Abstract
Adolescence is associated with characteristic behavioral patterns as well as with substantial neuronal pruning and re-organization of the brain. Recent research has determined that the effects of various centrally active drugs differ in adolescents and adults. This study examined the motor effects of two prototypic antipsychotics in adult [> postnatal day 70 (PN70)] and adolescent (PN30-PN39) rats. Rats were injected daily with saline, 0.3 mg/kg haloperidol, or 10 mg/kg clozapine for 10 days and activity and catalepsy were measured. Adolescents of both sexes were less sensitive to the cataleptic effects of haloperidol than were adults. Male adolescents were also less sensitive to the cataleptic effects of clozapine, although this difference was transitory. In contrast, female adults showed decreased sensitivity to clozapine's effects, differing from all other groups. These results suggest that adolescents of both sexes may be less sensitive to the extrapyramidal motor effects of haloperidol. Translational implications of the clozapine results are less clear; however, results suggest that developmental differences in neurochemical systems affected by clozapine that are also related to motor behavior may play a role. These results also emphasize the importance of age and sex as determinants of the pharmacological effects of these antipsychotics.
Keywords: Adolescent, Antipsychotics, Catalepsy, Clozapine, Haloperidol, Locomotion, Sex Differences
1.0 Introduction
Antipsychotics are prescribed for children and adolescents during the course of treatment for a wide variety of psychiatric disorders and other medical conditions, including psychosis, bipolar disorder, Tourette's syndrome, sedation during surgery, and antiemesis (1, 2); however, research to determine whether the effects of these drugs are different during development is sparse. Because this type of research cannot be done in human adolescents, rats and other animal models are typically used. In rats, adolescence occurs from approximately postnatal days 28 to 42 (PN28-PN42) (3). During this time period, rats display a characteristic pattern of behaviors (e.g., increased risk taking, novelty seeking, and increased orientation towards peers) that has been observed in adolescent mammals of many species, including humans. In addition, preclinical research results to date have suggested that substantial pruning and re-organization of the dopamine system occurs over the course of development to adulthood, and particularly, during adolescence [for a review, see (3)]. Both typical and atypical antipsychotics (i.e., antipsychotics that have high vs. low liability for producing extrapyramidal motor side effects, respectively) block dopamine D2 receptors in the brain and their affinities for doing so are positively correlated with their clinical potencies (4, 5). Hence, it would not be surprising if ongoing dopamine receptor changes resulted in age differences in responses to antipsychotic administration. Further, these observed developmental differences in the dopamine system may be most likely to be expressed in behaviors for which strong dopaminergic involvement has been demonstrated (e.g., motor behavior), albeit other neurotransmitter systems also modulate motor behavior. To this end, the purpose of this study was to determine whether or not the effects of haloperidol and clozapine (prototypic typical and atypical antipsychotics, respectively) on locomotor activity and catalepsy were age-related. Although clozapine does not produce extrapyramidal motor effects in humans, we have reported previously that it produces motor suppression and catalepsy in adult rodents (6). Nevertheless, the purpose here was not to model extrapyramidal effects per se, but rather to use motor activity and catalepsy as behavioral measures to examine age and sex differences that may be related to underlying developmental and/or hormonal differences in the functioning of neurotransmitter systems that are in the process of re-organization during adolescence, albeit information gained from these experiments may also have relevance for motor disorders such as idiopathic or antipsychotic-induced parkinsonism.
2.0 Materials and methods
2.1 Animals
Male and female Long-Evans rats were ordered from a commercial breeder (Harlan, Dublin, VA) at ages of PN22−25 or > PN65. These rats would subsequently serve as adolescent or adult subjects, as described in the procedures section below. Upon arrival, rats were housed in clear plastic cages in same-sex, same-age pairs and allowed at least 5 days to habituate to the vivarium. Because of this adaptation period, adult rats did not begin testing until at least PN70. Except for daily test sessions, all rats received free access to food and water in their home cages. The studies reported in this manuscript were carried out in accordance with guidelines published in the Guide for the Care and Use of Laboratory Animals (7) and were approved by our Institutional Animal Care and Use Committee.
2.2 Apparatus
Clear plastic rat cages (22.5 cm width X 44 cm length X 20 cm height) were housed in sound-attenuating cabinets and were used as locomotor chambers. Each cabinet contained up to 12 chambers, with a maximum of 2 per shelf. Chambers did not contain bedding and were wiped with alcohol solution between sessions. Sessions occurred in darkness (i.e., with the cabinet doors closed). A cage rack system with 4 X 8 equally spaced photocell beams on the X- and Y-axes (Lafayette Instrument, Lafayette, IN) was placed around each chamber (4.5 cm from bottom of cage) and locomotor activity was measured as total number of beam breaks for the entire session. The bar apparatus that was used to measure catalepsy-like behavior consisted of a 280 mm bolt (10 mm diameter) that was attached to a frame by eyebolts. Height of the bar was adjusted based upon the age of the rat (98 mm for adolescents and 130 mm for adults). Each bar apparatus was housed in its own box that was open in the front for experimenter access.
2.3 Procedure
Adolescent (PN30-PN39) and adult (>PN70) rats of both sexes were randomly assigned to receive daily injections of saline, 0.3 mg/kg haloperidol, or 10 mg/kg clozapine for 10 consecutive days. Antipsychotic doses were chosen based upon results of dose-effect curves with each drug that were done as part of another (unpublished) study. In this unpublished study, we found that the chosen doses of haloperidol and clozapine produced catalepsy across a 10-day dosing period whereas lower doses of each drug did not reliably alter the amount of time spent on the bar as compared to saline levels. After at least 30 min habituation time in the lab, each rat was weighed and was injected with saline or with their assigned drug. Subsequently, at 30, 45 and 60 min after the initial injection, the rat was tested in the bar test. The times were chosen within a range that is typically used for adult male rodents, as information concerning onset of action and peak effect for female and adolescent rodents was limited. At each time point, the front paws of the rat were placed on the bar apparatus for 5-min. The total amount of time (in s) that both of the rat's front paws remained in contact with the bar during the 5-min session was recorded. If both of the rat's paws dropped from the bar, they were re-positioned as before. The session timer was stopped during the brief time needed for re-positioning. If the rat voluntarily removed its paws from the bar 10 times during the session, the session was stopped and amount of time on bar was recorded as 0. Invariably, this situation occurred during the first minute of the session and was almost always associated with saline treatment. After the final 5-min bar test (i.e., 65 min after injection), rats were placed into the locomotor chamber for a 15-min session. Locomotor activity was measured as total number of beam breaks for the entire session. After the session, rats were returned to their home cages and transported back to the animal facility. This procedure was repeated daily for 10 days. In order to complete all testing during the short duration of adolescence in rats (approximately 2 weeks), habituation to the locomotor chambers prior to drug administration was not included in the study design. Timing and sequence of tests and injections and handling procedures for the adolescent and adult rats were identical.
2.4 Drugs
Haloperidol (McNeil Pharmaceutical, Spring House, PA) was prepared by adding saline to a commercially available 5 mg/ml stock solution containing 1.8 mg methyl p-aminobenzoic acid (paraben), 0.2 mg propylparaben, and lactic acid. Clozapine (NIMH Chemical Synthesis Program, Bethesda, MD) was mixed in purified distilled water. All injections were administered intraperitoneally (i.p.) at a volume of 1 ml/kg.
2.5 Statistical Analysis
Locomotor activity was assessed as the total number of photocell beam breaks over the 15-min session. Catalepsy was defined as the amount of time both of the rat's forepaws were in contact with the elevated bar, as measured during 3 separate 5-min sessions. Catalepsy score was expressed as percentage of the total 5-min session duration. Mean (± SEM) values for each dependent variable were calculated separately for each sex, treatment condition, day, and age. Locomotor counts and catalepsy scores for each post-injection time point (30, 45, and 60 min) were analyzed separately for each antipsychotic with four-way age X sex X treatment condition (saline vs. drug) X day (repeated) split-plot ANOVAs. Significant two-factor interactions were further analyzed by Tukey post hoc tests (α=0.05) were used to compare individual means.
3.0 Results
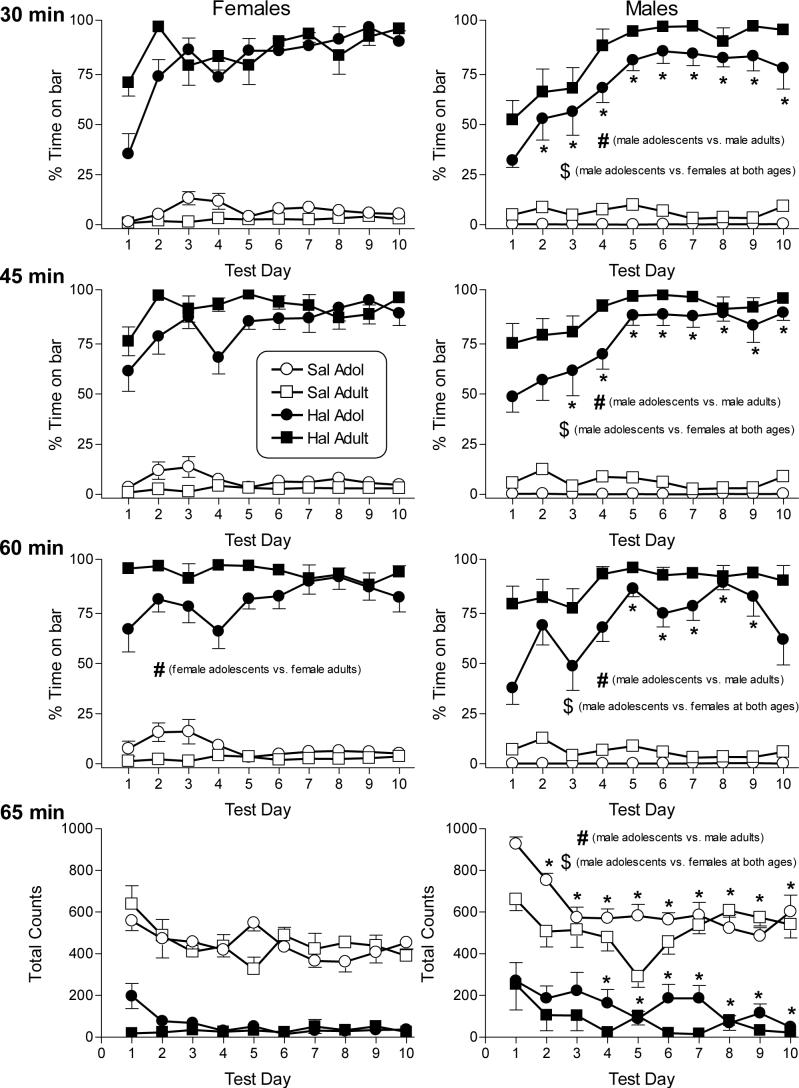
Figure 1 presents the effects of repeated dosing with saline and 0.3 mg/kg haloperidol on catalepsy (top three rows) and locomotor activity (bottom row) in adolescent (PN30-PN39) and adult female and male rats (left and right panels, respectively). The cataleptic effects of haloperidol were assessed at three time points (i.e., 30-, 45- and 60-min after injection). Data for each assessment time were analyzed separately. All comparisons described below for haloperidol-induced catalepsy refer to results of the separate overall 4-factor age X dose X sex X day ANOVAs performed at each assessment time, with Tukey post hoc tests (as necessary) used to specify the nature of significant interactions between combinations of two of the four variables. Based on these analyses, haloperidol produced significant catalepsy (compared to saline) in both sexes and at both ages on every day of the 10-day dosing period [dose X day interaction: F(9,288)=9.03, p<0.05 at 30-min; F(9,288)=5.38, p<0.05 at 45-min; F(9,288)=3.47, p<0.05 at 60 min]. Further, at each of the three assessment times, male adolescents were less cataleptic than male adults as well as less cataleptic than females of either age [age X sex interaction: F(1,32)=16.4, p<0.05 at 30-min; F(1,32)=14.6, p<0.05 at 45-min; F(1,32)=47.7, p<0.05 at 60-min]. In contrast, the magnitude of haloperidol-induced catalepsy in female adolescents and adults did not significantly differ at the two shorter post-injection assessments. Only at 60 min post-injection were female adolescents significantly less cataleptic than female adults. Hence, age-related differences in haloperidol-induced catalepsy were most prominent in males with male adolescents showing less catalepsy than male adults. The degree of haloperidol-induced catalepsy was also day-dependent, as indicated by the post hoc analysis of the dose X day interactions [F values for each assessment time presented above]. Whereas adult rats of both sexes showed almost maximal haloperidol-induced catalepsy near the end of the 10-day dosing regimen (i.e., ceiling effect), haloperidol-induced catalepsy was sub-maximal on the first few days of the dosing regimen in adolescent rats, particularly at the two earlier assessment times. This age-dependent sensitization to haloperidol's cataleptic effects was most evident in males.
Figure 1.
Effects of saline and haloperidol on catalepsy and locomotor activity in female and male adolescent and adult rats on 10 consecutive days. Catalepsy was assessed as percentage of time that the rat's forepaws remained in contact with a horizontal bar over each 5-min session. Three daily sessions were conducted 30 (1st row panels), 45 (2nd row panels), and 60 (3rd row panels) min after injection with saline (open symbols) or 0.3 mg/kg haloperidol (filled symbols). Haloperidol effects on locomotor activity, assessed as total number of photocell beam breaks during a 5-min session starting at 65 min following injection, are also shown (bottom panels). Each value represents the mean (± SEM) for 8−15 female adolescent and 9−10 female adult rats (left panels) and 11 male adolescent and 10 male adult rats (right panels). All indications of statistical significance are based upon results of Tukey post hoc analysis of significant interactions obtained in the overall 4-way (age X sex X dose X day) ANOVAs conducted on each of the four dependent measures. Haloperidol (0.3 mg/kg) produced significant catalepsy and decreased motor activity (as compared to saline) in rats of both sexes and ages on every day of the 10-day dosing regimen. # indicates significant difference between adolescent and adult rats of the same sex (age X sex interaction). $ indicates significant difference between rats of opposite sexes (age X sex interaction). * indicates significant difference from day 1 (dose X day interaction). All significance levels are reported at p<0.05.
In addition to its cataleptic effects, haloperidol suppressed locomotor activity during the 10-day dosing regimen. All comparisons described below refer to results of the overall 4-factor age X dose X sex X day ANOVA, with Tukey post hoc tests (as necessary) used to specify the nature of significant interactions between combinations of two of the four variables. Compared to saline, 0.3 mg/kg haloperidol significantly suppressed locomotion across every day of the 10-day dosing period [dose X day interaction: F(9,288)=2.3, p<0.05]. Age and sex differences were also observed. Adolescent males were significantly more active than adult males and than females of either age [age X sex interaction: F(1,32)=34.3, p<0.05], albeit differences were most pronounced following saline administration. Further, adult males were more active than female adults. In contrast, activity of female adults and adolescents did not significantly differ. With repeated exposure to the locomotor chambers, activity levels in saline- and haloperidol-treated rats decreased, suggesting habituation and/or sensitization to haloperidol's suppressive effects (with floor effect). Saline-treated rats were significantly more active on day 1 than on all of the other days whereas haloperidol-treated rats were significantly more active on day 1 than on days 4−10 [dose X day interaction: F(9,288)=2.3, p<0.05]. Again, these effects were more prominent in males [sex X day interaction: F(9,288)=2.13, p<0.05].
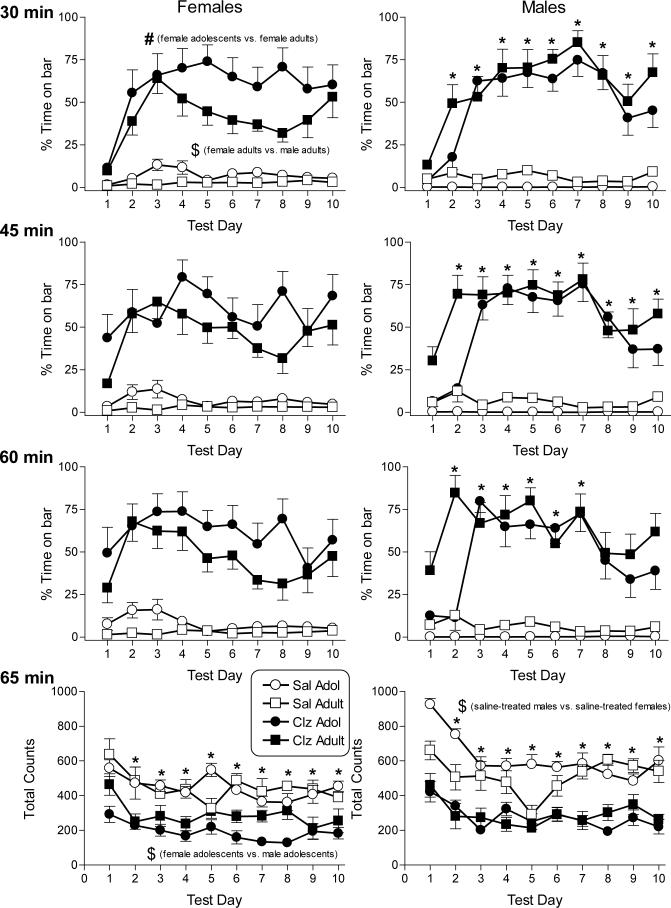
Figure 2 shows the effects of repeated dosing with saline and 10 mg/kg clozapine on catalepsy (top three rows) and locomotor activity (bottom row) in adolescent (PN30-PN39) and adult female and male rats (left and right panels, respectively). As with haloperidol, the cataleptic effects of clozapine were assessed at three time points (i.e., 30-, 45- and 60-min after injection). Data for each assessment time were analyzed separately. All comparisons described below for clozapine-induced catalepsy refer to results of the separate 4-factor age X dose X sex X day ANOVAs performed at each assessment time. Clozapine, like haloperidol, significantly increased the amount of time that forepaws remained on the bar (compared to saline) at all three time points in both sexes and at both ages and across all days with the exception of day 1 at the 30-min assessment time [dose X day interactions: F(9,243)=10.5, p<0.05 at 30 min; F(9,243)=6.26, p<0.05 at 45 min; F(9,243)=4.12, p<0.05 at 60 min]. At the 30-min assessment time, female adults exhibited significantly less clozapine-induced catalepsy than did female adolescents as well as less than male adults [age X sex interaction: F(1,27)=13.5, p<0.05]. In contrast, the magnitude of catalepsy observed in male adolescents and adults at this assessment time did not significantly differ nor did catalepsy in male adolescents differ from that seen in female adolescents. At later assessment times (i.e., 45 and 60 min), significant age differences in clozapine-induced catalepsy were not evident in either males or females. At all three assessment times, the degree of catalepsy induced by clozapine changed with repeated dosing. Significantly less catalepsy was observed on day 1 than on all other days at the 30- and 45-min assessment times whereas clozapine-induced catalepsy on day 1 was decreased compared to days 2−7 at the 60-min assessment time [F values above for dose X day interactions].
Figure 2.
Effects of saline and clozapine on catalepsy and locomotor activity in female and male adolescent and adult rats on 10 consecutive days. Catalepsy was assessed as percentage of time that the rat's forepaws remained in contact with a horizontal bar over each 5-min session. Three daily sessions were conducted 30 (1st row panels), 45 (2nd row panels), and 60 (3rd row panels) min after injection with saline (open symbols) or 10 mg/kg clozapine (filled symbols). Clozapine effects on locomotor activity, assessed as total number of photocell beam breaks during a 5-min session starting at 65 min following injection, are also shown (bottom panels). Each value represents the mean (± SEM) for 8−9 female adolescent and 9−10 female adult rats (left panels) and 11−13 male adolescent and 10 male adult rats (right panels). All indications of statistical significance are based upon results of Tukey post hoc analysis of significant interactions obtained in the overall 4-way (age X sex X dose X day) ANOVAs conducted on each of the four dependent measures. Clozapine (10 mg/kg) produced significant catalepsy and decreased motor activity (as compared to saline) in rats of both sexes and ages on every day of the 10-day dosing regimen with the one exception. Clozapine-induced catalepsy was not observed at the 30-min assessment time on day 1 (top panels). # indicates significant difference between adolescent and adult rats of the same sex (age X sex interaction). $ indicates significant difference between rats of opposite sexes (age X sex interaction). * indicates significant difference from day 1 (dose X day interaction). All significance levels are reported at p<0.05.
Clozapine's effects on locomotor activity were not as pronounced as its effects on catalepsy. Clozapine (compared to saline) decreased locomotion at both ages [age X dose interaction: F(1,27)=15.14, p<0.05] and in both sexes [dose X sex interaction: F(1,27)=7.15, p<0.05]. Sex differences in clozapine-induced suppression of locomotion were not evident, although saline-treated males were significantly more active than saline-treated females [sex X dose interaction: F(1,27)=7.15, p<0.05]. In contrast, significant age differences in clozapine's effect on locomotion were present, with lower activity observed in clozapine-treated adolescents than in adults [age X dose interaction: F(1,27)=15.14, p<0.05]. Further, although female adolescents were less active than their male age-mates, this sex difference in activity level was not observed in adults [age X sex interaction: F(1,27)=11.4, p<0.05]. As seen with haloperidol, activity was significantly decreased (compared to day 1) on days 2−10 in both adolescents and adults [age X day interaction: F(9,243)=6.72, p<0.05]. Significant age differences were transitory, occurring only at days 5 and 8.
4.0 Discussion
Results with haloperidol are consistent with previous reports that antipsychotics suppress motor activity and induce catalepsy in adult male rodents (6, 8, 9). Further, these results extend previous findings by showing that haloperidol also produces these effects in female rats and in adolescent rats of both sexes. In the present study, clozapine also decreased motor activity and produced catalepsy in all groups of rats. Despite similarities in antipsychotic effects across all groups, however, age and sex differences were apparent over the course of the 10-day dosing regimen, particularly for the catalepsy measure.
While most prior developmental research on antipsychotics has focused primarily on the consequences of pre- or perinatal administration of these drugs (10-12), a seminal study by Campbell and Baldessarini (13) examined the effects of haloperidol on catalepsy, ptosis and activity over the course of 1.5 years in male rats, with an earliest assessment age of 18 days. This study and a follow-up study with haloperidol and a phenothiazine antipsychotic perphenazine (14) reported decreasing sensitivity to the pharmacological effects of these antipsychotics during development from juvenile to adulthood, but a return to increased sensitivity with further age advancement. Other studies that have focused only on the periadolescent period have found that sensitivity to haloperidol-induced catalepsy tends to increase over the course of development from juvenile to adult (15, 16), as found in the present study. Interestingly, hyposensitivity to the locomotor stimulant and other behavioral effects of several monoamine agonists during periadolescence has also been reported (17-19). Although the reasons for discrepancies among findings of the different studies of haloperidol's effects are not completely clear, some points are notable. First, the ages at which the rats were assessed in each study differed. Specifically, in the long-term study, 32-day-old rats showed decreased sensitivity to haloperidol compared to 18-day-old rats and increased sensitivity compared to 56-day-old rats (13). By 110 days, however, sensitivity to haloperidol-induced effects was again decreased and was comparable to that seen in the 32-day-old rats. While the age of initial assessment of rats in the present study (i.e., PN30) was close to the age of the PN32 rats in the earlier study, adult rats in the present study were approximately PN70 at the beginning of testing (vs. PN56 or PN110). The follow-up study with haloperidol and perphenazine replicated the finding of greater sensitivity to haloperidol-induced catalepsy, ptosis and activity suppression in adolescent (PN30) rats compared to PN56 rats; however, PN100 rats were less sensitive than PN56 rats, which is in contrast with data reported for PN110 rats in the earlier study. Hence, discrepancies in the results of different studies may be at least partly attributable to differences in age inclusion. In addition, the way in which catalepsy was assessed (wire grid vs. bar test in the previous and present study, respectively) as well as the rat strain (Sprague-Dawley vs. Long-Evans) may also have contributed to divergent results, as each these factors has been associated with variation in the degree of drug-induced cataleptic response (20, 21).
Sex differences in the locomotor and cataleptic effects of haloperidol were also evident. Compared to males, female rats were more sensitive to the effects of haloperidol on both dependent measures, especially during the initial days of the dosing regimen. In addition, age differences in haloperidol's cataleptic effects in females were observed only at the 60-min assessment time. While this age-related difference in time course might be suggestive of quicker metabolism or elimination in female adolescents than in female adults, similar age differences across days in haloperidol's effects on motor activity were not observed despite its later time of measurement, suggesting that different rates of metabolism are not likely to account for these results. In addition, the observed age difference appears primarily related to peak haloperidol-induced catalepsy occurrence sooner in the dosing regimen for female adults vs. female adolescents. Nevertheless, age and sex differences in the pharmacokinetics of haloperidol in rodents are not well-investigated at the age range of rats included in the present study. A few studies, however, have reported pharmacodynamic differences in haloperidol's effects associated with advanced age (e.g., 2−3 times the maximum age of any of the rats in this study) [e.g., (22)]. For example, previous research with older adult rats has shown that age-related functional changes in dopamine receptors can attenuate the magnitude of haloperidol-induced catalepsy even in the presence of higher brain concentrations (23). Given that adolescence also represents a period of pruning and re-organization of the dopamine system, it is possible that a similar process may play a role in the differences between adolescent and adult rats observed here. Indeed, an in vivo imaging study of the brains never medicated human adolescents diagnosed with a psychotic disorder reported enhanced glucose utilization rates for haloperidol in thalamic brain areas in younger (13−15 years) vs. older (16−21 years) adolescents (24). Unfortunately, comparison to haloperidol metabolism in adult brains was not included, perhaps due to the difficulty of obtaining never medicated adult psychotic patients.
In contrast to the haloperidol results, analysis of age differences in the cataleptic effects of clozapine in females showed that, when age differences occurred, the direction of effect was opposite that observed for males as well as opposite of the age-related effects of haloperidol in females. Whereas male adolescents tended to be less sensitive than their adult counterparts to the cataleptic effects of high doses of both antipsychotics, female adolescents were significantly more sensitive to both of the motor effects of clozapine than were female adults. Further inspection of the data revealed that males of both ages and female adolescents showed similar magnitudes of clozapine-induced catalepsy. The fact that all of these groups were more sensitive to clozapine's cataleptic effects than were adult females suggests that the observed age differences in females were related to decreased cataleptic efficacy of clozapine in female adults rather than to increased propensity of clozapine to produce catalepsy in female adolescents. While this study was not designed to provide definitive mechanistic explanations of these behavioral observations, it is worthwhile to note that the profiles of neurochemical effects of clozapine and haloperidol differ. While both antipsychotics are dopamine antagonists, their affinities and kinetics differ at dopamine receptor subtypes (5, 25). For example, haloperidol has predominant affinity for dopamine D2 receptors whereas clozapine is more eclectic in its interactions with dopamine receptor subtypes (5, 25). In addition, chronic administration of haloperidol and clozapine may produce regionally specific effects on dopamine receptor subtypes (26, 27). Clozapine also blocks various non-dopamine receptors, including pronounced antagonism of 5-HT2A, histaminergic, alpha adrenergic and muscarinic receptors whereas haloperidol is more selective for dopamine antagonism (5, 28). Hence, adult females may be differentially sensitive to one or more of these neurochemical properties of clozapine that are not shared by haloperidol.
An obvious mechanistic explanation that could account for decreased sensitivity of female adults to clozapine's motor effects is hormonal differences. Results of experiments in which estradiol was administered to ovariectomized rats or in which phases of the estrous cycle were tracked have demonstrated a strong relationship between estrogen levels and alterations in dopaminergic and serotonergic neurotransmission in female rodents (29-32), suggesting that estrogen may modulate sex differences in the behavioral effects of drugs that affect these systems (e.g., clozapine). In addition, sex-dependent differences in brain development as well as in receptor densities that are independent of differences in sex steroids have been reported for several monoamine neurotransmitter systems (33-35).
In summary, both age and sex affect magnitude of the locomotor and cataleptic effects of high doses of haloperidol and clozapine. Male adolescents were consistently less sensitive to the cataleptic effects of both haloperidol and clozapine than were male adults, although these age differences were less pronounced and shorter in duration for clozapine than for haloperidol. In contrast, female adolescents (vs. female adults) showed decreased sensitivity to the cataleptic effects of haloperidol, but increased sensitivity to those of clozapine. This latter effect may be better conceptualized as decreased efficacy of clozapine in female adults, as the magnitude of clozapine's motor effects in this group was reduced compared not only to female adolescents, but also to males of both ages. These results have several potential implications for the use of antipsychotics in adolescents as well as for age and sex differences in functioning of the extrapyramidal motor system. For example, the finding that both male and female adolescents were less sensitive to the motor effects of a high dose of haloperidol than were same-sexed adults suggests that adolescents may initially be less susceptible to the motor side effects produced by this antipsychotic (although sensitization may develop at both ages with repeated administration). The motor effects of haloperidol are almost undoubtedly mediated by its action as a dopamine D2 antagonist. In contrast, clozapine has a much broader profile of neurochemical effects (5), resulting in an absence of extrapyramidal motor effects despite therapeutic efficacy. Hence, the mechanism through which clozapine produces its motor effects in this animal model is far less clear, but may involve nondopaminergic neurotransmission. The present results suggest that female adults may respond differently to clozapine than do male adults or adolescents of either sex and that these differences may be related to hormonal and developmental differences in neurochemical systems involved in motor behavior. Together, these results suggest that age and sex affect development and functioning of neurotransmitter systems involved in extrapyramidal motor control. Further, they suggest that age and sex are important variables to consider when prescribing antipsychotics to children and adolescents.
Acknowledgements
Research supported by National Institute of Mental Health grant MH-64771. Clozapine was generously provided by the NIMH Chemical Synthesis and Drug Supply Program. During the time this study was conducted, R.L.E. was on internship at VCU from the University of the West of England, Bristol, U.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenhill LL, Setterberg S. Pharmacotherapy of disorders of adolescents. Psychiatr. Clin. N. Amer. 1993;16:793–814. [PubMed] [Google Scholar]
- 2.Sylvester C. Psychopharmacology of disorders in children. Psychiatr. Clin. N. Amer. 1993;16:779–791. [PubMed] [Google Scholar]
- 3.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 4.McQuade RD, Duffy RA, Coffin VL, Barnett A. In vivo binding to dopamine receptors: a correlate of potential antipsychotic activity. Eur J Pharmacol. 1992;215(1):29–34. doi: 10.1016/0014-2999(92)90604-3. [DOI] [PubMed] [Google Scholar]
- 5.Schotte A, Janssen PF, Gommeren W, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996;124(1−2):57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- 6.Wiley JL, Martin BR. Cannabinoid pharmacological properties common to other centrally acting drugs. Eur. J. Pharmacol. 2003;471(3):185–193. doi: 10.1016/s0014-2999(03)01856-9. [DOI] [PubMed] [Google Scholar]
- 7.National Research Council . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- 8.Simon VM, Parra A, Minarro J, Arenas MC, Vinader-Caerols C, Aguilar MA. Predicting how equipotent doses of chlorpromazine, haloperidol, sulpiride, raclopride and clozapine reduce locomotor activity in mice. Eur Neuropsychopharmacol. 2000;10(3):159–64. doi: 10.1016/s0924-977x(00)00070-5. [DOI] [PubMed] [Google Scholar]
- 9.Bardin L, Kleven MS, Barret-Grevoz C, Depoortere R, Newman-Tancredi A. Antipsychotic-like vs cataleptogenic actions in mice of novel antipsychotics having D2 antagonist and 5-HT1A agonist properties. Neuropsychopharmacology. 2006;31(9):1869–79. doi: 10.1038/sj.npp.1300940. [DOI] [PubMed] [Google Scholar]
- 10.Cuomo V, Cagiano R, Coen E, Mocchetti I, Cattabeni F, Racagni G. Enduring behavioural and biochemical effects in the adult rat after prolonged postnatal administration of haloperidol. Psychopharmacology (Berl) 1981;74(2):166–9. doi: 10.1007/BF00432686. [DOI] [PubMed] [Google Scholar]
- 11.Shalaby IA, Spear LP. Chronic administration of haloperidol during development: later psychopharmacological responses to apomorphine and arecoline. Pharmacol Biochem Behav. 1980;13(5):685–90. doi: 10.1016/0091-3057(80)90012-x. [DOI] [PubMed] [Google Scholar]
- 12.Cuomo V, Cagiano R, Mocchetti I, Coen E, Cattabeni F, Racagni G. Behavioural and biochemical effects in the adult rat after prolonged postnatal administration of clozapine. Psychopharmacology (Berl) 1983;81(3):239–43. doi: 10.1007/BF00427270. [DOI] [PubMed] [Google Scholar]
- 13.Campbell A, Baldessarini RJ. Effects of maturation and aging on behavioral responses to haloperidol in the rat. Psychopharmacology (Berl) 1981;73(3):219–22. doi: 10.1007/BF00422406. [DOI] [PubMed] [Google Scholar]
- 14.Campbell A, Baldessarini RJ, Teicher MH. Decreasing sensitivity to neuroleptic agents in developing rats; evidence for a pharmacodynamic factor. Psychopharmacology (Berl) 1988;94(1):46–51. doi: 10.1007/BF00735879. [DOI] [PubMed] [Google Scholar]
- 15.Weihmuller FB, Bruno JP. Age-dependent plasticity in the dopaminergic control of sensorimotor development. Behav Brain Res. 1989;35(2):95–109. doi: 10.1016/s0166-4328(89)80110-x. [DOI] [PubMed] [Google Scholar]
- 16.Spear LP, Shalaby IA, Brick J. Chronic administration of haloperidol during development: behavioral and psychopharmacological effects. Psychopharmacology (Berl) 1980;70(1):47–58. doi: 10.1007/BF00432369. [DOI] [PubMed] [Google Scholar]
- 17.Brunell SC, Spear LP. Effects of acute ethanol or amphetamine administration on the acoustic startle response and prepulse inhibition in adolescent and adult rats. Psychopharmacology (Berl) 2006;186(4):579–86. doi: 10.1007/s00213-006-0380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Brain Res Dev Brain Res. 2002;138(1):27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- 19.Aberg M, Wade D, Wall E, Izenwasser S. Effect of MDMA (ecstasy) on activity and cocaine conditioned place preference in adult and adolescent rats. Neurotoxicol Teratol. 2007;29(1):37–46. doi: 10.1016/j.ntt.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell A, Baldessarini RJ, Cremens MC. Dose-catalepsy response to haloperidol in rat: effects of strain and sex. Neuropharmacology. 1988;27(11):1197–9. doi: 10.1016/0028-3908(88)90018-4. [DOI] [PubMed] [Google Scholar]
- 21.Sanberg PR, Pevsner J, Coyle JT. Parametric influences on catalepsy. Psychopharm. 1984;82:406–408. doi: 10.1007/BF00427696. [DOI] [PubMed] [Google Scholar]
- 22.Steinpreis RE, Salamone JD. Effects of acute haloperidol and reserpine administration on vacuous jaw movements in three different age groups of rats. Pharmacol Biochem Behav. 1993;46(2):405–9. doi: 10.1016/0091-3057(93)90371-y. [DOI] [PubMed] [Google Scholar]
- 23.Pizzolato G, Soncrant TT, Holloway HW, Rapoport SI. Reduced metabolic response of the aged rat brain to haloperidol. J Neurosci. 1985;5(11):2831–8. doi: 10.1523/JNEUROSCI.05-11-02831.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchsbaum MS, Haznedar MM, Aronowitz J, et al. FDG-PET in never-previously medicated psychotic adolescents treated with olanzapine or haloperidol. Schizophr Res. 2007;94(1−3):293–305. doi: 10.1016/j.schres.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 25.Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47(1):27–38. [PubMed] [Google Scholar]
- 26.Huang N, Ase AR, Hebert C, van Gelder NM, Reader TA. Effects of chronic neuroleptic treatments on dopamine D1 and D2 receptors: homogenate binding and autoradiographic studies. Neurochem Int. 1997;30(3):277–90. doi: 10.1016/s0197-0186(96)00093-9. [DOI] [PubMed] [Google Scholar]
- 27.Damask SP, Bovenkerk KA, de la Pena G, et al. Differential effects of clozapine and haloperidol on dopamine receptor mRNA expression in rat striatum and cortex. Brain Res Mol Brain Res. 1996;41(1−2):241–9. doi: 10.1016/0169-328x(96)00101-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Bymaster FP. The in vivo effects of olanzapine and other antipsychotic agents on receptor occupancy and antagonism of dopamine D1, D2, D3, 5HT2A and muscarinic receptors. Psychopharmacology (Berl) 1999;141(3):267–278. doi: 10.1007/s002130050834. [DOI] [PubMed] [Google Scholar]
- 29.Miller JC. Sex differences in dopaminergic and cholinergic activity and function in the nigro-striatal system of the rat. Psychoneuroendocrinology. 1983;8(2):225–36. doi: 10.1016/0306-4530(83)90059-8. [DOI] [PubMed] [Google Scholar]
- 30.Hafner H, Behrens S, De Vry J, Gattaz WF. An animal model for the effects of estradiol on dopamine-mediated behavior: implications for sex differences in schizophrenia. Psychiatry Res. 1991;38(2):125–34. doi: 10.1016/0165-1781(91)90038-q. [DOI] [PubMed] [Google Scholar]
- 31.Zhou W, Cunningham KA, Thomas ML. Estrogen regulation of gene expression in the brain: a possible mechanism altering the response to psychostimulants in female rats. Brain Res Mol Brain Res. 2002;100(1−2):75–83. doi: 10.1016/s0169-328x(02)00134-1. [DOI] [PubMed] [Google Scholar]
- 32.Fink G, Sumner BE, Rosie R, Grace O, Quinn JP. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325–44. doi: 10.1007/BF02088099. [DOI] [PubMed] [Google Scholar]
- 33.Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27(6):683–91. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- 34.Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8(6):1495–8. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- 35.Valencia-Sanchez A, Esparza-Avalos NS, Cruz ML, Ortega-Corona BG. Amine neurotransmitter levels in male and female rats through developmental periods. Arch Androl. 1997;39(1):79–83. doi: 10.3109/01485019708987905. [DOI] [PubMed] [Google Scholar]