Abstract
Objective
To determine if an elevated level of the complement activation fragment Bb in early pregnancy was associated with spontaneous preterm birth (SPTB) at less than 34 weeks gestation or SPTB between 34 and 37 weeks gestation (late SPTB).
Study Design
Prospective study of 784 women enrolled at < 20 weeks gestation.
Results
Following exclusions, 13 women (1.7%) had a SPTB at less than 34 weeks gestation and 25 (3.2%) a SPTB between 34 and 37 weeks gestation. Women with Bb in the top quartile were 4.7 times more likely to have an SPTB less than 34 weeks gestation as compared with women who had levels of Bb in the lower three quartiles (95% CI 1.5 to 14, P = 0.003). There was no association between Bb and late SPTB (RR= 0.8, 95% CI = 0.3 to 2).
Conclusions
A significant relationship was found between an elevated Bb in early pregnancy and SPTB < 34 weeks gestation. These results suggest that inflammatory events in early pregnancy are part of the pathogenic mechanisms of this condition.
Keywords: Inflammation, prematurity, early pregnancy
INTRODUCTION
Preterm (<37th weeks gestation) birth (PTB) is a complex adverse outcome of pregnancy, accounting for approximately 70% of perinatal mortality, and is a major predictor of long term neurologic morbidity 1-3. Despite intense research and clinical efforts, progress in preventing PTB has been disappointing 4. The incidence of PTB in the United States in 2005 was 12.7%. This represents an increase in the PTB rate of 20% since 19905, 6.
Intrauterine infection and or inflammation are strongly associated with preterm birth 7, 8. It is estimated that 40-50% of all PTB occur in mothers with intrauterine infection that may not be clinically evident 9, 10. It has been observed that the lower the gestational age at delivery the greater the likelihood of microbial invasion of the amniotic cavity11. It is unclear when the pathologic process for PTB is initiated. Infectious/inflammatory events may start early in pregnancy 12, 13, or chronic uterine infection may be present between pregnancies 14. Indeed, one of the strongest risk factors for a PTB is a previous preterm birth 15.
Notwithstanding the importance of infection in the natural history of PTB, the host’s response to infection or other potential inflammatory processes is also considered a critical element of the morbidity and mortality that accompanies this outcome 8, 16, 17. As one component of the host response, the complement system plays a pivotal role in innate immunity, the first line of defense against foreign antigens. The clinical importance of the complement system was discovered over 100 years ago by Jules Bordet 18. However, the important contribution of complement-mediated inflammatory events to a vast number of diseases has only emerged in recent times19. In point of fact, research in the animal model of the antiphospholipid antibody syndrome has illuminated the adverse pregnancy outcomes (intrauterine loss and intra-uterine growth restriction) that accompany excessive complement activation20, 21. Furthermore, very recent research from Lynch et al has demonstrated that women with elevated levels of the complement activation fragment Bb in early pregnancy are at a significantly increased risk of subsequently developing preeclampsia later in pregnancy22.
The complement system is a complex series of 30 proteins which forms one of the triggered enzyme systems that act sequentially to mediate inflammatory events 23, 24. The complement system has three main protective functions: defending the host against pyogenic infections, bridging innate and adaptive immunity and disposing of immune complexes and the products of inflammatory injury 25. Redirection of these functions towards self tissues underscores the pathogenesis of many diseases19, 21. Components of the complement system are found in the circulation, in body fluids and on the surface of tissues26. There are three pathways of complement activation: the classical, lectin and alternative pathway with different triggers for activation (Figure 1). The biological functions of complement are mediated through the production of activation fragments which initiate as well as amplify inflammation 27(Figure 1).
Figure 1. Classical, Lectin and Alternative Complement Pathways.
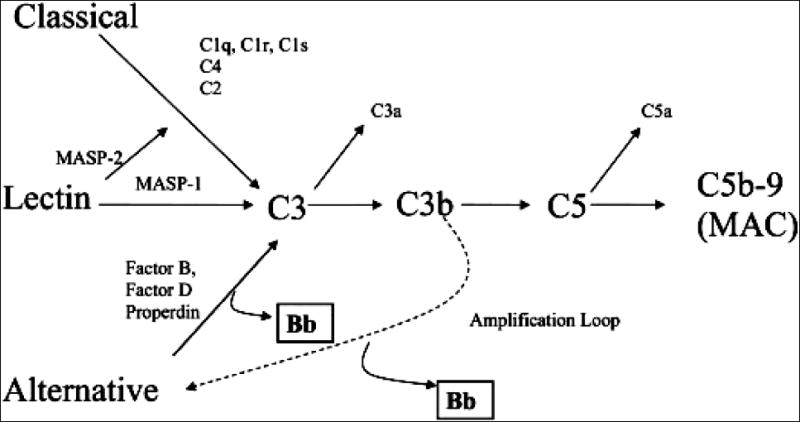
Complement has three initiating mechanisms known as the classical, lectin, and alternative pathways. The triggers for these pathways include: immune complexes, apoptotic bodies, mitochondrial membranes, mitochondrial cardiolipin, C-reactive protein, endothelial neoepitopes in ischemic tissue (classical pathway), carbohydrates on pathogens (lectin pathway) and “tickover19, 23”, polysaccharides, endotoxin, IgA immune complexes, C3 nephritic factor, absence or interference with regulatory proteins (alternative pathway) 24. Biological functions of the complement system are achieved through the production of activation fragments (e.g. C3a, C5a, C5b-9). The classical, lectin and alternative complement pathways converge to generate enzymes called C3 convertases which cleave C3 into C3b and C3a. C3b is a major effector molecule of the complement system. C3b binds to the surface of foreign cells and opsonizes the cells for phagocytosis. Through a series of reactions the membrane attack complex (MAC) is formed. This complex can insert into membranes and has the potential to damage cells. Factors B, D and Properdin initiate activation of C3 directly through the alternative pathway initiation complex or through the amplification loop when C3b is formed. The Bb activation product is derived from factor B and serves as a protease that is able to cleave additional C3 molecules. In the absence of Bb generation, no alternative pathway activation is possible. The production of C3b, triggered from engagement of the classical or lectin pathways is augmented through the alternative pathway amplification loop that is essential to generate pathogenic pro-inflammatory mediators in vivo 24.
Despite the strong literature suggesting a link between infection/inflammation and PTB and the central role complement plays in the pathogenesis of infectious disease and inflammatory disease there has been very little research examining the relationship between complement activation and PTB28, 29. The setting for the study reported here in was early (<20 weeks gestation) pregnancy and one of the specific aims of this prospective study was to determine if an elevated level of the complement activation fragment Bb at this time in pregnancy was associated with spontaneous preterm birth (SPTB) < 34 weeks gestation or late 30 (34-37 weeks) SPTB. The Bb activation fragment is primarily associated with alternative complement pathway activation but can also arise as a result of activation of either of the other complement pathways through the alternative complement pathway amplification loop (Figure 1). Bb is a protease generated during the sequential activation of both the alternative pathway and the activation loop and is required for further steps of the pathway. The alternative pathway and amplification loop are reviewed in detail elsewhere 23, 24.
MATERIAL AND METHODS
Study design
This prospective study was approved by the Colorado Multiple Institutional Review Board and is part of the Denver complement study started in June 2005 to examine the contribution of complement activation to adverse pregnancy outcomes (described in more detail elsewhere22). The women presented in this study represent all deliveries up to August 2007. Exclusion criteria were: multiple births, deliveries < 20 weeks gestation, medically indicated PTB, maternal medical disease (chronic hypertension, cardiac disease, and diabetes), congenital or chromosomal anomalies in the baby, and maternal pre-pregnancy uterine anomalies/abnormalities. The complement activation fragment Bb was the main explanatory variable of interest and was examined as a continuous and categorical variable (based on quartiles of Bb values for the entire cohort). We also examined Bb as a dichotomous variable (greater than the 75th versus less than the 75th percentile). Potential confounders included in the analysis included: race (African American versus others), parity (nulliparity versus multiparity), maternal age (over 35 versus less than 35 years), cigarette smoking at the time of conception (yes versus no), maternal educational status (≤ 12 versus >12 years), a past history of PTB (yes versus no) and the maternal prepregnant Body Mass Index (BMI) (kg/m2) ((over 30 (obese) versus less than 30)) calculated from the maternal prepregnant weight and height, and premature rupture of the fetal membranes (yes versus no). The primary outcome of the study was SPTB resulting from spontaneous rupture of the membranes or spontaneous labor after 20 and less than 37 completed weeks gestation 31. The spontaneous preterm births were further categorized as: 1) births at less than 34 weeks gestation and, 2) births between 34 and 37 weeks gestation (often referred to as “late” SPTB30, 32). Assessment of gestational age was based primarily on clinical assessment at the first visit and on early ultrasound examination in the majority of the cohort.
Study participants were recruited from the prenatal clinics at the University of Colorado Hospital (UCH) (n = 326), the Metro Community Practice Network (MCPN) clinic (a community-based prenatal clinic, n = 131) and the Platte River Prenatal Center, a faculty referral facility (n = 327). The study participants were referred to the complement study team by the prenatal care provider if the woman was less than 20 weeks gestation. Only 3% of women approached in this manner to be in the study have refused participation. The current loss to follow-up from the study is 3%. Following informed written consent, an interview was conducted with the study participant, and data were gathered on the maternal medical and obstetrical history. The deidentified data were entered into the complement study dataset. At the same visit plasma was drawn for Bb assay along with the routine prenatal laboratory tests. The women were followed prospectively throughout pregnancy. At delivery, outcome data were collected and entered into the complement study database. At this assessment the gestational age at blood draw at the recruitment visit was verified using results from ultrasound examinations. Outcomes on women who delivered outside UCH (n = 392) were obtained by a combination of administration of a detailed questionnaire within two weeks of the estimated date of delivery (by phone interview or by mail) and by review of the medical record. Medical record review was conducted on women who had complications of pregnancy and on a randomly selected group of controls with uncomplicated pregnancies (30% of the women who delivered outside UCH had a medical record review with patient consent)
Sample preparation and assay for complement activation fragment Bb
Each sample was centrifuged within an average time of 10 minutes (± 8 minutes range 1 to 57 minutes) from phlebotomy, the supernatant removed, aliquotted and placed in a freezer at -80°C. For women recruited outside UCH the sample was centrifuged immediately and the supernatant placed on dry ice for transportation back to the repository.
Complement activation fragment Bb was measured using a quantitative sandwich Enzyme-Linked ImmunoSorbent Assay (ELISA) that employs a monoclonal anti-human Bb as the capture antibody and an enzyme-conjugated goat anti-human Bb as the second antibody (Quidel, San Diego CA). Standards with known concentrations of Bb and high and low plasma controls were run on each ELISA plate. The addition of a chromogenic substrate causes a change in optical density (color) in each well of the ELISA plate. This change in OD was measured spectrophotometrically, and is a function of the amount of antigen (Bb) captured on the plate. The coefficient of variation for the in-house laboratory control was 10.2%. The inter and intra-assay coefficients of variation were 4.0% and 3.9% respectively. The detection limit was 0.01 ug/ml. The normal range for non-pregnant controls in our laboratory is 0 to 0.83 μg/ml. Approximately 100 consecutive samples were analyzed per month on women following completion of pregnancy. The technician performing the assay was blinded to the participant’s pregnancy outcome.
Statistical analysis
Univariable analysis was used to generate descriptive statistics for the cohort. Differences between categorical variables were examined using the chi-square or Fisher exact test (P < 0.05). The relative risk (RR) was used as a measure of association between dichotomous variables. Differences in means of continuous variables were tested using the Wilcoxon rank-sum and the Kruskal-Wallis test. Multivariable logistic regression analysis was used to determine the odds ratio (used as an approximation of the RR) of the main explanatory variable Bb for SPTB adjusted for other covariates.
RESULTS
Among the 964 women who delivered after 20 weeks gestation the overall incidence of PTB was 11.7% (n = 113). Fifty-six (5.8%) of these deliveries were SPTB and 57 (5.9%) were medically indicated PTB. Following the study’s exclusions 784 women remained in the analytic dataset. Among this group of women 38 (4.9%) had a SPTB, 13 (1.7%) had a SPTB < 34 weeks gestation (range 22 weeks and 3 days to 33 weeks and 5 days), 25 (3.2%) had a late SPTB, and 746 (95%) had a term delivery.
The general characteristics of the cohort are shown in Table 1. We saw significantly higher levels of the complement activation fragment Bb among women with SPTB < 34 weeks compared with women with late SPTB or term births (Table 1, Figure 2). Indeed, the mean and median levels of Bb were very similar among women with a late preterm or term delivery (Table 1 and Figure 2). Categorized across quartiles of Bb for the cohort we noted that women with a Bb in the upper quartile had an incidence of SPTB < 34 weeks of 62%. In contrast, the frequency of an elevated Bb in the upper quartile was lower and very similar among women with late SPTB (20%) and term birth (25%). We collapsed the lower 3 quartiles of Bb because of low numbers in the < 34 weeks SPTB stratum. With the analysis restricted to women with an SPTB < 34 weeks or term delivery we found that women with a level of Bb in the upper quartile were 4.7 times more likely to have an early PTB as compared with women term deliveries. (95% CI 1.5 to 14, P = 0.003).
Table 1.
General Characteristics of the Cohort –Overall and by Gestational Age at Delivery
| SPTB | |||||
|---|---|---|---|---|---|
| Total
N = 784 |
< 34 weeks
n = 13 |
34-37 weeks
n = 25 |
>=37 weeks
n = 746 |
P value | |
| Mean Bb ± SD | 0.7 ± 0.2 | 0.8 ± 0.17 | 0.69 ± 0.2 | 0.68 ± 0.2 | 0.047 |
| Median Bb | 0.66 | 0.83 | 0.61 | 0.67 | |
| Bb ≥75th percentile | 199 (25%) | 8 (62%) | 5 (20%) | 186 (25%) | 0.03 |
| 50 – 75th percentile | 193 (25%) | 2 (15%) | 4 (16%) | 187 (25%) | |
| 25-50th percentile | 202 (26%) | 2 (15%) | 12 (48%) | 188 (25%) | |
| <25th percentile | 190 (24%) | 1 (8%) | 4 (16%) | 185 (25%) | |
| Bb ≥ 75th Percentile | 199 (25%) | 8 (62%) | 5 (20%) | 186 (25%) | 0.01 |
| <75th Percentile | 585 (75%) | 5 (38%) | 20 (80%) | 560 (75%) | |
| Race/ Ethnicity Non Hispanic White | 510 (65%) | 5 (38%) | 17 (68%) | 488 (65%) | 0.08 |
| Hispanic | 192 (24%) | 5 (38%) | 4 (16%) | 183 (25%) | |
| African American | 37 (5%) | 2 (15%) | 1 (4%) | 34 (4.5%) | |
| Asian | 31 (4%) | 0 (0%) | 3 (12%) | 28 (4%) | |
| Other | 14 (2%) | 1 (8%) | 0 (0%) | 13 (2%) | |
| Nulliparity Yes | 343 (44%) | 6 (46%) | 11 (44%) | 326 (44%) | 1.0 |
| No | 441 (56%) | 7 (54%) | 14 (56%) | 420 (56%) | |
| Mean Maternal age ± SD | 32 ± 5 | 33 ± 6 | 33± 6 | 32 ± 6 | 0. 5 |
| Maternal age ≥ 35 | 338 (43%) | 6 (46%) | 15 (60%) | 317 (42%) | 0.2 |
| <35 | 446 (57%) | 7 (54%) | 10 (40%) | 429 (58%) | |
| Education ≤ 12 years | 238 (30%) | 7 (54%) | 9 (36%) | 222 (30%) | 0.1 |
| >12 years | 546 (70%) | 6 (46%) | 16 (64%) | 524 (70%) | |
| BMI ≥ 30 | 85 (11%) | 4 (31%) | 1 (4%) | 80 (11%) | 0. 04 |
| <30 | 699 (89%) | 9 (69%) | 24 (96%) | 666 (89%) | |
| History PTB Yes | 59 (7.5%) | 3 (23%) | 4 (16%) | 52 (7%) | 0.02 |
| No | 725 (92%) | 10 (77%) | 21(84%) | 694 (93%) | |
| Cigarette smoking Yes | 39 (5%) | 3 (23%) | 2 (8%) | 34 (4.5%) | 0.02 |
| No | 745 (95%) | 10 (77%) | 23 (92%) | 712 (95%) | |
| Mean gestational age blood draw (weeks) ± SD | 11.9 ± 2.5 | 11.03± 1.4 | 11.5± 2.4 | 12 ± 2.5 | 0.2 |
| Premature rupture of the membranes Yes | 42 (5%) | 10 (77%) | 17 (68%) | 15 (2%) | 0.0001 |
| No | 742 (95%) | 3 (23%) | 8 (32%) | 731 (98%) | |
Figure 2. Distribution of Bb levels by gestational age delivery.
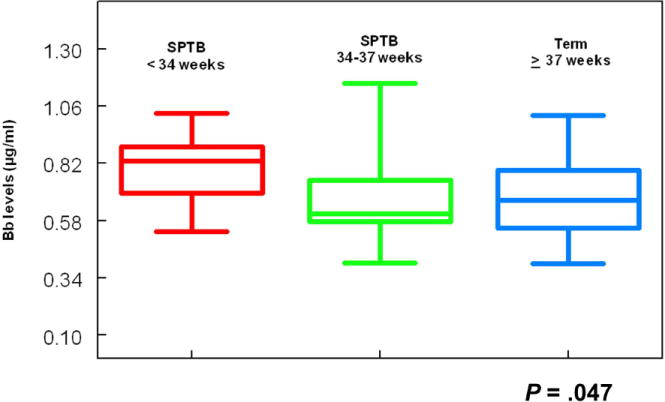
The figure demonstrates the levels of Bb in women who had an SPTB (< 34 weeks gestation n = 13), a SPTB (34-37 weeks gestation, n = 25) and a term delivery (after 37 weeks gestation, n = 746). The upper and lower ends of the box are the 75th and 25th percentiles. The line across the middle of the box represents the median level of Bb (μg/ml) in each group. The P value for the difference in the median levels of Bb between the SPTB < 34 weeks gestation and the term deliveries was 0.01. The P value for the difference in the median Bb levels between the SPTB < 34 weeks and the late SPTB was 0.09 (Wilcoxon rank-sum) and the P value for differences in Bb levels across the 3 categories of birth was 0.047 (Kruskal-Wallis test).
The multivariable logistic regression analysis is shown in Table 2. Adjusted for established risk factors for PTB, women with Bb in the top quartile were 4.3 times more likely (P = 0.02 ) to deliver a baby less than 34 weeks gestation compared with women who delivered a term baby. In this model, a history of PTB in a past pregnancy was the only other covariate significantly associated with early PTB. Backward selection was performed on this model (Table 2, Model B). Along with a high Bb, (adjusted OR = 4.5, P = 0.0095) the only other covariate remaining in this reduced model was cigarette smoking at the time of conception. A history of PTB was not significantly associated with SPTB < 34 weeks gestation in the reduced model. We had a closer look at the relationship between cigarette smoking and a history of PTB to identify if there was an association between these two covariates. We found that women with a history of PTB were more likely to be cigarette smokers at the time of conception. (RR = 2.7, 95% CI = 1.2 to 5.8, P = 0.01). In the full multivariable logistic model for late SPTB the adjusted OR of an elevated Bb for late SPTB was 0.8 (95% CI 0.3 to 2, P = 0.7). Measured as a continuous variable the adjusted odds ratio of Bb for early SPTB in the full and reduced models was 14.6 (95% CI 0.8 to 245, P = 0.06) and 22 (95% CI 1.5 to 332, P = 0.025) respectively.
Table 2.
Full and Reduced Multivariable Logistic Regression Model Showing the Unadjusted and Adjusted Odds Ratio of Complement Activation Fragment Bb and other Select Risk Factors for Spontaneous Preterm Birth at < 34 Weeks Gestation *
| Model A**
| ||||
|---|---|---|---|---|
| Unadjusted OR | Adjusted OR | 95% CI † | P Value ‡ | |
| Complement Bb (top quartile) | 4.8 | 4.3 | 1.3, 14 | 0.02 |
| Maternal age ≥ 35 | 0.8 | 2.3 | 0.6, 8 | 0.2 |
| African American | 3.8 | 1.4 | 0.2, 9 | 0.7 |
| Maternal BMI > 30 | 3.7 | 2.5 | 0.6, 9.5 | 0.2 |
| Nulliparity | 1.1 | 2.4 | 0.6, 9.5 | 0.2 |
| Educational Status < 12 years | 2.8 | 2.3 | 0.6, 8.5 | 0.2 |
| History of preterm birth | 4.0 | 6.2 | 1.2, 30 | 0.03 |
| Cigarette smoking | 6.2 | 3.9 | 0.8, 19 | 0.09 |
|
| ||||
|
Model B §
| ||||
| Complement Bb (top quartile) | 4.8 | 4.5 | 1.4, 14 | 0.0095 |
| Cigarette smoking | 6.2 | 5.5 | 1.4, 21 | 0.01 |
Women with late preterm birth are removed from the models
full multivariable logistic regression model
For the adjusted OR
Model B is the multivariable logistic regression model following backward selection of statistically unimportant variables from Model A.
In view of our previous research showing an association between Bb and preeclampsia we ran an additional model adjusting for the development of gestational hypertension or preeclampsia (we had no cases of preeclampsia among the women who had an early SPTB). The adjusted OR of Bb in the top quartile for early PTB was unchanged (OR = 4.3, 95% CI 1.3, 14, P = 0.02). Our choice of the lower boundary for SPTB was 20 weeks gestation. We ran an additional model with the lower boundary at 16 weeks gestation. With the addition of 5 cases to the group of 13 women with a SPTB < 34 weeks gestation the adjusted OR of Bb in the top quartile was 4.2 (95% CI 1.5 to 11, P = 0.005). The following are the results of the test characteristics of Bb for the outcome SPTB <3 4 weeks with an incidence of 1.71%; sensitivity 62%, specificity 75 %, positive predictive value 4.1%, negative predictive value 99%.
COMMENT
In this study of women with singleton pregnancies, we demonstrate a significant association between Bb in early pregnancy and SPTB < 34 weeks gestation. This suggests that inflammation linked to complement activation in early pregnancy may lead to this adverse pregnancy outcome. Indeed, adjusted for other established risk factors for PTB, women with levels of this activation fragment in the top quartile were 4.3 times more likely to deliver a baby born at less than 34 weeks gestation compared with women with lower levels of the activation fragment. The other covariate in the multivariable logistic model significantly associated with SPTB < 34 weeks was cigarette smoking and we found this variable was significantly associated with a history of PTB.
The etiology of PTB is heterogeneous. In order to provide a clearer picture of the pathogenesis four pathogenic pathways have been conceptualized. These pathways share a common final biological pathway of PTB leading to an inflammatory cascade with uterine contractions and cervical changes with or without premature rupture of the membranes. These pathogenic pathways include: premature activation of the maternal or fetal hypothalamic-pituitary-adrenal axis (i.e. stress); decidual-chorioamniotic or systemic inflammation; decidual hemorrhage (i.e. abruption); and pathological distention of the uterus due to multifetal gestations10, 33. Although complement activation may be linked to any of these four mechanisms of PTB, our study design (restriction to singleton births, exclusion of medically indicated PTB, see methods section) facilitated a close examination of the contribution of inflammation to PTB. One important finding of the analysis was that elevated levels of the alternative activation fragment Bb was predictive of births at less than 34 weeks gestational but not of late preterm births. This is in keeping with the findings of other researchers who have shown that spontaneous PTB in the earlier gestational window is strongly associated with intrauterine infection 8, 11. Interestingly, a similar observation was found in the Preterm Prediction Study with another marker of inflammation, granulocyte colony-stimulating factor. This cytokine was associated with SPTB at < 32 weeks gestation but not with late SPTB 15. These findings suggest that different mechanisms may be contributing to late SPTB. It is also noteworthy that some of the high Bb samples in our study were obtained as early as the first trimester suggesting that this activation fragment was linked to an early inflammatory event that remained clinically silent until the onset of preterm labor or PROM (Table 1). Of note when we dropped the lower boundary of PTB to 16 weeks gestation the association of an elevated Bb with SPTB < 34 weeks remained significant suggesting that there are maternal markers of SPTB present at this early gestational age. This long subclinical phase is in agreement with the results of other studies which found evidence of infection in the amniotic fluid as early as 16 weeks gestation in women who subsequently had prematurity-related adverse pregnancy outcomes 12. Indeed, other authors have recently shown that small fetal size at 10-19 weeks is associated with early PTB 34 and that women with a prior second-trimester loss are at significantly increased risk for spontaneous preterm birth and recurrent second-trimester loss in their next pregnancy 35. These observations suggest that researchers should consider a lower gestational boundary when examining early pregnancy markers of adverse pregnancy outcomes.
Although it is possible that this inflammatory event may be subclinical infection, it is also possible that the Bb activation fragment may be a marker of placental tissue necrosis or tissue ischemia and reperfusion or other problems in the early development of the placenta 16. These are events that trigger complement activation by exposing phospholipids and mitochondrial proteins (Figure 1). It is also noteworthy that damaged tissues may lack the complement regulatory proteins that prevent complement from attacking normal tissue 36. A non-microbial cause of inflammation would indeed be one explanation of why some women with histologic chorioamnionitis are culture negative 37. These concepts fit in nicely with the “danger model” of immunity which suggests that the host recognizes danger associated molecular patterns (DAMPS) irrespective of their nature (i.e. infections or non-infectious in origin) 38, 39.
Interestingly, Elimian et al examined singleton gestations (between 23 and 35 weeks) and correlated levels of complement in the amniotic fluid of 104 women with preterm labor with intact membranes and with no clinical signs of intrauterine infection. The median values of complement C3 were compared between the culture-positive and culture–negative groups. The prevalence of positive cultures was 11.5% (12/104). The culture-positive group had significantly higher levels of C3 than the culture-negative group28. In another study Soto et al. found that among women with preterm labor, those with intra-amniotic infection (positive amniotic fluid culture for microorganisms) had significantly higher median plasma levels of C5a than those without intra-amniotic infection 29. The results of these studies suggest that complement activation fragments may be a surrogate marker of subclinical infection.
There are limitations to this study, especially related to the fact that we have only information on one complement activation fragment from one early time point in pregnancy and the number of women with the outcome was low. It is a possible that these activation fragments may be even higher later in pregnancy in women who subsequently have an early SPTB. There is a need for a large pregnancy study to address the relationship of inflammatory markers including complement activation fragments measured at multiple points in pregnancy and the risk of SPTB5. In addition, a larger cohort would allow further stratification by gestational age among SPTB < 34 weeks gestation. We also note as a limitation that approximately 40% of the cohort delivered outside UCH. We obtained outcomes on these women with phone and mail administered questionnaires and review of a select group of medical records. We found an excellent correlation in the information from the questionnaires and the clinical information reported in the medical record. This can be attributed to the administration of the questionnaire close to the time of delivery. Notwithstanding these limitations, this study suggests that complement activation is a significant contributory factor to the pathogenesis of early SPTB. It is evident from studies on atypical hemolytic uremic syndrome, membranoproliferative glomerulonephritis type II and age–related macular degeneration 27, 40, 41 that excessive complement activation or genetically-related dysfunction of complement regulators can lead to local tissue injury and devastating disease for the host. These findings and the results of this study should hasten the need to develop therapeutic approaches that will target the mechanisms fundamental to tissue destruction in complement-related adverse clinical outcomes42-44.
Acknowledgments
We acknowledge Cynthia Marschner MT (ASCP) for performing the Bb assay, Henry Galan MD, William Goddard MD, Jocelyn Seelye B.S and Sarah Crowley B.A for facilitating use of the perinatal database, Joseph Piccoli B.S and Ted Wade PhD for data coordination at NJMRC, and the administrative, medical and nursing staff at the UCH, Platte River and MCPN clinics for their support of the complement study.
Funding: Dr Lynch is supported by NICHD grant K23 HD049684 and Newborn Hope Colorado. Dr Holers is supported by NIH R01 AI 55007 and Dr. Salmon is supported by NIH R01 AR49772.
Footnotes
Presentations: Presented at the Society for Gynecologic Investigation 55th Annual Scientific Meeting March 26-29, 2008, San Diego, CA, USA
Condensation A significant relationship was found between the complement activation fragment Bb in early pregnancy and spontaneous preterm birth at less than 34 weeks gestation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gibbs RS. The relationship between infections and adverse pregnancy outcomes: an overview. Ann Periodontol. 2001;6(1):153–63. doi: 10.1902/annals.2001.6.1.153. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buekens P, Klebanoff M. Preterm birth research: from disillusion to the search for new mechanisms. Paediatr Perinat Epidemiol. 2001;15 Suppl 2:159–61. doi: 10.1046/j.1365-3016.2001.00015.x. [DOI] [PubMed] [Google Scholar]
- 5.Green NS, Damus K, Simpson JL, Iams J, Reece EA, Hobel CJ, et al. Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol. 2005;193(3 Pt 1):626–35. doi: 10.1016/j.ajog.2005.02.106. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2005. Natl Vital Stat Rep. 2006;55(11):1–18. [PubMed] [Google Scholar]
- 7.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319(15):972–8. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7(4):259–74. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- 10.Lockwood CJ. Predicting premature delivery--no easy task. N Engl J Med. 2002;346(4):282–4. doi: 10.1056/NEJM200201243460412. [DOI] [PubMed] [Google Scholar]
- 11.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79(3):351–7. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Cassell GH, Davis RO, Waites KB, Brown MB, Marriott PA, Stagno S, et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16-20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis. 1983;10(4 Suppl):294–302. [PubMed] [Google Scholar]
- 13.Horowitz S, Mazor M, Romero R, Horowitz J, Glezerman M. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J Reprod Med. 1995;40(5):375–9. [PubMed] [Google Scholar]
- 14.Ghidini A, Salafia CM. Histologic placental lesions in women with recurrent preterm delivery. Acta Obstet Gynecol Scand. 2005;84(6):547–50. doi: 10.1111/j.0001-6349.2005.00694.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg RL, Andrews WW, Mercer BM, Moawad AH, Meis PJ, Iams JD, et al. The preterm prediction study: granulocyte colony-stimulating factor and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 2000;182(3):625–30. doi: 10.1067/mob.2000.104210. [DOI] [PubMed] [Google Scholar]
- 16.Caucheteux SM, Kanellopoulos-Langevin C, Ojcius DM. At the innate frontiers between mother and fetus: linking abortion with complement activation. Immunity. 2003;18(2):169–72. doi: 10.1016/s1074-7613(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 17.Gibbs RS, Davies JK, McDuffie RS, Jr, Leslie KK, Sherman MP, Centretto CA, et al. Chronic intrauterine infection and inflammation in the preterm rabbit, despite antibiotic therapy. Am J Obstet Gynecol. 2002;186(2):234–9. doi: 10.1067/mob.2002.119640. [DOI] [PubMed] [Google Scholar]
- 18.Bordet J, G O. Sur l’existences de substance sensibilisatrices dans la plupart des serum antimicrobiens. French. Ann Inst Pasteur. 1901;15:289–302. [Google Scholar]
- 19.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176(3):1305–10. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 20.Salmon JE, Girardi G, Holers VM. Complement activation as a mediator of antiphospholipid antibody induced pregnancy loss and thrombosis. Ann Rheum Dis. 2002;61(2):ii46–50. doi: 10.1136/ard.61.suppl_2.ii46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holers VM, Girardi G, Mo L, Guthridge JM, Molina H, Pierangeli SS, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002;195(2):211–20. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch AM, Murphy JR, Byers T, Gibbs RS, Neville MC, Giclas PC, et al. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol. 2008 doi: 10.1016/j.ajog.2007.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janeway C. Immunobiology: the immune system in health and disease. 6. New York: Garland Science; 2005. [Google Scholar]
- 24.Holers VM. Complement. In: RR R, editor. Principles and Practive of Clinical Immunology. St Louis, MO21.1-21.8: Mosby; 2001. [Google Scholar]
- 25.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344(14):1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 26.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171(3):715–27. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zipfel PF, Heinen S, Jozsi M, Skerka C. Complement and diseases: defective alternative pathway control results in kidney and eye diseases. Mol Immunol. 2006;43(12):97–106. doi: 10.1016/j.molimm.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Elimian A, Figueroa R, Canterino J, Verma U, Aguero-Rosenfeld M, Tejani N. Amniotic fluid complement C3 as a marker of intra-amniotic infection. Obstet Gynecol. 1998;92(1):72–6. doi: 10.1016/s0029-7844(98)00123-9. [DOI] [PubMed] [Google Scholar]
- 29.Soto E, Romero R, Richani K, Espinoza J, Nien JK, Chaiworapongsa T, et al. Anaphylatoxins in preterm and term labor. J Perinat Med. 2005;33(4):306–13. doi: 10.1515/JPM.2005.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engle WA. A recommendation for the definition of “late preterm” (near-term) and the birth weight-gestational age classification system. Semin Perinatol. 2006;30(1):2–7. doi: 10.1053/j.semperi.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Iams J, Creasy RK. Preterm labor and delivery. In: Creasy R, Resnik R, editors. Maternal-Fetal Medicine. 5. Philadelphia: Saunders; 2004. pp. 623–661. [Google Scholar]
- 32.Raju TN, Higgins RD, Stark AR, Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006;118(3):1207–14. doi: 10.1542/peds.2006-0018. [DOI] [PubMed] [Google Scholar]
- 33.Lockwood CJ, Kuczynski E. Risk stratification and pathological mechanisms in preterm delivery. Paediatr Perinat Epidemiol. 2001;2:78–89. doi: 10.1046/j.1365-3016.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- 34.Mercer BM, Merlino AA, Milluzzi CJ, Moore JJ. Small fetal size before 20 weeks’ gestation: associations with maternal tobacco use, early preterm birth, and low birthweight. Am J Obstet Gynecol. 2008 doi: 10.1016/j.ajog.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 35.Edlow AG, Srinivas SK, Elovitz MA. Second-trimester loss and subsequent pregnancy outcomes: What is the real risk? Am J Obstet Gynecol. 2007;197(6):581, e1–6. doi: 10.1016/j.ajog.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344(15):1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 37.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166(5):1515–28. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 38.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 39.Kohl J. The role of complement in danger sensing and transmission. Immunol Res. 2006;34(2):157–76. doi: 10.1385/IR:34:2:157. [DOI] [PubMed] [Google Scholar]
- 40.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102(20):7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38(7):450–71. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004;10(11):1222–6. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- 43.Holers VM. The complement system as a therapeutic target in autoimmunity. Clin Immunol. 2003;107(3):140–51. doi: 10.1016/s1521-6616(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 44.Kirschfink M. Targeting complement in therapy. Immunol Rev. 2001;180:177–89. doi: 10.1034/j.1600-065x.2001.1800116.x. [DOI] [PubMed] [Google Scholar]


