Abstract
An interlaboratory study was conducted to evaluate a method for determining total soy isoflavones in dietary supplements, dietary supplement ingredients, and soy foods. Isoflavones were extracted using aqueous acetonitrile containing a small amount of dimethylsulfoxide (DMSO) and all 12 of the naturally occuring isoflavones in soy were determined by high-performance liquid Chromatography (HPLC) with UV detection using apigenin as an internal standard. Fifteen samples (6 pairs of blind duplicates plus 3 additional samples) of soy isoflavone ingredients, soy isoflavone dietary supplements, soy flour, and soy protein products were successfully analyzed by 13 collaborating laboratories in 6 countries. For repeatability, the relative standard deviations (RSDr) ranged from 1.07 for samples containing over 400 mg/g total isoflavones to 3.31 for samples containing 0.87 mg/g total isoflavones, and for reproducibility the RSDR values ranged from 2.29 for samples containing over 400 mg/g total isoflavones to 9.36 for samples containing 0.87 mg/g total isoflavones. HorRat values ranged from 1.00 to 1.62 for all samples containing at least 0.8 mg/g total isoflavones. One sample, containing very low total isoflavones (<0.05 mg/g), gave RSDR values of 175 and a HorRat value of 17.6. This sample was deemed to be below the usable range of the method. The method provides accurate and precise results for analysis of soy isoflavones in dietary supplements and soy foods.
The 3 isoflavones daidzein, glycitein, and genistein are each found in soy in 4 forms. In the soybean, the 6″-O-malonyl-β-glucosides of the isoflavones are the predominant forms. The 6″-O-acetyl-β-glucoside-, β-glucoside-, and aglucone forms arise from degradation of the 6″-O-malonyl-β-glucosides during processing of soybeans and soy foods or during sample preparation and analysis. Soy isoflavone dietary supplement ingredients are produced and labeled using high-performance liquid chromatographic (HPLC) methods that quantify all 12 of the predominant isoflavones forms commonly found in soy. No analytical method which examines all of these forms has been validated for determination of total isoflavone content. This study was done to provide a validated method for reporting total unmodified isoflavone content of soy isoflavone dietary supplements and soy foods.
The method uses standards for the 3 glucoside forms and the 3 aglucone forms. The response factors for the malonyl and acetyl forms are calculated from the glucoside response factors.
Previous Methods
The current method is based on the 2001 publication from our laboratory, with some modifications (1). It uses extraction of isoflavones in acetonitrile-water without acidification and analyzes those samples directly by reversed-phase HPLC with UV detection. Apigenin is used as an internal standard. Acetyl and malonyl forms, which are either not readily commercially available or are very expensive, are identified using minimally processed soy flour, and quantified using the commercially readily available isoflavone-β-glucoside forms.
Method 2001.10 of AOAC INTERNATIONAL (2) uses saponification during sample preparation, so that only glucoside and aglycone forms are present during the HPLC analysis. Although this procedure simplifies the HPLC analysis, it modifies the isoflavones so that total content determined by this method does not match the results obtained when all 12 forms are analyzed. Total isoflavone aglycone content can be accurately determined by this method but total isoflavone content cannot be determined.
Collaborative Study
Study Design
Each laboratory was supplied 3 qualifying samples consisting of soy flour, heated soy flour, and a soy isoflavone dietary supplement concentrate. Laboratories were required to report successful results on those test samples prior to beginning the collaborative sample analysis.
Because of the expense of acquiring 7 standards, stock concentrated mixed standards and internal standard were supplied with the collaborative samples. Working standards were prepared by the collaborating laboratories.
Fifteen collaborative samples were provided to each laboratory. Three samples of concentrated soy isoflavone dietary supplement ingredients containing approximately 40–50% total isoflavones, one sample of concentrated soy isoflavone dietary supplement ingredients containing approximately 25% total isoflavones, one sample of water washed soy protein concentrate, and one sample of soy protein isolate were each supplied as blind duplicates (12 samples). Samples of soy flour, alcohol-washed soy protein concentrate, and soy isoflavone tablets were supplied as single samples without replication.
Collaborating Laboratories
Fifteen laboratories from 6 countries agreed to participate in the collaborative study. One of the 15 laboratories returned only the preliminary data. One laboratory returned data that were in obvious error, different from all the other laboratories, presumably due to errors in calculations. Data from those 2 laboratories were eliminated from the collaborative data prior to analysis.
Test Sample Preparation
Collaborative trial samples and suitability test samples were prepared from commercial isoflavone concentrates and from commercial soy protein products. Samples of concentrated soy isoflavone dietary supplement ingredients containing approximately 40–50% total isoflavones, soy flour, water-washed soy protein concentrate, alcohol-washed soy protein concentrate, soy protein isolate, and soy isoflavone tablets were acquired from Archer Daniels Midland Co. (Decatur, IL).
A sample of concentrated soy isoflavone dietary supplement ingredients containing approximately 25% total isoflavones was prepared in the laboratory by blending concentrated soy isoflavone dietary supplement ingredient containing about 40–50% total isoflavones with soy flour containing approximately 0.5% total isoflavones.
A suitability test sample of heated soy flour was prepared by heating soy flour in a shallow open pan in a laboratory oven at 120°C for 4 h.
Commercial samples were acquired as powders in 1 kg containers. No additional mixing or homogenization of the samples was done. Samples were distributed to labeled opaque heat-sealable bags, sealed in the laboratory and packaged in boxes for distribution to the collaborators. Homogeneity testing of the samples was not done, as previous experience with these types of samples indicated that samples of this sort are already sufficiently mixed.
Soy isoflavone dietary supplement tablets containing 50 mg total isoflavones/tablet were supplied by Archer Daniels Midland Co. Four bottles containing 60 tablets each were opened and mixed together, and 10 randomly chosen tablets were packed in heated-sealed opaque bags for distribution to the collaborators.
Single-Laboratory Validation Method Performance
System suitability
HPLC system used in the single-laboratory validation (SLV) work passed rigorous suitability testing on 3 consecutive days prior to use. Linearity of calibration was >0.99999 for all standards on all days for all systems. There was no significant change in the slopes of the calibration curves or the goodness of fit when data were forced through zero, allowing analysis to be performed forcing through zero. System precision, measured by 5 replicate injections of a test standard, had <0.2% coefficient of variation (CV) for all standards on all systems on all days. System accuracy was determined by how close the precision standard was to the expected values. Results were between 99.7 and 100.3% of the expected values for all results on all days.
Three different lots of a 40–50% soy isoflavone concentrate, one lot of isolated soy protein, and one lot of water-washed soy protein concentrate were used for the SLV testing. Accuracy was determined by spike recovery, adding standard solution to samples during preparation. Samples were analyzed by 2 analysts in one laboratory, and by a third analyst in a different laboratory, on 3 different HPLC systems over the course of several days.
Precision
The results from the nonspiked samples were used to determine precision and intermediate precision of the method. The soy isoflavone concentrate samples containing 40–50% total isoflavones had CVs of <0.5% for total isoflavones and total isoflavone aglycone content. HorRat values for those samples were all <0.5. The water-washed soy protein concentrate contained about 1.1 mg/g total isoflavones, had a CV of around 1% for total isoflavones and total isoflavone aglycones, and HorRat values of <0.5. The soy protein isolate contained <1 mg/g total isoflavones, had CVs of 5.2% for total isoflavones and 5.7% for total isoflavone aglycones, and HorRat values of 1.84 for both determinations. The samples all pass the HorRat test (<2) for reproducibility for total isoflavones and for aglucone equivalents for each isoflavone.
Accuracy
Accuracy was determined by subtracting the averaged results of the nonspiked samples from the result of the individual spiked sample to determine spike recovery. Spike recoveries ranged from a minimum of 97.9% to a maximum of 103.3%.
AOAC Official Method 2008.03 Total Soy Isoflavones in Dietary Supplements, Supplement Ingredients, and Soy Foods
High-Performance Liquid Chromatography with Ultraviolet Detection First Action 2008
[Applicable to the analysis of total soy isoflavones and isoflavone aglycone equivalents in commercial dietary supplements, soy isoflavone concentrates used to make dietary supplements, and soy foods containing at least 0.5 mg/g total isoflavones. This method is not applicable to dietary supplements or other products containing red clover (Trifolium pratense L.) or kudzu (Pueraria lobata) as several types of isoflavones found in those products are not found in soy, and are not included in this analysis. Dietary supplement formulations containing apigenin cannot be determined by the method due to interference with the internal standard.]
See Table 2008.03A for the results of the interlaboratory study supporting acceptance of the method.
Table 2008.03A.
Interlaboratory study results for determination of total soy isoflavones in dietary supplements, supplement ingredients, and soy foods
| Sample | A–F | B–H | C–E | D–G | J | K–Q | L–P | M | N | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total isoflavones, mg/g
| ||||||||||
| Total number of laboratories | p | 13 | 13 | 13 | 12 | 13 | 11 | 11 | 11 | 12 |
| Total number of replicates | Sum(n(L)) | 25 | 25 | 26 | 23 | 13 | 22 | 21 | 11 | 12 |
| Overall mean of all data (grand mean) | x̄ | 411.8 | 485.3 | 450.2 | 257.7 | 82.5 | 1.80 | 0.87 | 6.14 | 0.022 |
| Repeatability standard deviation | sr | 4.39 | 6.38 | 5.99 | 5.30 | 0.05 | 0.03 | |||
| Reproducibility standard deviation | sR | 9.44 | 11.35 | 12.00 | 6.73 | 3.31 | 0.11 | 0.08 | 0.30 | 0.040 |
| Repeatability relative standard deviation | RSDr | 1.07 | 1.32 | 1.33 | 2.06 | 2.70 | 3.31 | |||
| Reproducibility relative standard deviation | RSDR | 2.29 | 2.34 | 2.67 | 2.61 | 4.01 | 6.05 | 9.36 | 4.86 | 175 |
| HorRat value | 1.00 | 1.05 | 1.18 | 1.07 | 1.38 | 1.17 | 1.62 | 1.14 | 17.6 | |
|
| ||||||||||
| Total isoflavone aglycones, mg/g
| ||||||||||
| Total number of laboratories | p | 13 | 13 | 13 | 12 | 13 | 11 | 11 | 11 | 12 |
| Total number of replicates | Sum(n(L)) | 25 | 25 | 26 | 23 | 13 | 22 | 21 | 11 | 12 |
| Overall mean of all data (grand mean) | x̄ | 256.1 | 302.9 | 279.7 | 160.3 | 51.32 | 1.03 | 0.53 | ||
| Repeatability standard deviation | sr | 2.74 | 3.98 | 3.72 | 3.30 | 0.026 | 0.02 | 3.31 | 0.014 | |
| Reproducibility standard deviation | sR | 5.65 | 7.00 | 7.29 | 4.21 | 2.02 | 0.064 | 0.05 | ||
| Repeatability relative standard deviation | RSDr | 1.07 | 1.32 | 1.33 | 2.06 | 2.53 | 2.89 | 0.17 | 0.022 | |
| Reproducibility relative standard deviation | RSDR | 2.21 | 2.31 | 2.61 | 2.63 | 3.94 | 6.22 | 9.00 | 5.16 | 164 |
| HorRat value | 0.90 | 0.97 | 1.08 | 1.00 | 1.26 | 1.10 | 1.44 | 1.10 | 15.2 | |
Notes: Blind replicates A–F, B–H, D–G, and L–P each had had one outlier result by Cochran’s test that was eliminated from the statistical analysis; blind replicates C–E had no outliers; Laboratory 3 results were outliers by Single Grubb’s test for blind replicates D–G, K–Q, and L–P and was eliminated from the statistical analysis; sample M, Laboratory 5 was an outlier by Grubb’s test and was eliminated from the statistical analysis. Laboratory 13 did not follow the method for samples K–N, P, and Q. Laboratory 13 data was eliminated from the statistical analysis of the soy food samples (blind replicates K–Q, L–P, M, and N) because the sample amount specified in the method was not used for the analysis. Sample N contained too little isoflavones for successful analysis.
A. Principle
Test samples are extracted at room temperature 1 h in acetonitrile–water–dimethylsulfoxide (DMSO; 58.5 + 39.0 + 2.5) in the presence of an internal standard, diluted with water to lower the organic concentrations, and centrifuged to remove insoluble materials. The extracts are filtered, isoflavone malonyl glucosides, acetyl glucoside, glucosides, and aglycones are separated on a C18 reversed-phase column with an acetonitrile–water mobile phase using gradient elution, and are determined by UV detection at 260 nm. Total isoflavones are calculated as the sum of the 12 forms (malonyl daidzin, malonyl glycitin, malonyl genistin, acetyl daidzin, acetyl glycitin, acetyl genistin, daidzin, glycitin, genistin, daidzein, glycitein, genistein) on an as-is basis. Aglycone equivalents of daidzein, glycitein, and genistein are calculated from the 12 isoflavone forms. The method uses standards for the 3 glucoside forms and the 3 aglucone forms. The response factors for the malonyl and acetyl forms are calculated from the glucoside response factors.
B. Apparatus
High-performance liquid chromatographic (HPLC) system.—With solvent degasser, binary gradient pumping, gradient mixer, injector capable of 5 μL injection (either autosampler or manual), column oven, UV detector at 260 nm, and data analysis system.
Chromatographic column.—End-capped, high carbon load (15–20%) octadecylsilane (ODS; C18) derivatized silica reversed-phase HPLC column, pore size from 100 to 125 Å, is recommended. Recommended dimensions: 250 × 3 mm with 5 μm packing. Alternately, a 250 × 4.6 mm column with 5 μm packing may be used. Columns of 150 mm length may also provide adequate resolution at smaller particle size (<3.5 μm). Column choice is critical in achieving desired resolution. The column must pass the system qualification requirements (see J and K).
Guard column.—Low dead volume C18 guard cartridge.
Analytical balance.—Readability 0.01 mg preferred.
Pipets.—Adjustable volume pipets to accurately deliver 0.500 mL.
Bottles.—Brown glass, 125 mL screw-cap, for stock standards.
Vials.—Brown glass, 22 mL, for working standards,
Volumetric flasks.—50 mL glass, Class A.
Test tubes.—Glass, screw-cap, 20 × 125 mm.
Mixer.—Inversion or “wrist” type mixer preferred.
Graduated cylinder.—20 or 25 mL.
Centrifuge.—For centrifuging 20 × 125 mm glass tubes at 200 × g.
Syringe filters.—For preparation of HPLC extracts: 0.45 μm PVDF, 13 or 25 mm diameter. Hydrophilic polypropylene or PTFE filters are acceptable. Nylon filters are not acceptable for this method.
Syringes.—All plastic, 3 or 5 mL.
HPLC vials.—Appropriate to the HPLC injection system
Mortar and pestle.
C. Reagents
Water.—High purity, deionized water, filtered through a 0.45 μm filter.
Acetonitrile.—HPLC grade.
Phosphoric acid.—85%, HPLC grade.
DMSO.—>99.9% purity.
Dilution solvent.—40% Acetonitrile. Add 600 mL water to 400 mL acetonitrile, and mix thoroughly. Use as the dilution solvent and as the needle rinse solvent if an autosampler is used with the HPLC system, or to clean syringes between manual injections if a manual HPLC injector is used.
Standards.—Obtain the soy isoflavone glucosides daidzin, glycitin, genistin; the aglycones daidzein, glycitein, genistein; and the internal standard apigenin, from commercial sources. Use purity of the compounds stated in certificates of analysis for each standard for correcting the concentration of the standards.
Internal standard solution.—2 mg/mL apigenin in DMSO. Weigh 0.2 g apigenin into a 125 mL brown bottle. Add 100 mL DMSO, and dissolve. Stable at least 6 months if stored tightly closed at room temperature. This is sufficient internal standard solution for analysis of 200 test samples. Larger batches should be prepared if analyzing a large number of samples. The same batch of internal standard solution must be used for all standards and samples in an analysis set.
Stock standard solution.—Accurately weigh to the nearest 0.01 mg, the following into a tared 50 mL volumetric flask: Daidzin 100 mg, glycitin 25 mg, genistin 100 mg, daidzein 10 mg, glycitein 10 mg, and genistein 10 mg. Record the exact weights after addition of each compound. Determine the weight W(I) of each component by subtracting the weight after addition from the weight before addition. Dilute to volume with DMSO, mix to dissolve, and record the total weight of the solution W(s). Transfer to a brown screw-cap bottle for storage at room temperature. Kept in a brown bottle, tightly closed, the stock standard solution is stable at least 6 months at room temperature.
Calculate the exact concentrations in mg/g of each component as follows:
where (I) is one of the 6 isoflavone standards.
Working standards.—Prepare working standard solutions by pipetting stock standard solution and internal standard solution into a tared 20 × 125 screw-cap test tube as indicated in Table 2008.03B. Tare the tube, add the indicated amount of stock standard, and determine the weight of standard added W(n). Add the internal standard by volume using the same pipettor that will be used for preparing samples. Add dilution solvent using a graduated cylinder. Transfer working standard solutions to brown screw-cap 22 mL vials for storage. Working standard solutions are stable at least 2 months at room temperature.
Table 2008.03B.
Preparation of working standards in 20 ×125 mm test tubes
| Working standard | Stock standard solution, mL | Internal standard solution, mL | Dilution solvent (to volume of 20 mL), mL |
|---|---|---|---|
| Blank | 0 | 0.500 | 19.5 |
| 1 | 0.500 | 0.500 | 19.0 |
| 2 | 1.000 | 0.500 | 18.5 |
| 3 | 1.500 | 0.500 | 18.0 |
| 4 | 2.000 | 0.500 | 17.5 |
| 5 | 2.500 | 0.500 | 17.0 |
Calculate the concentration of each component of the working standards as follows:
The resulting standards should be approximately the concentrations shown in Table 2008.03C.
Table 2008.03C.
Approximate concentrations of working standards
| Working standard | Daidzin, mg/mL | Glycitin, mg/mL | Genistin, mg/mL | Daidzein, mg/mL | Glycitein, mg/mL | Genistein, mg/mL | Apigenin, mg/mL |
|---|---|---|---|---|---|---|---|
| 1 | 0.050 | 0.0125 | 0.050 | 0.005 | 0.005 | 0.005 | 0.050 |
| 2 | 0.100 | 0.0250 | 0.100 | 0.010 | 0.010 | 0.010 | 0.050 |
| 3 | 0.150 | 0.0375 | 0.150 | 0.015 | 0.015 | 0.015 | 0.050 |
| 4 | 0.200 | 0.0500 | 0.200 | 0.020 | 0.020 | 0.020 | 0.050 |
| 5 | 0.250 | 0.0625 | 0.250 | 0.025 | 0.025 | 0.025 | 0.050 |
Mobile phase A.— Water containing 0.05% phosphoric acid. Thoroughly mix 1.0 mL phosphoric acid into 2.0 L water. Filter if necessary prior to use.
Mobile phase B.—HPLC grade acetonitrile.
Malonyl and acetyl isoflavone standards.—Do not use malonyl and acetyl isoflavone standards for this procedure. Use the glucoside standards for the determination of malonyl and acetyl forms. Determine the retention times and response factors for malonyl and acetyl isoflavones as in F and H.
D. Preparation of Test Solutions
Note: Products containing significant amounts of acetyl and malonyl isoflavones should be analyzed within 4 h of extraction. Malonyl and acetyl forms may not be stable in solution.
Grind solid materials to a fine powder in a mortar and pestle, and mix.
Weigh approximately 1 g soy protein or other soy product, approximately 20 mg soy isoflavone concentrate (40% isoflavones), or an amount to contain about 8–10 mg total isoflavones, but no more than 1 g total, into tared 20×150 mm screw-cap test tube. An analytical balance with readability to 0.01 mg should be used for isoflavone concentrates (10–100% isoflavones) and other concentrated test materials. Record sample weight S(w). Do not exceed 10 mg total isoflavones as the solubility of the isoflavones in the extraction solvent is limited.
Add exactly 0.500 mL internal standard solution, and swirl to wet the sample. Add 8.00 mL acetonitrile and swirl or shake to completely suspend solids. Add 5.00 mL high-purity deionized water, and mix. Ensure the entire sample is off the walls of the tube by vigorous agitation if necessary.
Mix 60 min on a wrist shaker or inversion mixer, or mix manually by vigorously inverting the tube several times at least once every 5 min over the 60 min extraction. (This step is not necessary for products that dissolve completely on initial mixing.)
Add 6.5 mL deionized water, and shake to mix.
Centrifuge 10 min at 200 × g to pellet insoluble residue. (This step is not necessary for products that completely dissolve.)
Remove a portion of the supernatant and filter though a syringe filter, B(m), into an HPLC sample vial.
E. Chromatography
Injection volume.—5 μL. Do not use larger injection volumes.
Flow rate.—0.65 mL/min for 3 mm id column: 1.5 mL/min for 4.6 mm id column.
Detection.—UV absorbanceat 260 nm.
Column temperature.—40°C.
Gradient elution.—Initial 10% B, with no hold time after injection, linear gradient to 30% B over 60 min, 3 min wash at 90% B, 10 min equilibration at 10% B.
F. Identification of Malonyl and Acetyl Isoflavones
(a) Pattern matching method
Compare to soy products known to contain high levels of malonyl and/or acetyl isoflavones. Pattern comparison to test samples known to contain high levels of malonyl or acetyl isoflavones can identify these forms in soy products. This procedure relies on the fact that the native form of isoflavones in soy is the malonyl form, and that the malonyl forms can be converted to the acetyl forms.
Acquire a minimally processed dry soy product (such as a high enzyme activity soy flour) or soy beans that are ground and defatted in the laboratory. To prepare defatted ground soybeans in the laboratory, grind about 5–10 g soybeans in a laboratory mill. Transfer the powdered beans to a plastic 50 mL screw-cap test tube and add about 30 mL hexanes. Cap and shake 1–2 min. Allow to settle, decant the liquid, and discard. Add 10 mL hexanes to the tube, cap, shake 1–2 min, and allow to settle. Decant the liquid and discard, hi a fume hood, transfer the wet powder to a watch glass and allow the residual hexanes to evaporate.
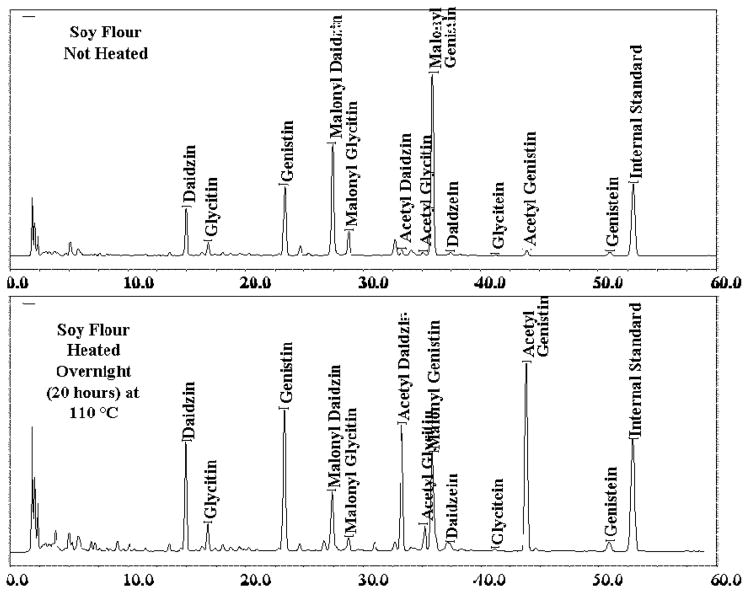
Split the dry product into 2 portions. Heat 1 portion in a shallow dish in an oven at 110–120°C for a minimum of 2 h to a maximum of 20 h to decarboxylate some of the malonyl isoflavones to acetyl isoflavones. Extract and chromatograph both samples as in D and E. Determine the retention times for the 6 known isoflavone components and for the internal standard from the standards previously run. Determine the retention times of the malonyl and acetyl isoflavones by matching the pattern expected for these analytes, as follows.
Identify the peaks for malonyl daidzin, malonyl glycitin, and malonyl genistin from the chromatogram of the soy product which was not heated. Malonyl daidzin should be the first significant peak after the genistin peak, and should have a retention time of about 25 min, as shown in Figure 2008.03A. Malonyl glycitin will follow the malonyl daidzin peak at about 26.5 min, and is typically about 25–30% of the size of the malonyl daidzin peak. Malonyl genistin should be the largest peak in the chromatogram, and should elute close to daidzein at about 33.5 min. Record the retention times for malonyl daidzin, malonyl glycitin, and malonyl genistin from the chromatogram of soy product that was not heated.
Figure 2008.03A.
Chromatogram of malonyl and acetyl isoflavones.
Identify the acetyl isoflavone peaks from the chromatogram of the heated soy product. Acetyl daidzin will be the first large peak in the chromatogram following malonyl glycitin, and will elute at about 31.5 min. Acetyl glycitin will elute close to (usually just before) malonyl genistin, and should be about 25–30% of the area of the acetyl daidzin peak. Resolution of acetyl glycitin from malonyl genistin is one of the critical parameters in performing the method. The acetyl genistin peak should be the largest peak in the chromatogram and should elute at about 41–42 min.
(b) Commercial standards
If malonyl and acetyl isoflavone standards are available, prepare them similarly to the glucoside standards, C(h) and (i). Malonyl and acetyl isoflavones are not stable in solution and must be analyzed within 4 h of preparation. Use commercial standards only for identifying the malonyl and acetyl isoflavone peaks, not for calibration.
G. Determination of Peak Areas and Calibration of Standards
Integrate peak area for quantitation. Use the apigenin peak as the internal standard.
Construct standard curves, plotting calibration standard concentration of each standard against the area ratio of the standard peak to the internal standard peak, using linear regression.
Calculate concentrations of the isoflavone glucoside and aglucone forms directly from their respective standard curves. Calculate the concentrations of the malonyl and acetyl glucoside forms from the glucoside standards, as described in H.
Calculate the amount of each of the 12 isoflavones in the original test sample as follows, where X is one of the 12 isoflavone forms:
Check for complete recovery of the internal standard peak in all test solutions by comparing peak area of the internal standard peak in each test chromatogram against the chromatograms of the standards.
H. Calibration of the Acetyl and Malonyl Isoflavones
Identity.—Identify the malonyl and acetyl isoflavone peaks as described in F.
Determination of malonyl and acetyl response factors.—Calibrate the HPLC system as described in G. Determine the internal standard response factors (amount/area ratio) for the 3 glucoside forms (daidzin, glycitin, and genistin). Note that some HPLC software calculates response factors as amount/area while others calculate area/amount.
Calculate the response factors (RF) of the malonyl and acetyl forms from the response factor of the respective glucoside forms, daidzin, glycitin, and genistin by multiplying (for amount/area RFs) or dividing (for area/amount RFs) the response factor for the glucoside by the ratio of the molecular weight of the malonyl or acetyl form to the glucoside form. The molecular weights of the isoflavone glucoside and acetyl- and malonyl-forms are shown in Table 2008.03D. Conversion factors from glucoside response factor to acetyl or malonyl glucoside response factors are shown in Table 2008.03E.
Table 2008.03D.
Molecular weights of soy isoflavones
| Aglycone | Glucoside | Acetyl glucoside | Malonyl glucoside | |
|---|---|---|---|---|
| Daidzein | 254.24 | 416.38 | 458.41 | 502.42 |
| Glycitein | 284.26 | 446.40 | 488.44 | 532.45 |
| Genistein | 270.24 | 432.38 | 474.41 | 518.42 |
Table 2008.03E.
Calibration conversion factors for acetyl and malonyl isoflavones
| Reference glucoside | Acetyl conversion factor | Malonyl conversion factor |
|---|---|---|
| Daidzin | 1.101 | 1.207 |
| Glycitin | 1.094 | 1.193 |
| Genistin | 1.097 | 1.199 |
The preferred method is to calculate the concentration of each malonyl or acetyl isoflavone as if it were a glucoside, then multiply the resulting concentration by the conversion factor to obtain actual concentration.
Many software packages can perform this correction. If the software uses amount/area as a response factor, the conversion factor is a multiplier. If the software calculates response factor as area/amount, the conversion factor is a divisor.
The concentration (mg/mL) of a malonyl- or acetyl-isoflavone is calculated as follows:
where XI is the acetyl or malonyl form of the isoflavone I, IS is the internal standard area, and RF is the internal standard response factor (amount/area ratio) of the isoflavone glucoside I.
For example, if the internal standard amount/area ratio response factor for genistin is 200, the area of the malonyl genistin peak is 400 000 and the area of the internal standard is 1 000 000, the concentration of malonyl genistin would be:
I. Calculations
(a) Total isoflavones
Calculate total isoflavones in mg/g by adding together the concentrations of the 12 isoflavone forms in the samples. Results may be expressed as analyzed in mg/g or may be divided by 10 to give percent by weight.
(b) Isoflavone aglycone equivalents
Isoflavone molecular weights are shown in Table 2008.03D and conversion factors are in Table 2008.03E. Aglycone equivalents are calculated by multiplying the aglycone, glucoside, acetyl glucoside, and malonyl glucoside concentrations for each isoflavone by their respective conversion factors and adding together the results.
For example, calculate daidzein equivalents as follows:
Calculate total isoflavone aglycone equivalents in mg/g by adding together the concentrations of the daidzein, glycitein, and genistein aglycone equivalents calculated in the samples. Results may be expressed as analyzed in mg/g or may be divided by 10 to give percent by weight.
J. HPLC System and Column Performance Criteria Qualification
This procedure is required for qualifying an HPLC column or system not previously used for the analysis.
(a) Daidzin peak shape
Prepare standards as specified in C(i). Using working standard 3, inject 5 μL onto the HPLC column.
Carefully examine the daidzin peak (the first standard peak to elute). The peak should be symmetrical and should not show a leading shoulder or a chair-like appearance. A leading shoulder or a chair-like appearance indicates excessive injection volume. Check to be certain the injection volume was set to 5 μL. If full loop injection was used, the problem could be caused by excessive dead volume where the loop attaches to the injector. Replace loop to correct this problem. Use of a larger diameter column (4.6 vs 3 mm) may also correct the problem.
(b) Resolution of internal standard
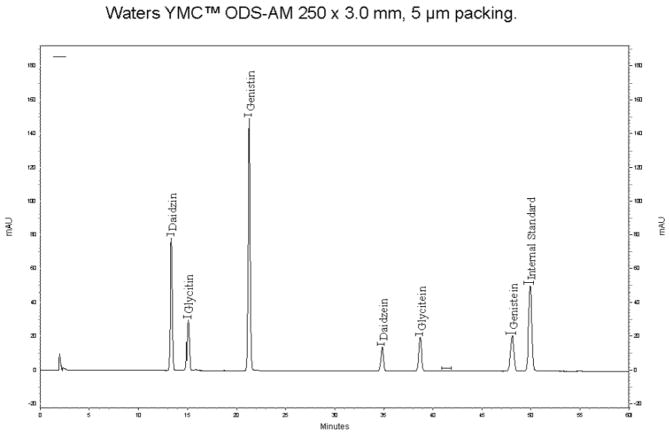
On the same chromatogram, examine the peaks for genistein and apigenin. Determine the retention time for the apigenin internal standard peak from the blank that was prepared along with the working standards. Apigenin and genistein should be baseline resolved from one another (R > 1.4).
Figure 2008.03B shows an example of a good chromatogram of a standard.
Figure 2008.03B.
Standard chromatogram.
(c) Critical resolution (acetyl gfycitin, malonyl genistin, and daidzein)
Prepare a heated soy product as described in F(a). Prepare and analyze the sample and ensure that the peaks for acetyl glycitin, malonyl genistin, and daidzein are fully resolved from one another, as shown in Figure 2008.03A.
Some HPLC columns are not capable of resolving these compounds and are not suitable for this method.
Some HPLC columns will change the elution order of these peaks. These columns can be used for the method provided the peaks are correctly identified and fully resolved from one another (see F for peak identification).
One cause of poor resolution in this region may be inadequate mixing of the A and B solvents on the HPLC system, which will be evident as peaks that are too broad and which vary in retention time from run to run. This may be corrected by addition of a mixer.
K. System Suitability
System suitability is a required procedure to ensure the HPLC system is working correctly.
Before running any test solutions, demonstrate the repeatability and lack of carryover of the HPLC system as follows:
System artifacts.—As the first 2 injections of the day, analyze the blank standard twice in succession. Inspect the 2 chromatograms for artifact peaks from the HPLC system Artifacts in the first chromatogram, absent in the second, indicate any buildup of impurities on the system. Artifacts present in both runs indicate impurities expected in every run. If the first chromatogram shows artifact peaks but the second chromatogram does not, inject a blank solution as the first sample in every analytical set. The presence of artifact peaks indicates impurities in the HPLC solvents, the needle wash system, or carryover in the injection system. These problems, if present, should be corrected.
Carryover.—Inject standard 5 and then the blank. Carefully examine the blank injection for carryover peaks. Calculate the carryover of any peaks seen in the blanks as a percentage of the concentration found in standard 5. Carryover of standard 5 to the blank inj ection should be <0.1 %.
Linearity of the standard curve.—Analyze each of the 5 standards, and construct a standard curve. The R2 of each standard curve should be >0.9990. If this linearity is not achieved, prepare fresh standards.
System precision.—Analyze 5 replicate analyses of standard 3. Calculate the concentration of each analyte and calculate the mean, standard deviation, and relative standard deviation (RSD) of the results. The RSD for all peaks should be ideally <0.2%. If the RSD is >0.5%, the system is not usable and the cause of the problem should be found and corrected.
L. Data Analysis and Reporting
Report each of the 12 isoflavones and the total isoflavone content.
Calculate and report aglycone equivalents for each isoflavone if desired.
M. Notes
(a) Reversed-phase HPLC column
As indicated in the method, any C18 reversed-phase HPLC column which passes the performance criteria requirements is acceptable. Many C18 columns on the market today are not capable of this analysis, so column choice is critical in achieving desired resolution.
The following columns have been tested with this method and worked acceptably:
Waters YMC-Pack™ ODS-AM™, 250 × 3.0 mm, 5 μm (recommended); 250 × 4.6 mm, 5 μm or 150 × 3.0 mm, 3 μm. Waters Xterra® RPi8, 3.0 × 150 mm 3.5 μm. Thermo BETASIL® C18 3.0 × 250 mm, 5.0 μm. Mac-Mod ACE® C18, 250 × 3.0 mm, 5 μm or 250 × 4.6 mm, 5 μm.
(b) Standard preparation
Note that the bulk standard is made by weight and is transferred to the working standards by volume, but concentrations are calculated by weight. This is done to improve the accuracy of the standards. Using weight in preparing and diluting bulk standards is essential to accurate results. Internal standards are transferred to the working standards with the same pipettor used for preparing the samples. Care must be used at this step to ensure volumetric transfer accuracy. Standards prepared in this manner usually have linear regression correlation coefficients (R2) values of 0.99999 or better.
Reference: J. AOACInt. 91, 489(2008).
Results and Discussion
Interlaboratory Study Results
Acceptable analytical data were submitted by 13 collaborating laboratories. Individual calculated results for total isoflavones are shown in Table 1 and total isoflavone aglycones are shown in Table 2. Statistical data from the collaborative results are shown in Table 2008.03A.
Table 1.
Interlaboratory data: total isoflavones, mg/g
| 40–50% Soy isoflavone concentrate blind replicates | 40–50% Soy isoflavone concentrate blind replicates | 40–50% Soy isoflavone concentrate blind replicates | 25% Soy isoflavone concentrate blind replicates | Soy isoflavone tablets | Isolated soy protein blind replicates | Water-washed soy protein concentrate blind replicates | Soy flour | Alcohol -washed soy protein concentrate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample
|
|||||||||||||||
| Lab | A | F | B | H | C | E | D | G | J | K | Q | L | P | M | N |
| 1 | 415.7 | 418.2 | 481.2 | 477.8 | 442.3 | 453.0 | 258.9 | 256.7 | 79.9 | 1.74 | 1.74 | 0.83 | 0.83 | 5.93 | 0.013 |
| 2 | 406.2 | 401.2 | 482.7 | 462.1 | 438.0 | 425.2 | 239.3 | 254.1 | 76.5 | 1.85 | 1.83 | 0.91 | 0.91 | 6.07 | 0.028 |
| 3 | 431.7 | 428.1 | 498.2 | 498.6 | 468.7 | 464.5 | 287.7a | 285.9a | 88.0 | 2.50a | 2.55a | 1.61a | 1.58a | 6.81 | 0.028 |
| 4 | 404.7 | 401.5 | 475.0 | 472.8 | 446.3 | 441.7 | 257.8 | 253.1 | 81.7 | 1.67 | 1.69 | 0.81 | 0.80 | 5.76 | 0.011 |
| 5 | 412.5 | 415.6 | 494.0 | 490.1 | 467.5 | 456.0 | 247.0 | 258.5 | 78.5 | 1.69 | 1.77 | 0.67 | 0.78 | 4.22a | 0.000 |
| 6 | 399.7 | 394.8 | 475.6 | 473.6 | 440.6 | 430.9 | 251.6 | 250.0 | 81.1 | 1.88 | 1.91 | 0.95 | 0.96 | 6.29 | 0.060 |
| 7 | 408.1 | 423.1 | 493.7 | 489.5 | 447.6 | 443.6 | 260.4 | 261.0 | 81.2 | 1.63 | 1.69 | 1.58b | 0.75 | 5.88 | 0.003 |
| 8 | 422.7 | 415.9 | 475.0 | 487.9 | 447.9 | 453.0 | 260.0 | 259.8 | 83.4 | 2.00 | 2.04 | 0.92 | 0.94 | 6.43 | 0.000 |
| 9 | 404.8 | 410.2 | 487.2 | 478.9 | 465.1 | 449.0 | 262.5 | 260.4 | 83.8 | 1.70 | 1.88 | 0.93 | 0.99 | 5.90 | 0.114 |
| 10 | 410.5 | 415.2 | 484.7 | 493.6 | 445.0 | 448.6 | 261.4 | 262.6 | 83.8 | 1.82 | 1.79 | 0.84 | 0.85 | 6.27 | −0.053 |
| 11 | 419.9 | 414.3 | 481.3 | 483.5 | 453.6 | 450.7 | 263.5 | 263.4 | 82.3 | 1.77 | 1.83 | 0.89 | 0.90 | 6.10 | 0.053 |
| 12 | 414.6 | 442.6b | 501.0 | 514.3 | 469.6 | 470.1 | 257.5 | 272.8 | 87.9 | 1.88 | 1.89 | 0.92 | 0.95 | 6.50 | 0.036 |
| 13 | 400.4 | 404.7 | 479.4 | 254.6b | 447.8 | 439.5 | 255.2 | 464.9b | 84.6 | 1.47c | 1.40c | 0.61c | 0.53c | 6.04c | 0.000c |
Eliminated single Grubbs’ test outlier.
Eliminated Cochran’s test outlier.
Laboratory 13 eliminated for the soy food samples as the method was not followed during analysis.
Table 2.
Interlaboratory data: total isoflavone aglycones, mg/g
| 40–50% Soy isoflavone concentrate blind replicates | 40–50% Soy isoflavone concentrate blind replicates | 40–50% Soy isoflavone concentrate blind replicates | 25% Soy isoflavone concentrate blind replicates | Soy isoflavone tablets | Isolated soy protein blind replicates | Water-washed soy protein concentrate blind replicates | Soy flour | Alcohol -washed soy protein concentrate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample
|
|||||||||||||||
| Lab | A | F | B | H | C | E | D | G | J | K | Q | L | P | M | N |
| 1 | 258.6 | 260.2 | 300.7 | 298.5 | 275.0 | 281.6 | 161.0 | 159.7 | 49.8 | 0.99 | 0.99 | 0.51 | 0.51 | 3.19 | 0.010 |
| 2 | 252.7 | 249.6 | 301.4 | 288.5 | 272.1 | 264.2 | 148.7 | 157.9 | 47.6 | 1.07 | 1.06 | 0.55 | 0.56 | 3.30 | 0.016 |
| 3 | 267.1 | 264.9 | 309.6 | 309.8 | 289.5 | 287.0 | 177.2a | 176.1a | 54.4 | 1.42a | 1.45a | 0.95a | 0.93a | 3.70 | 0.016 |
| 4 | 251.8 | 249.8 | 296.8 | 295.4 | 277.4 | 274.5 | 160.3 | 157.4 | 50.9 | 0.95 | 0.95 | 0.49 | 0.48 | 3.10 | 0.006 |
| 5 | 256.8 | 258.7 | 308.7 | 306.2 | 290.6 | 283.4 | 153.7 | 160.9 | 48.9 | 0.96 | 1.00 | 0.42 | 0.47 | 2.29a | 0.000 |
| 6 | 248.6 | 245.5 | 297.0 | 295.7 | 273.8 | 267.8 | 156.4 | 155.3 | 50.4 | 1.07 | 1.09 | 0.57 | 0.58 | 3.40 | 0.036 |
| 7 | 253.8 | 263.2 | 308.1 | 305.5 | 278.1 | 275.7 | 162.0 | 162.4 | 50.4 | 0.92 | 0.95 | 0.87b | 0.45 | 3.16 | 0.003 |
| 8 | 262.9 | 258.6 | 296.7 | 304.6 | 278.4 | 281.6 | 161.7 | 161.6 | 51.9 | 1.13 | 1.15 | 0.55 | 0.56 | 3.47 | 0.000 |
| 9 | 251.5 | 254.9 | 304.1 | 298.9 | 288.7 | 278.7 | 162.9 | 161.6 | 52.1 | 0.97 | 1.07 | 0.56 | 0.59 | 3.18 | 0.065 |
| 10 | 255.5 | 258.4 | 302.6 | 308.4 | 276.7 | 278.9 | 162.6 | 163.4 | 52.2 | 1.03 | 1.01 | 0.51 | 0.51 | 3.39 | −0.028 |
| 11 | 261.1 | 257.7 | 300.4 | 301.8 | 281.8 | 280.0 | 163.8 | 163.7 | 51.2 | 1.01 | 1.04 | 0.54 | 0.54 | 3.28 | 0.030 |
| 12 | 258.2 | 275.6b | 313.2 | 321.5 | 292.0 | 292.3 | 160.4 | 169.8 | 54.8 | 1.07 | 1.08 | 0.56 | 0.57 | 3.51 | 0.022 |
| 13 | 249.2 | 251.9 | 299.5 | 158.4b | 278.4 | 273.2 | 158.8 | 290.5b | 52.7 | 0.83c | 0.79c | 0.37c | 0.33c | 3.24c | 0.000c |
Eliminated single Grubbs’ test outlier.
Eliminated Cochran’s test outlier.
Laboratory 13 eliminated for the soy food samples as the method was not followed during analysis.
The isoflavone concentrates used for formulating dietary supplement ingredients gave very good results in the collaborative study (blind replicate sample pairs A–F, B–H, C–E, and D–G). HorRat values were close to 1 for all these samples.
Isoflavone dietary supplement tablets (sample J) had HorRat values <2. None of the laboratories were outliers by the Grubb’s test. The method is useful for this sort of product.
Data from Laboratory 13 was eliminated from the statistical analysis of the soy food samples (K–N, P, and Q) because the method was not followed. Significantly less sample was used for the analysis than the amount specified in the method.
The blind duplicate samples of isolated soy protein (samples K–Q) and water-washed soy protein isolate (samples L–P) had acceptable results with HorRat values well below 2.
The single sample of soy flour (sample M) had a HorRat value <2. In this minimally processed soy product, the malonyl isoflavone forms make up 80% of the total isoflavones.
The single sample of alcohol-washed soy protein (sample N) contained very low amounts of isoflavones. Most laboratories had extreme difficulty with this sample, and the results were not acceptable.
Collaborators’ Comments
The collaborators found a number of minor errors in the method, which were corrected and distributed to all collaborators.
Two of the laboratories had difficulty with achieving the needed HPLC resolution. In one case, replacement of the HPLC column corrected the problem, in the other, maintenance on the HPLC corrected the problem.
Recommendations
Based on the results of the collaborative study, it is recommended that the method be adopted Official First Action for analysis of total soy isoflavones in dietary supplements, dietary supplement ingredients, and processed soy foods containing at least 0.5 mg/g total isoflavones.
Table 2008.03F.
Aglycone conversion factors for soy isoflavones
| Aglycone | Glucoside | Acetyl glucoside | Malonyl glucoside | |
|---|---|---|---|---|
| Daidzein | 1.000 | 0.611 | 0.555 | 0.506 |
| Glycitein | 1.000 | 0.637 | 0.582 | 0.534 |
| Genistein | 1.000 | 0.625 | 0.570 | 0.521 |
Acknowledgments
We thank the following collaborators who participated in the study:
Bronwyn G. Hughes and Larry D. Lawson, Silliker Laboratories (formerly Plant Bioactives Research Institute), Orem, UT
Boqiang Fu, National Institute of Metrology, Beijing, China
Kailas Thakker, Analytical Solutions Inc., Durham, NC
Kurt Young and April Hall, Nutra Manufacturing, Greenville, SC
Klaus Reif, PhytoLab GmbH & Co. KG, Vestenbergsgreuth, Germany
A. Nilmini Wijewickreme and Cathy Sun, Cantest Ltd., Burnaby, BC, Canada
Andy Kohn, Covance Laboratories, Madison, WI
Peter Platteschor, Nutrilab B.V., Rijswijk NBr, The Netherlands
Pierluigi Delmonte, U.S. Food and Drug Administration, College Park, MD
Mengbin Zhao, Yanfen Shang, and Fang Zhou, Beijing Glorious-Land Technology Analytical Center Co. Ltd, Beijing, China
Lori B. Tremblay and Raul Sanchez, Green Mountain Laboratories, Inc., Montpelier, VT
Brian Mateja and Vida Popov-Rihter, Deibel Laboratories, Lincolnwood, IL
Renaud LeBouquin and G. Morice, Lareal, Vannes, France
Jan Swope and Rita Devore, Archer Daniels Midland Co., Decatur, IL
Footnotes
Collaborators: P. Delmonte, R. Devore, B. Fu, A. Hall, B. Hughes, A. Kohn, L. Lawson, R. LeBouquin, B. Mateja, G. Morice, P. Platteschor, V. Popov-Rihter, K. Reif, R. Sanchez, Y. Shang, C. Sun, J. Swope, K. Thakker, L. Tremblay, A.N. Wijewickreme, K. Young, M. Zhao, F. Zhou
The recommendation was approved by the Methods Committee on Dietary Supplements as First Action. See “Official Methods Program Actions” (2008) Inside Laboratory Management, March/April issue.
References
- 1.Griffith AP, Collison MW. J Chromatogr A. 2001;913:397–413. doi: 10.1016/s0021-9673(00)01077-3. [DOI] [PubMed] [Google Scholar]
- 2.Official Methods of Analysis. 18. AOAC INTERNATIONAL; Gaithersburg, MD: 2005. Method 2001.10. [Google Scholar]