Abstract
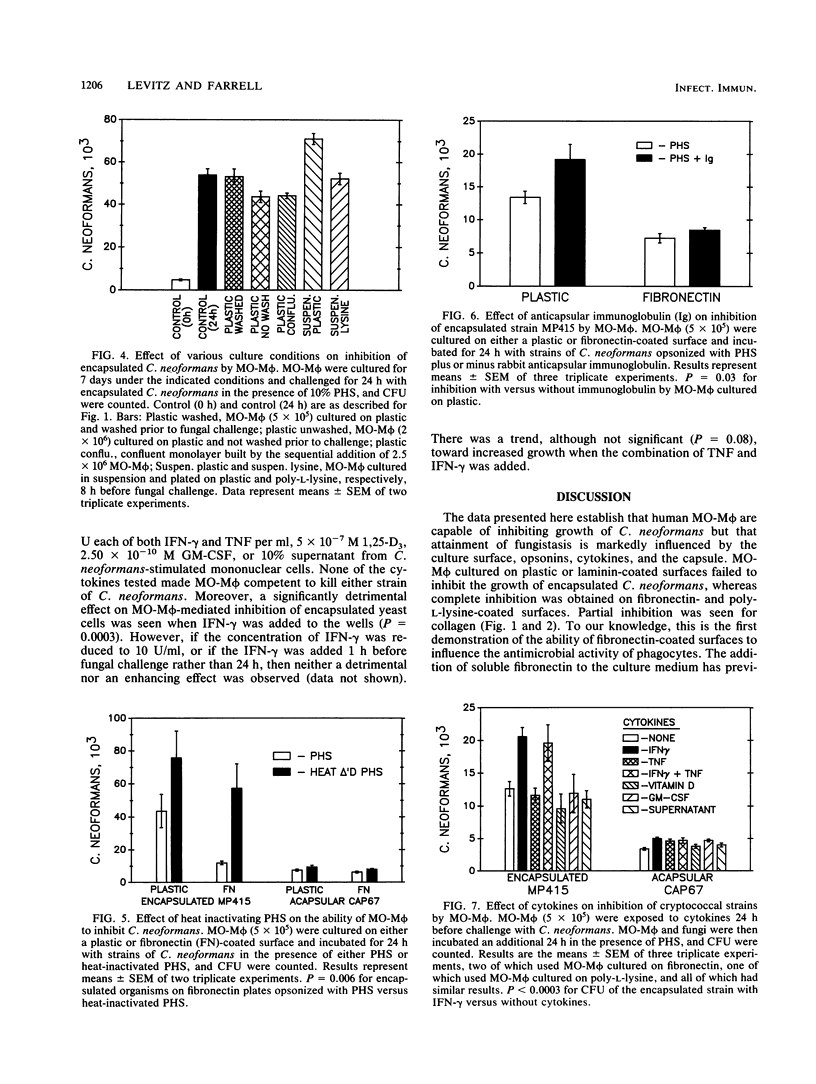
Despite a presumed critical role of macrophages in the host response to cryptococcal infections, previous studies have failed to show growth inhibition of encapsulated Cryptococcus neoformans by human peripheral blood cultured monocyte-derived macrophages (MO-M phi). Here, we examined whether MO-M phi could be induced to inhibit growth of an encapsulated strain and an isogenic acapsular mutant strain of C. neoformans. MO-M phi were cultured in microwells, and inhibition was measured by comparing CFU at 0 and 24 h after fungal challenge. MO-M phi cultured on plastic surfaces failed to inhibit growth of the encapsulated strain, even in the presence of pooled human serum and/or anticapsular antibody. Moreover, the presence of anticapsular antibody significantly enhanced fungal growth. However, if MO-M phi were cultured on surfaces coated with fibronectin or poly-L-lysine (but not laminin or collagen) and yeast cells were opsonized with pooled human serum, then complete growth inhibition occurred. Preincubation with various concentrations of tumor necrosis factor, granulocyte macrophage colony-stimulating factor, 1,25-dihydroxycholecalciferol, or supernatants from C. neoformans-stimulated lymphocytes failed to activate macrophages for enhanced antifungal activity. The addition of gamma interferon resulted in a significant loss of growth inhibition. For the acapsular strain, complete growth inhibition was observed regardless of the choice of culture surface, opsonins, or cytokines. Fungicidal activity, as measured by a significant decrement in CFU compared with the initial inoculum, was not observed under any conditions tested. These data demonstrate that macrophages are capable of inhibiting cryptococcal growth but that this capacity is markedly influenced by the culture surface, opsonins, cytokines, and the fungal capsule.
Full text
PDF

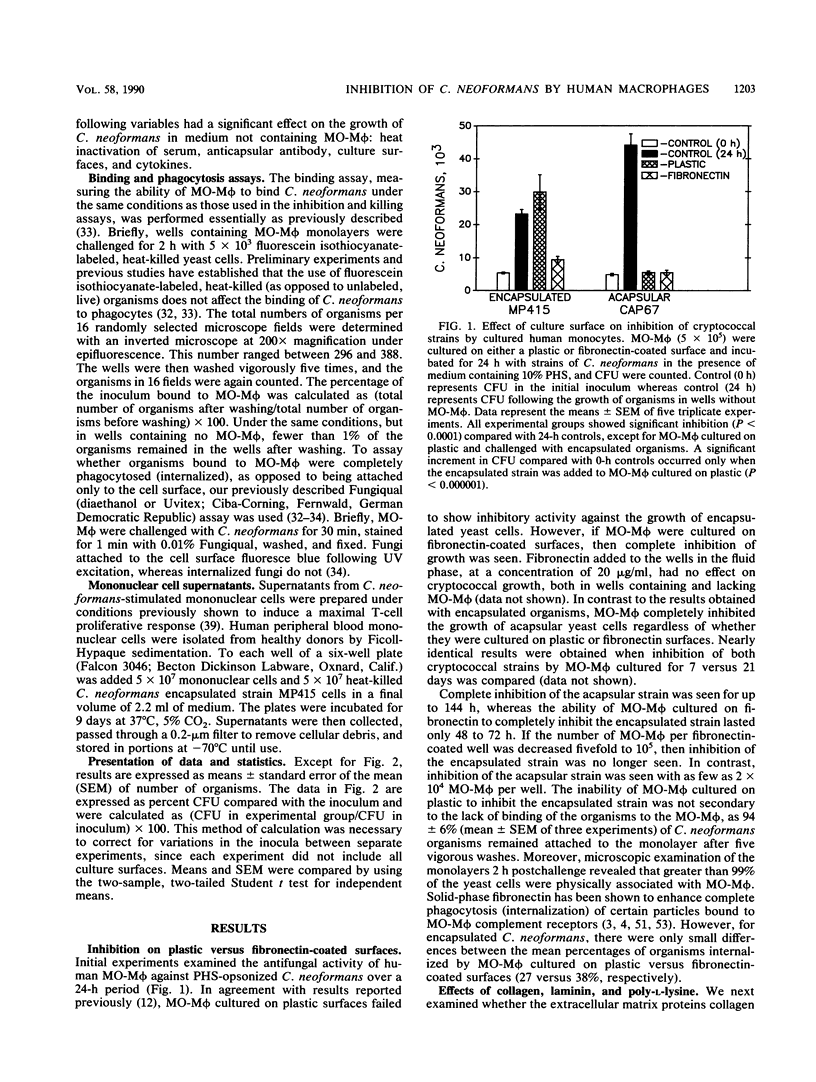
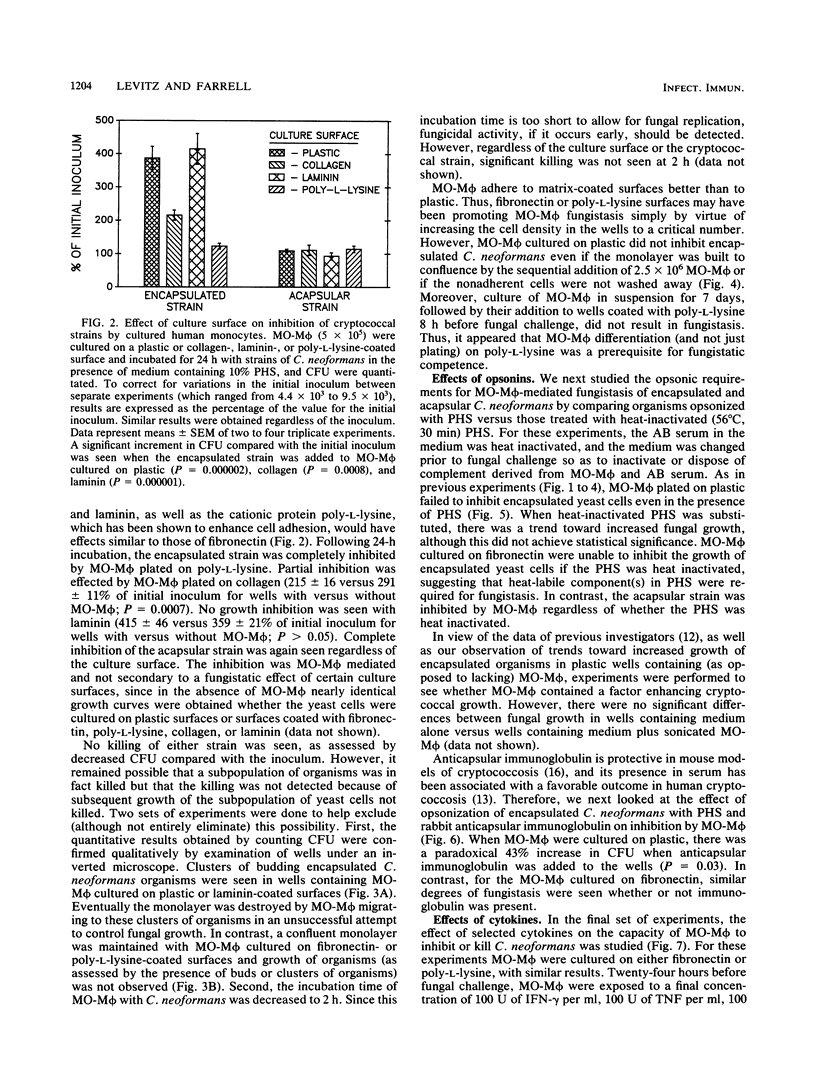
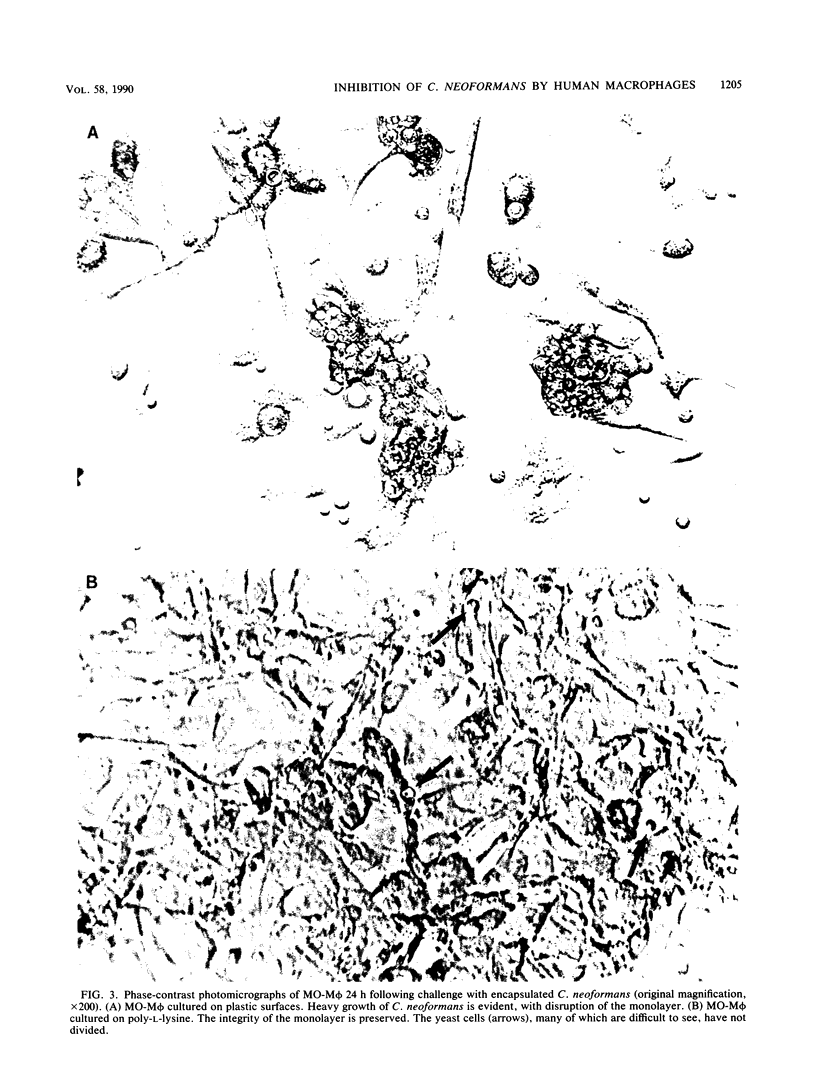



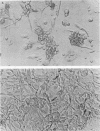
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- Bohnsack J. F., Kleinman H. K., Takahashi T., O'Shea J. J., Brown E. J. Connective tissue proteins and phagocytic cell function. Laminin enhances complement and Fc-mediated phagocytosis by cultured human macrophages. J Exp Med. 1985 May 1;161(5):912–923. doi: 10.1084/jem.161.5.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Goodwin J. L. Fibronectin receptors of phagocytes. Characterization of the Arg-Gly-Asp binding proteins of human monocytes and polymorphonuclear leukocytes. J Exp Med. 1988 Mar 1;167(3):777–793. doi: 10.1084/jem.167.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J. The role of extracellular matrix proteins in the control of phagocytosis. J Leukoc Biol. 1986 May;39(5):579–591. doi: 10.1002/jlb.39.5.579. [DOI] [PubMed] [Google Scholar]
- Cohen D. A., Stotelmyer N. L., Kaplan A. M. Induction of functional Fc receptors in P388 leukemia cells. Requirement for multiple differentiation signals. Exp Cell Res. 1985 Apr;157(2):511–519. doi: 10.1016/0014-4827(85)90136-3. [DOI] [PubMed] [Google Scholar]
- Crowle A. J., Ross E. J., May M. H. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1987 Dec;55(12):2945–2950. doi: 10.1128/iai.55.12.2945-2950.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. F., Clifford D. P., Hoidal J. R., Repine J. E. Opsonic requirements for the uptake of Cryptococcus neoformans by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1982 Jun;145(6):870–874. doi: 10.1093/infdis/145.6.870. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Allison A. C. Nature of the effector cells responsible for antibody-dependent cell-mediated killing of Cryptococcus neoformans. Infect Immun. 1976 Sep;14(3):716–720. doi: 10.1128/iai.14.3.716-720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect Immun. 1973 Feb;7(2):231–236. doi: 10.1128/iai.7.2.231-236.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974 Feb;80(2):176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., May J. E., Kane M. A., Frank M. M., Bennett J. E. The role of the classical and alternate complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol. 1974 Jun;112(6):2260–2270. [PubMed] [Google Scholar]
- Douvas G. S., Looker D. L., Vatter A. E., Crowle A. J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985 Oct;50(1):1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromer F., Charreire J., Contrepois A., Carbon C., Yeni P. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect Immun. 1987 Mar;55(3):749–752. doi: 10.1128/iai.55.3.749-752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Schwamberger G., Kaufmann S. H. Fungicidal activity of IFN-gamma-activated macrophages. Extracellular killing of Cryptococcus neoformans. J Immunol. 1989 May 1;142(9):3219–3224. [PubMed] [Google Scholar]
- Fromtling R. A., Shadomy H. J., Jacobson E. S. Decreased virulence in stable, acapsular mutants of cryptococcus neoformans. Mycopathologia. 1982 Jul 23;79(1):23–29. doi: 10.1007/BF00636177. [DOI] [PubMed] [Google Scholar]
- Gentry L. O., Remington J. S. Resistance against Cryptococcus conferred by intracellular bacteria and protozoa. J Infect Dis. 1971 Jan;123(1):22–31. doi: 10.1093/infdis/123.1.22. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Macrophage-mediated fungistasis in vitro: requirements for intracellular and extracellular cytotoxicity. J Immunol. 1986 Jan;136(2):672–680. [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest. 1985 Aug;76(2):508–516. doi: 10.1172/JCI112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD D. H. Some factors which affect the initiation of growth of Cryptococcus neoformans. J Bacteriol. 1961 Sep;82:430–435. doi: 10.1128/jb.82.3.430-435.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson E. S., Ayers D. J., Harrell A. C., Nicholas C. C. Genetic and phenotypic characterization of capsule mutants of Cryptococcus neoformans. J Bacteriol. 1982 Jun;150(3):1292–1296. doi: 10.1128/jb.150.3.1292-1296.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Gaudernack G. In vitro differentiation of human monocytes. Differences in monocyte phenotypes induced by cultivation on glass or on collagen. J Exp Med. 1982 Oct 1;156(4):1101–1114. doi: 10.1084/jem.156.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitz D. J., Johnson C. R., Kobayashi G. S., Medoff G., Little J. R. Growth inhibition of Cryptococcus neoformans by cloned cultured murine macrophages. Cell Immunol. 1984 Oct 15;88(2):489–500. doi: 10.1016/0008-8749(84)90180-1. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Follette J. L. Opsonization of encapsulated Cryptococcus neoformans by specific anticapsular antibody. Infect Immun. 1981 Mar;31(3):978–984. doi: 10.1128/iai.31.3.978-984.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Highison B., Stratton C. J. Localization on encapsulated Cryptococcus neoformans of serum components opsonic for phagocytosis by macrophages and neutrophils. Infect Immun. 1984 Feb;43(2):574–579. doi: 10.1128/iai.43.2.574-579.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Polacheck I., Popkin T. J. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J Bacteriol. 1982 Jun;150(3):1414–1421. doi: 10.1128/jb.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. C., Fauci A. S. Immunologic reconstitution in the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Nov;103(5):714–718. doi: 10.7326/0003-4819-103-5-714. [DOI] [PubMed] [Google Scholar]
- Levitz S. M., DiBenedetto D. J., Diamond R. D. A rapid fluorescent assay to distinguish attached from phagocytized yeast particles. J Immunol Methods. 1987 Jul 16;101(1):37–42. doi: 10.1016/0022-1759(87)90213-4. [DOI] [PubMed] [Google Scholar]
- Levitz S. M., DiBenedetto D. J. Differential stimulation of murine resident peritoneal cells by selectively opsonized encapsulated and acapsular Cryptococcus neoformans. Infect Immun. 1988 Oct;56(10):2544–2551. doi: 10.1128/iai.56.10.2544-2551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., DiBenedetto D. J. Paradoxical role of capsule in murine bronchoalveolar macrophage-mediated killing of Cryptococcus neoformans. J Immunol. 1989 Jan 15;142(2):659–665. [PubMed] [Google Scholar]
- Lo S. K., Ryan T. J., Gilboa N., Lai L., Malik A. B. Role of catalytic and lysine-binding sites in plasmin-induced neutrophil adherence to endothelium. J Clin Invest. 1989 Sep;84(3):793–801. doi: 10.1172/JCI114238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani B., Rabinovitch M., Nussenzweig V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG). J Exp Med. 1972 Apr 1;135(4):780–792. doi: 10.1084/jem.135.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. A. Extracellular matrix assembly. Annu Rev Cell Biol. 1988;4:183–207. doi: 10.1146/annurev.cb.04.110188.001151. [DOI] [PubMed] [Google Scholar]
- Miller G. P., Kohl S. Antibody-dependent leukocyte killing of Cryptococcus neoformans. J Immunol. 1983 Sep;131(3):1455–1459. [PubMed] [Google Scholar]
- Miller G. P., Puck J. In vitro human lymphocyte responses to Cryptococcus neoformans. Evidence for primary and secondary responses in normals and infected subjects. J Immunol. 1984 Jul;133(1):166–172. [PubMed] [Google Scholar]
- Monga D. P. Role of macrophages in resistance of mice to experimental cryptococcosis. Infect Immun. 1981 Jun;32(3):975–978. doi: 10.1128/iai.32.3.975-978.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Murray H. W., Hillman J. K., Rubin B. Y., Kelly C. D., Jacobs J. L., Tyler L. W., Donelly D. M., Carriero S. M., Godbold J. H., Roberts R. B. Patients at risk for AIDS-related opportunistic infections. Clinical manifestations and impaired gamma interferon production. N Engl J Med. 1985 Dec 12;313(24):1504–1510. doi: 10.1056/NEJM198512123132403. [DOI] [PubMed] [Google Scholar]
- Murray H. W. Interferon-gamma, the activated macrophage, and host defense against microbial challenge. Ann Intern Med. 1988 Apr;108(4):595–608. doi: 10.7326/0003-4819-108-4-595. [DOI] [PubMed] [Google Scholar]
- Nabavi N., Murphy J. W. Antibody-dependent natural killer cell-mediated growth inhibition of Cryptococcus neoformans. Infect Immun. 1986 Feb;51(2):556–562. doi: 10.1128/iai.51.2.556-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Prendergast T. J., Wiebe M. E., Stanley E. R., Platzer E., Remold H. G., Welte K., Rubin B. Y., Murray H. W. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984 Aug 1;160(2):600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Textor J. A., Vann J. M., Mosher D. F. Role of fibronectin in human monocyte and macrophage bactericidal activity. Infect Immun. 1985 Mar;47(3):629–637. doi: 10.1128/iai.47.3.629-637.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supino R., Gibelli N., Zunino F. Induction of differentiation of Friend murine erythroleukemia cells by poly-L-lysine and daunorubicin-poly-L-lysine conjugate. J Natl Cancer Inst. 1986 Aug;77(2):453–457. [PubMed] [Google Scholar]
- Takada Y., Wayner E. A., Carter W. G., Hemler M. E. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J Cell Biochem. 1988 Aug;37(4):385–393. doi: 10.1002/jcb.240370406. [DOI] [PubMed] [Google Scholar]
- Weinberg P. B., Becker S., Granger D. L., Koren H. S. Growth inhibition of Cryptococcus neoformans by human alveolar macrophages. Am Rev Respir Dis. 1987 Nov;136(5):1242–1247. doi: 10.1164/ajrccm/136.5.1242. [DOI] [PubMed] [Google Scholar]
- Wright S. D. Cellular strategies in receptor-mediated phagocytosis. Rev Infect Dis. 1985 May-Jun;7(3):395–397. doi: 10.1093/clinids/7.3.395. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Craigmyle L. S., Silverstein S. C. Fibronectin and serum amyloid P component stimulate C3b- and C3bi-mediated phagocytosis in cultured human monocytes. J Exp Med. 1983 Oct 1;158(4):1338–1343. doi: 10.1084/jem.158.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D. Methods for the study of receptor-mediated phagocytosis. Methods Enzymol. 1986;132:204–221. doi: 10.1016/s0076-6879(86)32009-3. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]