Abstract
The mechanisms underlying adjuvant effects are under renewed scrutiny because of the enormous implications for vaccine development. Additionally, new low-toxicity adjuvants are sought to enhance vaccine formulations. Muramyl dipeptide (MDP) is a component of the peptidoglycan polymer and was shown to be an active but low-toxicity component of complete Freund’s adjuvant, a powerful adjuvant composed of mycobacteria lysates in an oil emulsion. MDP activates cells primarily via the cytosolic NLR family member Nod2 and is therefore linked to the ability of adjuvants to enhance antibody production. Accordingly, we tested the adjuvant properties of the MDP-Nod2 pathway. We found that MDP, compared to the TLR agonist lipopolysaccharide, has minimal adjuvant properties for antibody production under a variety of immunization conditions. We also observed that the oil emulsion incomplete Freund’s adjuvant (IFA) supplanted the requirements for the TLR pathway independent of the antigen. Surprisingly, we observed that Nod2 was required for an optimal IgG1 and IgG2c response in the absence of exogenous TLR or NLR agonists. Collectively, our results argue that oil emulsions deserve greater attention for their immunostimulatory properties.
Keywords: adjuvant, Nod-like receptor, Toll-like receptor
1. Introduction
Adjuvants are essential for the efficacy of the majority of vaccines, especially those based on subunits of pathogens [1]. At present, alum (aluminium hydroxide) is the most widely used adjuvant. Other adjuvants that sequester antigen in physically restricted areas termed ‘depots’ have been approved for human use (e.g. MF59 oil emulsion for influenza vaccines) [2, 3]. Because of the key role adjuvants play in vaccines, recent efforts have focused both on new adjuvant development to harness the Toll-like receptor (TLR) signaling pathways, and in understanding the mechanisms of action of existing adjuvant formulations such as alum [4–6]. TLR agonists such as CpG derivatives [1, 7] or non-toxic variants of TLR4-activating lipids are being pursued as adjuvants [8] while alum has recently been shown to initiate IL-1β secretion by the activation of the NLR protein NLRP3 (also known as Cryopyrin or Nalp3) inflammasome. Activation of IL-1β is therefore linked to the adjuvant effect of alum, although the mechanisms involved in this pathway remain unresolved [9, 10] and may specific to the antigens used with alum [11].
Muramyl dipeptide (MDP) is a component of bacterial peptidoglycan. Decades of research on peptidoglycan sub-structures have concluded that MDP is the ‘minimal’ component of the peptidoglycan polymer that can activate an inflammatory response [12–15]. MDP is made up of a single muramic acid residue covalently conjugated to a dipeptide stem consisting of L-alanine and D-glutamine. While mammalian cells recognize peptidoglycan by a combination of different cell surface receptors, MDP seems to be detected by the cytosolic NLR family member Nod2 [16, 17]. Indeed, mice or cells deficient in Nod2 cannot respond to exogenous MDP, as read-out by the production of cytokines and chemokines [18–21]. Therefore, Nod2 appears to be a specific ‘detector’ for MDP. However, the physiological role of the Nod2-MDP pathway remains unresolved because Nod2-deficient mice have a narrow range of phenotypes [18–21]. In addition, MDP is a very weak activator of Nod2-expressing cells (compared to conventional TLR agonists) [19, 21–25]. Most intriguingly, some humans with predicted loss-of-function mutations in Nod2 that limit responses to MDP are linked to Crohn’s Disease, a disease of excessive gut inflammation [26–28]. However, lack of responsiveness to MDP is inconsistent with the hyper-inflammatory response observed in the intestines of Crohn’s Disease patients [17, 29]. Complicating these studies is the fact that MDP has also been shown to ‘activate’ other NLR proteins including Nalp1 (NLRP1) [30, 31], and NLRP3 [32, 33]. Thus, the precise functions of Nod2, MDP recognition and the roles of these processes are under intense investigation.
The conclusion that MDP has limited inflammatory properties was first reached through studies designed to reduce the toxicity of gram-positive cell walls by sequential isolation of small peptidoglycan sub-fragments that retained some of the properties of peptidoglycan [14]. Coincident with peptidoglycan dissection, other researchers tested the response of animals and humans to injected MDP and concluded that MDP has limited toxicity [34–36]. However, it should be pointed out that radiolabeled MDP is rapidly excreted in the urine when administered systemically suggesting MDP in aqueous solutions does not have sufficient time to be trapped by immune cells and subsequently activate them [37]. By contrast to MDP, polymers of peptidoglycan (containing MDP as part of the structure) have powerful, and often lethal, pro-inflammatory effects [38, 39]. The idea that the toxicity of larger bacterial cell wall polymers could be reduced to a non-toxic fragment led to the notion that MDP could function as an adjuvant [14, 15]. Indeed, Lederer and colleagues suggested that the potent adjuvant properties of complete Freund’s adjuvant can be recapitulated by addition of MDP to the incomplete Freund’s adjuvant (IFA) oil emulsion [13].
Taking these observations into account, we sought to systematically test the ability of MDP to function as an adjuvant in different immunization regimes. We also employed mice deficient in Nod2 as a control that have greatly reduced responses to MDP. Our results do not support the idea that MDP is a useful adjuvant. Instead, we found evidence of a remarkable complexity of responses to different adjuvant-antigen combinations, especially IFA.
2. Materials and methods
2. 1 Mice
Male and female C57BL/6, Nod2-deficient mice [21] backcrossed 10 generations to C57BL/6, and MyD88-deficient mice of 4–8 weeks old were used. For T cell proliferation assays, Nod2−/− mice were crossed to OTII transgenic mice (Jackson Laboratories) and backcrossed to generate Nod2−/−; OTII or Nod2+/+; OTII mice. Animal use adhered to National Institute of Health Animal Care Guidelines and was approved by the St. Jude Animal Care and Use Committee (PJM, Principal Investigator).
2.2 Antibodies and Reagents
Alkaline phosphatase (AP) conjugated antibodies anti-IgM, anti-IgG1, anti-IgG2b and anti-IgG2c were from Southern Biotech. The alkaline phosphate (pNPP) liquid substrate was purchased from Sigma. Lipopolysaccharide (LPS) (Escherichia coli O127:B8), Incomplete Freund’s adjuvant (IFA) was from Sigma, highly purified synthetic muramyl dipeptide (MDP, N-acetylmuramyl-L-alanyl-D-isoglutamine) was from InvivoGen and Human Serum Albumin for human injection (HSA) was from Baxter. The synthetic peptides [N-ISQAVHAAHAEINEAGRKPNLSSKRSELSQLS-COOH] (TB-OTII-NE236) and the biotinylated peptide [Biotin-KPNLSSKRSELSQLS-COOH] (NE236BIO) were synthesized by Invitrogen. The TB-OTII-NE236 protein sequence ISQAVHAAHAEINEAGR corresponds to the OTII T cell epitope, while the sequence KPNLSSKRSELSQLS corresponds to the Measles virus hemagglutinin B cell epitope.
2.3 Immunizations
Mice were immunized intraperitoneally with different antigen-adjuvant combinations: HSA alone, HSA+MDP, HSA+LPS and HAS+LPS+MDP, IFA+TB-OTII-NE236, IFA+TB-OTII-NE236+MDP; IFA+TB-OTII-NE236 + LPS, IFA+TB-OTII-NE236+ LPS+MDP. The concentrations used per mouse were: MDP 100µg, LPS 5µg, HSA 50µg and TB-OTII-NE236 100 µg. Animals (generally 5 per group) were boosted twice at the second and fourth weeks after prime. Serum samples were obtained two weeks after priming and two weeks after each boost.
2.4 ELISA
Microtiter plates were coated with streptavidin (20µg/mL) overnight at 4°C. Plates were washed with PBS containing 2% Tween and the biotinylated peptide NE236BIO (1.25 µg/mL) added. After 1.5 h of incubation, plates were washed and blocked with PBS containing 10% bovine calf serum (dilution buffer) for 2 h and then washed extensively. The test serum was diluted in dilution buffer and incubated for 1.5 h at 37°C. Plates were washed and the AP-conjugated antibodies diluted (1:2000) in dilution buffer were added and incubated at room temperature for 1.5 h. Finally, plates were washed extensively and the substrate solution added. The results were measured at ELISA reader optical density of 405 nm after 15 min. For the detection of antibodies anti-HSA microplates were coated with HSA (2 µg/mL) and incubated ON at 4°C.
2.5 Statistical analysis
The Mann-Whitney test was used within Graphpad Prism software. A p value <0.05 was considered significant.
3. Results
3.1 Overall evaluation of MDP adjuvant effect using HSA as an antigen
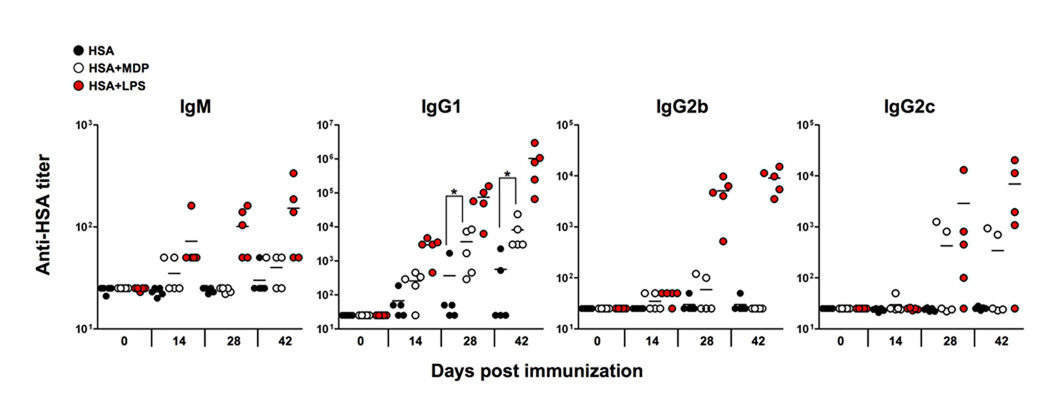
Previous reports suggesting that MDP can act as an adjuvant without additional lipid or alum-based antigen depots were have been described using HSA as a model antigen [19, 40]. For our studies we used an HSA preparation certified for human intravenous injection and devoid of contaminating microbial compounds that could confound our analysis of the adjuvant effect of MDP. By comparison to MDP, we used HSA mixed with LPS as a positive control noting that TLR agonists have adjuvant effects [1, 7, 40]. Following an immunization-boost regime, we measured the titer of HSA-specific antibody isotypes present in the sera of mice over time. Our analysis of the antibody response to HSA in the presence of MDP (Fig. 1) indicates that MDP is a weak adjuvant compared to LPS. MDP had no statistically significant effect on production of HSA-specific IgG2b or IgG2c, and marginally increased anti-HSA IgG1 production at days 14 and 28 (p=0.0317 and p=0.0079, respectively), compared to HSA alone.
Fig. 1.
Antibody production by C57BL/6 mice immunized with HSA in the presence of MDP or LPS. Mice were primed at day 0 and boosted at days 14 and 21. At 42 days, mice were bled and sacrificed. All serum time points were processed at the same time to determine the HSA-specific titers for each isotype. Each point represents the serum antibody titer to HSA for an individual mouse and titer values represent reciprocal serum dilution yielding half-maximal signal. *, p<0.05.
MDP activates cells primarily via the Nod2 pathway, independently of the TLR pathways [16, 17]. Indeed, limited studies have suggested that MDP had an adjuvant effect via the Nod2 pathway [19]. We next performed a similar study to that shown in Fig. 1 but this time comparing C57BL/6 mice or Nod2−/− mice immunized with HSA alone, HSA and MDP or HSA and LPS (Fig. 2). The analysis of the sera from these mice recapitulated the experiment shown in Fig. 1 in that MDP was a weak adjuvant compared to LPS. However, the adjuvant effect of MDP for HSA-specific IgG1 in Nod2-deficient mice was reduced, confirming the notion that Nod2 is essential for the pathway that senses MDP. Furthermore, we could find no evidence that MDP synergistically enhanced anti-HSA antibody production when mixed with LPS: a test of the ‘MDP synergy’ effect that is routinely performed when testing cellular responses to MDP [19–21, 25] (Supplemental Fig. 1). Collectively our studies suggest that MDP by itself has a marginal adjuvant effect for IgG1 and by itself cannot stimulate IgG2b or IgG2c isotype production, consistent with studies performed in the 1970s [41]. However, the weak effect of MDP in stimulating IgG1 production was Nod2-dependent.
Fig. 2.
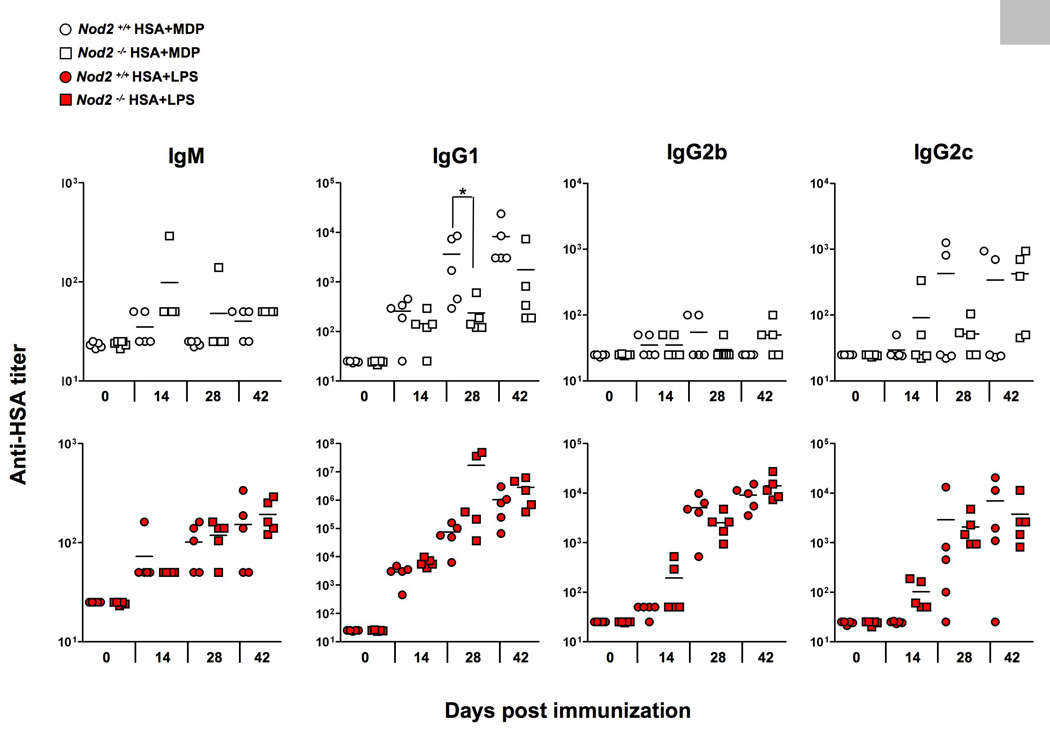
Antibody production by C57BL/6 and Nod2-deficient mice immunized with HSA in the presence or in the absence of MDP or LPS. Mice were immunized as described in Fig. 1 and serum anti-HSA titers determined by ELISA. *, p<0.05.
3.2 Effect of MDP emulsified in IFA
We tested whether the absence of Nod2 intrinsically affected the ability of APCs to present antigen. Control or Nod2-deficient APC were pulsed with different concentrations of TB-OTII-NE236 peptide and mixed to control or Nod2-deficient T cells derived from OTII TCR transgenic background, which specifically recognize the OTII peptide. Ag-specific T cell proliferation was measured. Under all conditions we could not detect any role for Nod2 in the Ag-presentation process. (Supplemental Fig. 2).
We next tested if MDP promoted the production anti-TB-OTII-NE236 antibodies by injecting TB-OTII-NE236 and MDP together as we had done for HSA (Fig. 1,Fig. 2). No specific antibodies of any isotype were produced under these conditions (data not shown) in contrast to the HSA+MDP experiments shown in Fig.1 where MDP did have some adjuvant effect for anti-HSA IgG1 antibodies. We next considered whether MDP could act as an adjuvant when trapped in an ‘antigen depot’ such as IFA [1]. We observed that IFA mixed with TB-OTII-NE236 was sufficient to promote a strong antibody response measured by increased production of IgM, IgG1, IgG2b and Ig2c over time (Fig. 3). Surprisingly, the addition of MDP or LPS to the IFA-TB-OTII-NE236 mixture did not have any significant effect on the ability of mice to make specific antibodies beyond that observed with TB-OTII-NE236 and IFA. Collectively, these data argue that IFA alone is a strong adjuvant when used with the TB-OTII-NE236 peptide and that MDP has no effect on enhancing the IFA response.
Fig. 3.
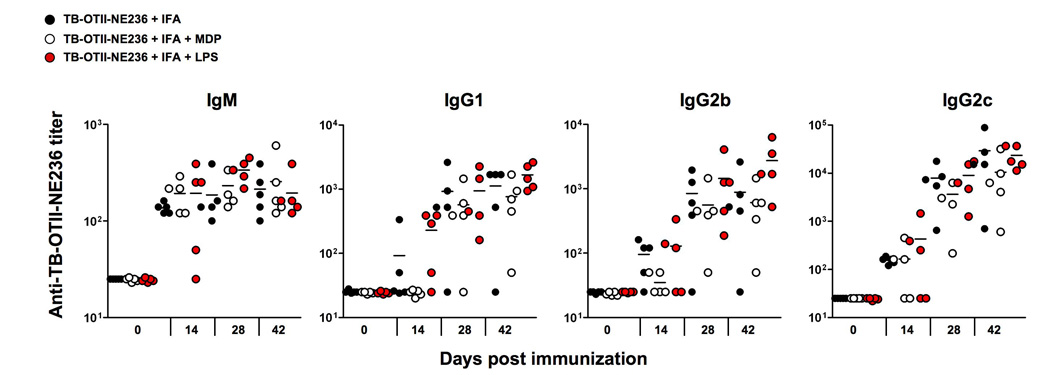
Antibody production by C57BL/6 mice after peritoneal injection with TB-OTII-NE236 peptide plus IFA in the presence or in the absent of MDP or LPS. Mice were immunized at day 0 and boosted at days 14 and 21 with TB-OTII-NE236 in IFA alone, IFA and LPS or IFA and MDP. Each point represents the serum antibody titer to NE236 for an individual mouse and titer values represent reciprocal serum dilution yielding half-maximal signal.
We confirmed these findings by checking if IFA was also a powerful adjuvant for an antibody response to HSA. We injected HSA alone or HSA emulsified in IFA and measured the anti-HSA antibody response over time (Supplemental Fig. 3). We observed that, like the TB-OTII-NE236 peptide, IFA was sufficient for inducing antigen-specific production of all isotypes.
3.3 Nod2 is required for an optimal anti-TB-OTII-NE236 response in the presence of IFA
We wondered if Nod2 played a role in the IFA response using TB-OTII-NE236 as an antigen because preliminary control experiments suggested that IFA responses were reduced in Nod2−/− mice (data not shown). In subsequent experiments reported here we did not use any exogenous MDP emulsified into IFA. Rather, we injected control or Nod2−/− mice with IFA-TB-OTII-NE236 or IFA-TB-OTII-NE236 mixed with LPS as a positive control. We then determined the titers of each TB-OTII-NE236-specific antibody isotype . Surprisingly, we found that Nod2 was partially required for IgG1 and IgG2c production, compared to controls because anti-TB-OTII-NE236 titers were reproducibly lower in the absence of Nod2 (Fig. 4). By contrast, Nod2−/− mice were indistinguishable from control mice when IFA was emulsified with LPS and TB-OTII-E236 supporting previous data showing that deficiency in Nod2 has no obvious effects on the TLR4 pathway and Nod2-deficient mice to do have any obvious defects on lymphocyte function. Taken together, these data argue that like NLRP3 for alum, Nod2 plays a partial role in the optimal adjuvant response to IFA.
Fig. 4.
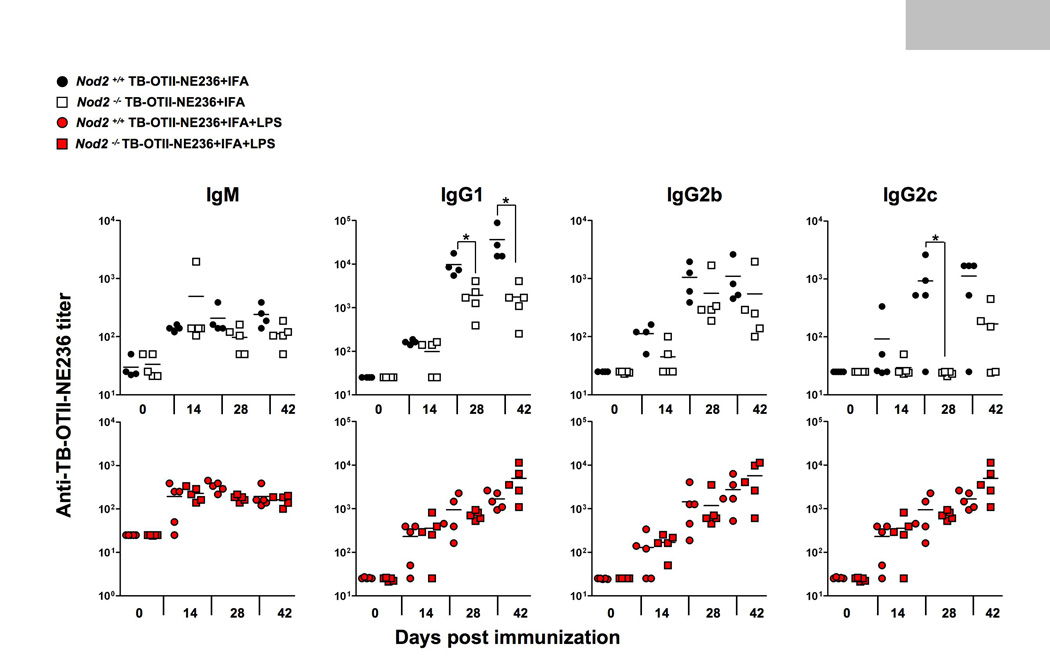
Antibody production by C57BL/6 or Nod2-deficient mice immunized with TB-OTII-NE236 peptide plus IFA (top graphs) or IFA+LPS (lower graphs). Mice were immunized as described in Fig. 1 and serum titers to NE236 measured by isotype-specific ELISA. *, p<0.05.
3.4 Role of MyD88 in the response to antigens emulsified in IFA
The preceding results suggested that IFA was a sufficiently strong adjuvant, regardless of added LPS or MDP. This led us to test if TLR and IL-1β signaling via MyD88 was needed for an antibody response when mixed with IFA. The role of TLR signaling in promoting B cell responses has recently been debated, with arguments made for and against a critical role of MyD88 signaling (and by extension, TLR and other receptors that use MyD88 such as IL-1, IL-18 and IL-33) [42] in antibody generation [40, 43]. In parallel with our analysis of Nod2 in adjuvant responses, we tested the antibody response to TB-OTII-NE236 emulsified in IFA in mice lacking MyD88 (Fig. 5). We did not observe any essential role of MyD88 in the response to TB-OTII-NE236 emulsified in IFA, excluding an essential role for MyD88 signaling via the IL-1, IL-18 or IL-33 receptors in the IFA response. Therefore, Nod2 is required for the optimal response to IFA, independently of MyD88.
Fig. 5.
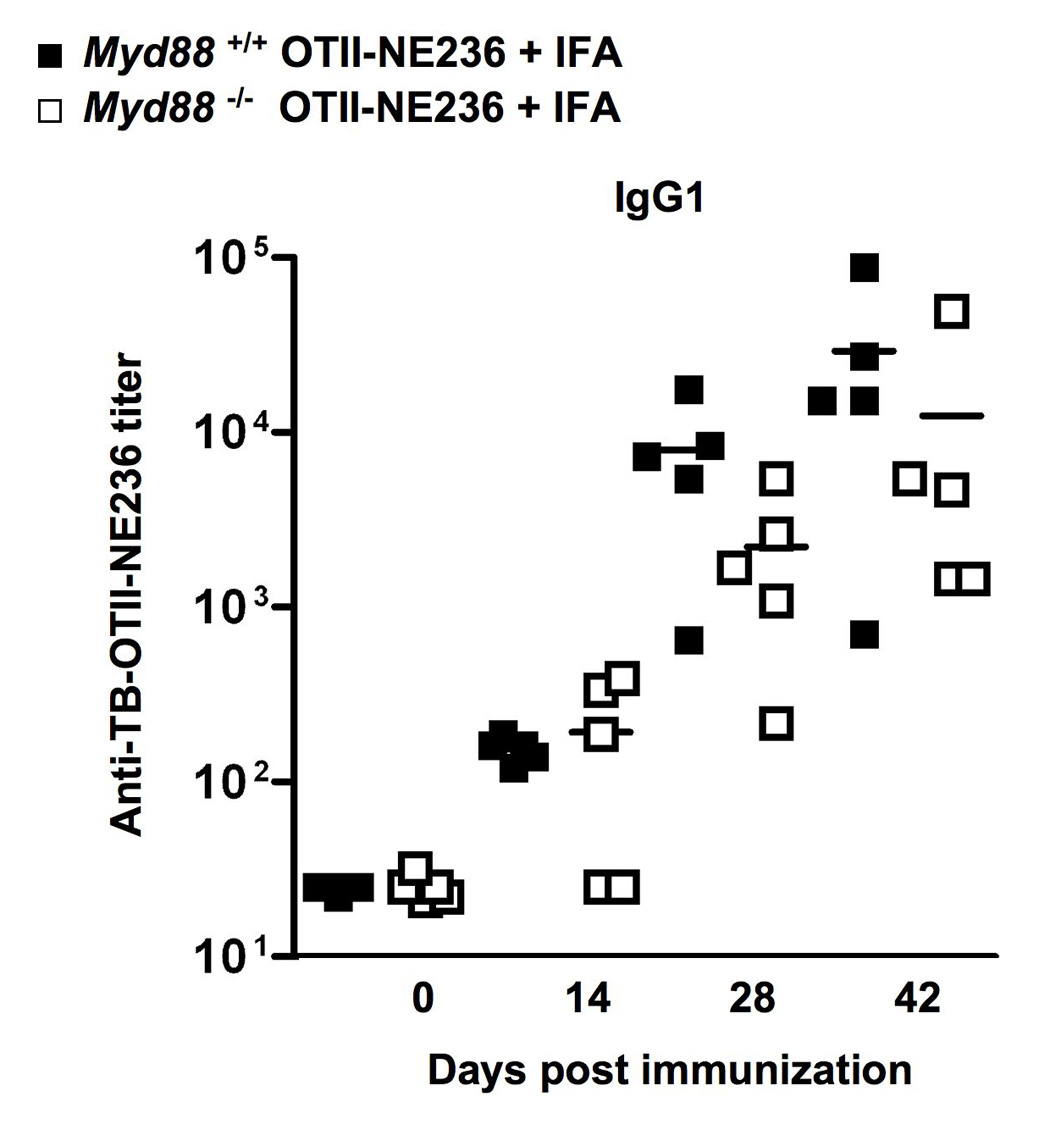
Immunization of control (Myd88+/+) or MyD88-deficient mice immunized with TB-OTII-NE236 peptide plus IFA. Mice were immunized as described in Figs 1. Shown are IgG1 antibody titers specific for NE236. Antigen-specific IgM, IgG2b and IgG2c responses were also determined in these experiments and did not show any significant differences comparing control or Myd88−/− mice.
4. Discussion
Our data suggests that MDP is a poor adjuvant and caution should be used should MDP be considered for inclusion in test adjuvant preparations for use in human studies. However, we cannot exclude the possibility that MDP derivatives, where lipids or carbohydrates are chemically added to MDP, could be tested for enhanced adjuvant effects [24]. Our findings also suggest that MDP, either alone or in combination with LPS, does not efficiently enhance antibody responses to two model antigens, HSA and TB-OTII-NE236. These studies argue against suggestions that MDP is the ‘minimal’ peptidoglycan component of CFA capable of engendering a strong antigen-specific B cell response at least to the highly purified antigens used here [14]. We suggest that these older studies, ensconced in the literature, are most likely the result of contamination of MDP preparations with bacterial components that stimulate the TLR pathways.
An unexpected finding of our systematic studies of the role of Nod2 in MDP-driven antibody production was that responses to IFA and antigen (without the addition of MDP) were reduced in the absence of Nod2. We noted that recent data has shown that another NLR member, NLRP3 (previously called cryopyrin or Nalp3) plays a critical role in the adjuvant response to alum, the most commonly used adjuvant in humans [4, 5, 9]. However, a more recent study has shown that NLRP3 is not required for the alum adjuvant effect when HSA was used as the antigen [11]. Exposure of myeloid-lineage cells to alum causes the release of IL-1β in an NLRP3-dependent way, suggesting that the NLRP3 ‘inflammasome’ is activated by alum to subsequently activate caspase-1 and then IL-1β secretion [9]. Thus, IL-1β might be considered a key player in the adjuvant response. However, the underlying mechanism of alum-mediated activation of IL-1β production and how IL-1β (or other caspase-1 processed cytokines) promotes an adjuvant effect remains unresolved.
The potential role of IL-1β (or other known caspase-1 dependent cytokines such as IL-18 or IL-33) does not agree with data suggesting that MyD88 is not required for adjuvant effects as shown by detailed studies in mice lacking both MyD88 and Trif (and therefore no TLR or IL-1R signaling) [43]. By contrast, Medzhitov and colleagues have provided evidence that shows MyD88 in B cells is essential for antibody production when antigens are co-administered with TLR agonists [40]. It is important to recognize that the studies of the Medzhitov and Gavin groups differ substantially in the immunization route, antigen, adjuvants and assay methods. The interpretation of our studies using HSA (data not shown) or TB-OTII-NE236 in combination with different adjuvants suggests that the MyD88-dependent promotion of an antibody response depends on the antigen-adjuvant combination and the route of injection: thus, we were able to recapitulate previous data [43] showing that MyD88 was not essential for a normal antibody response to HSA (data not shown), or the TB-OTII-NE236 peptide when mixed with LPS (LOM, unpublished data). Additionally, we found that the IFA adjuvant effect was independent of MyD88 (Fig. 5), similar to findings observed in IFA-immunized MyD88-Trif doubly-deficient mice [43].
More surprising, however, were our results that showed that Nod2 was required for an optimal IgG1 and IgG2c response to TB-OTII-NE236 in the presence of IFA. We favor a model where Nod2 is involved, in part, in the coordinated immune and inflammatory response driven by oil emulsions. Recent studies have suggested that Nod2 forms complexes with NLRP1, another NLR member that seems to be involved in MDP sensing [30, 31]. Given these data, it appears that NLR proteins have the potential for multiple types of interactions with each other, some of which might be involved in detecting cellular damage elicited by IFA. Multiple mechanisms must however, be required to detect the presence of IFA: these mechanisms, and the reason for the potency of IFA remain an open question. We also note that even though IFA was abandoned for human use some years ago due to toxicity, there is significant interest in revisiting the use of IFA and other oil-based adjuvants such as MF59 in humans for new vaccine development [44]. Establishing the pathways that respond to oil emulsions will be a necessary first step in determining how best to harness their potent adjuvant properties.
Supplementary Material
Acknowledgments
We thank Drs. Thirumala-Devi Kanneganti and Fabio Re for discussions and comments on the manuscript. This work was supported by the Cancer Center CORE grant P30 CA21765 and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McKee AS, Munks MW, Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007;27(5):687–690. doi: 10.1016/j.immuni.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Dupuis M, McDonald DM, Ott G. Distribution of adjuvant MF59 and antigen gD2 after intramuscular injection in mice. Vaccine. 1999;18(5–6):434–439. doi: 10.1016/s0264-410x(99)00263-7. [DOI] [PubMed] [Google Scholar]
- 3.O'Hagan DT, Wack A, Podda A. MF59 is a safe and potent vaccine adjuvant for flu vaccines in humans: what did we learn during its development? Clin Pharmacol Ther. 2007;82(6):740–744. doi: 10.1038/sj.clpt.6100402. [DOI] [PubMed] [Google Scholar]
- 4.Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205(4):869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007;178(8):5271–5276. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 6.Seubert A, Monaci E, Pizza M, O'Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180(8):5402–5412. doi: 10.4049/jimmunol.180.8.5402. [DOI] [PubMed] [Google Scholar]
- 7.Lanzavecchia A, Sallusto F. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr Opin Immunol. 2007;19(3):268–274. doi: 10.1016/j.coi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316(5831):1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 9.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008 doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Willingham SB, Ting JP, Re F. Cutting edge: Inflammasome activation by alum and alum's adjuvant effects are mediated by NLRP3. J Immunol. 2008 doi: 10.4049/jimmunol.181.1.17. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38(8):2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adam A, Ciorbaru R, Ellouz F, Petit JF, Lederer E. Adjuvant activity of monomeric bacterial cell wall peptidoglycans. Biochem Biophys Res Commun. 1974;56(3):561–567. doi: 10.1016/0006-291x(74)90640-8. [DOI] [PubMed] [Google Scholar]
- 13.Adam A, Petit JF, Lefrancier P, Lederer E. Muramyl peptides. Chemical structure, biological activity and mechanism of action. Mol Cell Biochem. 1981;41:27–47. doi: 10.1007/BF00225295. [DOI] [PubMed] [Google Scholar]
- 14.Ellouz F, Adam A, Ciorbaru R, Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- 15.Strominger JL. Bacterial cell walls, innate immunity and immunoadjuvants. Nat Immunol. 2007;8(12):1269–1271. doi: 10.1038/ni1207-1269. [DOI] [PubMed] [Google Scholar]
- 16.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27(4):549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6(1):9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 18.Barreau F, Meinzer U, Chareyre F, Berrebi D, Niwa-Kawakita M, Dussaillant M, et al. CARD15/NOD2 is required for Peyer's patches homeostasis in mice. PLoS ONE. 2007;2(6):e523. doi: 10.1371/journal.pone.0000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307(5710):731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 20.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 21.Pauleau AL, Murray PJ. Role of nod2 in the response of macrophages to toll-like receptor agonists. Mol Cell Biol. 2003;23(21):7531–7539. doi: 10.1128/MCB.23.21.7531-7539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinsner A, Boveri M, Hareng L, Brown GC, Coecke S, Hartung T, et al. Highly purified lipoteichoic acid induced pro-inflammatory signalling in primary culture of rat microglia through Toll-like receptor 2: selective potentiation of nitric oxide production by muramyl dipeptide. J Neurochem. 2006;99(2):596–607. doi: 10.1111/j.1471-4159.2006.04085.x. [DOI] [PubMed] [Google Scholar]
- 23.Parant MA, Pouillart P, Le Contel C, Parant FJ, Chedid LA, Bahr GM. Selective modulation of lipopolysaccharide-induced death and cytokine production by various muramyl peptides. Infect Immun. 1995;63(1):110–115. doi: 10.1128/iai.63.1.110-115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uehori J, Fukase K, Akazawa T, Uematsu S, Akira S, Funami K, et al. Dendritic cell maturation induced by muramyl dipeptide (MDP) derivatives: monoacylated MDP confers TLR2/TLR4 activation. J Immunol. 2005;174(11):7096–7103. doi: 10.4049/jimmunol.174.11.7096. [DOI] [PubMed] [Google Scholar]
- 25.Wolfert MA, Murray TF, Boons GJ, Moore JN. The origin of the synergistic effect of muramyl dipeptide with endotoxin and peptidoglycan. J Biol Chem. 2002;277(42):39179–39186. doi: 10.1074/jbc.M204885200. [DOI] [PubMed] [Google Scholar]
- 26.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8(6):458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 27.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117(3):514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 29.Murray PJ. NOD proteins: an intracellular pathogen-recognition system or signal transduction modifiers? Curr Opin Immunol. 2005;17(4):352–358. doi: 10.1016/j.coi.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25(5):713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 31.Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1{beta} secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14(21):1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 33.Pan Q, Mathison J, Fearns C, Kravchenko VV, Da Silva Correia J, Hoffman HM, et al. MDP-induced interleukin-1beta processing requires Nod2 and CIAS1/NALP3. J Leukoc Biol. 2007;82(1):177–183. doi: 10.1189/jlb.1006627. [DOI] [PubMed] [Google Scholar]
- 34.Azuma I. Synthetic immunoadjuvants: application to non-specific host stimulation and potentiation of vaccine immunogenicity. Vaccine. 1992;10(14):1000–1006. doi: 10.1016/0264-410x(92)90108-v. [DOI] [PubMed] [Google Scholar]
- 35.Andren J, Andrews K, Brown L, Chidgey J, Geary N, King MG, et al. Muramyl peptides and the functions of sleep. Behav Brain Res. 1995;69(1–2):85–90. doi: 10.1016/0166-4328(95)00004-d. [DOI] [PubMed] [Google Scholar]
- 36.Shoham S, Krueger JM. Muramyl dipeptide-induced sleep and fever: effects of ambient temperature and time of injections. Am J Physiol. 1988;255(1 Pt 2):R157–R165. doi: 10.1152/ajpregu.1988.255.1.R157. [DOI] [PubMed] [Google Scholar]
- 37.Parant M, Parant F, Chedid L, Yapo A, Petit JF, Lederer E. Fate of the synthetic immunoadjuvant, muramyl dipeptide (14C-labelled) in the mouse. Int J Immunopharmacol. 1979;1(1):35–41. doi: 10.1016/0192-0561(79)90028-6. [DOI] [PubMed] [Google Scholar]
- 38.Fillon S, Soulis K, Rajasekaran S, Benedict-Hamilton H, Radin JN, Orihuela CJ, et al. Platelet-activating factor receptor and innate immunity: uptake of gram-positive bacterial cell wall into host cells and cell-specific pathophysiology. J Immunol. 2006;177(9):6182–6191. doi: 10.4049/jimmunol.177.9.6182. [DOI] [PubMed] [Google Scholar]
- 39.Tuomanen EI, Austrian R, Masure HR. Pathogenesis of pneumococcal infection. N Engl J Med. 1995;332(19):1280–1284. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- 40.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438(7066):364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 41.Leclerc C, Audibert F, Chedid L. Influence of a synthetic adjuvant (MDP) on qualitative and quantitative changes of serum globulins. Immunology. 1978;35(6):963–970. [PMC free article] [PubMed] [Google Scholar]
- 42.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9(1):143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 43.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314(5807):1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller LH, Saul A, Mahanty S. Revisiting Freund's incomplete adjuvant for vaccines in the developing world. Trends Parasitol. 2005;21(9):412–414. doi: 10.1016/j.pt.2005.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.