Abstract
Human ghrelin is transported across the blood-brain barrier (BBB) of normal mice. Here, we studied the effects of triglycerides, obesity, and starvation in retired breeder mice maintained on a high fat diet, mice age-matched to the retired breeders but maintained on normal chow, and 8 week old mice maintained on breeder chow. The rate of ghrelin transport across the BBB was studied by both the intravenous administration method of multiple-time regression analysis and by the brain perfusion method. We found: 1) obese, aged mice lost the ability to transport intravenously administered ghrelin across the BBB, resulting in an inverse relation between body weight and ghrelin BBB permeability; 2) serum triglycerides promoted transport of intravenously administered ghrelin across the BBB, whereas epinephrine had no effect; 3) fasting tended to promote ghrelin transport across the BBB as most readily shown in brain perfusion studies; 4) evidence suggested that a serum factor promoted ghrelin transport in 8 week old mice. Overall, these results show that serum factors and physiological states influence the rate at which ghrelin is transported across the blood-brain barrier.
Keywords: Blood-brain barrier, ghrelin, fasting, starvation, triglycerides, obesity, satiety
Introduction
Ghrelin is a 28 amino acid peptide produced mostly by the fundus of the stomach [17]. Ghrelin has effects on appetite mediated largely through effects at the hypothalamus through a nitric oxide dependent mechanism [10] and on cognition mediated largely through effects at the hippocampus [9]. Blood-borne ghrelin must cross the blood-brain barrier (BBB) in order to reach its receptors in the hippocampus and hypothalamus [8;9]. Ghrelin crosses the BBB by an elaborate interplay between non-saturable transport, saturable brain-to-blood transport, and saturable blood-to-brain transport that depends on both the primary amino acid structure of ghrelin and the presence or absence of the octanoyl group [8]. For example, human ghrelin is transported in both the blood-to-brain and the brain-to-blood directions by saturable transporters.
Other feeding-related hormones that are produced in the periphery but act in the brain, such as leptin and insulin, are also transported across the BBB by their own selective transporters [5;6]. These systems are know to be highly regulated. For example, leptin transport is affected by epinephrine, triglycerides, obesity, fasting/starvation, and in the nondiabetic state by glucose and insulin [1;2;7;12;13;13]. Insulin transport is affected by obesity, starvation, triglycerides, and the diabetic state [4;11;16]. Here, we determined the effects of obesity, starvation, and serum triglycerides on the blood-to-brain transport of human ghrelin.
Methods
Radioactive Labeling and Purification of Human Ghrelin: Human ghrelin (1) was purchased from Phoenix Pharmaceuticals Inc. (Phoenix, TX). Ghrelin was radioactively labeled with 131I (I-Ghr) by the chloramine-T method and purified by reverse-phase HPLC on a C18 column.
Animals
All studies were approved by the local Animal Care and Use Committee and were performed in an AAALAC approved facility and used male CD-1 mice from our in-house (VA medical center) colony. Retired breeders [6–8 mo old mice used as breeders and maintained on breeder chow (Teklad Mouse Breeder Diet 8626; Harlan/Teklad, Madison, WI, contains 10% fat) since weaning], aged-matched non-breeders (referred to as the “aged-matched group) maintained on regular chow (Lab Diet 5001 PMI Nutrition, Brentwood, MO, contains 5% fat), and young (8 week old) mice maintained on regular chow were used in the studies. As indicated, mice were fasted for 48 h before study or fed ab lib up to the time of study.
Multiple-time Regression Analysis
Mice were anesthetized with ip urethane (4.0 g/kg) and the left jugular vein and right carotid artery exposed. 0.2 ml of Ringer’s lactated solution (LR) with 1% bovine serum albumin (BSA) containing 105 cpm of I-Ghr was injected into the jugular vein. Where indicated in a study of retired breeders, 30 microg/mouse was included in the iv injection. Blood was collected from the carotid artery and the whole brain removed 1–20 min after the jugular injection, a time period when the radioactivity represents intact I-Ghr [8].[6] Blood was centrifuged at 5000 g for 10 min at 4 °C and the serum collected. The whole brain was cleaned of large vessels and weighed after discarding the pituitary and pineal gland. Levels of radioactivity in brain and serum were measured in a gamma counter and brain/serum ratios (µl/g) calculated.
Multiple-time regression analysis (13) (14) was used to calculate the blood to brain unidirectional influx rate (Ki). The brain/serum ratios were plotted against their respective exposure times (Expt). Expt was calculated from the formula:
where Cp is the level of radioactivity in serum and Cpt is the level of radioactivity in serum at time t. Expt corrects for the clearance of peptide from the blood. Ki with its error term is measured as the slope for the linear portion of the relation between the brain/serum ratios and Expt and the Y intercept of the linearity measures Vi, the distribution volume in brain at t = 0,so that the equation describing the linear portion of the relation between brain/serum ratios and Expt is:
Brain Perfusion
Mice were anesthetized with ip urethane (4.0 g/kg). The thorax was opened, the heart was exposed, both jugulars severed, and the descending thoracic aorta clamped. A 26 gauge butterfly needle was inserted into the left ventricle of the heart and Zlokovic’s [19] buffer containing I-Ghr [2(10)5 cpm/ml] was infused at a rate of 2 ml/minute for 5 minutes [14]. The exact cpm infused was determined on a 1 ml aliquot of perfusion fluid. After perfusion, the brain was removed as above and brain/perfusion ratios calculated. The unidirectional influx rate (Ki) was calculated by regressing the brain/perfusion ratio against perfusion time.
Preparation of Triglycerides and Epinephrine
Epinephrine (Sigma, St. Louis, MO) was diluted in the vehicle used for iv injection above in multiple-time regression studies at a dose of 40 nmol/mouse. Triglycerides (Sigma Chemical Co, St. Louis, MO ) and L-α-phosphatidylcholine (Sigma) were each dissolved in chloroform, mixed, and dried under a stream of nitrogen gas. Zlokovic’s buffer was added and the material vigorously mixed, homogenized, and alternatively frozen in liquid nitrogen and thawed in a warm water bath for 12 cycles. Material was either immediately diluted to the desired concentration with LR containing I-Ghr and injected iv or stored at −20 °C for use within 48 h. On day of study, anesthetized mice received iv injections of 0.2 ml of LR containing 1% BSA and 105 cpm of I-Ghr with or without the triglyceride emulsion. Carotid artery blood and brains were obtained 20 min later and results expressed as brain/serum ratios.
Statistics
Linear regression was performed by the least squares method and the slopes of the lines (the Ki’s) compared using the Prism 5.0 statistical package (GraphPad, Inc., San Diego, CA). Two means were compared by t-test. More than two means in one category were compared by one-way analysis of variance (ANOVA) followed by Newman-Keuls post-test. Mor than two means in two categories were compared by two-way ANOVA followed by the Bonferroni post-test (Prism 5.0). Statistical significance was taken as p<0.05.
Results
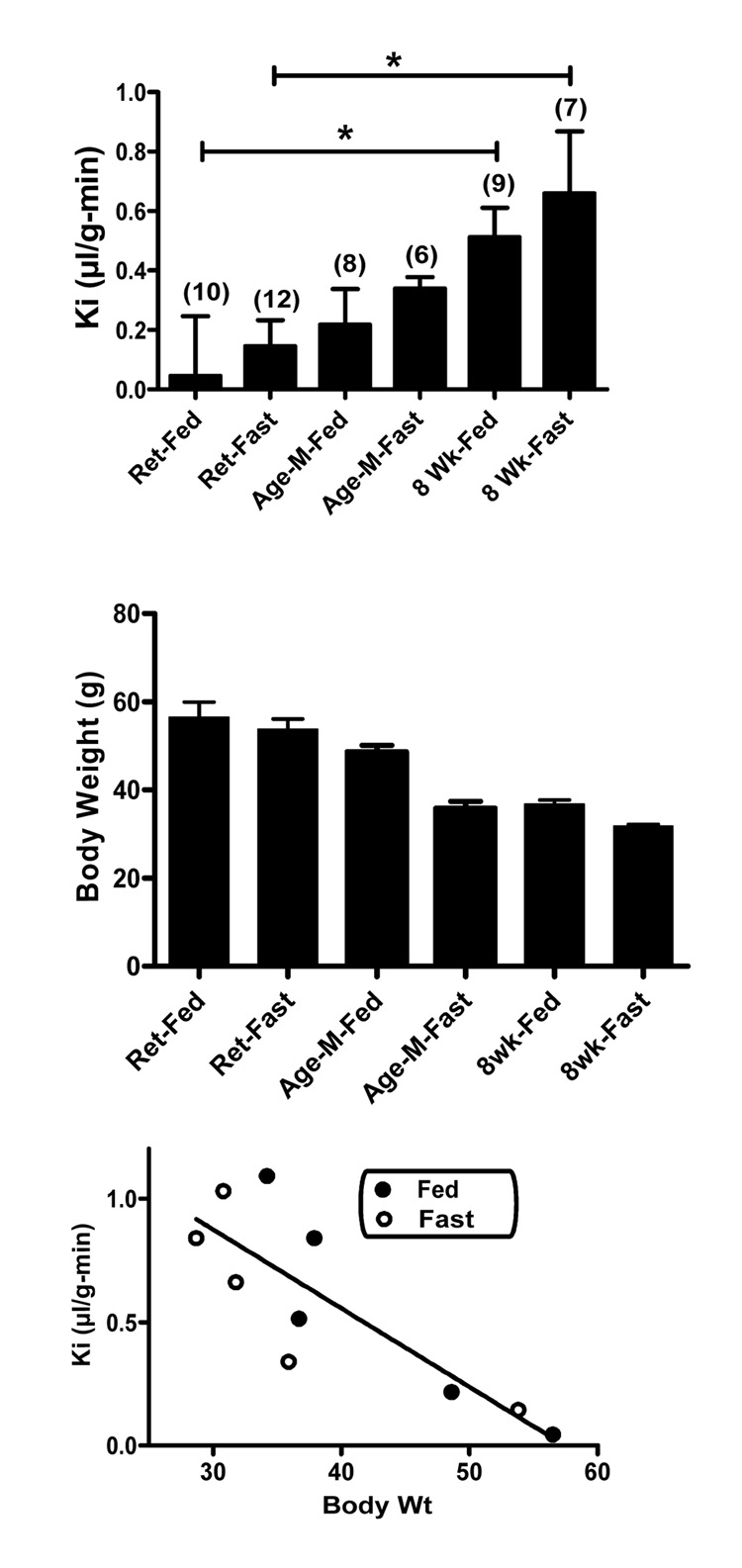
In the first experiment, we used multiple-time regression analysis to measure the rate of I-Ghr transport across the mouse BBB in retired breeders, age-matched controls , and 8 week old controls. These groups contained mice fed ad lib until study (the “Fed” group) and mice that had been fasted for 48 h (the “Fasted” group) before study. A two-way ANOVA had as its independent variables "Pretreatment" (retired breeders, age-matched controls, 8 week old controls) and "Intervention" (Fed vs Fasted) and found a significant effect for Pretreatment [F(2,40) = 21.7, p<0.01], but not for Fed vs Fasted status nor for interaction between the two independent variables. The Bonferroni post-test showed statistical differences (p<0.05) between the retired breeder-fed and 8-week old fed groups and between the retired breeder-fasted and 8 week old-fasted groups (Figure 1, upper panel). Although fasting was associated with an arithmetic increase in Ki in comparison to fed animals in all three Pretreatment groups, none of the differences reached statistical significance.
Figure 1.
Ghrelin transport in fasted and fed mice. Upper panel compares unidirectional influx rates (Ki) from blood to brain in fed (Fed) or fasted (Fast) retired breeders (Ret), mice aged-matched to the retired breeders but not fed breeder chow (Age-M), and 8 week old (8 Wk) mice (n/group shown in parentheses). The fed or fasted 8 week old mice had higher rates on ghrelin transport into the brain and retired fed mice *p<0.05). Middle panel shows body weights for these groups. For clarity, statistical differences among groups are not shown, but include, Retired Fed vs Age-Matched and 8 week Fed, Retired Fasted vs Age-Matched and 8 week Fasted, and Age-Matched Fed vs Age-Matched Fasted. Lower panel plots the Ki and body weights of the top to panels and results from 4 other groups (fed and fasted 8 week and age-matched controls). It shows that there was a statistically significant inverse relation for these groups between body weight and Ki.
The low rate of I-Ghr uptake seen in the retired breeders group suggested that the saturable aspect of ghrelin transport has been lost in this group. To further test this, we measured influx rate in another set of retired breeders-fed in which 30 microg of unlabeled ghrelin, a dose which inhibits the saturable transport of ghrelin across the BBB [8], was or was not included in the iv injection of I-Ghr. The uptake rate measured for I-Ghr between these two groups was not different (data not shown), showing that the saturable transport of exogenous, intravenously administered I-Ghr across the BBB was lost in these mice.
The lack of a statistical difference between age-matched to retired breeder and 8 week old mice suggested that body weight/obesity was affecting ghrelin transport rather than age. Figure 1 middle panel shows the body weights of the groups shown in the upper panel and suggests an inverse relation between Ki and body weight. To test this, we plotted the influx rate of I-Ghr into brain against body weight for the 6 groups shown in figure 1 upper panel plus two more pairs of age-matched-to retired breeder fed/fasted and 8 week fed/fasted mice (Figure 1, lower panel). There was a statistically significant correlation between rate of I-Ghr transport across the BBB and body weight: r = 0.837, n = 10, p<0.05.
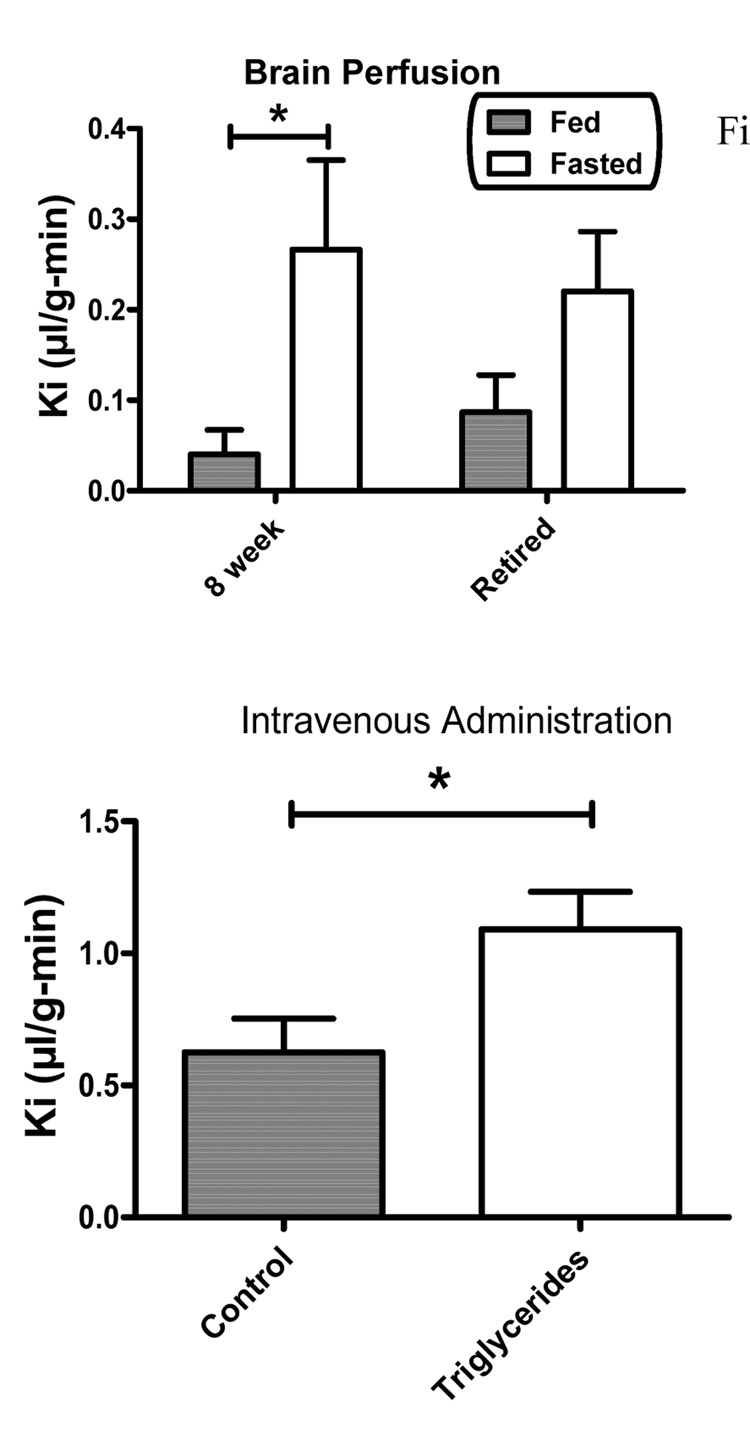
Brain perfusion was used to study the effects of 48 h fasting on I-Ghr transport in the absence of serum factors. Two-way ANOVA showed an effect for Fed/Fasted state [F(1,39) = 17.1, p<0.01)] but not for Pretreatment nor for interaction (Figure 2, upper panel). The Bonferroni post-test showed a significant difference between the Fed and Fasted groups for the 8 week old mice but not for the retired breeders. It was also noted that the uptake rate of I-Ghr across the BBB in the 8 week old-Fed group was much lower in this brain perfusion experiment (figure 2, upper panel) than in the intravenous injection group (Figure 1, upper panel).
Figure 2.
Upper panel compares Ki as assessed by brain perfusion in fed 8 week ( n = 15), fed retired breeder (n = 15), fasted 8 week (n = 11) and fasted retired breeder (n = 6) mice. Fasting significantly increased transport of ghrelin in 8 week old mice. Lower panel shows that intravenous injection of ghrelin with the triglyceride triolein increased ghrelin transport across the BBB (n = 11/group).
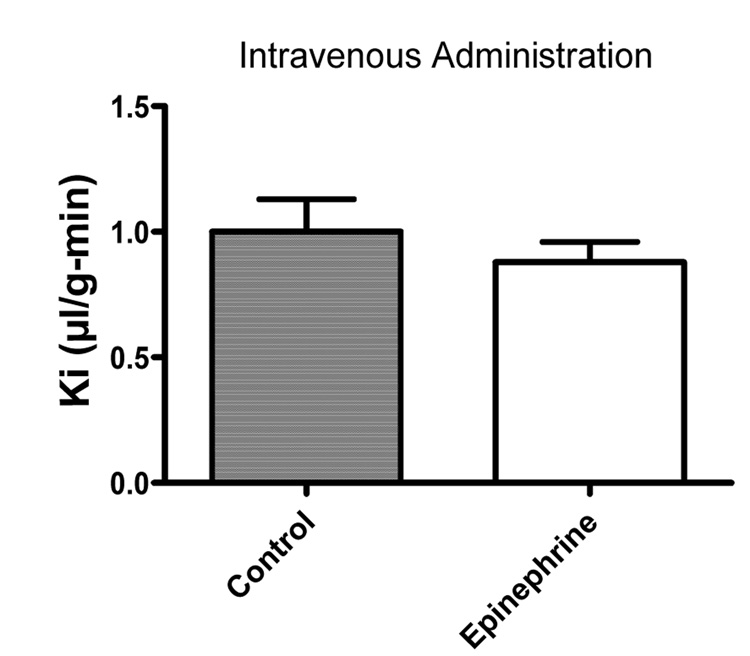
We used multiple-time regression analysis to test the effects of epinephrine and of the triglyceride triolein on the BBB transport of intravenously administered I-Ghr in 8 week old mice. Inclusion of triolein at the dose of 7.2 mg/mouse enhanced the transport of I-Ghr across the BBB by over 70%, from 0.63 +/− 0.13 microl/g-min (n = 10) to 1.09 +/− 0.14 microl/g-min (n = 10), a statistically significant difference: F(1,16) = 5.74, p<0.05); figure 2, lower panel. Epinephrine had no effect on I-Ghr uptake.
Discussion
Previous work has shown that ghrelin transport across the BBB is controlled by a complex of BBB transporters. Transport is affected by the primary amino acid structure of ghrelin as well as the presence or absence of octanol [8]. Human ghrelin, for example, crosses the BBB in both the brain-to-blood and blood-to-brain directions by saturable transporters. Permeability to ghrelin also varies among brain regions, suggesting that the number or affinity of ghrelin transporters varies among various regions of the BBB [9]. Here, we found evidence that blood-borne and other factors also affect the permeability of the BBB to ghrelin. Our major finding here were: 1) obese, aged mice lost the ability to transport intravenously administered ghrelin across the BBB, resulting in an inverse relation between body weight and ghrelin BBB permeability; 2) serum triglycerides promoted transport of intravenously administered ghrelin across the BBB, whereas epinephrine had no effect; 3) fasting tended to promote ghrelin transport across the BBB as most readily shown in brain perfusion studies; 4) evidence suggested that a serum factor promoted ghrelin transport in 8 week old mice. Each of these findings are discussed in detail below.
We found that retired breeder mice transported little ghrelin across the BBB in comparison to 8 week old mice. Mice that were aged matched to retired breeders but weighed considerably less had a transport rate that was intermediate between that of the retired breeders and 8 week old mice and without a statistically significant difference to either. Hence, obesity is likely a more important factor than age as a cause of decreased ghrelin transport. Consistent with this, we found an inverse relation between body weight and the transport rate of ghrelin across the BBB.
A similar inverse relation exists between obesity and the rate of transport across the BBB for leptin. In that case, serum triglyceride levels can at least partially explain the inverse relation as they inhibit the transport of leptin across the BBB. Here, we found that triglycerides promoted, rather than inhibited, the rate of ghrelin transport across the BBB. Triglycerides, then, have opposite effects on ghrelin and leptin transport. By inhibiting the transport of the anorectic leptin into brain while stimulating the transport of the orectic ghrelin, triglycerides would act to stimulate feeding. Promoting feeding makes teleological sense if triglycerides evolved as a signal to the brain of starvation, as we have previously suggested [3].
However, brain signaling as mediated by circulating factors is likely complex as triglycerides also promote insulin transport across the BBB [16] and insulin is thought to have anorectic actions within the brain [18]. Similarly, here we found no effect of epinephrine on ghrelin transport, whereas previously we found that epinephrine stimulates leptin transport across the BBB [1]. Although it is unclear which other circulating factors will be found to modify the BBB transport of feeding hormones, it seems clear that the influence of circulating factors places the BBB transport of feeding hormones into a pathophysiological context.
Since triglycerides increase ghrelin transport, we would predict that prolonged fasting should increase the transport of ghrelin across the BBB. Although we saw arithmetic increases in transport of intravenously administered ghrelin across the BBB in all the groups tested, this increase only reached statistical significance in the 8 week old mice studied by brain perfusion. This suggests that whatever the promoting factor is in starvation, it is attenuated by circulating factors and in obese mice. It may be that increases in serum ghrelin with starvation [15] or the already elevated level of triglycerides in obese mice attenuates the starvation effect.
We also noted that the rate of transport of ghrelin across the BBB in 8 week old mice was much lower when studied by brain perfusion than by intravenous injection. This is unusual as results obtained by the brain transfusion and iv methods usually agree. When they disagree, it is usually the intravenous method which produces the lower rate of transport, reflecting self-inhibition of transport by endogenous ligand, binding to serum proteins, and other inhibitory factors. The higher transport found here for ghrelin after intravenous administration suggests that some serum factor is promoting ghrelin transport. Triglycerides could be one such factor, although a second serum factor would than need to be invoked to explain the loss of transport in obese mice.
In conclusion, we found that ghrelin transport across the BBB is decreased in obese mice. Fasting/starvation and serum factors, including triglycerides, promote ghrelin transport across the BBB. It remains unclear whether the loss of ghrelin transport across the BBB in obesity is caused by the loss of the serum factor promoting ghrelin transport or is caused by the introduction of an inhibitory factor. Overall, these results show that ghrelin transport across the BBB is influenced by circulating and other factors that modify its transport under various pathophysiologic conditions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banks WA. Enhanced leptin transport across the blood-brain barrier by a1-adrenergic agents. Brain Res. 2001;899:209–217. doi: 10.1016/s0006-8993(01)02242-9. [DOI] [PubMed] [Google Scholar]
- 2.Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 3.Banks WA, Farr SA, Morley JE. The effects of high fat diets on blood-brain barrier transport of leptin: Failure or Adaptation? Physiol Behav. 2006;88:244–248. doi: 10.1016/j.physbeh.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 4.Banks WA, Jaspan JB, Kastin AJ. Effect of diabetes mellitus on the permeability of the blood-brain barrier to insulin. Peptides. 1997;18:1577–1584. doi: 10.1016/s0196-9781(97)00238-6. [DOI] [PubMed] [Google Scholar]
- 5.Banks WA, Jaspan JB, Kastin AJ. Selective, physiological transport of insulin across the blood-brain barrier: Novel demonstration by species-specific enzyme immunoassays. Peptides. 1997;18:1257–1262. doi: 10.1016/s0196-9781(97)00198-8. [DOI] [PubMed] [Google Scholar]
- 6.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 7.Banks WA, King BM, Rossiter KN, Olson RD, Olson GA, Kastin AJ. Obesity-inducing lesions of the central nervous system alter leptin uptake by the blood-brain barrier. Life Sci. 2001;69:2765–2773. doi: 10.1016/s0024-3205(01)01346-7. [DOI] [PubMed] [Google Scholar]
- 8.Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 9.Diano S, Farr SA, Benoit SE, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschop MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nature Neuroscience. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 10.Gaskin FS, Farr SA, Banks WA, Kumar VB, Morley JE. Ghrelin-induced feeding is dependent on nitric oxide. Peptides. 2003;24:913–918. doi: 10.1016/s0196-9781(03)00160-8. [DOI] [PubMed] [Google Scholar]
- 11.Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–1533. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- 12.Kastin AJ, Akerstrom V. Fasting, but not adrenalectomy, reduces transport of leptin into the brain. Peptides. 2000;21:679–682. doi: 10.1016/s0196-9781(00)00195-9. [DOI] [PubMed] [Google Scholar]
- 13.Kastin AJ, Akerstrom V. Glucose and insulin increase the transport of leptin through the blood-brain barrier in normal mice but not in streptozotocin-diabetic mice. Neuroendocrinology. 2001;73:237–242. doi: 10.1159/000054640. [DOI] [PubMed] [Google Scholar]
- 14.Shayo M, McLay RN, Kastin AJ, Banks WA. The putative blood-brain barrier transporter for the b-amyloid binding protein apolipoprotein J is saturated at physiological concentrations. Life Sci. 1996;60:L115–L118. doi: 10.1016/s0024-3205(96)00685-6. [DOI] [PubMed] [Google Scholar]
- 15.Uneo N, Asakawa A, Inui A. Blunted metabolic response to fasting in obese mice. Endocrine. 2007;32:192–196. doi: 10.1007/s12020-007-9016-z. [DOI] [PubMed] [Google Scholar]
- 16.Urayama A, Banks WA. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology. 2008;149:3592–3597. doi: 10.1210/en.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocrine Rev. 2004;25:426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 18.Woods SC, Seeley RJ, Baskin DG, Schwartz MW. Insulin and the blood-brain barrier. Current Pharmaceutical Design. 2003;9:795–800. doi: 10.2174/1381612033455323. [DOI] [PubMed] [Google Scholar]
- 19.Zlokovic BV, Lipovac MN, Begley DJ, Davson H, Rakic L. Slow penetration of thyrotropin-releasing hormone across the blood-brain barrier of an in situ perfused guinea pig brain. J Neurochem. 1988;51:252–257. doi: 10.1111/j.1471-4159.1988.tb04864.x. [DOI] [PubMed] [Google Scholar]