinal directions across the daily trials. Animals that failed to locate the submerged platform during the 120-s trial were manually placed on the platform for 30 s. Performance during training was evaluated as average latency to find the platform for each of the training days. Two probe trials separated by a 4-min rest period were conducted the day after the last training trial. The platform was removed from the tank, and the swim pattern was recorded for 60 s using a video tracking system (Chromotrack 3.0, San Diego Instruments, San Diego, CA). Performance on the probe trials was scored as percent time spent in each of the four pool quadrants. After the two probe trials, the animals’ ability to navigate to the platform when the platform was rendered visible by exposing it 1.5 cm above water level and marking it with colored tape was assessed. During visible-platform testing, animals were released into the tank from each of the four cardinal points as described previously for the training trials. Performance on the visible-platform trials was evaluated as average latency to the platform.
Statistical analyses
Immunohistochemistry and behavioral data were analyzed utilizing Stat View (SAS Institute, Cary, NC). Data from groups of males or females of similar age were pooled only if no significant effect of date of testing and/or gender on the dependent variables of interest were found. Pooled data then were analyzed for effects of genotype and, if applicable, trial and age. Three values for velocity (cm/s) from the open field test were discarded as outliers based on Grubb’s test; no other values were omitted from any other datasets. Data were evaluated by χ2 tests, two-tailed t-tests, paired t-tests, multifactorial or repeated-measures ANOVAs, followed by Bonferroni post-hoc testing and individual contrasts when appropriate. The differences among means were accepted as significant at the P < 0.05 level.
RESULTS
Immunohistochemistry
Brain tissue from 20-month old male B6.129 Blmhtm1Geh/J null mice and littermate wild-type controls was stained with Nissl and also probed for GFAP to assess brain morphology, neuronal density, and astrogial architecture. No obvious differences in size or gross brain morphology were observed with Nissl staining. Neuronal density appeared similar throughout the brain, including in the hippocampus and the layers of the cerebral cortex (Figure 1A and B). GFAP immunohistochemistry revealed a global increase in GFAP immunoreactivity in the brains of the null animals compared to their wild-type littermates. GFAP-stained cells in the brains of the null animals were frequently hypertrophied and poorly organized in comparison to those in the brains of the control animals (Figure 2A–D). In cerebral cortex layers II–V, GFAP-positive cells were seen infrequently in wild-type tissue, whereas clusters of hypertrophied cells were noted in null animals. In the CA1 and CA3 regions of the hippocampus, we found a 40 % increase in the number of GFAP-stained cells in mid- and ventral hippocampal tissue from null animals compared to tissue from wild-type controls (Figure 2E).
Figure 1. Nissl staining of B6.129 Blmhtm1Geh/J null and wild-type controls.
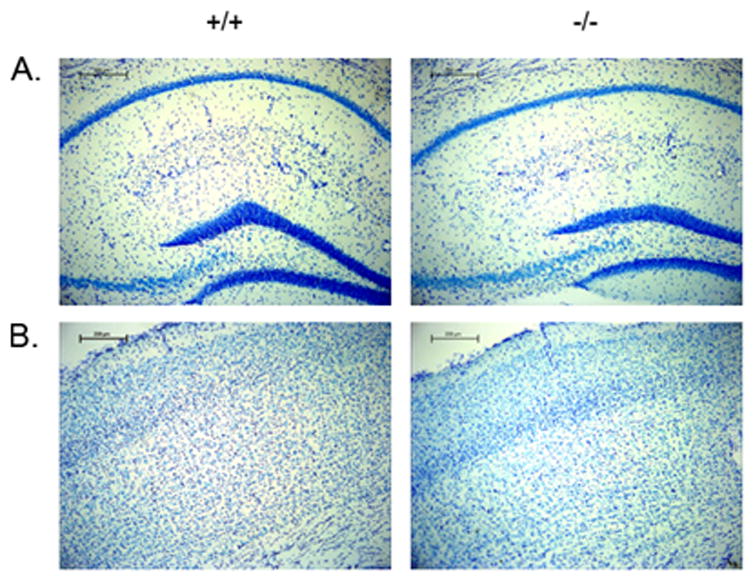
Serially sectioned 4 % paraformaldehyde fixed brain tissue from male aged littermate wild-type and BLMH null B6.129 Blmhtm1Geh/J mice were stained with Nissl. A. Nissl staining of the hippocampus. B. Nissl staining of cerebral cortex. (N = 3 +/+, −/−).
Figure 2. GFAP staining in cortex and hippocampus of B6.129 Blmhtm1Geh/J null and wild-type controls.
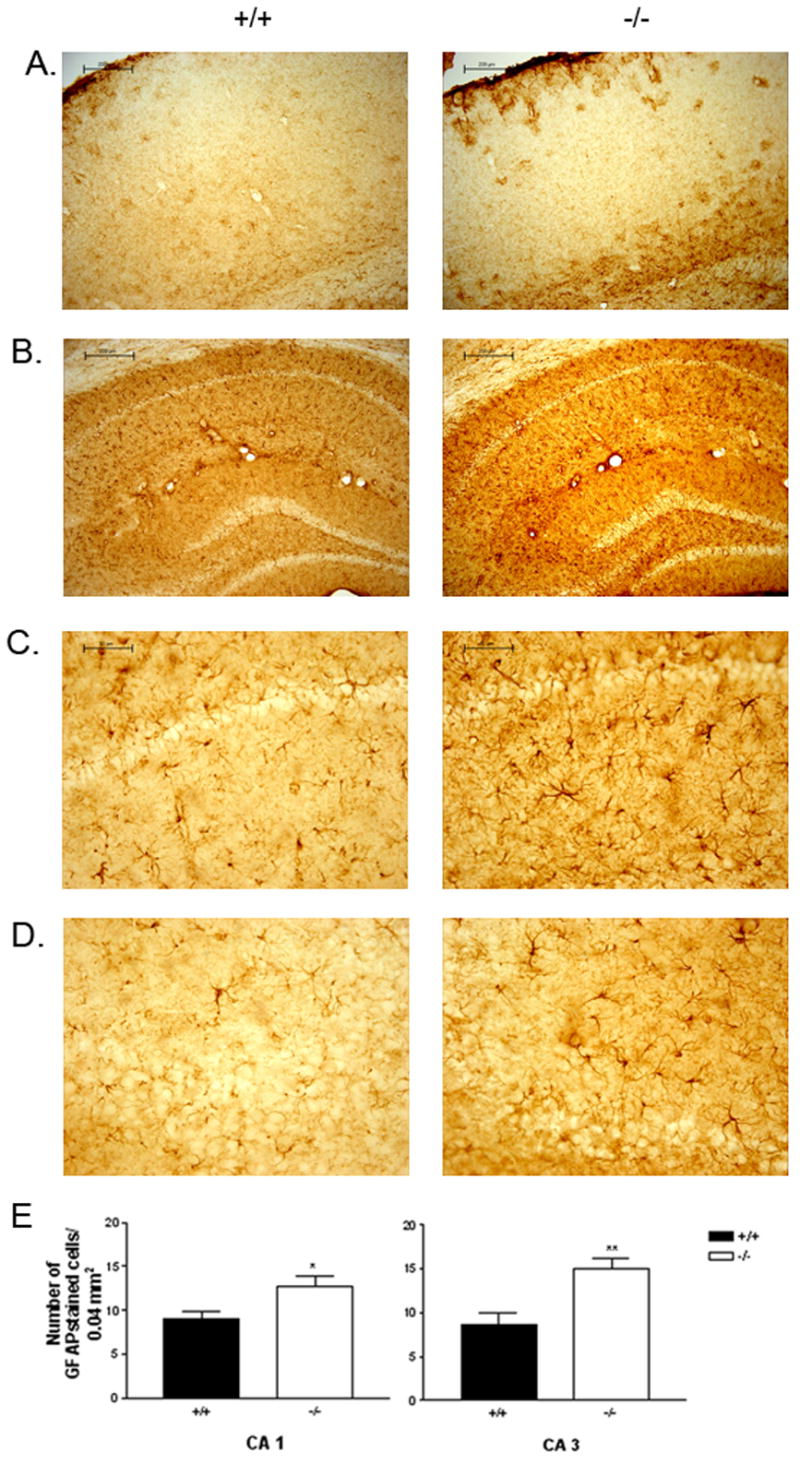
Serially sectioned 4 % paraformaldehyde-fixed brain tissue from littermate male aged wild-type and BLMH null B6.129 Blmhtm1Geh/J mice were immunostained with GFAP to assess astrogliosis. Representative GFAP staining of the cortex (A), hippocampus (B), CA 1 region of hippocampus (C) and the CA3 region of hippocampus (D) (N = 3 +/+, −/−). Bar graph representation of the number of GFAP stained cells in the CA1 and CA3 regions of the hippocampus from wild-type and null hybrid mice (E). *P < 0.05, t-test for independent groups; **P < 0.005, t-test for independent groups).
F1 Hybrid Animals
Hybrid crosses provide a standardized genetic background and eliminate the homozygosity of alleles that can result in significant variation in targeted deletion phenotype in inbred murine strains (Owen et al., 1997; Ingram et al., 1999). For behavioral phenotyping, hybrid [129S6-Blmhtm1Geh/J X B6.129 Blmhtm1Geh/J]F1 Blmh null and wild type mice were bred in accordance with the recommendations of the Banbury Conference (Banbury Conference, 1997). Genotype distribution of the F1 hybrids did not deviate from the expected Mendelian ratio (χ2 = 4.62, P > 0.10). Although null hybrid animals often appeared smaller at weaning, they were otherwise indistinguishable in gross appearance from wild-type littermates. Body weight at 4 to 5 months was significantly lower for null animals (males: −/− = 30.8 ± 0.7 (SEM) g, N = 7; +/+ = 35.9 ± 0.9 g, N = 8; t (13) = 4.13, P < 0.005, t-test for independent groups), but differences between genotypes in body weight did not persist in animals older than 8 months. Brain immunohistrochemistry of 18- to 20-month old littermate hybrid mice revealed no gross abnormalities in null animals except for the presence of hypertrophied GFAP positive astroglia. Astrogliosis was less pronounced in the hybrid animals. In hippocampus, a 15% increase in GFAP positive astroglia was observed in the CA1 region of the null animals in comparison to wild type controls (+/+ = 6.6 ± 0.51, −/− = 9.0 ± 0.44, t(15) = 3.13, P < 0.05), although no significant differences were observed in the CA3 region (+/+ = 6.2 ± 0.73, −/− = 5.8 ± 0.56, P > 0.05, n.s.).
Behavioral studies
Rotarod
At 4 to 5 months of age, the performance of male [129S6-Blmhtm1Geh/J X B6.129 Blmhtm1Geh/J]F1 hybrid animals significantly improved over the 7-days of rotarod testing (repeated-measures ANOVA, F(6,78) = 41.0, P < 0.0001) and did not vary between genotypes (+/+ N = 8, −/− N = 7) (P > 0.05, n.s.) or show a significant trial x genotype interaction (P>0.05, n.s.) (Figure 3A). The same male animals retested at 14 to 15 months of age again showed significant improvement across trials (F(6,72) = 3.9, P < 0.005), but surprisingly, a significant main effect of genotype now emerged (F(1,12)=6.3, P<0.05) (Figure 3B). The trial x genotype interaction was not significant (P > 0.05, n.s.). Post-hoc testing revealed significantly longer latencies to fall for aged male null mice compared to their wild-type littermates on days 1 and 3 of the rotarod testing. Naïve aged female mice (20 to 23 months of age, +/+ N = 6, −/− N = 6) then were evaluated to confirm the differences between genotypes in rotarod performance observed in aged males. We again found a significant main effect of trial (F(5,50) = 4.6, P < 0.005) and of genotype (F(1,10) = 17.1, P < 0.005). The trial x genotype interaction, however, was significant as well (F(5, 50) = 3.9, P < 0.005) (Figure 3C). Post-hoc tests showed that the aged female null mice had longer latencies to fall than the wild-type controls on days 1, 3 and 5 of testing. The role of BLMH in the neuromuscular system is uncharacterized, but related calpain proteases have been shown to have significant physiological functions in the neuromuscular system (Huang et al., 2001).
Figure 3. Rotarod testing of BLMH null F1 hybrids and littermate controls.

Young and aged F1 hybrid BLMH null and wild-type mice were tested over seven days on an accelerating rotarod apparatus (3–19 rpm). Latency to fall from the apparatus was measured daily during a 180-s trial and represented as mean ± SEM for each genotype. A. Seven-day accelerating rotarod test for 4-to 5-month old young males (+/+ N = 8, −/−N = 7). B. Seven-day accelerating rotarod retesting for males at 14 to 15 months of age (+/+ N = 8, −/− N = 7). C. Seven-day accelerating rotarod test for naïve 20- to 23- month old female mice (+/+ N = 6, −/− N = 6). * Post-hoc test P< 0.05.
Open field testing
Spontaneous exploratory behavior and locomotion were evaluated using the open field paradigm. No significant differences between genotypes were observed for any of the parameters tested in either pooled male and female young (4 to 7 months of age, +/+ N = 15, −/− N = 18) or pooled male and female aged mice (18 to 23 months of age, +/+ N = 13, −/− N = 11) (Table 1). Rearing behavior was significantly more prominent in young compared to aged animals (F(1,53)=5.7, P < 0.05, two-way ANOVA), but no main effect of genotype (P > 0.05, n.s.) or genotype x age interaction (P > 0.05, n.s.) was observed. With respect to all other measures, no significant differences between young and aged mice were found.
Table 1. Open field testing of BLMH null and wild-type F1 hybrids.
Young and aged BLMH null and wild-type mice were individually evaluated for 10 min in an open field apparatus. The distance, location, and duration of travel bouts and rearing, and occurrence of stereotypical behavior were recorded and are represented for pooled male and female animals in the table as mean ± SEM. Rearing behavior differed significantly between young and aged animals (P < 0.05)*.
| YOUNG MICE | AGED MICE* | |||
|---|---|---|---|---|
| +/+ (n=15) | −/− (n=18) | +/+ (n=12) | −/− (n=10) | |
| Velocity (cm/s) | 34.4 ± 1.5 | 31.8 ± 0.9 | 33.4 ± 2.0 | 33.8 ± 2.9 |
| Time traveled (s) | 41.9 ± 3.8 | 45.1 ± 4.5 | 32.6 ± 7.1 | 43.4 ± 8.2 |
| Time at rest (s) | 468.2 ± 7.0 | 462.9 ± 7.4 | 477.3 ± 14.5 | 471.5 ± 13.6 |
| Stereotypical behavior (s) | 83.2 ± 3.1 | 85.2 ± 3.4 | 79.3 ± 5.6 | 83.7 ± 6.4 |
| Rearing (s)* | 22.8 ± 4.9 | 16.0 ± 3.0 | 10.0 ± 3.6 | 9.9 ± 4.0 |
| Percent time in center | 14.4 ± 3.3 | 10.5 ± 1.8 | 14.7 ± 4.4 | 16.6 ± 7.4 |
Contextual fear conditioning
In the contextual fear conditioning paradigm, pooled male and female young (2–6 months, +/+ N = 17, −/− N = 19) as well as pooled male and female aged mice (17–23 months, +/+ N = 14, −/− N = 13) displayed significant conditioning to the context (young animals, F(1, 34) = 150.4, P < 0.0001, repeated measures ANOVA; aged animals, F(1, 25) = 257.2, P < 0.0001) and to the tone (young animals, F(1, 34) = 205.0, P < 0.0001; aged animals, F(1,25) = 135.0, P < 0.0001). No significant difference due to genotype was observed in either pooled young animals (P>0.05, n.s.) or pooled aged animals (P>0.05, n.s.), and no significant interaction between genotype and trial was seen in either age group. These data suggest that deletion of BLMH does not impact negatively on fear conditioning, including hippocampus-dependent fear conditioning in either young or aged animals.
Independent of genotype, aged animals displayed a higher level of freezing to the context (F(1,59) = 13.5, P < 0.0005), the tone (F(1,59) = 8.7, P < 0.005), as and the new context during the tone test (F(1,59) = 58.9, P<0.0005) in comparison to young animals. Because of the elevated levels of freezing behavior by aged mice in the fear conditioning paradigm, animals were tested for light-dark exploration to assess anxiety as indicated by percent of time spent in the darkened chamber of the test apparatus. No significant differences were noted between genotypes (P> 0.05, n.s.) or age groups (P > 0.05, n.s.), and no significant genotype x age interaction was observed (P > 0.05, n.s.) (data not shown). Although we did not find evidence for increased levels of unconditional anxiety in aged animals with this paradigm, we cannot exclude the possibility of subtle differences between the age groups as all animals tended to display a relatively strong preference for the dark chamber.
Water maze testing
The ability of mice to acquire and recall spatial information was assessed by escape latency in the Morris water maze. The first group of young male animals was tested under different testing conditions than the other groups of young animals; consequently, their data were not included in the pooled analyses. Pooled male and female young animals (5–7 months, +/+ N = 12, −/− N = 13) exhibited a significant reduction in escape latency over the training period (F(4,92) = 20.6, P < 0.005, repeated-measures ANOVA), but neither the genotype effect (P> 0.05, n.s.) nor or the trial x genotype interaction was significant (P > 0.05, n.s.) (Figure 5A). Pooled male and female aged animals (17–23 months, +/+ N = 10, −/− N = 12) also displayed significant improvement over the training trials (F(4,80) = 19.3, P < 0.005), and, as was the case with the young animals, neither the genotype effect (P > 0.05, n.s.) nor the genotype x trial interaction was significant (P > 0.05, n.s.) (Figure 5B).
Figure 5. Acquisition phase of water maze testing for BLMH null and littermate controls.
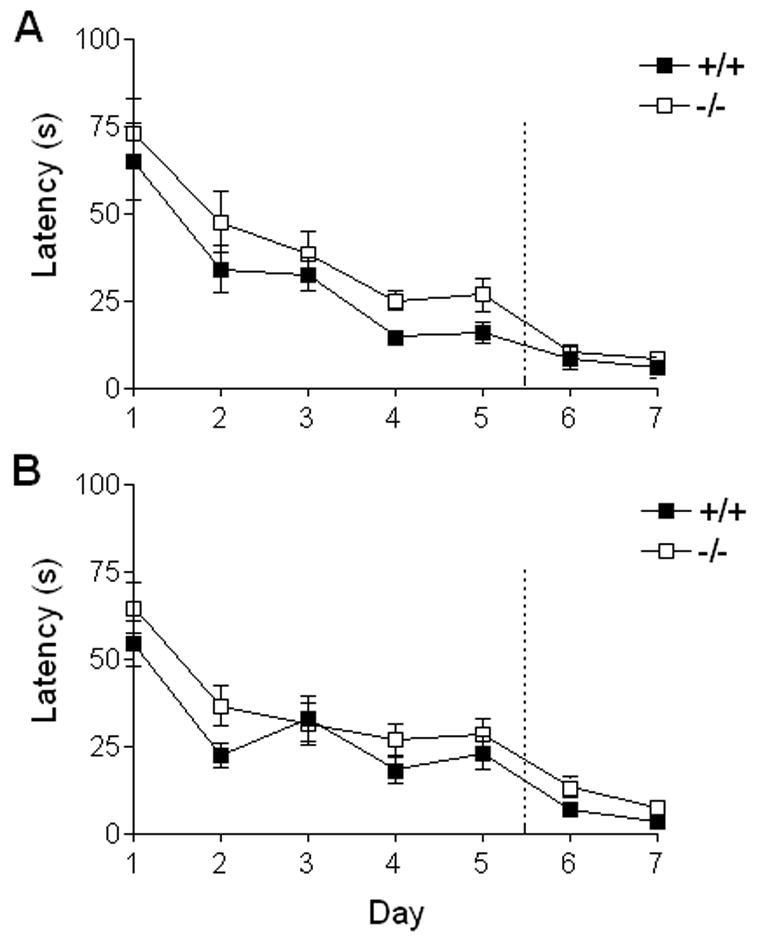
Young and aged F1 hybrid BLMH null and wild-type animals were evaluated for learning and memory of spatial information using the water maze paradigm. On days 1–5, each animal received four training trials in which the animal was allowed to swim freely about a pool in which a submerged, hidden platform was located. On days 6 and 7, the task was modified in that the platform was made visible. Average escape latency from the water was measured for each animal in s and is represented for pooled male and female mice as mean ± SEM. A. Latency results for young animals across the 5 days of training and the visible-platform testing (days 6 and 7) (+/+ N = 12, −/− N = 13). B. Latency results for aged animals across the 5 days of training and the visible-platform testing (days 6 and 7). (+/+ N = 10, −/− N = 12).
After the five days of training with the submerged platform, each animal was evaluated for retention of the platform location by probe trial testing. Analysis of percent dwell time in the target and control quadrants revealed a significant main effect of quadrant dwell time in pooled young animals (F(3,69)=53.5, P < 0.0001, repeat measures ANOVA) (Figure 6A). All young animals showed preference for the target quadrant over the control quadrants, which is indicative of some degree of learning about the platform location. Although a main genotype effect was not observed (P > 0.05, n.s.), a significant quadrant x genotype interaction was noted (F(3,69) = 4.8, P < 0.005, repeateded measures ANOVA). Post-hoc testing showed the percent dwell time differed between genotypes only in the target quadrant (P < 0.005), with young wild-type mice displaying a greater preference for the target quadrant than young null mice. Pooled aged animals also displayed preference for the target quadrant over the control quadrants (F(3,60) = 11.7, P < 0.0001, repeated measures ANOVA) (Figure 6B), but showed no significant genotype effect (P > 0.05, n.s.) nor a significant genotype x quadrant interaction (P > 0.05, n.s.).
Figure 6. Probe trial testing of BLMH null and littermate controls.
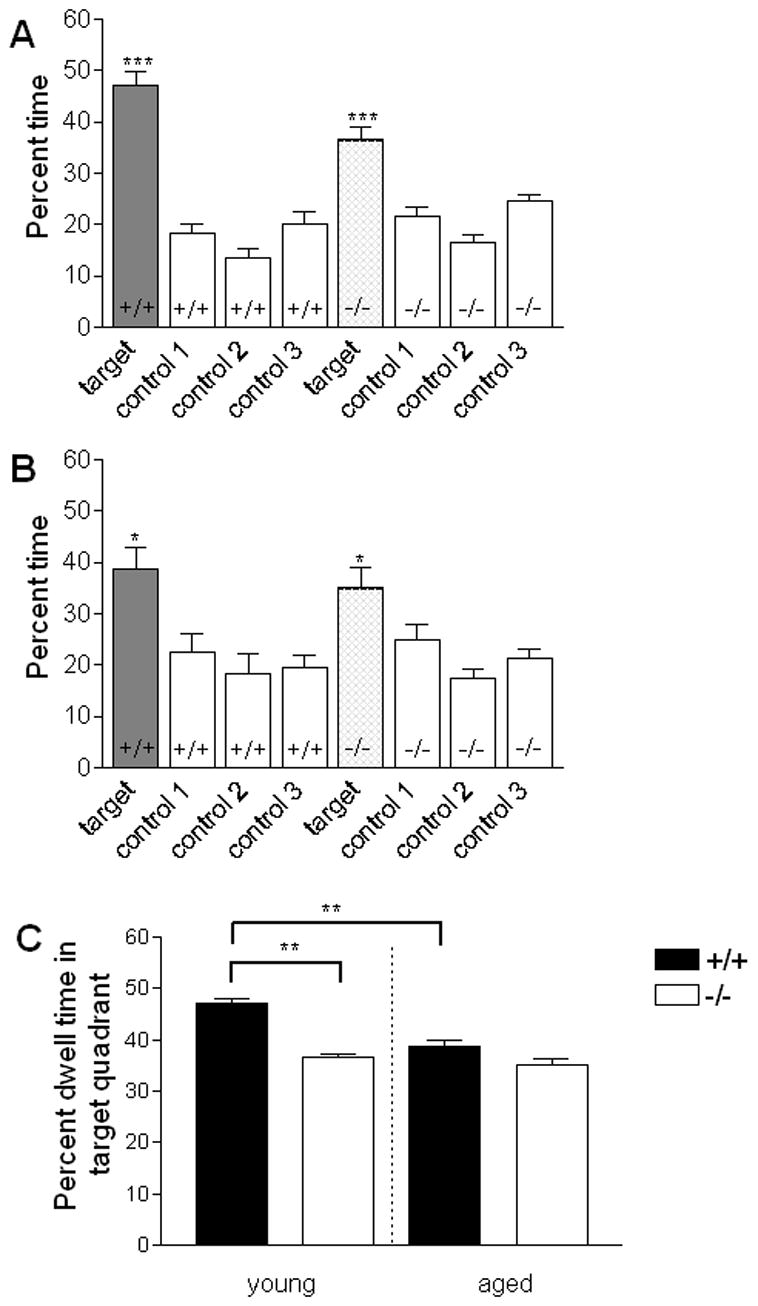
Probe trials were conducted to evaluate retention of the submerged platform location following 5 days of training. Young and aged F1 hybrid BLMH null and wild-type animals were allowed to swim freely for 60 s in the water tank with the platform removed. Time spent in each quadrant was recorded in s and expressed as mean (± SEM) percent time in the respective quadrants. Target represents the quadrant that formerly contained the submerged platform. A. Percent time spent in each of the 4 test quadrants by pooled male and female young mice. B. Percent time spent in each of the 4 test quadrants by pooled male and female aged animals. Animals in all groups tested show preference for the target quadrant over control quadrants. C. Percent time in the target quadrant for wild-type and BLMH null mice across age. Young null animals spent significantly less time in the target quadrant than did young wild-type controls (P < 0.005). In addition, aged wild-type animals spent significantly less time in the target quadrant than young wild-type mice (P < 0.005). * P<0.05, **P < 0.005, *** P < 0.001
Analysis of target quadrant dwell time only confirmed our previous observation that young null animals spend significantly less percent time in the target quadrant than their littermate controls (t(23)=9.1, P<0.005; t-test for independent groups) (Figure 6C). Interestingly, no significant difference in percent time spent in the target quadrant was observed between genotypes among the aged mice (P > 0.05, n.s.) (Figure 6C). These data suggest that the robustness spatial memory as inferred by dwell time in target quadrant is poorer in null animals than littermate controls and that this phenotypic difference is not observed among aged animals.
The presence of a genotype effect among young but not aged animals prompted testing for differential changes in performance across age. Analysis of percent dwell time in the target quadrant across age and genotype showed a nearly significant genotype effect (F(1,43) = 3.9, P = 0.06, two way ANOVA) and a trend for a main effect of age (F(1, 43) = 3.1, P = 0.08), but no age x genotype interaction (P < 0.1). Consideration of an age effect in each genotype separately, however, revealed a significant decline in the percent of time spent in the target quadrant among wild-type animals (t(20) = 5.7, P < 0.005, two-tailed t-test for independent groups), but not among null animals (P > 0.05, n.s.) (Figure 6C). Thus, aging appears to affect performance in wild-type but not BLMH null animals.
Following the probe trials, the animals were evaluated on two consecutive days for their ability to locate the platform after it was made visible. The sensory-motor requirements of the task are similar when the platform is visible vs. submerged; however, in contrast to the version of the task with the platform submerged, performance on the version with the platform visible does not depend on hippocampus-mediated memory function. Visible platform testing revealed no significant differences in escape latency due to genotype in either pooled young (P > 0.5, n.s., repeat measures ANOVA) or aged animals (P > 0.5, n.s., repeat measures ANOVA) (Figure 5A and B). Analysis of swim speeds during the probe trials revealed that although aged animals swam significantly slower than did young animals (young = 22.63 ± 0.42 (SEM) cm/s N = 25; aged = 18.68 ± 0.57 cm/s, N = 22; F(1,43) = 30.13, P < 0.0001), there was no significant difference between age matched genotypes in swim speed (P>0.05, n.s.) or genotype x age interaction (P > 0.05, n.s.). Overt differences in response to sensory function and swim performance do not appear to account for changes in probe trial performance in age matched null and wild-type animals.
DISCUSSION
Immunohistochemical analysis and behavioral phenotyping of mice with a targeted deletion of the neutral cysteine protease BLMH revealed significant differences between animals lacking the BLMH gene and wild-type littermate controls. An increase in GFAP-positively stained astroglia was observed in the brains of aged null mice compared to littermate controls. Furthermore, behavioral analyses revealed selective changes in performance. In the water maze task, young null animals displayed less preference for the target quadrant than did wild-type controls which can be interpreted as differences in robustness of spatial memory between genotypes. Although the knockout model cannot exclude the possibility of phenotypic effects related to disruption or modification of neighboring genes, or expression of the neomycin selection marker, these data are consistent with a non-redundant physiological role for BLMH in the CNS.
Murine models provide an in vivo system for examining age-related changes in the CNS that serve as a context for the occurrence of neurodegenerative disease, and may even contribute to its development. Age-related changes in complex tasks, such as the spatial version of the water maze, are well documented and have been shown to occur as a function of strain (Gower et al., 1993; Ingram et al., 1999: Frick et al., 2000; Magnusson et al., 2003). The wild-type F1 hybrids performed well in all behavioral paradigms evaluated, consistent with hybrid vigor (Owen et al., 1997), and displayed few significant age-related behavioral changes. The most notable age-related change was the decline in water maze probe trial performance. We found this decline in performance to be comparable in male and female mice, although others have observed gender differences in the occurrence of age-related changes in spatial cognition (Frick et al., 2000; Benice et al., 2006). This apparent discrepancy may be attributable, at least in part, to differences in the types of mouse strains tested in the various studies. In addition to age-related changes in probe trial performance, an increase in generalized freezing in contextual fear conditioning was observed. Similar results in the contextual fear paradigm were noted for aged 22-month old vs. 7-month old Wistar rats (Doyere et al., 2000). The reason for the increased freezing in the aged animals is not known, but the results from the open field and the light-dark paradigms suggest that it probably was not due to overall greater anxiety in aged animals.
Immunohistochemical evaluation of the CNS of aged B6.129 Blmhtm1Geh/J wild and null animals revealed significant global astrogliosis, as indicated by increased GFAP staining in the null animals. A similar, but less pronounced astrogliosis was also observed in the aged F1 hybrid [129S6-Blmhtm1Geh/J X B6.129 Blmhtm1Geh/J] animals. The finding of astrogliosis in the BLMH null mice is significant because it suggests the presence of CNS pathology or changes in neuronal-glial interactions that underlie the astrocyte’s primary role in the maintenance of the neural microenvironment (Bjorklund et al., 1985; Newcombe et al., 1986; Hansen et al., 1987; Steward et al., 1991). In this context it is important to note that astrocytes are involved in homocysteine metabolism (Enokido et al., 2005), and that homocysteine has been suggested as a neurotransmitter amino acid (Lehmann et al., 1988; Benz et al., 2004). Furthermore, homocysteine accumulation is toxic to neurons (Kruman et al., 2000) and BLMH may play a role in protecting cells against homocysteine toxicity (Zimny et al., 2006).
In three independently tested cohorts of young F1 hybrid [129S6-Blmhtm1Geh/J X B6.129 Blmhtm1Geh/J] animals, deletion of BLMH was associated with reduced dwelling in the target quadrant of the water maze during probe trials. This effect could not be attributed to diminished locomotor activity or visual impairment based on the swim speed evaluation and performance in the rotatod, open field, and the water maze paradigm when the platform was visible. Although subtle differences in sensorimotor or emotional function cannot be ruled out entirely as a possible explanation for the difference in relative dwell time in the target quadrant, the most parsimonious conclusion is that deletion of BMLH is associated with a mild impairment in spatial memory function in young animals.
Although both the water maze task and the contextual fear conditioning paradigm frequently are used as tests of hippocampus-dependent associative learning and memory, the lack of correlated findings in the two paradigms is consistent with observations by others suggesting that the relative dependence on the integrity of the hippocampus differs between these two tasks (Owen et al., 1997; Burwell et al., 2004). For instance, hippocampal lesions have been shown to cause significantly greater impairment of water maze performance than of contextual fear conditioning, which suggests differences in the neural systems required for performance in the two paradigms (Good and Honey, 1997; Logue et al., 1997).
In the water maze probe trials, a trend for an age-dependent decline in accurate performance was observed. Analysis of dwell time in the target quadrant across age for wild-type animals only revealed a significant decline of performance consistent with age-dependent changes in performance on this task observed by others (Gower et al., 1993; von Bohlen und Halbach et al., 2006). Interestingly, no significant change across age was observed when a similar analysis was applied to the data of the young and aged null animals only. This lack of an age effect is unlikely to have been due to a floor effect, as the aged mutant mice, similar to the wild-type controls, continued to exhibit a slight preference for the target quadrant. The lack of a comparable age-dependent decline in BMLH null mice is currently unclear; however, it raises the intriguing possibility that the absence of BLMH may attenuate age-dependent deterioration of the kind of cognitive function as is assessed with the water maze task. At minimum, the finding suggests that aging does not result in significant progression of the spatial memory impairment observed in the younger animals.
Although a role for BLMH in AD remains unclear, the recent discovery of a putative physiological function for BLMH in intracellular metabolism of homocysteine thiolactone (Zimny et al., 2006) presents a potential mechanism by which the cysteine protease could influence the pathophysiology of the disease. In several animal models of diet-induced homocysteinemia, selective impairment in spatial learning and memory were observed using a water maze paradigm (Bernardo et al., 2006; Troen et al., 2006). Furthermore, in studies with APP overexpressing mice, homocysteinemia appeared to induce memory impairments (Bernardo et al., 2006). Polymorphisms of BLMH may increase the risk of developing AD through subtle changes in enzyme levels or efficiency, which, in turn, results in changes in intracellular levels of homocysteine. Future studies on the relationship between BLMH and homocysteine metabolism in brain may help define the role of this unique cysteine protease in AD.
Figure 4. Contextual fear conditioning of BLMH null F1 hybrids and littermate controls.

Contextual fear conditioning was evaluated in young and aged F1 hybrid BLMH null and wild-type mice. On the day of training, animals were exposed for 2 min to the training context (train-context) and a 30-s tone (train–tone). Immediately following the tone, the animals received a 0.75-mA scrambled foot shock. Tone and shock pairing was repeated once after a 2-min interval. Twenty-four hours later, the animals were evaluated for freezing when exposed to the original training context (context) or when exposed to the tone in a novel context (tone). Data are represented for pooled male and female mice by genotype as mean ± SEM. Aged animals showed significantly more freezing than young animals to the context (P < 0.0005) and the tone (P < 0.005) during testing.
Acknowledgments
The authors wish to thank Dr. Edward Dixon and his staff at the Safar Center of the University of Pittsburgh for assistance with the Morris water maze. The authors also wish to thank Dr. Gregg Homanics for assistance with the rotarod testing, and Heather Herman and Eloise Peet for assistance with behavior testing. Grant support from NIH (AG18557), the Alzheimer’s Disease Research Center, and PA DOH-SAP#4100027294 is acknowledged and appreciated.
Abbreviations
- BLMH
bleomycin hydrolase
- AD
Alzheimer’s disease
- GFAP
glial fibrillary acid protein
- CNS
central nervous system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–23. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Benz B, Grima G, Do KQ. Glutamate-induced homocysteic acid release from astrocytes: possible implications in glia-neuron signaling. Neuroscience. 2004;124:377–386. doi: 10.1016/j.neuroscience.2003.08.067. [DOI] [PubMed] [Google Scholar]
- Bernardo A, McCord M, Troen AM, Allison JD, McDonald MP. Impaired spatial memory in APP-overexpressing mice on a homocysteinemia-inducing diet. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.05.035. In press. [DOI] [PubMed] [Google Scholar]
- Bjorklund H, Eriksdotter-Nilsson M, Dahl D, Rose G, Hoffer B, Olson L. Image analysis of GFA-positive astrocytes from adolescence to senescence. Exp Brain Res. 1985;58:163–170. doi: 10.1007/BF00238964. [DOI] [PubMed] [Google Scholar]
- Bromme D, Rossi AB, Smeekens SP, Anderson DC, Payan DG. Human bleomycin hydrolase: molecular cloning, sequencing, functional expression, and enzymatic characterization. Biochemistry. 1996;35:6706–6714. doi: 10.1021/bi960092y. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Saddoris MP, Bucci DJ, Wiig KA. Corticohippocampal contributions to spatial and contextual learning. J Neurosci. 2004;24:3826–3836. doi: 10.1523/JNEUROSCI.0410-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapot-Chartier MP, Rul F, Nardi M, Gripon JC. Gene cloning and characterization of PepC, a cysteine aminopeptidase from Streptococcus thermophilus, with sequence similarity to the eucaryotic bleomycin hydrolase. Eur J Biochem. 1994;224:497–506. doi: 10.1111/j.1432-1033.1994.00497.x. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Doyere V, Gisquet-Verrier P, de Marsanich B, Ammassari-Teule M. Age-related modifications of contextual information processing in rats: role of emotional reactivity, arousal and testing procedure. Behav Brain Res. 2000;114:153–165. doi: 10.1016/s0166-4328(00)00223-0. [DOI] [PubMed] [Google Scholar]
- Duff K, Rao MV. Progress in the modeling of neurodegenerative diseases in transgenic mice. Curr Opin Neurol. 2001;14:441–447. doi: 10.1097/00019052-200108000-00003. [DOI] [PubMed] [Google Scholar]
- Enenkel C, Wolf DH. BLH1 codes for a yeast thiol aminopeptidase, the equivalent of mammalian bleomycin hydrolase. J Biol Chem. 1993;268:7036–7043. [PubMed] [Google Scholar]
- Enokido Y, Suzuki E, Iwasawa K, Namekata K, Okazawa H, Kimura H. Cystathionine beta-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J. 2005;19:1854–1856. doi: 10.1096/fj.05-3724fje. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Velasco G, Campo E, Lopez-Otin C. Cloning and expression analysis of human bleomycin hydrolase, a cysteine proteinase involved in chemotherapy resistance. Cancer Res. 1996;56:1746–1750. [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Good M, Honey RC. Dissociable effects of selective lesions to hippocampal subsystems on exploratory behavior, contextual learning, and spatial learning. Behav Neurosci. 1997;111:487–493. doi: 10.1037//0735-7044.111.3.487. [DOI] [PubMed] [Google Scholar]
- Gower AJ, Lamberty Y. The aged mouse as a model of cognitive decline with special emphasis on studies in NMRI mice. Behav Brain Res. 1993;57:163–173. doi: 10.1016/0166-4328(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Hansen LA, Armstrong DM, Terry RD. An immunohistochemical quantification of fibrous astrocytes in the aging human cerebral cortex. Neurobiol Aging. 1987;8:1–6. doi: 10.1016/0197-4580(87)90051-0. [DOI] [PubMed] [Google Scholar]
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s Disease. Trends Pharmacol Sci. 1991;12:383–8. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Hardy J. and Higgins G.A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–5. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wang KK. The calpain family and human disease. Trends Mol Med. 2001;7:355–362. doi: 10.1016/s1471-4914(01)02049-4. [DOI] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK, Jucker M. Developing mouse models of aging: a consideration of strain differences in age-related behavioral and neural parameters. Neurobiol Aging. 1999;20:137–145. doi: 10.1016/s0197-4580(99)00033-0. [DOI] [PubMed] [Google Scholar]
- Joshua-Tor L, Xu HE, Johnston SA, Rees DC. Crystal structure of a conserved protease that binds DNA: the bleomycin hydrolase, Gal6. Science. 1995;269:945–950. doi: 10.1126/science.7638617. [DOI] [PubMed] [Google Scholar]
- Kajiya A, Kaji H, Isobe T, Takeda A. Processing of amyloid beta-peptides by neutral cysteine protease bleomycin hydrolase. Protein Pept Lett. 2006;13:119–123. doi: 10.2174/092986606775101562. [DOI] [PubMed] [Google Scholar]
- Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, Mattson MP. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci. 2000;20:6920–6926. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Tsai C, Wood PL. Homocysteic acid as a putative excitatory amino acid neurotransmitter: I. Postsynaptic characteristics at N-methyl-D-aspartate-type receptors on striatal cholinergic interneurons. J Neurochem. 1988;51:1765–70. doi: 10.1111/j.1471-4159.1988.tb01157.x. [DOI] [PubMed] [Google Scholar]
- Lazo JS, Humphreys CJ. Lack of metabolism as the biochemical basis of bleomycin-induced pulmonary toxicity. Proc Natl Acad Sci U S A. 1983;80:3064–3068. doi: 10.1073/pnas.80.10.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefterov IM, Koldamova RP, Lazo JS. Human bleomycin hydrolase regulates the secretion of amyloid precursor protein. FASEB J. 2000;14:1837–1847. doi: 10.1096/fj.99-0938com. [DOI] [PubMed] [Google Scholar]
- Lefterov IM, Koldamova RP, Lefterova MI, Schwartz DR, Lazo JS. Cysteine 73 in bleomycin hydrolase is critical for amyloid precursor protein processing. Biochemistry. 2001;283:994–999. doi: 10.1006/bbrc.2001.4860. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Scruggs B, Aniya J, Wright KC, Ontl T, Xing Y, Bai L. Age-related deficits in mice performing working memory tasks in a water maze. Behav Neurosci. 2003;117:485–495. doi: 10.1037/0735-7044.117.3.485. [DOI] [PubMed] [Google Scholar]
- Malherbe P, Faull RL, Richards JG. Regional and cellular distribution of bleomycin hydrolase mRNA in human brain: comparison between Alzheimer’s diseased and control brains. Neurosci Lett. 2000;281:37–40. doi: 10.1016/s0304-3940(00)00802-8. [DOI] [PubMed] [Google Scholar]
- Montoya SE, Aston CE, DeKosky ST, Kamboh MI, Lazo JS, Ferrell RE. Bleomycin hydrolase is associated with risk of sporadic Alzheimer’s disease. Nat Genet. 1998;18:211–212. doi: 10.1038/ng0398-211. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Namba Y, Ouchi Y, Takeda A, Ueki A, Ikeda K. Bleomycin hydrolase immunoreactivity in senile plaque in the brains of patients with Alzheimer’s disease. Brain Res. 1999;830:200–202. doi: 10.1016/s0006-8993(99)01435-3. [DOI] [PubMed] [Google Scholar]
- Neumann H, Cavalie A, Jenne DE, Wekerle H. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- Newcombe J, Woodroofe MN, Cuzner ML. Distribution of glial fibrillary acidic protein in gliosed human white matter. J Neurochem. 1986;47:1713–1719. doi: 10.1111/j.1471-4159.1986.tb13079.x. [DOI] [PubMed] [Google Scholar]
- Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80:1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Bagli M, Jessen F, Frahnert C, Rao ML, Maier W, Heun R. Confirmation of the association between bleomycin hydrolase genotype and Alzheimer’s disease. Mol Psychiatry. 2000;5:213–215. doi: 10.1038/sj.mp.4000656. [DOI] [PubMed] [Google Scholar]
- Paylor R, Tracy R, Wehner J, Rudy JW. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci. 1994;108:810–817. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- Raina AK, Takeda A, Nunomura A, Perry G, Smith MA. Genetic evidence for oxidative stress in Alzheimer’s disease. Neuroreport. 1999;10:1355–1357. doi: 10.1097/00001756-199904260-00036. [DOI] [PubMed] [Google Scholar]
- Rall GF, Mucke L, Oldstone MB. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I-expressing neurons in vivo. J Exp Med. 1995;182:1201–1212. doi: 10.1084/jem.182.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ, 3rd, Kandel ER, Duff K, Kirkwood A, Shen J. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- Schwartz DR, Homanics GE, Hoyt DG, Klein E, Abernethy J, Lazo JS. The neutral cysteine protease bleomycin hydrolase is essential for epidermal integrity and bleomycin resistance. Proc Natl Acad Sci U S A. 1999;96:4680–4685. doi: 10.1073/pnas.96.8.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebti SM, Mignano JE, Jani JP, Srimatkandada S, Lazo JS. Bleomycin hydrolase: molecular cloning, sequencing, and biochemical studies reveal membership in the cysteine proteinase family. Biochemistry. 1989;28:6544–6548. doi: 10.1021/bi00442a003. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PWF, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s Disease. New Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- Stolze L, Schirle M, Schawarz G, Schoter C, Thompson M, Hersh L, Kalbacher H, Stevanovic S, Rammensee H, Schild H. Two new proteases in the MHC class I processing pathway. Nat Immunol. 2000;1:413–418. doi: 10.1038/80852. [DOI] [PubMed] [Google Scholar]
- Steward O, Torre ER, Tomasulo R, Lothman E. Neuronal activity up-regulates astroglial gene expression. Proc Natl Acad Sci U S A. 1991;88:6819–6823. doi: 10.1073/pnas.88.15.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels E, Urban NN, Gonzales-Burgos GR, Kanterewicz BI, Barrionuevo G, Chu CT, Oury TD, Klan E. Impairment of long-term potentiation and associative memory in mice that overexpress superoxide dismutase. J Neurosci. 2000;20:7631–7639. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troen AM, Shukitt-Hale B, Chao WH, Albuquerque B, Smith DE, Selhub J, Rosenberg J. The cognitive impact of nutritional homocysteinemia in Apolipoprotein-E deficient mice. J Alzheimers Dis. 2006;9:381–92. doi: 10.3233/jad-2006-9403. [DOI] [PubMed] [Google Scholar]
- Umezawa H, Maeda K, Takeuchi T, Okami Y. New antibiotics, bleomycin A and B. J Antibiot (Tokyo) 1966;19:200–209. [PubMed] [Google Scholar]
- Umezawa H, Takeuchi T, Hori S, Sawa T, Ishizuka M, Ichikawa T, Komai T. Studies on the mechanism of antitumor effect of bleomycin on squamous cell carcinoma. J Antibiot. 1972;25:409–420. doi: 10.7164/antibiotics.25.409. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Zacher C, Gass P, Unsicker K. Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice. J Neurosci Res. 2006;83:525–31. doi: 10.1002/jnr.20759. [DOI] [PubMed] [Google Scholar]
- Zheng W, Johnston SA, Joshua-Tor L. The unusual active site of Gal6/bleomycin hydrolase can act as a carboxypeptidase, aminopeptidase, and peptide ligase. Cell. 1998;93:103–109. doi: 10.1016/s0092-8674(00)81150-2. [DOI] [PubMed] [Google Scholar]
- Zimny J, Sikora M, Guranowski A, Jakubowski H. Protective mechanisms against homocysteine toxicity: the role of bleomycin hydrolase. J Biol Chem. 2006;281:22485–22492. doi: 10.1074/jbc.M603656200. [DOI] [PubMed] [Google Scholar]


