Abstract
A multilaboratory collaborative study was conducted on a high-performance liquid chromatographic (HPLC) method utilizing UV detection, previously validated using AOAC single-laboratory validation guidelines for determination of hydrastine and berberine in goldenseal (Hydrastis canadensis L.) raw materials, extracts, and dietary supplements at levels ranging from 0.4 to 6% (w/w). Nine collaborating laboratories determined the hydrastine and berberine content in 8 blind samples. Sample materials included powdered botanical raw materials, whole root material, and 4 finished product dietary supplements containing either goldenseal powdered root material or extract. The materials were extracted with an acidified water and acetonitrile solution. HPLC analyses of the extracts were performed on a C18 column using UV detection at 230 nm. Results for powdered root material and capsule products ranged from about 0.2% (w/w) for each alkaloid to about 4% (w/w) for each alkaloid. Liquid tincture results were approximately 4000–5000 μg/mL for each alkaloid. Reproducibility relative standard deviations (RSDR) for hydrastine ranged from 2.68 to 6.65%, with HorRat values ranging from 0.77 to 1.89. RSDR for berberine ranged from 5.66 to 7.68%, with HorRat values ranging from 1.32 to 2.12. All finished products containing goldenseal extract yielded HorRat values <2.0. Based on these results, the method is recommended for Official First Action for determination of hydrastine and berberine in goldenseal raw materials and dietary supplement finished products containing powdered goldenseal and goldenseal extract.
Goldenseal (Hydrastis canadensis L.) is a perennial herb in the Ranunculaceae family native to southeastern Canada and northeastern United States (1). Its roots and rhizomes, which internally are bright yellow in color, have been used as a traditional medicine for the treatment of infection, inflammation, and as an immune system booster (1–3). It is taken orally to treat upper respiratory infections and gastrointestinal tract disorders, and is commonly found in commercial products in combination with Echinacea purpurea (L.) Moench (1–4). Modern herbalists consider it an alternative, anticatarrhal, anti-inflammatory, antiseptic, astringent, bitter tonic, laxative, and muscular stimulant (5–8). The commercial popularity of this herb has resulted in the overharvesting of wild goldenseal, and it is now listed in the Convention on International Trade in Endangered Species (CITES) of Wild Fauna and Flora (1,9).
The bioactive components of goldenseal are believed to be a series of isoquinoline alkaloids including hydrastine, 1.5–4.0%, w/w; berberine, 0.5–6.0%, w/w; and canadine, 0.5–1.0%; w/w; (10–17; Figure 1). With the popularity of this herb and the resulting overharvesting of it, economic adulteration with visually similar but less expensive species has become more prevalent (1,18). These adulterants include Chinese goldthread [Coptis japonica (Thunb.) Makino], Oregon grape root (Berberis aquifolium Pursh), Barberry (Berberis spp.), Yellow dock root (Rumex spp.), and Yellow root (Xanthorhiza simplicissima Marshall; 2, 17). In addition, Coptis chinensis has been sold as “Chinese Goldenseal,” and has been identified as a goldenseal adulterant in the U.S. market (19). While most of these adulterants contain berberine, both hydrastine and canadine are unique to Hydrastis canadensis L. (2, 17).
Figure 1.
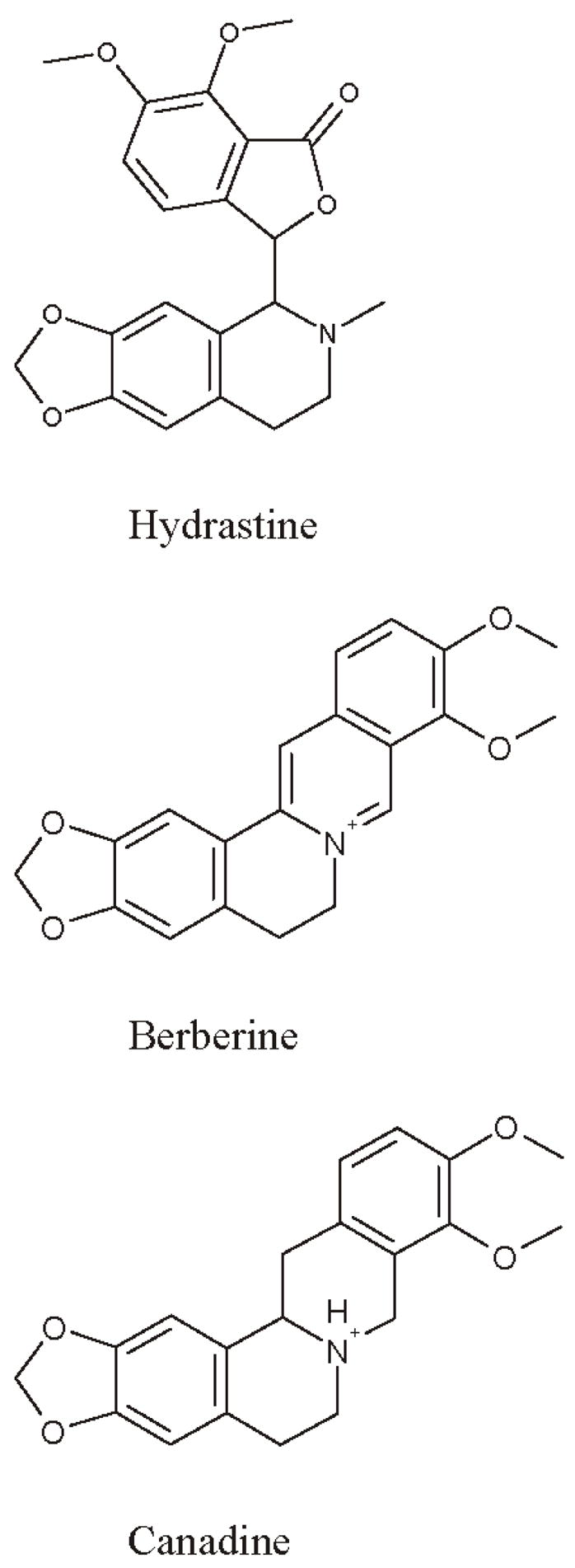
Chemical structure of goldenseal isoquinoline alkaloids.
Although several high-performance liquid chromatographic (HPLC) methods have been developed for the quantitation of berberine and hydrastine in goldenseal products (17, 18, 20–28), no Official Method of Analysis currently exists for these materials. A collaborative study of an HPLC-UV method for the determination of hydrastine and berberine in goldenseal raw materials and dietary supplement finished products was conducted after a single-laboratory validation (SLV) following AOAC guidelines was performed (29, 30). The collaborative study involved 8 blind materials submitted to 9 laboratories.
Single-Laboratory Validation
A complete description of the SLV study was previously published (29); results are summarized here.
Concentration Range
A 5-point calibration curve covering from 10 to 150 μg/mL each of hydrastine and berberine demonstrated that the method is linear over this range with coefficients of determination, R2, of 0.9995 and 0.9998, respectively.
Accuracy
To assess recovery of each analyte, solid hydrastine and berberine reference standards were spiked in triplicate at a concentration of 2% (w/w) into a root sample. The spiked samples were extracted as per the method and analyzed. Average recoveries were 87.8 ± 3.6% for hydrastine and 93.2 ± 3.2% for berberine.
Repeatability
Repeatability of the method was evaluated by performing triplicate extractions on 3 days (9 total replicates) on 250 mg of goldenseal powders, extracts, capsules, and tablet matrixes (8 in total) and 9 replicate dilutions (triplicates on 3 days) of goldenseal tincture. Repeatability relative standard deviation (RSDr) was found to be 0.9–2.7% for hydrastine and berberine, with HorRat values ranging from 0.26 to 0.88. A summary of the repeatability data is presented in Table 1.
Table 1.
Single-laboratory validation repeatability data
| Hydrastine
|
Berberine
|
|||||
|---|---|---|---|---|---|---|
| Material3 | Average, % (w/w) | RSDr found | HorRatr | Average, % (w/w) | RSDr found | HorRatr |
| GS-001 | 3.04 | 1.3 | 0.38 | 2.90 | 0.9 | 0.26 |
| GS-005 | 0.17 | 2.5 | 0.48 | 0.17 | 2.7 | 0.52 |
| GS-006 | 2.37 | 1.4 | 0.40 | 3.05 | 1.6 | 0.47 |
| GS-007 | 4.05 | 1.4 | 0.43 | 3.45 | 1.2 | 0.35 |
| GS-008 | 2.99 | 1.5 | 0.44 | 3.01 | 2.1 | 0.62 |
| GS-009 | 3.64 | 1.9 | 0.58 | 2.79 | 1.5 | 0.44 |
| GS-010 | 2.24 | 2.2 | 0.88 | 3.08 | 2.1 | 0.62 |
| GS-011 | 2.30 | 2.0 | 0.57 | 3.05 | 1.9 | 0.56 |
|
| ||||||
| Ethanol tincture | Average, ppm | RSDr found | HorRatr | Average, ppm | RSDr found | HorRatr |
|
| ||||||
| GS-003 | 3810 | 2.4 | 0.52 | 6209 | 0.65 | 2.8 |
GS-001 = Capsules containing goldenseal ground root powder; GS-003 = goldenseal liquid herbal extract (ethanol tincture); GS-005 = capsules containing Echinacea purpurea root extract with goldenseal root extract; GS-006 = goldenseal root powder; GS-007 = goldenseal ground root powder; GS-008 = goldenseal root, ground to 80 mesh in originating laboratory; GS-009 = goldenseal root, ground to 80 mesh in originating laboratory; GS-010 = goldenseal root powder, certified organic; GS-011 = Goldenseal root powder, certified organic.
Limit of Detection/Limit of Quantitation
The limits of quantitation (LOQ) for hydrastine and berberine were found to be 4.2 and 1.3 μg/mL in solution, respectively, corresponding to approximately 340 and 100 μg/g in the test material. The limits of detection (LOD) were calculated to be 1.3 and 0.4 μg/mL in solution, respectively, or 100 and 32 μg/g in the test material. Eight replicates of mixed standard 1 were made and the standard deviation (SD) of each analyte was calculated. The LOD was calculated as 3 times the SD and the LOQ was calculated as 10 times the SD (31,32).
Collaborative Study
Study Design
The HPLC-UV method was provided to 9 laboratories participating in the collaborative study. Each laboratory was sent 8 materials as blind samples. These materials consisted of 4 goldenseal root powder samples, one dietary supplement capsule product containing goldenseal root powder, one dietary supplement capsule product containing goldenseal extract, one dietary supplement tablet product containing Echinacea and goldenseal extracts, and one goldenseal ethanol tincture. Test materials are described in Table 2. Random identification numbers were assigned to each sample. Each sample was blinded in terms of composition and concentration of hydrastine and berberine.
Table 2.
Interlaboratory study results for goldenseal test materials
| Results, % (w/w) by Lab No.a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Materialb | Analyte | 1 | 2 | 3 | 4 | 5 | 7 | 8 | 9 |
| LPP-01-E01 | Hydrastine | 2.92 | 3.01 | 3.07 | 3.20 | 2.90 | 2.89 | 3.04 | 3.24 |
| Berberine | 2.66 | 2.66 | 3.00 | 3.10 | 3.03 | 2.58 | 2.92 | 2.89 | |
| LPP-01-E02 | Hydrastine | 2.56 | 2.61 | 2.57 | 2.70 | 2.57 | 2.61 | 2.63 | 2.76 |
| Berberine | 2.73 | 2.52 | 2.63 | 3.00 | 2.83 | 2.58 | 2.75 | 2.64 | |
| LPP-01-E03 | Hydrastine | 0.19 | 0.19 | 0.16 | 0.18 | 0.18 | 0.17 | 0.18 | 0.19 |
| Berberine | 0.18 | 0.18 | 0.17 | 0.18 | 0.18 | 0.16 | 0.15 | 0.17 | |
| LPP-01-E04 | Hydrastine | 2.35 | 2.56 | 2.51 | 2.54 | 2.39 | 2.31 | 2.44 | 2.51 |
| Berberine | 2.99 | 2.82 | 3.31 | 3.40 | 3.23 | 2.96 | 3.20 | 3.17 | |
| LPP-01-E05 | Hydrastine | 2.87 | 3.15 | 2.98 | 3.20 | 2.89 | 2.71 | 3.04 | 3.20 |
| Berberine | 2.76 | 2.95 | 3.22 | 3.40 | 3.20 | 2.79 | 3.06 | 3.05 | |
| LPP-01-E06 | Hydrastine | 3.45 | 3.69 | 3.71 | 3.70 | 3.49 | 3.23 | 3.60 | 3.77 |
| Berberine | 2.72 | 2.50 | 2.94 | 3.10 | 3.03 | 2.63 | 2.82 | 2.92 | |
| LPP-01-E07 | Hydrastine | 2.11 | 2.48 | 2.43 | 2.40 | 2.24 | 2.09 | 2.37 | 2.45 |
| Berberine | 2.74 | 3.04 | 3.28 | 3.20 | 3.31 | 2.84 | 3.10 | 3.18 | |
| LPP-01-E08c | Hydrastine | 3910 | 4010 | 4350 | 3910 | 4030 | 4370 | 4150 | 3980 |
| Berberine | 5340 | 6030 | 5940 | 5370 | 5730 | 4820 | 5460 | 5050 | |
Laboratory 6 was unable to meet system suitability requirements.
LPP-01-E01 = Capsules, goldenseal ground root powder, 550 mg/capsule; LPP-01-E02 = capsules, goldenseal extract, 250 mg/capsule; LPP-01-E03 = capsules, Echinacea purpurea root extract (680 mg) with goldenseal root extract (100 mg); LPP-01-E04 = goldenseal root powder; LPP-01-E05 = goldenseal root, ground to 80 mesh in originating laboratory; LPP-01-E06 = goldenseal root, ground to 80 mesh in originating laboratory; LPP-01-E07 = goldenseal ground root powder (certified organic); LPP-01-EOS = goldenseal ethanol tincture.
Results for LPP-01-EOS in μg/mL.
Collaborators
Nine laboratories agreed to participate in the collaborative study and received materials to conduct the study. The laboratories represented raw material, dietary supplement, and pharmaceutical finished product manufacturers, and contract analytical laboratories.
Test Sample Preparation
Root samples were ground to approximately 80 mesh in a Retsch Ultra Centrifugal Mill (Newton, PA) and homogenized prior to shipment. Powdered raw material samples were tested as-is. For tablet and hardshell capsule finished product dosage forms, a minimum of 20 dosage units were combined and powdered by the participating laboratories to reduce variations due to sample inhomogeneity.
Standards
Berberine chloride dihydrate and hydrastine reference standards were obtained from ChromaDex (Santa Ana, CA) and provided to each of the collaborating laboratories. The berberine chloride dihydrate had an assigned purity of 73.1% corrected for water content, and the hydrastine had an assigned purity of 99.0%. Palmatine and canadine reference standards, which were used for identification and system suitability purposes, were provided as solutions at concentrations of approximately 1000 μg/mL each in water–acetonitrile (10 + 90, v/v).
Test Material Homogeneity
Two of the root sample raw materials were obtained as whole roots and ground in a Retsch centrifugal mill to approximately 80 mesh. No additional homogeneity testing was performed on these materials. Multiple bottles of each of the same lot of the tablet, hardshell capsule, and ethanol tincture finished product dosage forms were combined, and subsamples of each of these composites were distributed to the laboratories.
Preparation and Shipment of Samples
Eight test materials were shipped to each of the collaborating laboratories. A sufficient amount of each finished product test material was packaged in suitable sized high-density polyethylene bottles by the Study Director. The bottles/vials were labeled with random identification codes. Reference standards were shipped in 1.7 mL amber glass vials. Test materials were shipped overnight at ambient temperature to the collaborating laboratories. Upon receipt, the laboratories were instructed to store the test materials at room temperature. The hydrastine reference standard was to be stored in a desiccator at refrigerated temperature (4°C) until use. The berberine chloride dihydrate reference standard was to be stored in a desiccator at room temperature until use. Palmatine and canadine reference standard solutions were to be stored refrigerated until use.
AOAC Official Method 2008.04 Hydrastine and Berberine in Goldenseal Raw Materials, Extracts, and Dietary Supplements
High-Performance Liquid Chromatography with UV First Action 2008
(Applicable for the determination of hydrastine and berberine content in goldenseal raw materials and dietary supplements containing goldenseal.)
See Table 2008.04A for the results of the interlaboratory study supporting acceptance of the method.
Table 2008.04A.
Interlaboratory study results for hydrastine and berberine in goldenseal raw materials, extracts, and dietary supplements
| Hydrastine
|
Berberine
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Materiala | Average (%,w/w) | sR | RSDR, % | HorRat | Average (%, w/w) | sR | RSDR, % | HorRat |
| LPP-01-E01 | 3.03 | 0.132 | 4.38 | 1.29 | 2.86 | 0.196 | 6.86 | 2.01 |
| LPP-01-E02 | 2.63 | 0.0703 | 2.68 | 0.44 | 2.71 | 0.153 | 5.66 | 1.64 |
| LPP-01-E03 | 0.181 | 0.0103 | 5.75 | 1.11 | 0.175 | 0.0118 | 6.86 | 1.32 |
| LPP-01-E04 | 2.45 | 0.0931 | 3.80 | 1.09 | 3.14 | 0.195 | 6.22 | 1.85 |
| LPP-01-E05 | 3.01 | 0.176 | 5.86 | 1.73 | 3.05 | 0.219 | 7.17 | 2.12 |
| LPP-01-E06 | 3.58 | 0.180 | 5.03 | 1.53 | 2.83 | 0.205 | 7.24 | 2.12 |
| LPP-01-E07 | 2.32 | 0.154 | 6.65 | 1.89 | 3.09 | 0.204 | 6.62 | 1.96 |
| LPP-01-E0 | 4502b | 203 | 4.52 | 1.00 | 6021 | 462 | 7.68 | 1.78 |
LPP-01-E01 = Capsules containing goldenseal ground root powder, 550 mg/capsule; LPP-01-E02 = capsules containing goldenseal extract, 250 mg/capsule; LPP-01-E03 = capsules containing Echinacea purpurea root extract (680 mg) with goldenseal root extract (100 mg); LPP-01-E04 = goldenseal root powder; LPP-01-E05 = goldenseal root, ground to 80 mesh in originating laboratory; LPP-01-E06 = goldenseal root, ground to 80 mesh in originating laboratory; LPP-01-E07 = goldenseal ground root powder (certified organic); LPP-01-E08 = goldenseal ethanol tincture.
Results in μg/g. Original results reported in μg/mL by laboratories and converted to μg/g using density of 0.908 g/mL.
A. Priniciple
Hydrastine and berberine are extracted from the matrixes with a mixture of water–acetonitrile–phosphoric acid (70 + 30 + 0.1, v/v/v). The resulting solutions are then subjected to reversed-phase high-performance liquid chromatographic (HPLC) analysis using a C18 column and UV detection. Quantitation is performed using hydrastine and berberine chloride dihydrate external standards and a 5-point calibration curve.
B. Apparatus
Note: Equivalent apparatus may be substituted. All volumetric pipets and volumetric flasks are Class A.
LC system.—Suitable HPLC system equipped with pump, autosampler, and UV detector. HPLC operating conditions: column temperature, 40°C; mobile phase flow rate, 0.75 mL/min; injection volume, 10 μL; detection, 230 nm.
LC column.—Agilent (Santa Clara, CA) Zorbax Eclipse XDB-C18, 4.6 ×150mm, 3.5 μm particle size, 80 Å pore size, equipped with guard column, or equivalent.
Analytical balance.—Readability, ±0.1 mg.
Ultrasonication bath.
Vortex mixer.
Centrifuge.—Table-top, capable of centrifuging 50 mL tubes at 5000 rpm.
Low actinic glass (LAG) volumetric flasks.—50, 100, and 200 mL.
pH Meter.—Calibrated between pH 4.1 and 7.0.
Syringe filters.—PTFE, 0.2 μm pore.
LC injection vials.—2 mL, with teflon-coated caps.
Syringe.—Luer-Lok™, 3 or 10 mL.
C. Reagents
Note: Chemicals from other suppliers meeting the specifications may also be used. Acetonitrile and triethylamine are flammable and should be stored away from heat and flames.
Solvents.—Acetonitrile, HPLC grade; water, HPLC grade.
Ammonium formate.—Purity ≥96%.
Formic acid.—Purity ≥98%.
o-Phosphoric acid, 85%.—HPLC grade.
Triethylamine.—Purity ≥99% by gas chromatography (GC).
Mobile phase A.—For every 1000 mL mobile phase A, dissolve 1.61 g ammonium formate in 1 L water. Adjust pH to 3.8 with formic acid.
MobilephaseB.—For every 1000 mL mobile phase B, add 1 mL triethylamine to 1 L acetonitrile. Mix well.
Extraction solvent.—For every liter of extraction solvent, mix 700 mL water, 300 mL acetonitrile, and 1 mL phosphoric acid. Allow to equilibrate to room temperature before use.
Diluent.—For every 100 mL diluent, mix 90 mL acetonitrile with 10 mL water and mix well. Allow to equilibrate to room temperature before use.
Reference standards.—(1R,9S)-(−)-β-Hydrastine.— AHP grade (ChromaDex, Santa Ana, CA). Berberine chloride dihydrate.—AHP grade (ChromaDex). DL-Canadine (tetrahydroberberine).—ChromaDex. Palmatine chloride (tetramethoxyprotoberberine chloride).—ChromaDex.
D. Preparation of Test Solutions
(a) Preparation of standard solutions.—Berberine stock solution
Prepare a 1000 μg/mL solution of berberine by dissolving approximately 13.7 mg (record exact weight) of berberine chloride dihydrate (73.1% berberine) in exactly 10 mL diluent (volumetric pipets or glassware must be used). Mix on a Vortex mixer and/or sonicate at room temperature until all solid material has been dissolved. Store at +4°C and protect from light.
Hydrastine stock solution
Prepare a 1000 μg/mL solution of hydrastine by dissolving approximately 10.1 mg (record exact weight) of (1R,9S)-(−)-β-hydrastine (99.0% pure) in exactly 10 mL standard diluent (volumetric pipets or glassware must be used). Mix on a Vortex mixer and/or sonicate at room temperature until all solid material has been dissolved. Store at +4°C.
Mixed stock solution
Using the provided canadine and palmatine chloride stock solutions and the berberine and hydrastine stock solutions, prepare a mixed stock solution containing 250 μg/mL berberine and hydrastine and 125 μg/mL palmatine and canadine. Store this mixed stock solution at +4°C, protected from light.
Calibration standard solutions
Prepare calibration solutions as shown in Table 2008.04B by pipeting the indicated amount of mixed stock standard solution into an HPLC autosampler vial and adding the specified amount of diluent with a calibrated automatic pipettor.
Table 2008.04B.
Preparation of calibration solutions
| Standard | Volume mixed stock solution, μL | Volume Extraction solvent, μL | Expected concn hydrastine and berberine, mg/mL |
|---|---|---|---|
| Mix Std 1 | 1 00 Mix Std 4 | 900 | 0.01 |
| Mix Std 2 | 1 00 Mix Std 6 | 900 | 0.02 |
| Mix Std 3 | 200 | 800 | 0.05 |
| Mix Std 4 | 400 | 600 | 0.10 |
| Mix Std 5 | 600 | 400 | 0.15 |
| Mix Std 6 | 800 | 200 | 0.20 |
(b) Preparation of sample test solutions.—Raw materials, hardshell capsules, and tablets
For root samples, grind the material so that it passes through an 80 mesh sieve. For capsules samples, combine the contents of 20 capsules and mix well. For tablet samples, crush 20 tablets using a mortar and pestle and mix well.
Accurately weigh about 250 mg (±20 mg) of test sample into a 50 mL plastic screw-cap centrifuge tube. Volumetrically pipet 20.0 mL extraction solvent into the centrifuge tube, cap, and mix on a Vortex mixer for 10 s. Sonicate the slurry for 10 min at room temperature, then mix on a Vortex mixer for another 10 s. Centrifuge the mixture for 5 min at 5000 rpm. Dilute 200 μL supernatant with 800 μL diluent in an HPLC autosampler vial. Label as Test Solution.
Tinctures
Pipet 1 mL tincture into a 10 mL volumetric flask and dilute to volume with diluent. Filter a portion of this solution through a 0.2 μm PTFE syringe filter into an HPLC autosampler vial. Label as Test Solution.
E. Determination
(a) System suitability test
Equilibrate the LC system with the mobile phase for at least 10 min until a stable baseline is obtained. Make 5 replicate 10 μL injections of Mixed Standard No. 5. The system is considered suitable if (1) the capacity factor (k′) for hydrastine is not <1.0, (2) the resolution between palmatine and berberine is ≥1.0, (3) the RSD of the peak area for both hydrastine and berberine is ≤2.0%, (4) the tailing factor, T, of canadine (the latest eluting component) is ≤2.0, and (5) the number of theoretical plates for canadine is at least 5000.
(b) Calibration
Make single 10 μL injections of Mixed Standard Solutions 1–5 (do not inject Mixed Standard Solution No. 6). Calculate the slope, y-intercept, and r2 value for the calibration curve for both hydrastine and berberine. The r2 value should be ≥0.995.
(c) Injection
Make single 10 μL injections of each test solution.
F. Calculations
The amount of each alkaloid in the test material in percent (w/w) is calculated as follows (excluding tinctures):
where P0 = peak area of the analyte (hydrastine or berberine) in sample chromatogram; b0 = y-intercept of calibration curve for the analyte; m0 = slope of calibration curve for the analyte; V = volume of Test Solution 1, in mL; D = dilution factor (5); W = sample weight, in mg.
The amount of each alkaloid in the tincture test material in mg/mL is calculated as follows:
where P0 = peak area of the analyte (hydrastine or berberine) in sample chromatogram; b0 = y-intercept of calibration curve for the analyte; m0 = slope of calibration curve for the analyte: D = dilution factor of Test Solution 1 (10).
Milligrams per tablet (mg/tab) is calculated from percent (w/w) as follows:
where TW = average tablet weight in milligrams.
Milligrams per capsule (mg/cap) is calculated from percent (w/w) as follows:
where FW = average capsule fill weight in milligrams.
Reference: J. AOAC Int. 91, 694(2008).
Results and Discussion
Nine laboratories agreed to participate in the collaborative study. Eight laboratories submitted acceptable data before the submission deadline. The remaining laboratory experienced instrumental problems and was unable to meet the system suitability requirements; therefore, the data were not used.
Collaborative Study Results
Test samples were assigned random codes prior to shipment to collaborators, and then decoded when results were returned. Results, in percent (w/w) of hydrastine and berberine in the test material, for each of the 8 blind samples are presented in Table 2. Table 2008.04A presents a statistical summary of the results. Reproducibility standard deviations (sR), reproducibility relative standard deviations (RSDR), and HorRat values are presented in these tables. The HorRat was calculated as RSDR (observed)/RSDR (predicted), where the RSDR (predicted) is calculated using the equation
where C is the measured analyte concentration in decimal mass units (33, 34). Because materials were not submitted as blind duplicates, overall repeatability standard deviation (sr) and RSD could not be calculated; however, the extensive SLV repeatability study provides information on the method repeatability. Grubbs and double Grubbs tests were used to remove statistical outliers where appropriate; however, no outliers were observed using these tests.
Collaborators’ Comments
Laboratory 6 was unable to meet system suitability requirements due to problems with injector reproducibility; therefore, data from this laboratory were not used. Laboratory 7 noted that extracted samples were left overnight prior to analysis. Several laboratories noted difficulty in obtaining adequate resolution between palmatine and berberine on columns other than those specified in the method.
Performance Characteristics
Hydrastine RSDR for all materials was acceptable, with HorRat values ranging from 0.77 to 1.9. The berberine reproducibility was acceptable for finished product dosage forms containing goldenseal extract, with HorRat values ranging from 1.3 to 1.8 for these materials. HorRat values for materials containing goldenseal root powder were slightly higher, with values ranging from 1.8 to 2.1, and an average HorRat value for these materials of 2.0. No Grubbs outliers were identified for any material; therefore, all data were used.
Recommendations
On the basis of the results of the study and the SLV results obtained for the method, it is recommended that the method be adopted Official First Action for the determination of hydrastine and berberine in goldenseal powdered raw materials and finished product dietary supplements containing goldenseal extracts or root powder.
Acknowledgments
This work was funded in part by Health Canada’s Natural Health Products Research Program and the Investment Agriculture Foundation of British Columbia, through the Agri-Food Futures Fund, Health Product and Functional Food Program, a joint venture between Agriculture and Agri-Food Canada and the British Columbia Ministry of Agriculture and Lands. The British Columbia Innovation Council delivers the initiative. Support for the collaborative study was provided by Nutri-Net Canada, a collaborative initiative among regional and national industry, trade and research organizations, and government bodies dedicated to advancing the development of the functional food and natural health product sector in Canada. Nutri-Net Canada is funded by Agriculture and Agri-Food Canada under the Science and Innovation Broker Program.
We gratefully acknowledge the following collaborators for participation in the study: Chuck Chang, Natural Factors Laboratory, Burnaby, BC, Canada
Changdong Jin, Labs-Mart Inc., Edmonton, AB, Canada
Ron Kuriyedath, SGS Canada Inc., Vancouver, BC, Canada
Sean Lidstone, Enviro-Test Laboratories, Edmonton, AB, Canada
Lan Ly, Applied Chemistry Laboratories, Vancouver, BC, Canada
Yuan-Chun Ma, Canadian Phytopharmaceutical Corp., Richmond, BC, Canada
Phillip Sigmund, Hypex Technologies Inc., Saltspring Island, BC, Canada
Ron Smith, Natural Factors Nutritional Products, Coquitlam, BC, Canada
Nilmini Wijewickreme, CanTest Ltd, Burnaby, BC, Canada
Footnotes
The recommendation was approved by the Methods Committee on Dietary Supplements as First Action. See “Official Methods Program Actions,” (2008) Inside Laboratory Management, March/April issue.
Contributor Information
Paula N. Brown, Natural Health Products Research Group, Technology Centre and School of Health Sciences, British Columbia Institute of Technology, 3700 Willingdon Ave, Burnaby, BC, Canada V5G 3H2
Mark C. Roman, Tampa Bay Analytical Research, Inc., 10810 72nd St, Suite 206, Largo, FL 33777
References
- 1.Borchers AT, Keen CL, Stern JS, Gershwin EM. Am J Clin Nutr. 2000;72:339–347. doi: 10.1093/ajcn/72.2.339. [DOI] [PubMed] [Google Scholar]
- 2.American Herbal Pharmacopoeia and Therapeutic Compendium. Goldenseal Root (Hydrastis canadensis): Standard of Analysis, Quality Control, and Therapeutics. Santa Cruz, CA: 2001. pp. 10–18. [Google Scholar]
- 3.Scazzocchio F, Cometa MF, Palmery M. Fitoterapia. 1998;69:58–59. [Google Scholar]
- 4.Schieffer GW, Kohn M. J Liq Chromatogr Rel Technol. 2002;25:263–274. [Google Scholar]
- 5.Tierra M. The Way of Herbs. Pocket Books; New York, NY: 1998. [Google Scholar]
- 6.Grieve M. A Modern Herbal. Dover Publications, Inc.; New York, NY: 1971. [Google Scholar]
- 7.Mills S, Bone K. Principles and Practice of Phytotherapy. Churchill Livingstone; Philadelphia, PA: 2000. [Google Scholar]
- 8.North Carolina Medicinal Herbs: Goldenseal. University of North Carolina; Chapel Hill: http://www.med.unc.edu/phyrehab/ncmedicinalherbs/goldenseal/Goldenseal-hp.pdf. [Google Scholar]
- 9.Foster S, Tyler VE. Tyler’s Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedies. The Haworth Herbal Press; Binghamton, NY: 1999. [Google Scholar]
- 10.Budzinzki JW, Foster BC, Vandenhoek S, Arnason JT. Phytomedicine. 2000;7:273–282. doi: 10.1016/S0944-7113(00)80044-6. [DOI] [PubMed] [Google Scholar]
- 11.Chadwick LR, Wu CD, Kinghorn AD. J Liq Chromatogr Rel Technol. 2001;24:2445–2453. [Google Scholar]
- 12.Gentry EJ, Jampani HB, Keshavarz-Shokri A, Morton MD, Vander Velde D, Kelikepalli H, Mitscher LA. J Nat Prod. 1998;61:1187–1193. doi: 10.1021/np9701889. [DOI] [PubMed] [Google Scholar]
- 13.Kim JS, Tanaka K, Shoyama Y. Analyst. 2004;129:87–91. doi: 10.1039/b311304c. [DOI] [PubMed] [Google Scholar]
- 14.Lin HL, Liu TY, Wu CW, Chi CW. Br J Cancer. 1996;81:416–422. doi: 10.1038/sj.bjc.6690710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messana I, LaBua R, Galeffi C. Gazz Chim Ital. 1980;110:539–543. [Google Scholar]
- 16.Zeng X, Zeng X. Biomed Chromatogr. 1999;13:442–444. doi: 10.1002/(SICI)1099-0801(199911)13:7<442::AID-BMC908>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 17.Betz JM, Musser SM, Larkin GM. Differentiation Between Goldenseal (Hydrastis canadensis L.) and Possible Adulterants by LC/MS. Proceedings of the 39th Annual Meeting of the American Society of Pharmacognosy; Orlando, FL. July 19–23.1998. [Google Scholar]
- 18.Chen CM, Chang HC. J Chromatogr B. 1995;665:117–123. doi: 10.1016/0378-4347(94)00517-9. [DOI] [PubMed] [Google Scholar]
- 19.Chung B. Natural Plant Extracts Export Market Opportunities in the USA. Barton; Australia: 2000. RIRDC Publication No. 00/51. [Google Scholar]
- 20.Abourashed EA, Khan IA. J Pharm Sci. 2003;90:817–822. doi: 10.1002/jps.1035. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Fitzloff JF. J Pharm Pharmacol. 2002;54:435–439. doi: 10.1211/0022357021778510. [DOI] [PubMed] [Google Scholar]
- 22.United States Pharmacopeia–National Formulary (USP 30-NF25) 2007. The United States Pharmacopeial Convention, Inc.; Rockville, MD: pp. 2389–2390. [Google Scholar]
- 23.Weber HA, Zart MK, Ferguson SL, Greaves JG, Clark AP, Harris RK, Overstreet D, Smith C. J Liq Chromatogr Rel Technol. 2001;24:87–95. [Google Scholar]
- 24.Weber HA, Zart MK, Hodges AE, Molloy HM, O’Brien BM, Moody LA, Clark AP, Harris RK, Overstreet JD, Smith CS. J Agric Food Chem. 2003;51:7352–7358. doi: 10.1021/jf034339r. [DOI] [PubMed] [Google Scholar]
- 25.Edwards DJ, Draper EJ. J Am Pharm Assoc. 2003;43:419–423. doi: 10.1331/154434503321831148. [DOI] [PubMed] [Google Scholar]
- 26.Weber HA, Zart MK, Hodges AE, White KD, Barnes SM, Moody LA, Clark AP, Harris RK, Overstreet JD, Smith CS. J AOAC Int. 2003;86:476–483. [PubMed] [Google Scholar]
- 27.Leone MG, Cometa MF, Palmery M, Saso L. Phytother Res. 1996;10:S45–S46. [Google Scholar]
- 28.Schieffer GW, Pfeiffer K. J Liq Chromatogr Rel Technol. 2001;24:2415–2427. [Google Scholar]
- 29.Brown PN, Paley LA, Roman MC, Chan M. Pharm Biol. 2008;46:135–144. [Google Scholar]
- 30.AOAC INTERNATIONAL. AOAC Guidelines for Single-Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals. Gaithersburg, MD: 2002. pp. 1–38. [Google Scholar]
- 31.Long GL, Winefordner JD. Anal Chem. 1983;55:712A–724A. [Google Scholar]
- 32.MacDougall D, Amore FJ, Cox GV, Crosby DG, Estes FL, Freeman DH, Gibbs WE, Gordon GE, Keith LH, Lal J, Langner RR, McClelland NI, Phillips WF, Pojasek RB, Sievers RE, Smerko RG, Wimert DC, Crummett WB, Amore FJ, Freeman DH, Libby R, Laitinen HA, Phillips WF, Reddy MM, Taylor JK. Anal Chem. 1980;52:2242–2249. [Google Scholar]
- 33.Horwitz W. Anal Chem. 1982;54:67A–76A. [Google Scholar]
- 34.Horwitz W, Albert R. J AOAC Int. 2006;89:1095–1109. [PubMed] [Google Scholar]


