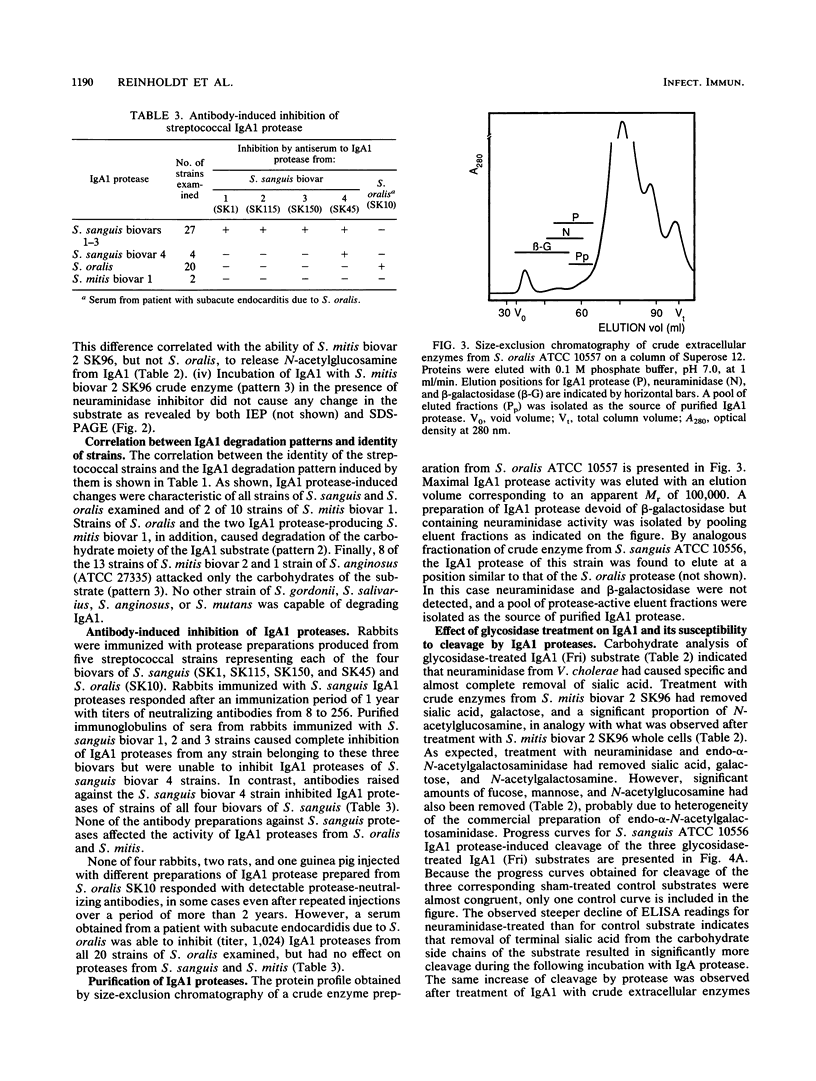
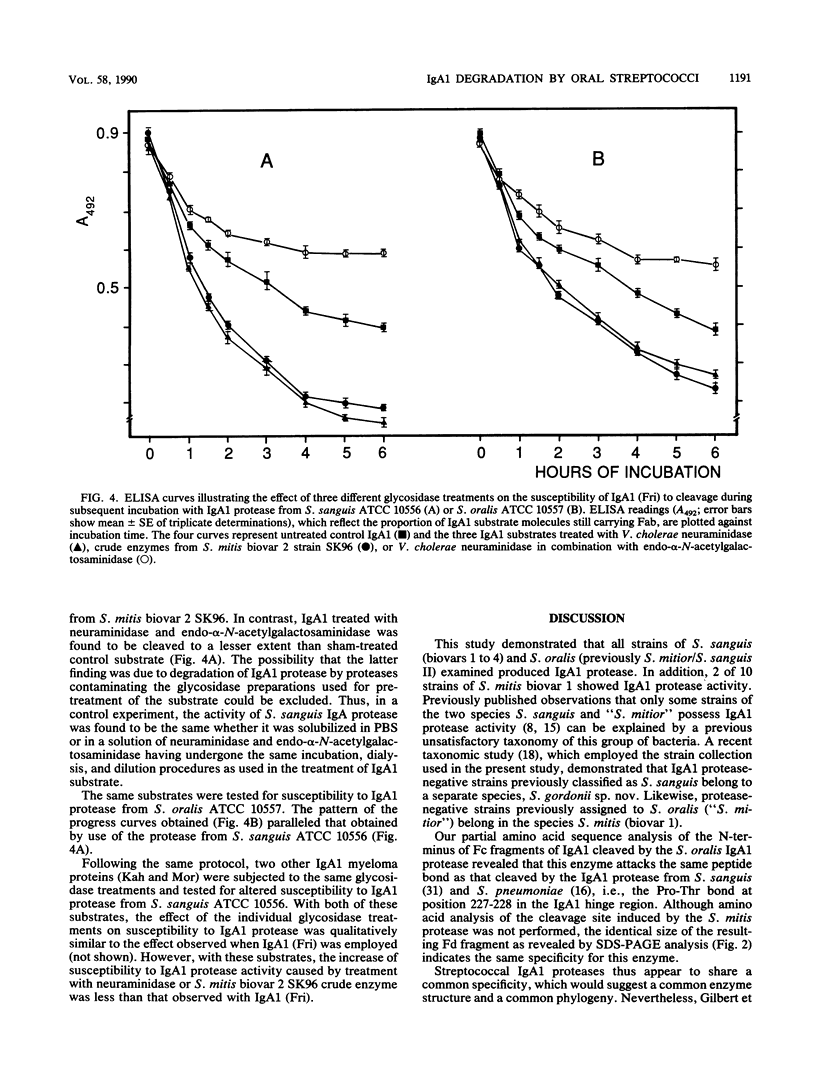
Abstract
Using a panel of 143 strains classified according to a novel taxonomic system for oral viridans-type streptococci, we reexamined the ability of oral streptococci to attack human immunoglobulin A1 (IgA1) molecules with IgA1 protease or glycosidases. IgA1 protease production was an exclusive property of all strains belonging to Streptococcus sanguis and Streptococcus oralis (previously S. mitior) and of some strains of Streptococcus mitis biovar 1. These are all dominant initiators of dental plaque formation. Degradation of the carbohydrate moiety of IgA1 molecules accompanied IgA1 protease activity in S. oralis and protease-producing strains of S. mitis biovar 1. Neuraminidase and beta-galactosidase were identified as extracellular enzymes in organisms of these taxa. By examination with enzyme-neutralizing antisera, four distinct IgA1 proteases were detected in S. sanguis biovars 1 to 3, S. sanguis biovar 4, S. oralis, and strains of S. mitis, respectively. The cleavage of IgA1 molecules by streptococcal IgA proteases was found to be influenced by their state of glycosylation. Treatment of IgA1 with bacterial (including streptococcal) neuraminidase increased susceptibility to protease, suggesting a cooperative activity of streptococcal IgA1 protease and neuraminidase. In contrast, a decrease in susceptibility was observed after extensive deglycosylation of the hinge region with endo-alpha-N acetylgalactosaminidase. The effector functions of IgA antibodies depend on the carbohydrate-containing Fc portion. Hence, the observation that oral streptococci may cleave not only the alpha 1 chains but also the carbohydrate moiety of IgA1 molecules suggests that the ability to evade secretory immune mechanisms may contribute to the successful establishment of these bacteria in the oral cavity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J., Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. I. Composition, glycopeptide isolation, and structure of the asparagine-linked oligosaccharide units. J Biol Chem. 1974 Nov 25;249(22):7260–7269. [PubMed] [Google Scholar]
- Baenziger J., Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974 Nov 25;249(22):7270–7281. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burton J., Wood S. G., Lynch M., Plaut A. G. Substrate analogue inhibitors of the IgA1 proteinases from Neisseria gonorrhoeae. J Med Chem. 1988 Aug;31(8):1647–1651. doi: 10.1021/jm00403a027. [DOI] [PubMed] [Google Scholar]
- Frandsen E. V., Theilade E., Ellegaard B., Kilian M. Proportions and identity of IgA1-degrading bacteria in periodontal pockets from patients with juvenile and rapidly progressive periodontitis. J Periodontal Res. 1986 Nov;21(6):613–623. doi: 10.1111/j.1600-0765.1986.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Gilbert J. V., Plaut A. G., Fishman Y., Wright A. Cloning of the gene encoding streptococcal immunoglobulin A protease and its expression in Escherichia coli. Infect Immun. 1988 Aug;56(8):1961–1966. doi: 10.1128/iai.56.8.1961-1966.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J. V., Plaut A. G., Longmaid B., Lamm M. E. Inhibition of microbial IgA proteases by human secretory IgA and serum. Mol Immunol. 1983 Sep;20(9):1039–1049. doi: 10.1016/0161-5890(83)90045-7. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Kilian M. Degradation of immunoglobulins A2, A2, and G by suspected principal periodontal pathogens. Infect Immun. 1981 Dec;34(3):757–765. doi: 10.1128/iai.34.3.757-765.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Holmgren K. Ecology and nature of immunoglobulin A1 protease-producing streptococci in the human oral cavity and pharynx. Infect Immun. 1981 Mar;31(3):868–873. doi: 10.1128/iai.31.3.868-873.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Kulhavy R., Tomana M., Butler W. T. IgA1 proteases from Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and Streptococcus sanguis: comparative immunochemical studies. J Immunol. 1980 Jun;124(6):2596–2600. [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Russell M. W. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol Rev. 1988 Jun;52(2):296–303. doi: 10.1128/mr.52.2.296-303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Reinholdt J., Nyvad B., Frandsen E. V., Mikkelsen L. IgA1 proteases of oral streptococci: ecological aspects. Immunol Invest. 1989 Jan-May;18(1-4):161–170. doi: 10.3109/08820138909112235. [DOI] [PubMed] [Google Scholar]
- Kilian M., Thomsen B., Petersen T. E., Bleeg H. Molecular biology of Haemophilus influenzae IgA1 proteases. Mol Immunol. 1983 Sep;20(9):1051–1058. doi: 10.1016/0161-5890(83)90046-9. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. J., Plaut A. G. Secretory immunity and the bacterial IgA proteases. Rev Infect Dis. 1981 May-Jun;3(3):521–534. doi: 10.1093/clinids/3.3.521. [DOI] [PubMed] [Google Scholar]
- Labib R. S., Calvanico N. J., Tomasi T. B., Jr Studies on extracellular proteases of Streptococcus sanguis. Purification and characterization of a human IgA1 specific protease. Biochim Biophys Acta. 1978 Oct 12;526(2):547–559. doi: 10.1016/0005-2744(78)90145-6. [DOI] [PubMed] [Google Scholar]
- Meindl P., Bodo G., Palese P., Schulman J., Tuppy H. Inhibition of neuraminidase activity by derivatives of 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. Virology. 1974 Apr;58(2):457–463. doi: 10.1016/0042-6822(74)90080-4. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Kilian M. Immunoglobulin A (IgA). Methods Enzymol. 1985;116:37–75. doi: 10.1016/s0076-6879(85)16005-2. [DOI] [PubMed] [Google Scholar]
- Mortensen S. B., Kilian M. Purification and characterization of an immunoglobulin A1 protease from Bacteroides melaninogenicus. Infect Immun. 1984 Sep;45(3):550–557. doi: 10.1128/iai.45.3.550-557.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose M., Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6632–6636. doi: 10.1073/pnas.80.21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyvad B., Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res. 1987 Oct;95(5):369–380. doi: 10.1111/j.1600-0722.1987.tb01627.x. [DOI] [PubMed] [Google Scholar]
- Plaut A. G. Microbial IgA proteases. N Engl J Med. 1978 Jun 29;298(26):1459–1463. doi: 10.1056/NEJM197806292982608. [DOI] [PubMed] [Google Scholar]
- Plaut A. G. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- Potier M., Mameli L., Bélisle M., Dallaire L., Melançon S. B. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-D-N-acetylneuraminate) substrate. Anal Biochem. 1979 Apr 15;94(2):287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- Putnam F. W., Liu Y. S., Low T. L. Primary structure of a human IgA1 immunoglobulin. IV. Streptococcal IgA1 protease, digestion, Fab and Fc fragments, and the complete amino acid sequence of the alpha 1 heavy chain. J Biol Chem. 1979 Apr 25;254(8):2865–2874. [PubMed] [Google Scholar]
- Reinholdt J., Kilian M. A sensitive enzyme-linked immunosorbent assay for IgA protease activity. J Immunol Methods. 1983 Oct 28;63(3):367–376. doi: 10.1016/s0022-1759(83)80010-6. [DOI] [PubMed] [Google Scholar]
- Reinholdt J., Kilian M. Interference of IgA protease with the effect of secretory IgA on adherence of oral streptococci to saliva-coated hydroxyapatite. J Dent Res. 1987 Feb;66(2):492–497. doi: 10.1177/00220345870660021801. [DOI] [PubMed] [Google Scholar]
- Reinholdt J., Krogh P., Holmstrup P. Degradation of IgA1, IgA2, and S-IgA by Candida and Torulopsis species. Acta Pathol Microbiol Immunol Scand C. 1987 Dec;95(6):265–274. doi: 10.1111/j.1699-0463.1987.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- Siemion I. Z., Pedyczak A., Burton J. Conformational preferences of the sequential fragments of the hinge region of the human IgA1 immunoglobulin molecule. Biophys Chem. 1988 Aug;31(1-2):35–44. doi: 10.1016/0301-4622(88)80006-1. [DOI] [PubMed] [Google Scholar]
- Theilade E., Theilade J., Mikkelsen L. Microbiological studies on early dento-gingival plaque on teeth and Mylar strips in humans. J Periodontal Res. 1982 Jan;17(1):12–25. doi: 10.1111/j.1600-0765.1982.tb01127.x. [DOI] [PubMed] [Google Scholar]
- Tomana M., Mestecky J., Niedermeier W. Studies on human secretory immunoglobulin A. IV. Carbohydrate composition. J Immunol. 1972 Jun;108(6):1631–1636. [PubMed] [Google Scholar]
- Tomana M., Niedermeier W., Mestecky J., Schrohenloher R. E., Porch S. Affinity chromatography of glycopeptides using concanavalin A. Anal Biochem. 1976 May 7;72:389–399. doi: 10.1016/0003-2697(76)90546-7. [DOI] [PubMed] [Google Scholar]
- Tomana M., Niedermeier W., Mestecky J., Skvaril F. The differences in carbohydrate composition between the subclasses of IgA immunoglobulins. Immunochemistry. 1976 Apr;13(4):325–328. doi: 10.1016/0019-2791(76)90342-6. [DOI] [PubMed] [Google Scholar]
- Tomana M., Niedermeier W., Spivey C. Microdetermination of monosaccharides in glycoproteins by gas-liquid chromatography. Anal Biochem. 1978 Aug 15;89(1):110–118. doi: 10.1016/0003-2697(78)90731-5. [DOI] [PubMed] [Google Scholar]
- Tomana M., Prchal J. T., Garner L. C., Skalka H. W., Barker S. A. Gas chromatographic analysis of lens monosaccharides. J Lab Clin Med. 1984 Jan;103(1):137–142. [PubMed] [Google Scholar]
- Toraño A., Tsuzukida Y., Liu Y. S., Putnam F. W. Location and structural significance of the oligosaccharides in human Ig-A1 and IgA2 immunoglobulins. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2301–2305. doi: 10.1073/pnas.74.6.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A., Diaz S. A neuraminidase from Streptococcus sanguis that can release O-acetylated sialic acids. J Biol Chem. 1983 Oct 25;258(20):12465–12471. [PubMed] [Google Scholar]





