Abstract
A growing number of brain–machine interfaces have now been developed that allow movements of an external device to be controlled using recordings from the brain. This work has been undertaken with a number of different animal models, as well as several human patients with quadriplegia. The resulting movements, whether of computer cursors or robotic limbs, remain quite slow and unstable compared to normal limb movements. It is an open question, how much of this instability is the result of the limited forward control path, and how much has to do with the total lack of normal proprioceptive feedback. We have begun preliminary studies of the effectiveness of electrical stimulation in the proprioceptive area of the primary somatosensory cortex (area 3a) as a potential means to deliver an artificial sense of proprioception to a monkey. We have demonstrated that it is possible for the monkey to detect brief stimulus trains at relatively low current levels, and to discriminate between trains of different frequencies. These observations need to be expanded to include more complex, time-varying waveforms that could potentially convey information about the state of the limb.
Index Terms: Brain–machine interface (BMI), intracortical microstimulation, proprioception
I. Introduction
A wide variety of brain–machine interfaces (BMIs) have been tested in rats, monkeys, and even human patients. These systems have allowed the subjects to control a robotic device, a cursor on a computer screen, or a 3-D virtual reality display [1]-[5]. However, in all these approaches, feedback was supplied entirely through the subject’s intact visual or auditory system. Human patients lacking proprioception have greatly impoverished movement, even if their motor capacity itself is undiminished [6]-[8]. Vision of the limbs improves these deficits but by no means eliminates them. Consequently, it must be assumed that the lack of proprioceptive feedback contributes to the slow, unstable movements that characterize current motor BMI applications.
It is essential that together with the development of more sophisticated efferent interfaces, we also begin to develop afferent interfaces that would deliver information about the state of a controlled limb to the subject by means of direct activation of the central nervous system. This study represents an initial attempt to use electrical stimulation in the proprioceptive region (area 3a) of the primary somatosensory cortex to convey a conscious perception to a monkey. However, far less is known of the physiology of area 3a compared to the tactile portion, 3b. In part, this may be because of its location at the fundus of the central sulcus. We have now shown that a monkey can detect and discriminate between electrical stimuli of different frequencies in 3a. Building on the success of these studies, it will be necessary to deliver more complicated stimulus patterns that mimic the natural proprioceptive input related to the state of the monkey’s limb.
II. Methods
A. Behavior
A male adult monkey (Macaca Mulatta) was trained to make visually guided limb movements from a central target to targets displayed left and right of center on a video screen using a two-link, planar manipulandum. When the monkey positioned the cursor in the correct, illuminated target, it received a liquid reward. After the monkey gained proficiency at the visual task, the illumination of the two targets was paired with intracortical microstimulation (ICMS) trains of two different frequencies. Fig. 2(a) illustrates a series of three such trials, showing hand position and the times of ICMS and target onset and offset. The third trial was a “catch” trial, in which no ICMS occurred. The higher frequency stimulus was paired with the left target, the lower frequency with the right target. Frequency was selected as the stimulus variable for the monkey to discriminate because of the success of a very similar series of experiments in area 3b [9], [10]. The range of frequencies used (15–300 Hz) is within the range of firing rates used to encode limb movement in 3a [11].
Fig. 2.
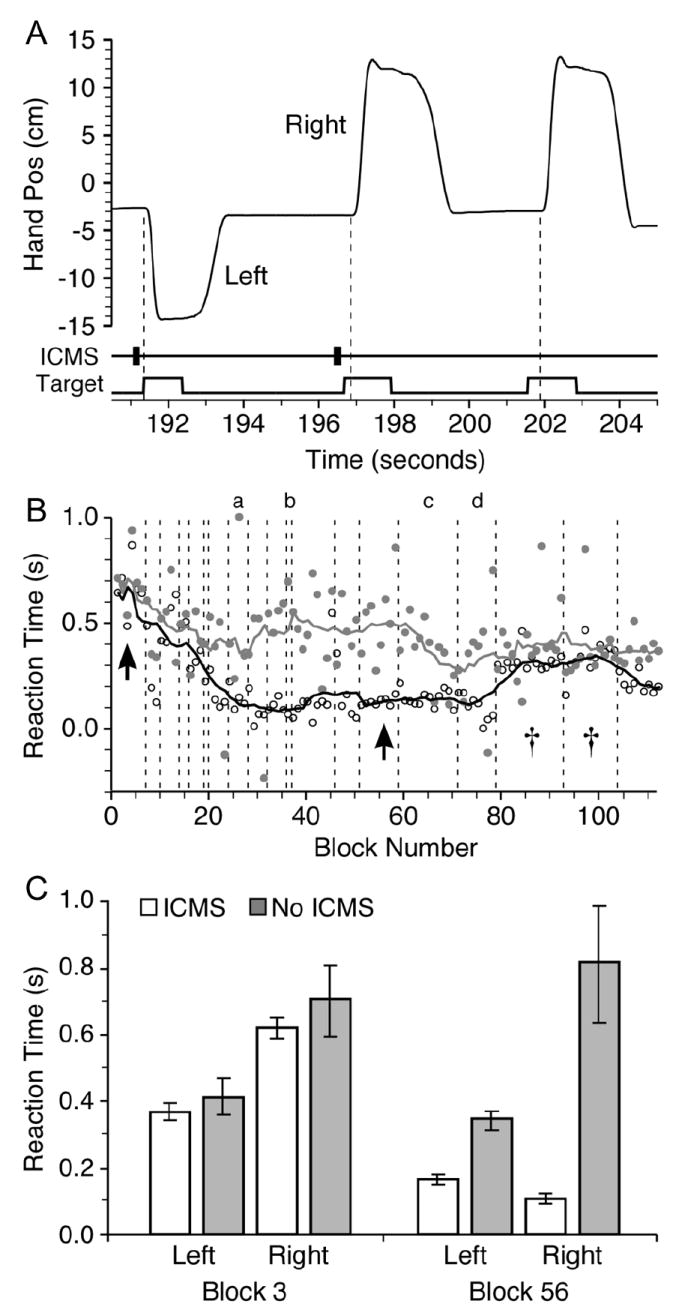
Behavioral effects of ICMS delivered to area 3a. A: Time course of three hand movements triggered by visual target plus ICMS, or visual target alone (catch trials). Movement onset indicated by dashed lines. B: Mean RT between target appearance and movement onset for blocks of catch trials (gray circles) and ICMS trials (open circles). Black and gray lines are 11-block moving averages of regular and catch trials, respectively. C: Mean RT ± SEM for early (3) and late (56) blocks (shown by arrows in B). Late block ANOVA was significant across the four target/cue conditions (F = 46.2; p < 10 – 16) and post-hoc Student’s t-tests indicate that RTs to stimulus trials were significantly less than those to catch trials (p < 10 – 4 and p < 10 – 11) for left and right targets, respectively.
Movement onset was defined as the instant at which hand speed exceeded the greater of either 1) 5% of the peak speed of the movement or 2) four standard deviations above the mean speed for the previous 200 ms. Since the electrical stimulation preceded the illumination of the visual targets, consistently faster reaction times for trials in which ICMS was delivered were indicative of the monkey’s ability to detect the stimulus. Subsequently, in some blocks the visual targets were eliminated and the monkey’s response to ICMS-only trials was observed, in order to evaluate the monkey’s ability to discriminate the two stimulus frequencies.
Trials were organized into blocks consisting generally of between 50–85 (25th and 75th percentile) trials. All of the blocks recorded on a given day comprised a single session. Blocks with and without visual cues were interspersed to avoid extinction of the ICMS-target association.
B. Structural MRI and Surgery
After training, the monkey was anesthetized with Nembutal, and a T1 structural MRI with a 0.8×0.8×0.9 mm voxel size was taken to assess the structure of the central sulcus. MRI data were imported into the Visualization Toolkit (VTK; Kitware, Clifton Park, NY) using custom python code. The relevant region was extracted and an isosurface rendered by VTK corresponding roughly to the boundary between white and gray matter.
The location of the recording sites in the microdrive coordinates were overlaid on the MRI-generated isosurface. Relative positioning was calculated by aligning the recording chamber coordinate system so as to preserve distances from anatomical landmarks (e.g., central sulcus and interhemispheric fissure) observable both in the MRI and on the brain surface.
A stainless steel recording chamber was implanted above the central sulcus, centered just medial to the spur of the arcuate sulcus, approximately 15 mm lateral to the midsagittal plane and contralateral to the hand used for the task. All methods were conducted in accordance with a protocol approved by the Northwestern University Institutional Animal Care and Use Committee.
C. Recording and Stimulation
Single neuron recording and ICMS were conducted using either epoxy-coated tungsten or glass-coated Pt-Ir micro-electrodes and a hydraulically actuated Narishige microdrive. Single neurons were amplified and filtered and discriminated using a digital neuronal acquisition processing system (Tucker-Davis Technologies, Alachua, FL). Stimulus trains consisted of charge balanced, 200 μs, biphasic pulses, with current between 30 and 50 μA, and train duration between 200 and 500 ms. Single phase charge density on the electrode was between 1.0 and 1.6 mC/cm2 depending on stimulus parameters. Although a number of parameters were varied throughout a given session, they were unchanged within any given block.
D. Statistics
To evaluate the monkey’s ability to detect the ICMS, an analysis of variance (ANOVA) was run on the reaction times under the four conditions (i.e., left and right targets with and without stimulation) for blocks in which visual stimulation was provided. If the ANOVA indicated a significant variation (α = .05) across the trial conditions, post-hoc Student’s t-tests were run on the stimulus and catch trials for each target (α = .05).
To evaluate the monkey’s ability to discriminate between stimuli, responses were evaluated for blocks in which visual stimulation was not provided. A percent correct significantly better than chance (α = .05; cumulative binomial test) indicated that the monkey could distinguish the two stimuli. The probability of a correct response under the null hypothesis was taken as
| (1) |
Where P[m = 1] indicates the probability that the monkey moved to the left target on a given trial, and P[t = 1] indicates the probability that the left target was correct on a given trial.
III. Results
The experiments described here included two primary components. The first goal was to test the ability of the monkey to detect small electrical stimuli delivered to the somatosensory cortex. The second was to test the monkey’s ability to discriminate between stimuli of different frequencies.
A. Identification of Cortical Areas
Electrodes were advanced into the arm representations of the precentral and postcentral gyri, as well as the corresponding banks of the central sulcus. Regions were identified by their responses to tactile or proprioceptive stimuli and to ICMS. A region was considered to be in 3a, if it met all three of the following conditions: 1) a neuron recorded at that location responded to both active and passive movement of the proximal limb as determined by two concurring observers, 2) no muscular twitch was evoked by ICMS (11 pulses, 400 Hz, 30 μA), and 3) stereotaxic location of the recording site corresponded with the cortex surrounding the fundus of the central sulcus. Neurons in areas meeting these criteria typically had firing rates that were roughly proportional to joint velocity. These observations are consistent with those reported for 3a in earlier studies [11]. Recording sites posterior and dorsal to these locations that responded to tactile stimulation of the arm were considered putatively 3b, while sites at which twitches were evoked by ICMS were considered to be primary motor cortex, area 4.
Fig. 1 illustrates the distribution of recording and stimulation sites across the cortex. Over the course of the full experiment, we sampled much of the area that contained cells responsive to proprioceptive stimulation of the proximal arm. While a new site was generally selected at the beginning of each session, several of the more successful sites were repeated a small number of times. The view is from the lateral surface looking towards the midline. The plane at the back of the rendering shows a pseudocolored slice through the MRI to illustrate cortical thickness. As expected, proprioceptive sites (blue) were at the bottom of the central sulcus, with tactile sites (red) dorsally in the central sulcus and on the postcentral gyrus. Motor sites (yellow) were in the anterior bank and crown of the sulcus. Larger spheres indicate those sites that were used in the area 3a stimulation experiments reported below.
Fig. 1.
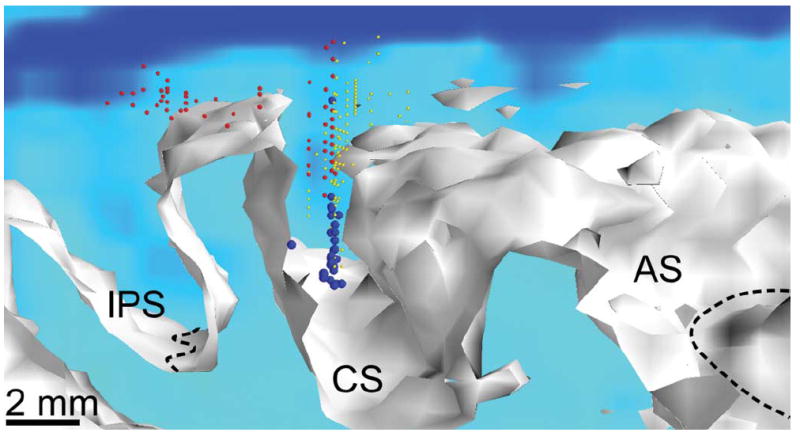
Locations of recording and stimulation sites surrounding central sulcus (CS). White surface corresponds to the boundary between cortex and underlying white matter. View is along the CS from the right side of the monkey looking towards the midline and slightly posterior. Dashed black lines indicate the bottom of the intraparietal (IPS) and arcuate sulci (AS). Red spheres indicate recording sites containing neurons with tactile properties, blue represents proprioceptive, and yellow represents motos sites. Large spheres represent those sites used in the stimulation experiments.
B. Detection of Electrical Stimulation
Fig. 2(a) illustrates a sequence of three trials, showing hand position, together with the times of the visual target appearance and the ICMS cue. Reaction time (RT) was measured between the appearance of the visual target and movement onset. Target onset was normally preceded by an ICMS train that began 300 ms earlier. This sequence above included two regular trials and one catch trial without ICMS. If the monkey responds more quickly in the presence of the ICMS, it is evidence that he is able to detect the electrical stimulation. RTs for the three trials shown in Fig. 2(a) were 9 ms, 198 ms, and 337 ms. The final (catch) trial was the slowest of the three trials, while the leftward (first) trial was the fastest of the three.
Fig. 2(b) shows RTs recorded during a series of experimental sessions like those illustrated in panel A. Dots indicate the mean RT within a single block, combining left and right movements. Open symbols indicate regular trials; gray symbols show catch trials. The corresponding black and gray curves represent 11-block running averages, and the dashed vertical lines demarcate sessions.
Fig. 2(c) shows statistics calculated for two of the blocks, indicated by the arrows in panel B. The bars indicate the mean latency within the block, and the error bars indicate the standard error across trials. During the initial five sessions, although there was apparently some general task-related learning (indicated by the reduction in RT for both regular and catch trials), there was no difference between the two types of trials. There was, however, a significant difference in RT between left and right movements, which may have been due to the higher frequency used for leftward movements.
By the beginning of the sixth session, a significant latency difference developed and was maintained for eleven sessions. During this period, the mean latency difference between regular and catch trials was 320 ms, approximately the latency between the ICMS and visual cues. During the sessions marked with s in Fig. 2(b), the latency difference fell below significance. In these sessions the electrode was placed in a location unique to these two sessions (dorsal outlier visible in Fig. 1). It is possible that neural activity in this location was less capable of evoking a conscious perception or that stimulation in this location caused an unnatural proprioceptive sensation that interfered with the monkey’s ability to initiate movements. A significant latency difference returned in the next and final session in this paradigm, which was conducted at a new location.
C. Discrimination Between Stimulus Frequencies
Within many sessions, we also conducted a direct test of the monkey’s ability to discriminate different ICMS frequencies, by eliminating the visual target cue from entire blocks of trials. During these ICMS-only blocks, the monkey’s choice of target was entirely dependent on his ability to discriminate the frequency of the stimulus train. Fig. 3 summarizes the results for four representative sessions. These sessions are designated a–d on Fig. 2(b). The results of the ICMS-only blocks were analyzed by calculating the percentage of correct responses within each block. Fig. 3 indicates the mean and standard error across the ICMS-only blocks within each session. Dashed lines indicate the 95% confidence level for performance significantly above chance across each entire session (see statistical methods). As stated above, these ICMS-only blocks were not included in the summary plot shown in Fig. 2, nor in the latency statistics summarizing those results.
Fig. 3.
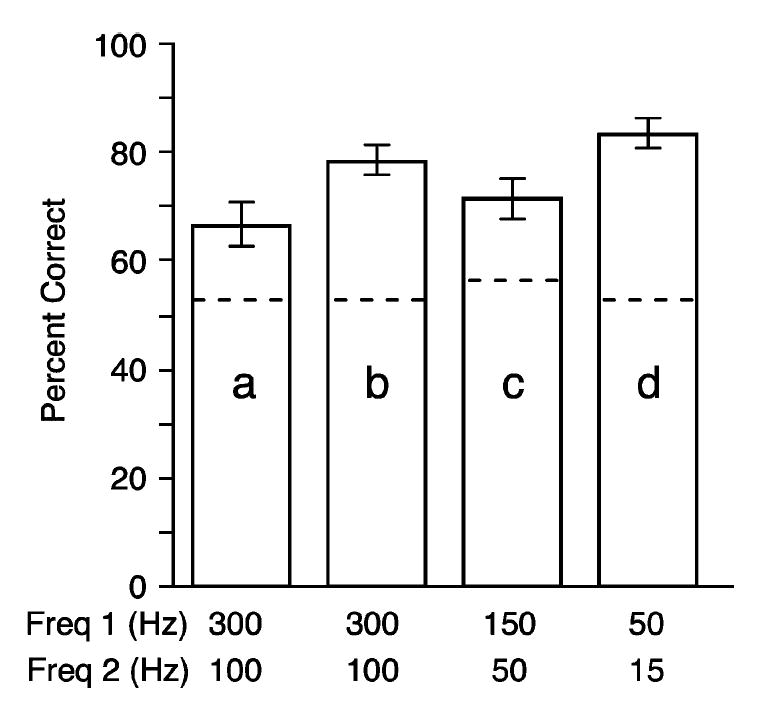
Average percent correct responses for ICMS frequency discrimination within four different sessions. Each example represents a different electrode site and pair of frequencies. Error bars indicate standard error across blocks within the session. Dashed lines indicate 95% confidence intervals above chance performance. Sessions are indicated with the letters a, b, c, and d in Fig. 2(b).
IV. Discussion
Our results are an initial attempt to test the feasibility of electrically activating area 3a, the area of cortex principally associated with the proprioceptive sense. We have demonstrated that a monkey subject can learn to detect such stimuli and to recognize the frequency of a given stimulus, based on the memory of previous stimuli. The percentage correct, although well above the chance level, was also well below the level achieved when visual target cues were available, which was essentially 100%. This was probably in part due to the fact that aligning a visually displayed cursor with a target is a simpler task than is moving the cursor to a target associated with an arbitrary somatosensory stimulus. The fact that the ICMS was relatively brief, while the targets remained illuminated throughout the trial would also have made the ICMS-only task more difficult. The percentage of correct responses across sessions was largely unrelated to the magnitude of the frequency difference, suggesting that even greater differences would not have increased the success rate, which was apparently related to a variety of other factors. Consistently shorter reaction times for movements to the left target may have been due to this target’s having been paired with higher frequency stimulation, or simply to behavioral preferences of the monkey.
Although this is the first demonstration of such effects within proprioceptive cortex, other primary sensory areas have been used for analogous experiments. Most closely related is the work of Romo and colleagues, who trained monkey subjects to discriminate between stimulus trains of different frequencies applied to area 3b, the tactile portion of the primary somatosensory cortex [9].
Attempts have also been made to deliver visual and auditory percepts by electrical stimulation of primary visual [12], [13] and auditory [14], [15] cortex, respectively. This work has been undertaken in an effort to develop visual and auditory prostheses, which might restore a crude sense of vision or hearing to blind or deaf patients. Neither project has been nearly as successful as the cochlear implant, used by approximately 110 000 patients world-wide for the treatment of complete sensorineural hearing loss [6]. The greater success of the cochlear implant is undoubtedly due to the simpler sensory maps found in the periphery and the further processing that occurs as signals are propagated centrally. The disadvantage of stimulating in the periphery is that such methods would be inapplicable to sensory loss due to a more central lesion.
Our results demonstrate the ability of a monkey to detect ICMS within area 3a, to discriminate different frequencies of stimulation, and to use this information as a basis for decision making with a latency that is very similar to that of vision. These results were not at all a foregone conclusion. However, much work remains to be done before a functional neuroprosthesis could be attempted. Stimulus current and charge density, while similar to that used acutely by other groups [10], [12] are outside of the limits proposed for chronic use (for review, see [16]). Like the cochlear implant, a useful proprioceptive prosthesis would need to deliver continuously varied stimulation of multiple electrodes in order to encode time-varying information about limb state. Under these conditions, much lower intensity, more focal stimulus trains would clearly be appropriate.
Our understanding of the maps and signals representing proprioception within the cortex are far more limited than for the senses of touch, vision, or audition. Considerably more work is needed to expand our knowledge of area 3a, through both recording and stimulation studies. An important question is of the relative importance of the cerebellum and area 3a in processing proprioceptive feedback. Both are likely to play important roles through extensive interconnections with primary motor cortex. An interesting possibility would be to stimulate within the cuneate nucleus. In addition to the potential advantage of its more peripheral location, it would provide input to both the cerebellum and S1. The potential relevance for brain machine interface applications should provide additional motivation for such studies.
Acknowledgments
This work was supported by the National Institutes of Health under Grant NINDS NS048845.
Biographies

Brian M. London received the B.S. degree in electrical engineering from Columbia University, New York, NY, in 2002. Currently, he is a doctoral candidate in the Northwestern University Interdisciplinary Neuroscience Program.

Luke R. Jordan received the B.A. degree in biology from Goshen College, Goshen, IN, in 2002.
Currently, he is a Research Technologist in Dr. L. Miller’s laboratory in the Physiology Department of the Feinberg School of Medicine, Northwestern University, Chicago, IL.

Christopher R. Jackson received the B.S. degree in finance and accounting from Miami University in 2002. Currently, he is a medical student at the University of Michigan Medical School, Ann Arbor.

Lee E. Miller received the B.A. degree in physics from Goshen College, Goshen, IN, in 1980, and the M.S. degree in biomedical engineering and the Ph.D. degree in physiology from Northwestern University, Evanston, IL, in 1983 and 1989, respectively. He completed two years of postdoctoral training in the Department of Medical Physics, University of Nijmegen, The Netherlands.
He is currently an Associate Professor in the Departments of Physiology and Biomedical Engineering at Northwestern University. His primary research interests are in the cortical control of muscle activity and limb movement, and in the development of brain–machine interfaces that attempt to mimic normal physiological systems.
Contributor Information
Brian M. London, Department of Physiology, and the Neuroscience Institute, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611 USA (e-mail: bml573@northwestern.edu)
Luke R. Jordan, Department of Physiology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611 USA (e-mail: lukerj@northwestern.edu)
Christopher R. Jackson, University of Michigan Medical School, Ann Arbor, MI 48109 USA
Lee E. Miller, Departments of Physiology and Biomedical Engineering, and the Neuroscience Institute, Northwestern University, Chicago, IL 60611 USA (e-mail: lm@northwestern.edu)
References
- 1.Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Instant neural control of a movement signal. Nature. 2002 Mar 14;416:141–142. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- 2.Chapin JK, Moxon KA, Markowitz RS, Nicolelis MA. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat Neurosci. 1999 Jul;2:664–670. doi: 10.1038/10223. [DOI] [PubMed] [Google Scholar]
- 3.Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002 Jun 7;296:1829–1832. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- 4.Carmena JM, Lebedev MA, Crist RE, O’Doherty JE, Santucci DM, Dimitrov D, Patil PG, Henriquez CS, Nicolelis MA. Learning to control a brain-machine interface for reaching and grasping by primates. PLoS Biol. 2003 Nov;1:E42. doi: 10.1371/journal.pbio.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gage GJ, Ludwig KA, Otto KJ, Ionides EL, Kipke DR. Naive coadaptive cortical control. J Neural Eng. 2005 Jun;2:52–63. doi: 10.1088/1741-2560/2/2/006. [DOI] [PubMed] [Google Scholar]
- 6.Abbott A. Neuroprosthetics: In search of the sixth sense. Nature. 2006;442:125–127. doi: 10.1038/442125a. [DOI] [PubMed] [Google Scholar]
- 7.Sanes JN, Mauritz KH, Dalakas MC, Evarts EV. Motor control in humans with large-fiber sensory neuropathy. Human Neurobiol. 1985;4:101–114. [PubMed] [Google Scholar]
- 8.Sainburg RL, Poizner H, Ghez C. Loss of proprioception produces deficits in interjoint coordination. J Neurophysiol. 1993 Nov;70:2136–2147. doi: 10.1152/jn.1993.70.5.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romo R, Hernandez A, Zainos A, Salinas E. Somatosensory discrimination based on cortical microstimulation. Nature. 1998 Mar 26;392:387–390. doi: 10.1038/32891. [DOI] [PubMed] [Google Scholar]
- 10.Romo R, Hernandez A, Zainos A, Brody CD, Lemus L. Sensing without touching: Psychophysical performance based on cortical microstimulation. Neuron. 2000 Apr;26:273–278. doi: 10.1016/s0896-6273(00)81156-3. [DOI] [PubMed] [Google Scholar]
- 11.Gardner EP, Costanzo RM. Properties of kinesthetic neurons in somatosensory cortex of awake monkeys. Brain Res. 1981 Jun 15;214:301–319. doi: 10.1016/0006-8993(81)91196-3. [DOI] [PubMed] [Google Scholar]
- 12.Bradley DC, Troyk PR, Berg JA, Bak M, Cogan S, Erickson R, Kufta C, Mascaro M, McCreery D, Schmidt EM, Towle VL, Xu H. Visuotopic mapping through a multichannel stimulating implant in primate V1. J Neurophysiol. 2005 Mar;93:1659–1670. doi: 10.1152/jn.01213.2003. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt E, Bak M, Hambrecht F, Kufta C, O’Rourke D, Vallabhanath P. Feasibility of a visual prosthesis for the blind based on intracortical microsimulation of the visual cortex. Brain. 1996;119:507–522. doi: 10.1093/brain/119.2.507. [DOI] [PubMed] [Google Scholar]
- 14.Otto KJ, Rousche PJ, Kipke DR. Cortical microstimulation in auditory cortex of rat elicits best-frequency dependent behaviors. J Neural Eng. 2005 Jun;2:42–51. doi: 10.1088/1741-2560/2/2/005. [DOI] [PubMed] [Google Scholar]
- 15.Rousche PJ, Otto KJ, Reilly MP, Kipke DR. Single electrode micro-stimulation of rat auditory cortex: An evaluation of behavioral performance. Hear Res. 2003 May;179:62–71. doi: 10.1016/s0378-5955(03)00081-9. [DOI] [PubMed] [Google Scholar]
- 16.Merrill DR, Bikson M, Jefferys JG. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J Neurosci Methods. 2005 Feb 15;141:171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]


