Abstract
Two virulence factors produced by the periodontopathogen Aggregatibacter actinomycetemcomitans are leukotoxin, a secreted lipoprotein that kills human polymorphonuclear leukocytes and macrophages, and poly-N-acetylglucosamine (PGA), a surface polysaccharide that mediates intercellular adhesion, biofilm formation and detergent resistance. In this study we examined the roles of leukotoxin and PGA in protecting A. actinomycetemcomitans cells from killing by the human macrophage cell line THP-1. Monolayers of THP-1 cells were infected with single-cell suspensions of a wild-type A. actinomycetemcomitans strain, or of isogenic leukotoxin or PGA mutant strains. After 48 h, viable bacteria were enumerated by dilution plating, macrophage morphology was evaluated microscopically, and macrophage viability was measured by a Trypan blue dye exclusion assay. The number of A. actinomycetemcomitans CFUs increased approximately 2-fold in wells infected with the wild-type strain, but decreased by approximately 70–90% in wells infected with the leukotoxin and PGA mutant strains. Infection with the wild-type or leukotoxin mutant strain caused a significant decrease in THP-1 cell viability, whereas infection with the PGA mutant strain did not result in any detectable changes in THP-1 viability. Pre-treatment of wild-type A. actinomycetemcomitans cells with the PGA-hydrolyzing enzyme dispersin B rendered them sensitive to killing by THP-1 cells. We concluded that both leukotoxin and PGA are necessary for evasion of macrophage killing by A. actinomycetemcomitans.
Keywords: Aggregatibacter actinomycetemcomitans, biofilm, dispersin B, HL-60, J774-1, leukotoxin, macrophage, poly-N-acetylglucosamine, THP-1
1. Introduction
The gram-negative bacterium Aggregatibacter actinomycetemcomitans (formerly Actinobacillus actinomycetemcomitans) has been implicated as a causative agent of several forms of severe periodontitis in humans [1]. In vitro, A. actinomycetemcomitans produces several factors that may contribute to its ability to colonize the oral cavity and cause disease. These include surface-exposed autotransporter proteins that bind to human buccal epithelial cells and type V collagen [2–4]; adhesive type IV pili and exopolysaccharides that mediate surface attachment, autoaggregation and biofilm formation [5, 6]; and several exotoxins, including leukotoxin and cytolethal distending toxin, that kill host cells [7].
One of the best studied A. actinomycetemcomitans virulence factors is leukotoxin, a 114-kDa secreted lipoprotein that belongs to the RTX family of pore-forming bacterial toxins [8]. A. actinomycetemcomitans leukotoxin has been shown to kill polymorphonuclear leukocytes (PMNs) and macrophages isolated specifically from humans and Old World primates [9]. Human subjects harboring highly-leukotoxic strains of A. actinomycetemcomitans are more likely to develop periodontitis than are subjects harboring minimally-leukotoxic strains [10]. These findings suggest that leukotoxin may play a role in host cell killing and immune evasion in vivo. It has recently been shown that leukotoxin is also capable of lysing human erythrocytes in vitro [11]. Although numerous studies have confirmed that leukotoxin-producing strains of A. actinomycetemcomitans can kill host cells, only one study demonstrated that the leukotoxicity of A. actinomycetemcomitans strains correlates with their ability to avoid phagocytic killing [12]. In this study, a highly-leukotoxic strain of A. actinomycetemcomitans (strain HK1519) was more resistant to killing by human PMNs than was a minimally-leukotoxic strain (strain Y4). However, both A. actinomycetemcomitans strains used in this study were “smooth-colony” variants, which arise spontaneously upon repeated subculture of A. actinomycetemcomitans clinical isolates in broth [13]. Smooth-colony variants are usually deficient in intercellular aggregation and biofilm formation, phenotypes that are universally associated with fresh clinical isolates. The reason for using smooth-colony variants of A. actinomycetemcomitans in studies on phagocytic killing is that even small aggregates of cells formed by autoaggregating clinical strains are too large to be efficiently phagocytosed [14]. Therefore, a role for leukotoxin in immune evasion by biofilm-forming, clinical strains of A. actinomycetemcomitans has not yet been demonstrated.
A. actinomycetemcomitans also produces poly-N-acetylglucosamine (PGA), a surface polysaccharide that mediates intercellular adhesion, biofilm formation, and detergent resistance [6, 15, 16]. PGA is produced by several other pathogens including Actinobacillus pleuropneumoniae, Escherichia coli, Yersinia pestis, Bordetella spp., Staphylococcus aureus and S. epidermidis [6, 17–21]. Functions ascribed to PGA in other bacteria include abiotic surface attachment, intercellular adhesion, biofilm formation, epithelial cell attachment, and resistance to killing by antibiotics, antimicrobial peptides and human PMNs [21–28].
The purpose of the present study was to determine whether leukotoxin and PGA protect cells of a biofilm-forming, clinical strain of A. actinomycetemcomitans against macrophage killing. In this report we describe the antimicrobial activity of the human macrophage cell line THP-1 against A. actinomycetemcomitans clinical strain IDH781, and isogenic, biofilm-forming leukotoxin- and PGA-deficient mutant strains. To overcome the problem of intercellular autoaggregation, we utilized a filtration protocol to prepare single-cell suspensions from tightly adherent A. actinomycetemcomitans biofilms. Our results suggest that both leukotoxin and PGA play a key role in immune cell evasion by clinical strains of A. actinomycetemcomitans.
2. Results
2.1 Phenotypes of A. actinomycetemcomitans strains
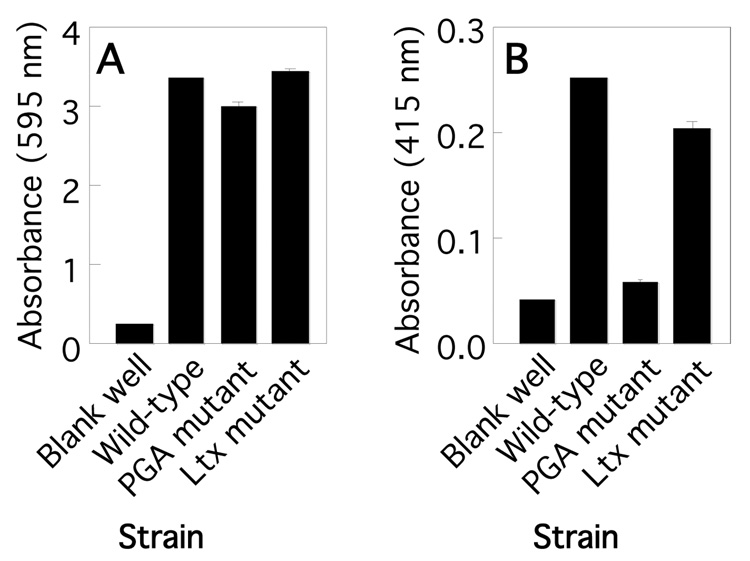
In the present study we utilized a wild-type A. actinomycetemcomitans clinical isolate (strain IDH781 [29]), and isogenic leukotoxin and PGA mutant strains. The leukotoxin mutant strain contained a transposon insertion in the leukotoxin structural gene ltxA [30] and the PGA mutant strain contained a transposon insertion in pgaC, which encodes the integral membrane glycosyltransferase responsible for the polymerization and secretion of PGA [6]. All three strains produced biofilms in 96-well microtiter plates as determined by using a crystal violet binding assay (Fig. 1A). In all cultures, the broth remained optically clear and contained <1% of the CFUs in the well. Biofilm colonies produced by the leukotoxin and PGA mutant strains exhibited a morphology that was very similar to the morphology exhibited by wild-type biofilm colonies (data not shown). These data are consistent with the results of previous studies demonstrating that PGA is not essential for biofilm formation by A. actinomycetemcomitans in the 96-well microplate assay [6, 15].
Fig. 1.
Phenotypes of wild-type and isogenic leukotoxin (Ltx) and PGA mutant A. actinomycetemcomitans strains. (A) Biofilm formation in 96-well microtiter plates. Biofilms were stained with crystal violet dye. The amount of bound crystal violet (absorbance at 595 nm) is proportional to biofilm biomass. (B) Quantitation of PGA production in wild-type and mutant biofilms. Biofilms were stained with Congo red dye. The amount of bound Congo red (absorbance at 415 nm) is proportional to PGA production. All values in panels A and B indicate the mean and standard deviation for triplicate wells.
The amount of PGA in wild-type and mutant A. actinomycetemcomitans biofilms was measured by staining the biofilms with Congo red dye, which binds to PGA [6]. As expected, biofilms produced by the PGA mutant strain bound significantly less Congo red dye than did wild-type biofilms (Fig. 1B). The amount of Congo red bound by biofilms produced by the leukotoxin mutant was the same as the amount bound by wild-type biofilms, indicating that the leukotoxin mutant strain was not deficient in PGA production.
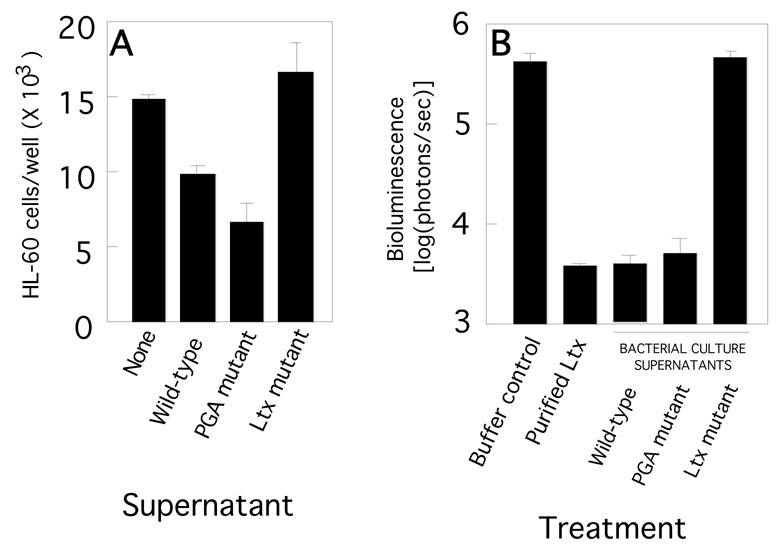
The amount of leukotoxin produced by all three A. actinomycetemcomitans strains was measured by treating the leukotoxin-sensitive cell line HL-60 for 6 h with bacterial culture supernatants, and then measuring HL-60 cell viability by Trypan blue exclusion (Fig. 2A). As expected, supernatants from the A. actinomycetemcomitans wild-type strain killed significantly more HL-60 cells than did supernatants from the leukotoxin mutant strain. Supernatants from the PGA mutant strain also killed significantly more HL-60 cells than did supernatants from the leukotoxin mutant strain, indicating that the PGA mutant strain was not deficient in leukotoxin production. The amount of HL-60 killing exhibited by PGA mutant supernatant was not significantly different from the amount exhibited by wild-type supernatant (P > 0.1). To confirm that the wild-type and PGA mutant strains produce an equivalent amount of leukotoxin, supernatants from both strains were tested in an bioluminescent HL-60 killing assay (Fig. 2B). Both supernatants killed bioluminescent HL-60 as efficiently as purified leukotoxin (400 µg/ml). Pre-treatment of wild-type and PGA mutant cells with DNase I did not result in the release of additional leukotoxin into the supernatant (data not shown), indicating that leukotoxin is probably not associated with cell-surface nucleic acids in either strain. [31].
Fig. 2.
HL-60 cell killing assay. (A) HL-60 cells were treated with supernatants from wild-type or mutant A. actinomycetemcomitans biofilms. After 6 h, the number of viable HL-60 cells was counted in a hemocytometer by using Trypan blue exclusion. (B) Killing of bioluminescent HL-60 cells by purified leukotoxin (400 µg/ml) and A. actinomycetemcomitans wild-type, PGA mutant and leukotoxin supernatants. Bioluminescent HL-60 cells were subjected to the indicated treatment for 4.5 h and cell viability [log9photons/sec)] was measured in an IVIS 50 imaging system. All values in panels A and B indicate the mean and standard deviation for triplicate wells.
2.2 Both leukotoxin- and PGA-deficient A. actinomycetemcomitans cells are sensitive to THP-1 killing
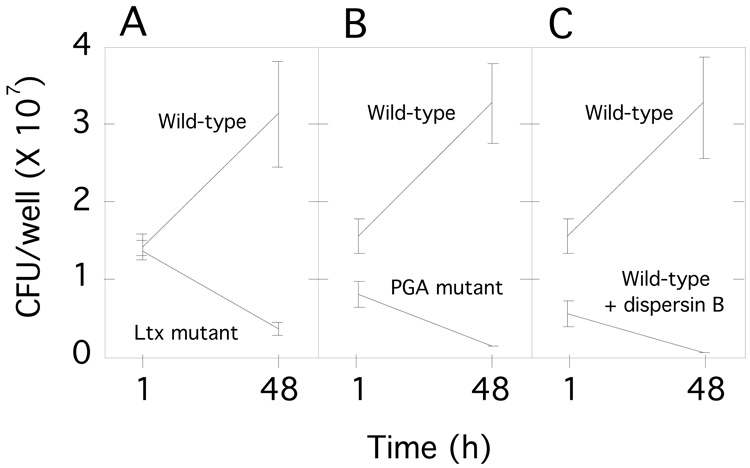
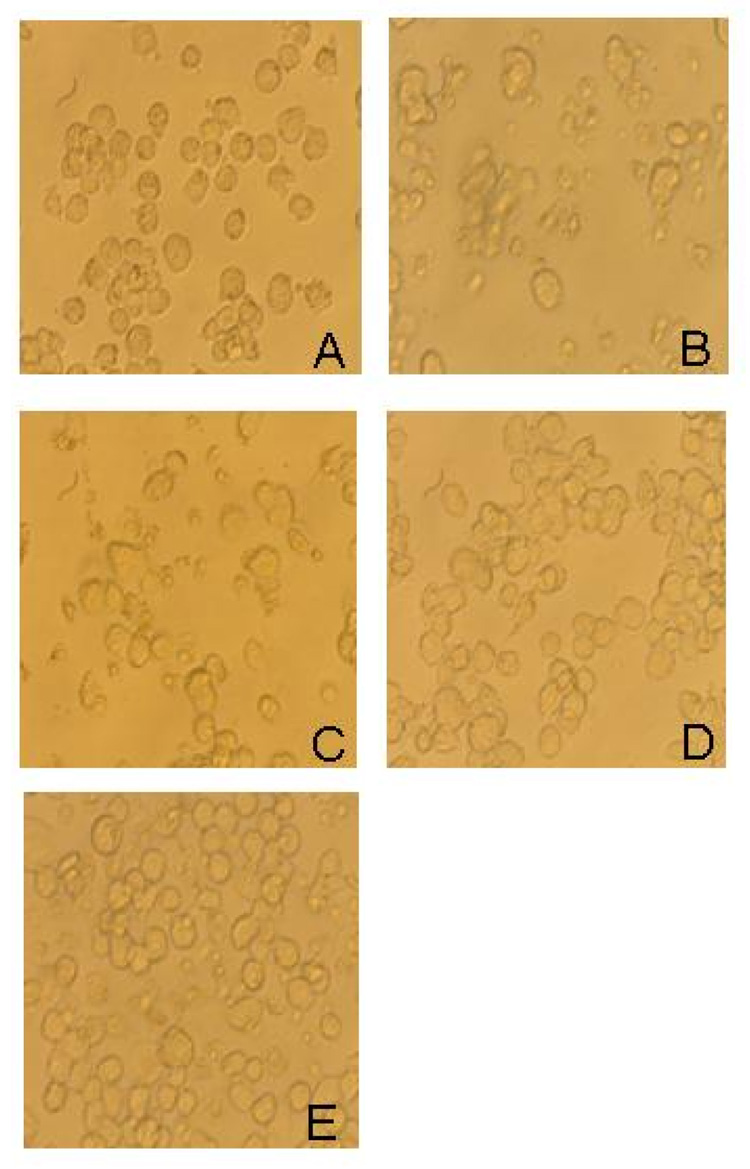
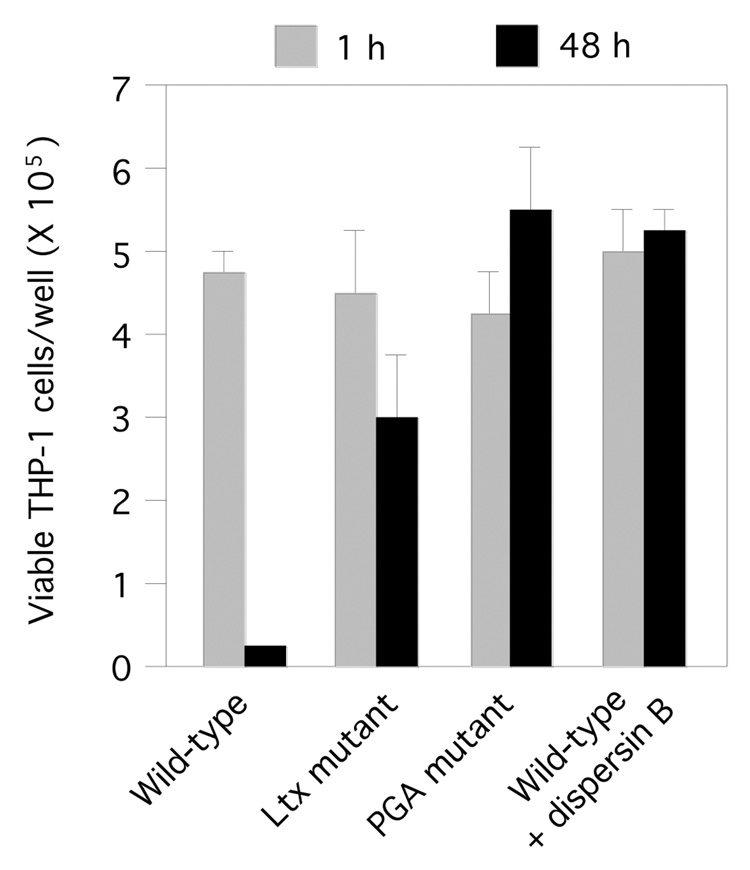
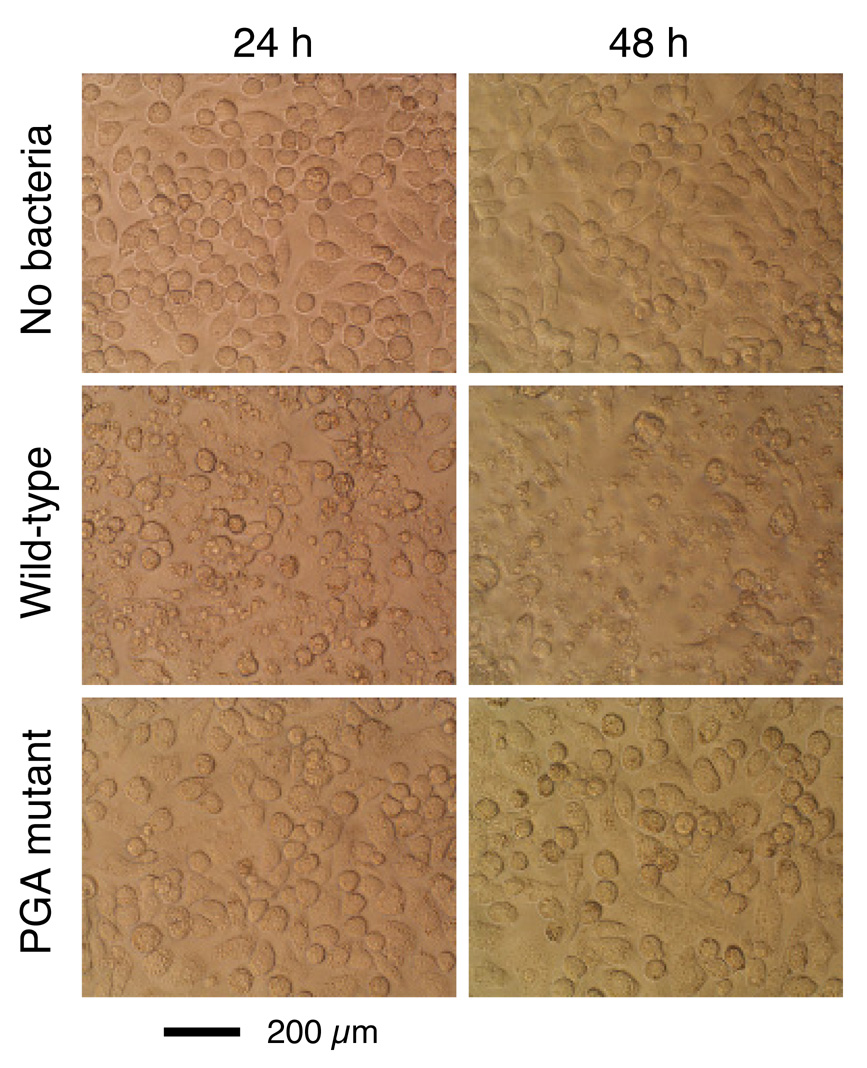
We infected monolayers of human THP-1 macrophages with single cell suspensions of wild-type, leukotoxin mutant, and PGA mutant A. actinomycetemcomitans strains. All infections were carried out at a ratio of 10–30 bacterial cells per macrophage. The total number of viable bacteria in each well was enumerated by lysing macrophages with water and determining the CFUs by dilution plating. After 48 h, the number of A. actinomycetemcomitans CFUs increased approximately 100% in wells infected with the wild-type strain, but decreased by approximately 70–90% in wells infected with the leukotoxin and PGA mutant strains (Fig. 3A and B). Infection with the wild-type strain caused significant alterations in the morphology of THP-1 cells, including cell shrinkage and cell detachment (Fig. 4A and B), and a significant decrease in THP-1 cell viability (Fig. 5). In contrast, infection with the leukotoxin mutant resulted in only moderate alterations in THP-1 morphology (Fig. 4C), and viability (Fig. 5). Infection with the PGA mutant strain did not result in any detectable changes in THP-1 morphology (Fig. 4D), or viability (Fig. 5).
Fig. 3.
Survival of A. actinomycetemcomitans in THP-1 macrophage cultures. (A and B) THP-1 cells were infected with wild-type, leukotoxin mutant, or PGA mutant A. actinomycetemcomitans strains. The number of CFUs/well was measured after 1 h and 48 h. (C) THP-1 cells were infected with wild-type A. actinomycetemcomitans cells, or wild-type cells pre-treated with the PGA-hydrolyzing enzyme dispersin B. All values in panels A-C indicate the mean and standard deviation for triplicate wells.
Fig. 4.
Micrographs of uninfected human THP-1 macrophages (A) or THP-1 macrophages infected with wild-type (B), leukotoxin (Ltx) mutant (C) or PGA mutant (D) A. actinomycetemcomitans strains. Panel (E) shows THP-1 macrophages infected with wild-type A. actinomycetemcomitans cells that were pretreated with dispersin B.
Fig. 5.
Viability of human THP-1 macrophages infected with wild-type, leukotoxin (Ltx) mutant, and PGA mutant A. actinomycetemcomitans strains. Values indicate the mean number of viable THP-1 cells per well for triplicate wells. Error bars indicate standard deviation.
2.3 Depolymerization of PGA renders wild-type A. actinomycetemcomitans cells sensitive to THP-1 killing
To confirm that PGA is involved in evasion of macrophage killing, we treated wild-type A. actinomycetemcomitans cells with the PGA-hydrolyzing enzyme dispersin B prior to THP-1 infection. Wild-type A. actinomycetemcomitans cells pre-treated with dispersin B exhibited the same phenotype as PGA mutant cells, resulting in a significant decrease in bacterial CFUs (Fig. 3C), with little change in THP-1 cell morphology (Fig. 4E), or viability (Fig. 5). Pre-treatment of wild-type and leukotoxin mutant cells with dispersin B did not result in the release of additional leukotoxin into the supernatant (data not shown), indicating that leukotoxin is probably not associated with cell-surface PGA in either strain.
2.4 PGA-deficient A. actinomycetemcomitans cells are sensitive to killing by mouse macrophages
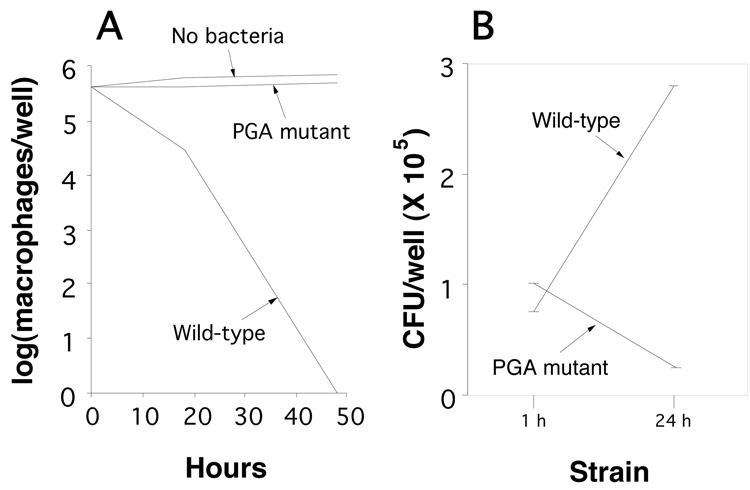
To further confirm that PGA protects A. actinomycetemcomitans cells from macrophage killing, we infected monolayers of murine J774A.1 macrophages with single-cell suspensions of wild-type and PGA mutant strains, and then measured macrophage viability and CFU/well values after 1 and 24 h (Fig. 6). All infections were carried out at a ratio of 10–30 bacterial cells per macrophage. Infection with the wild-type strain caused a significant decrease in J774A.1 viability, whereas infection with the PGA mutant strain did not significantly affect J774A.1 viability (Fig. 6A). The CFU/well values increased in wells infected with the wild-type strain, but not in wells infected with the PGA mutant strain (Fig. 6B). Wild-type A. actinomycetemcomitans cells, but not PGA mutant cells, caused significant alteration in J774A.1 morphology, consistent with macrophage cell death (Fig. 7).
Fig. 6.
Infection of murine J774A.1 macrophages with wild-type and PGA mutant A. actinomycetemcomitans strains. (A) Viability of J774A.1 macrophages after 24 and 48 h. (B) CFU/well values after 1 and 24 h. Values in panels A and B indicate the mean for duplicate wells.
Fig. 7.
Micrographs of uninfected mouse J774A.1 macrophages and macrophages infected with wild-type and PGA mutant A. actinomycetemcomitans cells.
3. Discussion
In the present study we demonstrated that cells of a wild-type, biofilm-forming strain of A. actinomycetemcomitans were resistant to killing by the human macrophage cell line THP-1. These results are consistent with those of previous studies demonstrating that A. actinomycetemcomitans cells are resistant to killing by human PMNs [14, 32–34]. Infection of THP-1 cells with wild-type A. actinomycetemcomitans cells resulted in a highly reproducible 2-fold increase in bacterial CFUs, and a concomitant decrease in THP-1 cell viability. Since A. actinomycetemcomitans cells did not replicate in RPMI-FBS culture medium, these findings suggest that A. actinomycetemcomitans cells may be able to replicate within THP-1 cells. This hypothesis is consistent with data from previous studies demonstrating that A. actinomycetemcomitans cells can invade human epithelial and endothelial cells [35–37]. Further studies are needed to confirm this hypothesis.
Our findings indicate that an A. actinomycetemcomitans mutant strain that was deficient in leukotoxin production was severely compromised in its ability to survive in THP-1 cultures. These finding provide direct evidence that leukotoxin plays a role in protecting A. actinomycetemcomitans cells from macrophage killing. Since the infection experiments carried out in the present study utilized washed, single-cell suspensions of bacteria, and were performed at a relatively low multiplicity of infection, these results suggest that leukotoxin can protect A. actinomycetemcomitans at the cellular level.
Our findings indicate that an A. actinomycetemcomitans mutant strain deficient in PGA production was also severely compromised in its ability to survive in THP-1 and J774A.1 cultures. In addition, depolymerization of PGA rendered wild-type A. actinomycetemcomitans cells sensitive to THP-1 killing. These findings confirm that PGA is a surface-associated polymer that can protect A. actinomycetemcomitans at the cellular level. Our findings are consistent with those of previous studies demonstrating that surface-associated PGA protects S. aureus and S. epidermidis from phagocytic killing [23, 26–28]. PGA has also been shown to mediate resistance to killing by antibiotics [24], detergents [16] and antimicrobial peptides [28]. PGA, therefore, may act through a general mechanism whereby it binds to, or electrostatically repulses, immune modulators and antimicrobial agents, thereby preventing their access to the bacterial cell [28]. This hypothesis is supported by the fact that purified, exogenously-added PGA can protect S. epidermidis cells from phagocytosis [25] and antibiotic killing [38]. Our findings indicate that PGA can protect A. actinomycetemcomitans from macrophage killing at the cellular level, which suggests that PGA may exert its protective effect inside the phagosome. Another interesting possibility is that the absence of PGA in the biofilm matrix may interfere with the ability of leukotoxin to bind to other cell surface components such as adhesive pili. Previous studies showed that leukotoxin is not associated with the cell surface of A. actinomycetemcomitans mutant cells that lacked adhesive type IV pili, another biofilm matrix component associated with autoaggregation [5, 39]. These possibilities will require further investigation.
4. Materials and methods
4.1. A. actinomycetemcomitans strains and culture conditions
A wild-type A. actinomycetemcomitans clinical strain (IDH781, serotype d [29]), an isogenic leukotoxin mutant strain (IDH781 ltxA∷R6Kγori/KAN [30]), and an isogenic PGA mutant strain (IDH781 pgaC∷R6Kγori/KAN; JK1048 [6]) were employed. Bacteria were grown in Tryptic soy broth supplemented with 6 g of yeast extract and 8 g of glucose per liter. All cultures were incubated statically at 37°C in 10% CO2.
4.2. Crystal violet and Congo red binding assays
Bacteria were grown in 96-well microtiter plates as previously described [6]. All three A. actinomycetemcomitans strains formed adherent biofilms that were firmly attached to the surface of the well after 24 h. Biofilms were washed with water and then stained for 1 min with Gram’s crystal violet (Fisher no. 23255960) or 1% Congo red dye. Wells were then rinsed with water and dried. Biofilms were destained with 200 µl of 33% acetic acid (for crystal violet) or 100% dimethylsulfoxide (for Congo red). The amount of bound dye was quantitated by measuring the absorbance of the dye solution at 595 nm (for crystal violet) or 415 nm (for Congo red).
4.3. HL-60 cell killing assay
The amount of leukotoxin produced by wild-type and mutant A. actinomycetemcomitans strains was measured by using a HL-60 cell killing assay as previously described.31 Briefly, cells of the human promyelocytic leukemia cell line HL-60 (no. CCL-240, American Type Culture Collection) were maintained in tissue culture flasks in RPMI-1640 medium supplemented with 10% fetal bovine serum (RPMI-FBS) at 37°C in 5% CO2. At the time of the assay, 1-ml aliquots of cells (approximately 105 cells/ml) were distributed into the wells of a 12-well tissue culture plate, and 100 µl of supernatants collected from 24-h-old biofilm cultures of wild type, leukotoxin mutant, or PGA mutant strains was added. After 6 h, the viability of the HL-60 cells was determined by trypan blue exclusion. At least 100 cells were counted for each sample. In some experiments, bioluminescent HL-60 cells cultured in 96-well microtiter plates were treated for 4.5 h with bacterial culture supernatants, and HL-60 viability was measured using an IVIS 50 imaging system as previously described [41].
4.4. THP-1 human macrophage cultures
Cells of the acute monocytic leukemia cell line THP-1 (no. TIB-202, American Type Culture Collection) were maintained in tissue culture flasks in RPMI-FBS at 37°C in 5% CO2. At the time of the assay, 105 cells were distributed in 12-well tissue culture plates and cultured overnight in the presence of 10 ng/ml phorbol-12-myristate 13-acetate to induce macrophage adherence and differentiation. Uniform monolayers of adherent THP-1 cultures were used for bacterial infections.
4.5. Infection of THP-1 cells with A. actinomycetemcomitans
Single-cell bacterial suspensions were prepared by using a previously described filtration protocol [42] with slight modifications. Briefly, wells of a 6-well tissue culture plate (Becton Dickenson no. 353046) were filled with 4 ml of broth containing 1–5 × 104 CFU/ml and incubated for 24 h. Biofilms were washed three times with phosphate buffered saline (PBS), scraped into a small volume of RPMI-FBS using a cell scraper, and then harvested by centrifugation. Cells were resuspended in RPMI-FBS, subjected to high-speed vortex agitation for 1 min, and then incubated statically for 10 min to allow large clumps of cells to settle to the bottom of the tube. The upper layer was then passed through a 5-µm pore-size polyvinylidene fluoride syringe filter (Millipore). This procedure results in a bacterial inoculum containing >99% single cells [42]. Bacterial cell suspensions were used for infections immediately after filtration. THP-1 macrophages were infected with A. actinomycetemcomitans cells at a ratio of 10–30 bacterial cells per THP-1 cell. Plates were incubated for 2 h, after which extracellular bacteria were removed by washing the cultures three times with PBS. Infected macrophages were maintained in RPMI-FBS and terminated after 1 or 48 h. Control experiments indicated that A. actinomycetemcomitans cells did not grow in RPMI-FBS during the 48-h infection period. Intracellular viability of A. actinomycetemcomitans strains was determined by lysing the infected macrophages with sterile distilled water, and then determining the number of CFUs by dilution plating as previously described [16]. Control experiments indicated that A. actinomycetemcomitans wild-type, PGA mutant and leukotoxin mutant strains were equally resistant to osmotic stress. All infection experiments were performed on numerous occasions with similar significant decreases in macrophage viability and CFU/well values.
4.6. Pretreatment of A. actinomycetemcomitans cells with dispersin B
Single-cell suspensions of wild-type A. actinomycetemcomitans cells were pre-treated for 15 min with phosphate buffered saline (PBS) containing 20 µg/ml of the PGA-hydrolyzing enzyme dispersin B (1,000 units per mg of protein [43]). Cells were washed twice with PBS and once with RPMI-FBS, and then re-filtered prior to THP-1 infection.
4.7. Infection of murine J774A.1 macrophages with A. actinomycetemcomitans
The murine macrophage cell line J774A.1 (no. TIB-67, American Type Culture Collection) was grown in Dulbecco’s modified eagle medium (DMEM) containing 10% fetal bovine serum, 2 mM glutamine and essential amino acids. Cells were maintained at 37°C in 5% CO2. For infection experiments, cells were aliquoted into the wells of a 12-well tissue culture plate (5 × 105 macrophages/well). After 16 h, cells were infected with A. actinomycetemcomitans at a ratio of 10–30 bacterial cells per macrophage as described above. After 1, 24 or 48 h, macrophage viability and CFU/well values were measured as described above.
4.8. Statistical analyses
The statistical significance of differences between means was calculated using a two-tailed Student’s t test. A P value of <0.05 was considered significant.
Acknowledgements
We thank Era Izano and Juan Crosby for technical assistance. Supported in part by a grant from the American Heart Association (to V.V.), and by NIH grants DE15124 (to J.B.K.) and DE16133 (to S. C.K.).
Abbreviations
- PGA
poly-β-1,6-N-acetyl-D-glucosamine
- PBS
phosphate buffered saline
- TSA
Tryptic Soy agar
- TSB
Tryptic Soy broth
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zambon JJ. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985;12:1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 2.Fine DH, Velliyagounder K, Furgang D, Kaplan JB. The Actinobacillus actinomycetemcomitans autotransporter adhesin Aae exhibits specificity for buccal epithelial cells of humans and Old World primates. Infect Immun. 2005;73:1947–1953. doi: 10.1128/IAI.73.4.1947-1953.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mintz KP. Identification of an extracellular matrix protein adhesin, EmaA, which mediates the adhesion of Actinobacillus actinomycetemcomitans cells to collagen. Microbiology. 2004;150:2677–2688. doi: 10.1099/mic.0.27110-0. [DOI] [PubMed] [Google Scholar]
- 4.Yue G, Kaplan JB, Furgang D, Mansfield KG, Fine DH. A second Aggregatibacter actinomycetemcomitans autotransporter adhesin exhibits specificity for buccal epithelial cells in humans and Old World primates. Infect Immun. 2007;75:4440–4448. doi: 10.1128/IAI.02020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kachlany SC, Planet PJ, DeSalle R, Fine DH, Figurski DH, Kaplan JB. flp-1, first representative of a new pilin gene subfamily, is required for nonspecific adherence of Actinobacillus actinomycetemcomitans. Mol Microbiol. 2001;40:542–554. doi: 10.1046/j.1365-2958.2001.02422.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan JB, Velliyagounder K, Ragunath C, Rohde H, Mack D, Knobloch JKM, et al. Genes involved in the synthesis and degradation of matrix polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae biofilms. J Bacteriol. 2004;186:8213–8220. doi: 10.1128/JB.186.24.8213-8220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson B, Nair SP, Ward JM, Wilson M. Molecular pathogenecity of the oral opportunistic pathogen Actinobacillus actinomycetemcomitans. Annu Rev Microbiol. 2003;57:29–55. doi: 10.1146/annurev.micro.57.030502.090908. [DOI] [PubMed] [Google Scholar]
- 8.Lally ET, Hill RB, Kieba IR, Korostoff J. The interaction between RTX toxins and target cells. Trends Microbiol. 1999;7:356–361. doi: 10.1016/s0966-842x(99)01530-9. [DOI] [PubMed] [Google Scholar]
- 9.Taichman NS, Simpson DL, Sakurada S, Cranfield M, DiRienzo J, Slots J. Comparative studies on the biology of Actinobacillus actinomycetemcomitans leukotoxin in primates. Oral Microbiol Immunol. 1987;2:97–104. doi: 10.1111/j.1399-302x.1987.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 10.Bueno L, Marcia PA, DiRienzo JM. Relationship between conversion of localized juvenile periodontitis-susceptible children from health to disease and Actinobacillus actinomycetemcomitans leukotoxin promoter structure. J Periodontol. 1998;69:998–1007. doi: 10.1902/jop.1998.69.9.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balashova NV, Crosby JA, Al Ghofaily L, Kachlany SC. Leukotoxin confers beta-hemolytic activity to Actinobacillus actinomycetemcomitans. Infect Immun. 2006;74:2015–2021. doi: 10.1128/IAI.74.4.2015-2021.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson A, Sandström G, Claesson R, Hänström L, Kalfas S. Anaerobic neutrophil-dependent killing of Actinobacillus actinomycetemcomitans in relation to bacterial leukotoxicity. Eur J Oral Sci. 2000;108:136–146. doi: 10.1034/j.1600-0722.2000.00790.x. [DOI] [PubMed] [Google Scholar]
- 13.Fine DH, Furgang D, Schreiner HC, Goncharoff P, Charlesworth J, Ghazwan G, et al. Phenotypic variation in Actinobacillus actinomycetemcomitans during laboratory growth: implications for virulence. Microbiology. 1999;145:1335–1347. doi: 10.1099/13500872-145-6-1335. [DOI] [PubMed] [Google Scholar]
- 14.Permpanich P, Kowolik MJ, Galli DM. Resistance of fluorescent-labelled Actinobacillus actinomycetemcomitans strains to phagocytosis and killing by human neutrophils. Cell Microbiol. 2006;8:72–84. doi: 10.1111/j.1462-5822.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 15.Izano EA, Sadovskaya I, Wang H, Vinogradov E, Ragunath C, Ramasubbu N, et al. Poly-N-acetylglucosamine mediates biofilm formation and detergent resistance in Aggregatibacter actinomycetemcomitans. Microb Pathogen. 2008;44:52–60. doi: 10.1016/j.micpath.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izano EA, Wang H, Ragunath C, Ramasubbu N, Kaplan JB. Detachment and killing of Aggregatibacter actinomycetemcomitans biofilms by dispersin B and SDS. J Dent Res. 2007;86:618–622. doi: 10.1177/154405910708600707. [DOI] [PubMed] [Google Scholar]
- 17.Cramton SE, Gerke C, Schnell NF, Nichols WW, Götz F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun. 1999;67:5427–5433. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irie Y, Preston A, Yuk MH. Expression of the primary carbohydrate component of the Bordetella bronchiseptica biofilm matrix is dependent on growth phase but independent of Bvg regulation. J Bacteriol. 2006;188:6680–6687. doi: 10.1128/JB.00605-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoh Y, Wang X, Hinnebusch BJ, Preston JF, III, Romeo T. Depolymerization of β-1,6-N-acetyl-D-glucosamine disrupts the integrity of diverse bacterial biofilms. J Bacteriol. 2005;187:382–387. doi: 10.1128/JB.187.1.382-387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, et al. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parise G, Mishra M, Itoh Y, Romeo T, Deora R. Role of a putative polysaccharide locus in Bordetella biofilm development. J Bacteriol. 2007;189:750–760. doi: 10.1128/JB.00953-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agladze K, Wang X, Romeo T. Spatial periodicity of Escherichia coli K-12 biofilm microstructure initiates during a reversible, polar attachment phase of development and requires the polysaccharide adhesin PGA. J Bacteriol. 2005;187:8237–8246. doi: 10.1128/JB.187.24.8237-8246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerca N, Jefferson KK, Oliveira R, Pier GB, Azeredo J. Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infect Immun. 2006;74:4849–4855. doi: 10.1128/IAI.00230-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izano EA, Sadovskaya I, Vinogradov E, Mulks M, Velliyagounder K, Ragunath C, et al. Poly-N-acetylglucosamine mediates biofilm formation and antibiotic resistance in Actinobacillus pleuropneumoniae. Microb Pathogen. 2007;43:1–9. doi: 10.1016/j.micpath.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson GM, Lee DA, Regelmann WE, Gray ED, Peters G, Quie PG. Interference with granulocyte function by Staphylococcus epidermidis slime. Infect Immun. 1986;54:13–20. doi: 10.1128/iai.54.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kropec A, Maira-Litrán T, Jefferson KK, Grout M, Cramton SE, Götz F, et al. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect Immun. 2005;73:6868–6876. doi: 10.1128/IAI.73.10.6868-6876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiro H, Muller E, Gutierrez N, Boisot S, Grout M, Tosteson TD, et al. Transposon mutants of Staphylococcus epidermidis deficient in elaboration of capsular polysaccharide/adhesin and slime are avirulent in a rabbit model of endocarditis. J Infect Dis. 1994;169:1042–1049. doi: 10.1093/infdis/169.5.1042. [DOI] [PubMed] [Google Scholar]
- 28.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, et al. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol. 2004;6:269–275. doi: 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 29.Haubek D, Poulsen K, Asikainen S, Kilian M. Evidence for absence in northern Europe of especially virulent clonal types of Actinobacillus actinomycetemcomitans. J Clin Microbiol. 1995;33:395–401. doi: 10.1128/jcm.33.2.395-401.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crosby JA, Kachlany SC. TdeA, a TolC-like protein required for toxin and drug export in Aggregatibacter (Actinobacillus) actinomycetemcomitans. Gene. 2007;388:83–92. doi: 10.1016/j.gene.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohta H, Hara H, Fukui K, Kurihara H, Murayama Y, Kato K. Association of Actinobacillus actinomycetemcomitans leukotoxin with nucleic acids on the bacterial cell surface. Infect Immun. 1993;61:4878–4884. doi: 10.1128/iai.61.11.4878-4884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cacchillo DA, Walters JD. Effect of ciprofloxacin on killing of Actinobacillus actinomycetemcomitans by polymorphonuclear leukocytes. Antimicrob Agents Chemother. 2002;46:1980–1984. doi: 10.1128/AAC.46.6.1980-1984.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holm A, Kalfas S, Holm SE. Killing of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus by human polymorphonuclear leukocytes in serum and saliva. Oral Microbiol Immunol. 1993;8:134–140. doi: 10.1111/j.1399-302x.1993.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi M, Kawasaki M, Yamashita Y, Nakashima K, Koga T. Role of capsular polysaccharide-like serotype-specific antigen in resistance of Actinobacillus actinomycetemcomitans to phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1995;63:4589–4594. doi: 10.1128/iai.63.12.4589-4594.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer DH, Sreenivasan PK, Fives-Taylor PM. Evidence for invasion of a human oral cell line by Actinobacillus actinomycetemcomitans. Infect Immun. 1991;59:2719–2726. doi: 10.1128/iai.59.8.2719-2726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudney JD, Chen R, Sedgewick GJ. Intracellular Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in buccal epithelial cells collected from human subjects. Infect Immun. 2001;69:2700–2707. doi: 10.1128/IAI.69.4.2700-2707.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schenkein HA, Barbour SE, Berry CR, Kipps B, Tew JG. Invasion of human vascular endothelial cells by Actinobacillus actinomycetemcomitans via the receptor for platelet-activating factor. Infect Immun. 2000;68:5416–5419. doi: 10.1128/iai.68.9.5416-5419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farber BF, Kaplan MH, Clogston AG. Staphylococcus epidermidis extracted slime inhibits the antimicrobial action of glycopeptide antibiotics. J Infect Dis. 1990;161:37–40. doi: 10.1093/infdis/161.1.37. [DOI] [PubMed] [Google Scholar]
- 39.Kachlany SC, Fine DH, Figurski DH. Secretion of RTX leukotoxin by Actinobacillus actinomycetemcomitans. Infect Immun. 2000;68:6094–6100. doi: 10.1128/iai.68.11.6094-6100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz R, Al Ghofaily L, Patel J, Balashova NV, Freitas AC, Labib I, et al. Characterization of leukotoxin from a clinical strain of Actinobacillus actinomycetemcomitans. Microb Pathogen. 2006;40:48–55. doi: 10.1016/j.micpath.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Isaza MP, Chau JT, Le A, Balashova NV, Patel JK, Salerno E, et al. A bioluminescence HL-60 cell line to assay anti-leukaemia therapeutics under physiological conditions. Luminescence [serial online] 2008;23(1):17–21. doi: 10.1002/bio.1010. Available at: http://www3.interscience.wiley.com. [DOI] [PubMed] [Google Scholar]
- 42.Kaplan JB, Fine DH. Biofilm dispersal of Neisseria subflava and other phylogenetically diverse oral bacteria. Appl Environ Microbiol. 2002;68:4943–4950. doi: 10.1128/AEM.68.10.4943-4950.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaplan JB, Ragunath C, Ramasubbu N, Fine DH. Detachment of Actinobacillus actinomycetemcomitans biofilms cells by an endogenous β-hexosaminidase activity. J Bacteriol. 2003;185:4693–4698. doi: 10.1128/JB.185.16.4693-4698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]