Abstract
A single laboratory validation (SLV) study was conducted for a liquid Chromatography (LC) method for the determination of total and all-trans-β-carotene in a variety of dietary supplements, including multivitamin tablets, softgels, capsules, and beadlet raw materials. Extraction variants were developed for the different types of supplements tested based upon the supplement type and level of β-carotene. Water dispersible formulations such as powders, emulsions, tablets, and capsules were enzymatically digested with protease and extracted with dichloromethane–ethanol. Oily suspensions were directly dissolved in dichloromethane–ethanol. After appropriate dilution or concentration, the extracts were chromatographed by using either a reversed-phase C18 column or, in products containing high amounts of α-carotene, a reversed-phase C30 column. The LC systems provided linear responses in the range of 0.1–50 μg β-carotene/mL. The main geometrical isomers of β-carotene (all-trans, 9-cis, 13-cis, and 15-cis) were well separated from each other and from other carotenoids such as α-carotene, cryptoxanthin, lutein, lycopene, and zeaxanthin. Duplicate determinations of total β-carotene performed by 2 technicians in 8 different test materials on 5 different days resulted in relative standard deviations of 1.2–4.4%. Recoveries determined for supplements and beadlet raw material spiked with β-carotene levels of 10 μg to 100 mg/test portion and 0.2–40%, respectively, ranged from 97.5 to 102.1%. On the basis of the accuracy, precision, and recovery results from the SLV study, the method is suggested for a collaborative study on the determination of total and all-trans-β-carotene in dietary supplements.
β-carotene is generally regarded as the most commercially important and widely used carotenoid. It is used as a food coloring agent, an antioxidant, and an important and safe pro-vitamin A source (1–3). β-carotene is currently incorporated in a wide variety of dietary supplements, including multivitamin, vitamin A, and antioxidant formulations. Recently, additional carotenoids, including lutein and lycopene, have been the subject of nutritional studies and are now also incorporated, both in combination products and in dietary supplements available to the general public. Spectrophotometry is still a common technique for the analysis of β-carotene in commercial product forms. Schierle et al. (4) reported a spectrophotometric procedure for the determination of total β-carotene in food additives with varying cis-/trans-ratios using an isobestic wavelength. However, spectrophotometric methods cannot differentiate between all-trans-β-carotene and cis-isomers of β-carotene, which may be formed during processing (5–12). Spectrophotometric analytical procedures are also not capable of determining β-carotene in combination products containing other carotenoids such as α-carotene, lutein, or lycopene. β-carotene and carotenoids in general have been intensively studied by liquid chromatography (LC), and procedures have been reported for the separation of β-carotene cis-/trans-isomers (13–18) and for β-carotene in supplements and foods (19, 20). Thus, a chromatographic procedure capable of separating all-trans-β-carotene from the corresponding cis-isomers and from other commercially used carotenoids was deemed necessary to properly determine the β-carotene content in dietary supplements.
METHOD
Test and Negative Control Materials
(a) Test materials
Dietary supplements test materials used in the study were chosen to represent the range of dietary supplements containing β-carotene currently available to the general public and included the following (1000 IUβ-carotene = 0.6 mg β-carotene; 21):
Multivitamin tablets; claim: vitamin A 3500 IU, 29% as β-carotene.
Softgels with β-carotene in soybean oil; claim: β-carotene 25 000 IU.
Capsules with β-carotene in fish oil; no claim for β-carotene.
Softgels with β-carotene from carrot oil extract; claim: vitamin A (as β-carotene ) 10 000 IU.
Tablets with β-carotene; claim: 25 mg β-carotene.
Capsules with β-carotene; claim 6 mg β-carotene.
Softgels with vitamin A (as β-carotene ) and vitamin E; claim: vitamin A 25 000 IU (as β-carotene).
Beadlets raw material with pure β-carotene; claim: 20% β-carotene.
(b) Negative control materials
Placebo multivitamin tablets, Reference No. 81.16.03.32 (DSM Formula No. 80.23) containing vitamin A acetate, vitamin D3, vitamin E, ascorbic acid, thiamin mononitrate, vitamin B2, nicotinamide, pyridoxine HCl, vitamin B12, folic acid, pantothenic acid, biotin, vitamin K1, iron fumarate, magnesium oxide, potassium iodide, zinc sulfate monohydrate, manganese sulfate monohydrate, copper sulfate anhydrous, chromium chloride hexahydrate, sodium selenite anhydrous, sodium molybdate dihydrate, calcium phosphate anhydrous, potassium chloride, silicon dioxide, cellulose, stearic acid, magnesium stearate, n-vinyl-2-pyrollidone. Coated with Sepifilm LP014 and Sepifilm 6097 Red (Seppic, Inc., Fairfield, NJ).
Lycopene 10% WS (water soluble) placebo beadlets, Lot UT02071003 32, containing fish gelatin, saccharose, vitamin C palmitate, maize oil, DL-α-tocopherol, water.
Test materials for dietary supplements were obtained from commercial sources and provided by AOAC INTERNATIONAL. β-carotene beadlets, reference materials, and the negative control materials were provided by DSM Nutritional Products (Basel, Switzerland). Test and negative control materials were stored at 5°C in the dark (refrigerator).
Principle
Water-dispersible formulations such as powders, emulsions, tablets, and capsules are digested with protease and extracted with dichloromethane and alcohol. Oily suspensions are dissolved directly in dichloromethane and alcohol. The extract is chromatographed on a C18 isocratic LC system that separates the predominant geometrical isomers of β-carotene from each other (Figure 1) and from other carotenoids such as all-trans-α-carotene, lycopene, cryptoxanthin, lutein, and zeaxanthin. In the case of products with relatively high α-carotene content, the cis-isomers of α-carotene can interfere. In this case, the extract is also chromatographed with a more selective but longer running C30 reversed-phase LC system that avoids this interference.
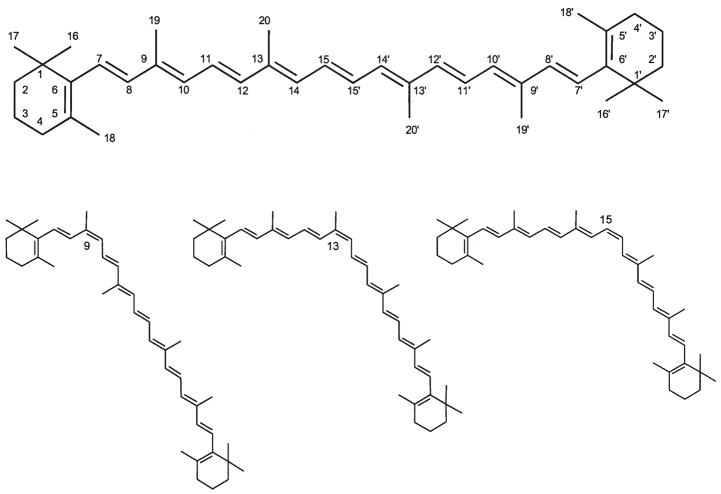
Figure 1.
The predominant geometrical isomers of β-carotene : all-trans-β-carotene, 9-cis-β-carotene, 13-cis-β-carotene, and 15-cis-β-carotene.
Scope
The method is suitable for the determination of all-trans and total β-carotene. The term total β-carotene comprises all-trans-β-carotene and all other compounds identified as cis-isomers of β-carotene. The method is applicable to the determination of 0.01 mg or greater of total β-carotene in tablets, capsules, and >0.2% β-carotene in oily or water-dispersible raw materials in the presence of other carotenoids such as lycopene, α-carotene, and xanthophylls.
Apparatus
Balances.—With readability of 0.01 mg, precision [standard deviation (SD)] of ±0.015 mg, capacity 205 g (AT261 DeltaRange; Mettler-Toledo, Nänikon-Uster, Switzerland); with readability of 0.01 g, precision (SD) of ± 0.005 g, capacity 2100 g (PM2000; Mettler-Toledo).
Spectrophotometer.—Dual beam, wavelength range of 190–900 nm, 1.5 nm fixed spectral bandwidth, wavelength accuracy of 0.07 nm (at 541.92 nm), wavelength reproducibility of 0.01 nm (Cary 50 Scan; Varian, Darmstadt, Germany).
Ultrasonic water bath.—150 W, 35 kHz, 4 L (TUC-160; Telsonic, Bronschhofen, Switzerland).
Rotary evaporator.—5–240 rpm, 20°–100°C (Rotavapor R-114, water bath R-480; Büchi Labortechnik, Flawil, Switzerland), connected to a vacuum pump with absolute minimum pressure of 10 mbar (PC 5; Vacuubrand, Wertheim, Germany).
Homogenizer.—750 W, 12 mm diameter dispersing aggregate [Polytron PT3100 (drive unit), PT-DA-3012/2T (aggregate); Kinematica, Lucerne, Switzerland].
Syringe.—Disposable, 2 mL (Henke-Sass, Wolf GmbH, Tuttlingen, Germany).
Filter.—Disposable, 0.45 μm pore size, 25 mm diameter, for organic solvents (Chromafil Type O-45/25: Machery-Nagel, Düren, Germany); 0.45 μm pore size, for organic solvents (Millipore, Bedford, MA; Type DVPP, 5 mm diameter).
LC system.—Consisting of online degasser (154, Gastorr; Omnilab, Mettmenstetten, Switzerland); gradient pump (L6200; Merck-VWR, Dietikon, Switzerland): autosampler with cooling unit (AS-1559; Jasco, available from Omnilab); column thermostat (Jetstream 2 Plus: Thermotechnic Products, A-2103 Langenzersdorf, Austria): UV-Vis detector (Jasco PU-2070 Plus, available from Omnilab); integrator (ATLAS™ Chromatography Data System; Thermo LabSystems, Manchester, UK, combined with Microsoft® Windows 2000 Terminal Server, Citrix MetaFrame).
Suplex PKB-100 LC column.—5 μm, 250 × 4.6 mm, Cat. No. 58934 (Supelco, Bellefonte, PA).
YMC-Pack C30 LC column.—5 μm, 250 × 4.6 mm, Product Code CT99S052546WT, available, e.g., from Stagroma (Wallisellen, Switzerland).
Reagents
All chemicals and solvents were analytical grade unless otherwise specified.
Chemicals.—Butylated hydroxytoluene (BHT, 2,6-di-tert-butyl-p-cresol): Purity ≥99%(Flukapurum grade): N-ethyldiisopropylamine: purity ≥98% (GC; Fluka purum grade).
Solvents.—Acetonitrile: purity ≥99.9% (Merck LC grade); tert-butyl methyl ether (tBME): purity ≥99.5% (Fluka puriss grade); water: demineralized.
Protex 6L.—Bacterial alkaline protease enzyme preparation in water. Synonyms: subtilisin, IUB 3.4.21.62, CAS 9014-01-1. EINECS 2327522, available from Genencor International, Inc. (Palo Alto, CA; www.genencor.com).
Ammonium acetate solution 0.2%.—Dissolve 0.50 g ammonium acetate in 250 mL water. Store solution at 5°C for no longer than 1 month.
Mobile phase A.—In 1 L volumetric flask, dissolve 50 mg BHT in 20 mL 2-propanol and add 0.2 mL N-ethyldiisopropylamine, 25 mL 0.2% ammonium acetate solution, 455 mL acetonitrile, and ca 450 mL methanol. Mixture cools and contracts. Let warm to room temperature and dilute to volume with methanol. Discard after 2 days.
Mobile phase B.—In 1 L volumetric flask, dissolve 100 mg vitamin C in ca 100 mL methanol (sonicate), add 100 mL tBME, and dilute to volume with methanol. Discard after 2 days.
β-carotene reference substance.—β-carotene, C40H56, FW 536.9, CAS 7235-40-7, purity ≥95% (LC), available under Product Code 11045800 from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Store under argon or nitrogen in a cool dark place. The compound deteriorates in the presence of oxygen.
Other carotenoids reference substances.—All-trans-lutein, all-trans-zeaxanthin, all-trans-β-cryptoxanthin, all-trans-lycopene, all-trans-α-carotene, all-trans-β-carotene’, and 9-cis-, 13-cis-, 15-cis-, 9,9′-di-cis, 9,15-di-cis-, 13,15-di-cis-β-carotene were synthesized at DSM Nutritional Products.
Calibration
Protect solutions from direct sun or UV light.
(a) Standard solutions
3 μg/mL β-carotene. Weigh with an accuracy of 0.01 mg ca 6 mg all-trans-β-carotene reference material into 100 mL volumetric flask, dissolve in about 20 mL tetrahydrofuran by treating in an ultrasonic bath for about 30s. Dilute to volume with tetrahydrofuran (60 μg/mL). From this standard stock solution, pipet exactly aliquots of 5 mL into two 100 mL volumetric flasks. Dilute to volume with cyclohexane in one flask [standard measuring solution, 3 μg/mL β-carotene in cyclohexane-tetrahydrofuran (95 + 5)]. Pipet 5 mL tetrahydrofuran into the other flask and dilute to volume with ethanol [standard working solution, 3 μg/mL β-carotene in ethanol-tetrahydrofuran (9+1)]. Store standard stock, measuring and working solutions at 5°C and use only on day of preparation.
(b) Spectrophotometry
Measure absorption of the standard measuring solution against cyclohexane at the maximum, ca 456 nm. Calculate concentration of β-carotene, Cstd, as follows:
here E is the absorption at the maximum, 2500 is the E1%, l cm of pure all-trans-β-carotene in cyclohexane, and 10 000 is the factor to convert % to mg/L.
Calculate the apparent E1%, l cm of the reference substance (ap E1%, l cm) used to prepare the standard solutions as follows:
where E is the absorption at the maximum, 10 000 is the factor to convert mg/L to %, 2 is the theoretical volume (in L) in hich the reference substance is dissolved, and W is the weight of the reference substance (in mg). The apparent E1%, 1 cm must exceed 2375 for a spectrophotometric purity >95%.
(c) LC of standard solution
Inject at least 6 aliquots of 20 μL of the standard working solution, (a), immediately after preparation into the LC system. Calculate the response factor of all-trans-β-carotene from the averaged total peak areas of the chromatograms and the spectrophotometrically measured β-carotene concentration as
where RFtrans is the response factor of all-trans-β-carotene, Astd is the mean total peak area of the standard chromatograms (Area Units, AU), and Cstd is the spectrophotometrically measured β-carotene concentration of the standard measuring solution [mg/L]. The peak area of all-trans-β-carotene must exceed 95% of the total peak area (i.e., chromatographic purity is >95%).
(d) LC response of control solutions
Control solutions are solutions of heat isomerized β-carotene, concentrations of which have been found to be stable at 5°C for at least 3 months in the dark. It is not necessary to recalibrate the LC system if the previous response factor has been shown to be valid by use of control solutions.
Preparation of the LC control solution.—Dissolve about 3 mg β-carotene reference substance and 1 g BHT in 50 mL tetrahydrofuran. Add 200 mL ethanol and reflux for 2 h in a water bath at 80°C. Cool, dilute to 500 mL with ethanol, and transfer solution to a dispenser bottle. Mix well, leave overnight at room temperature, and dispense solution into a large number of LC vials. Carefully seal the vials immediately after filling with Teflon/silicone septa, and store at ca 5°C in the dark.
Use of the LC control solution.—Measure the initial total β-carotene content of the control solution when the LC system is calibrated. Inject in parallel with the standard solution, at least 6 aliquots of 20 μL into the LC system. Calculate the mean β-carotene content of the control solution from the resulting chromatograms using the newly determined response factor. Subsequently inject the control solution together with each series of test extracts. The response factor is regarded as constant as long as the measured total β-carotene content of the control solution corresponds to the initial value within ±2%. As long as the response factor remains within these limits, the original response factor can be used for calculations. However, the LC system must be recalibrated if the measured total β-carotene content exceeds the tolerance.
Linearity of the LC response.—Dissolve 10 mg all-trans-β-carotene in 5 mL dichloromethane in 100 mL volumetric flask. Add 50 mL ethanol and reflux at 80°C in water bath for 2 h. Add ca 40 mL dichloromethane, bring to room temperature, and dilute to volume with dichloromethane. Dilute solution (100 μg/mL) with mixed solvent as shown in Table 1 to obtain β-carotene concentrations of 100–0.05 μg/mL.
Table 1.
Dilutions and concentrations
| Solution to be diluted, μg/ml | Dilution | Final concentration, μg/ml |
|---|---|---|
| 100 | 1 + 1 | 50 |
| 100 | 1 + 4 | 20 |
| 100 | 1 + 9 | 10 |
| 50 | 1 + 9 | 5 |
| 20 | 1 + 9 | 2 |
| 10 | 1 + 9 | 1 |
| 5 | 1 + 9 | 0.5 |
| 2 | 1 + 9 | 0.2 |
| 1 | 1 + 9 | 0.1 |
| 0.5 | 1 + 9 | 0.05 |
Inject solutions into the LC system and calculate the β-carotene content as in Calculations. The response must be linear for β-carotene concentrations of 0.1–50 μg/mL with a determination coefficient R2 > 0.995, and the back calculated concentration is within the target of ±10% of theoretical.
Sample Preparation
Sample preparation is dependent on the physical form of the material, the claimed content of β-carotene, and the weight of the dosage form.
(a) Mean weight per dose
Weigh 20 tablets or capsules and calculate the mean weight, WD. Use the mean weight to calculate the β-carotene content of test material.
(b) Number of tablets or capsules per assay
For content uniformity tests, take 1 tablet or the content of 1 capsule for each assay and perform 10 assays in parallel. Report individual results. For determination of the mean β-carotene content, take equivalent of content of 3 tablets or capsules for each assay and conduct 2 assays in parallel. Report the averaged result of the 2 assays.
(c) Preparation of test sample
Homogenize suspensions or emulsions by intensive stirring, e.g., with a glass rod. Crush tablets to a powder by grinding a tablet placed between the 2 halves of a folded weighing paper with a pestle. Empty capsules containing powder formulations and extract powder together with capsule shells. Use capsules containing liquid formulations as such.
Extraction
Protect extracts and solutions from direct sun or UV light.
(a) Oily solutions or suspensions
Accurately weigh test portion equivalent to ca 20 mg β-carotene, add 250 mg BHT, and rinse into 250 mL volumetric flask with 120 mL dichloromethane. Add 100 mL ethanol, and shake. Mixture cools and contracts. Let stand in dark until room temperature is reached (ca 2 h), dilute to volume with dichloromethane, and shake vigorously. Dilute aliquot in ratio of 1 + 9 with dichloromethane-ethanol (1 + 1) in volumetric flask, e.g., 5 to 50 mL. If necessary, filter the solutions through a 0.45 μm membrane. Inject 20 μL into LC system.
(b) Powders, beadlets, or emulsions
Accurately weigh test portion equivalent to about 10 mg β-carotene into 250 mL volumetric flask. Add 250 mg BHT, 0.5 mL Protex 6L, and 15 mL water. Sway to wet contents and place in ultrasonic bath at ca 50°C for 30 min, swirling after about 15 min. Add 100 mL ethanol to the warm suspension and shake vigorously. Add 135 mL dichloromethane, and shake again. Mixture cools and contracts. Let stand in dark until room temperature is reached (ca 2 h), dilute to volume with dichloromethane, shake vigorously, and let solids settle. Dilute an aliquot of the supernatant in a ratio of 1 + 9 with dichloromethane-ethanol (1 + 1) in a volumetric flask, e.g., 5 to 50 mL. If necessary filter the solutions through 0.45 μm membrane. Inject 20 μL into LC system.
(c) Tablets and capsules with test portion mass <5 g
Accurately weigh test portions of 1–3 tablets or capsules, intact or broken as in Sample Preparation (c), into 250 mL volumetric flask. Add 250 mg BHT, 0.5 mL Protex 6L, and 15 mL water. Sway to wet contents and place in ultrasonic bath at ca 50°C for 30 min, swirling after about 15 min. Add 100 mL ethanol to the warm suspension and shake vigorously. Add 120 mL dichloromethane and shake again. If clumps form, homogenize with rotation homogenizer, rinse with 15 mL dichloromethane, combining rinse with contents of volumetric flask. Mixture cools and contracts. Let stand in dark until room temperature is reached (ca 2 h), dilute to volume with dichloromethane, shake vigorously, and let solids settle. Proceed as follows:
β-carotene content of the test portion <0.1 mg.—Evaporate an aliquot, e.g., 50 mL, of the supernatant under reduced pressure at 50°C using rotary evaporator and 250 mL round-bottom flask. Dissolve dry residue in dichloromethane-ethanol (1 + 1) so that extract is concentrated by a factor of 10, e.g., dissolve residue from 50 mL in 5 mL.
β-carotene content of the test portion between 0.1 and 10 mg.—Use supernatant without dilution or concentration.
β-carotene content of test portion >10 mg.—Dilute aliquot of supernatant with dichloromethane-ethanol (1 + 1) so that β-carotene content of final solution is between 1 and 10 μg/mL. Filter solutions through 0.45 μm membrane, if necessary, and inject 20 μL into LC system.
(d) Tablets with mass of test portion >5 g
Accurately weigh test portions of 1–3 tablets prepared as in Sample Preparation (c), into weighed 100 mL volumetric flask. Add 1 g BHT, 1 mL Protex 6L, and 40 mL water. In the case of effervescent tablets, add water slowly and in small portions to avoid foaming over and wait for the next step until all gas has been released. Shaking and adding a few droplets of ethanol help to repress foaming. Place in ultrasonic bath at ca 50°C for 30 min, swirling after about 15 min. Add 50 mL ethanol to the warm suspension, let cool to room temperature, and dilute to volume with water. Weigh flask and contents. Shake vigorously and immediately pour 8–12 g suspension into a tared 100 mL volumetric flask using a funnel. Weigh the transferred aliquot of the suspension. Add 35 mL ethanol and shake. Add 35 mL dichloromethane and shake again. If clumps form, homogenize with rotation homogenizer, rinse with 15 mL dichloromethane, combining rinse with contents of volumetric flask. Mixture cools and contracts. Let stand in dark until room temperature is reached (ca 2 h), dilute to volume with dichloromethane, shake vigorously, and let solids settle. Proceed as follows:
β-carotene content of the test portion <0.5 mg.—Evaporate an aliquot, e.g., 50 mL, of supernatant under reduced pressure at 50°C using a rotary evaporator and 250 mL round-bottom flask. Dissolve dry residue in dichloromethane-ethanol (1 + 1) so that the extract is concentrated by a factor of 10, e.g., dissolve residue from 50 mL in 5 mL.
β-carotene content of the test portion between 0.5 and 50 mg.—Use supernatant without dilution or concentration.
β-carotene content of the test portion >50 mg.—Dilute aliquot of supernatant with dichloromethane-ethanol (1 + 1) so that β-carotene content of final solution is between 1 and 10 μg/mL. Filter the solutions through 0.45 μm membrane, if necessary, and inject 20 μL into LC system.
Chromatography
Test solutions are first injected into LC system A involving a C18 column. In this system, cis-isomers of α-carotene can interfere with the trans- and cis-isomers of β-carotene. If in chromatograms of LC system A, the peak area of all-trans-α-carotene exceeds the total peak area of β-carotene isomers by >5%, the test solution is analyzed by the more selective LC system B.
(a) LC system A
Conditions.—Column: Suplex pKb-100; column temperature 30°C; mobile phase A (see Reagents); flow rate 0.6 mL/min; pressure ca 33 bar; run time ca 30 min; injection volume 20 μL; autosampler temperature 15°C; detection at 448 nm.
Retention times.—All-trans-β-carotene: ca 20–25 min. Retention times relative to all-trans-β-carotene: all-trans-lutein 0.30; all-trans-zeaxanthin 0.32; all-trans-β-cryptoxanthin 0.58; all-trans-lycopene 0.67; nonidentified cis-lycopenes 0.69, 0.75, 0.79; 9′-cis-α-carotene 0.91; all-trans-α-carotene 0.93; 9-cis-α-carotene 0.98; 13-cis- and 13′-cis-α-carotene 1.03; nonidentified cis-α-carotene 1.08; all-trans-α-carotene 1.00; 9-cis-β-carotene 1.07; 13-cis-β-carotene 1.17; 15-cis-β-carotene 1.21. Other minor cis-isomers of β-carotene eluted in the range of the trans- and mono-cis-isomers of β-carotene (e.g., with relative retention times of 1.02 for 13,15-di-cis- β-carotene, 1.11 for 9,9′-di-cis- β-carotene, and 1.12 for 9,15-di-cis- β-carotene).
(b) LC system B
Conditions.—Column YMC-Pack C30; column temperature 30°C; mobile phase B (see Reagents); flow rate 0.9 mL/min (use 2 mL/min during flush): pressure ca 80 bar; total run time ca 65 min; injection volume 20 μL; autosampler temperature 15°C; detection at 445 nm. After elution of 9- cis-β-carotene, flush column with pure tBME at flow rate of 2 mL/min for 3 min and recondition column with mobile phase B for 5 min.
Retention times.—All-trans-β-carotene: ca 40–55 min. Retention times relative to all-trans-β-carotene: all-trans-lutein 0.22; all-trans-zeaxanthin 0.26; all-trans-β-cryptoxanthin 0.51: 13-cis-α-carotene 0.48; nonidentified cis-β-carotene 0.50; nonidentified cis-α-carotene 0.50; 13′-cis-α-carotene 0.53; 15-cis-β-carotene 0.56; 13,15-di-cis-β-carotene 0.58; 13-cis-β-carotene 0.61; 9,15-di-cis-β-carotene 0.64: nonidentified cis-β-carotene 0.73; all-trans-α-carotene 0.76; 9-cis-α-carotene 0.79; 9,9′-di-cis-β-carotene 0.91; 9′-cis-α-carotene 0.95; all-trans-β-carotene 1.00; 9-cis-β-carotene 1.12; lycopene isomers >1.20.
Calculations
Calculate contents of total and all-trans-β-carotene in test samples as follows:
where Ctot and Ctrans are the total and all-trans-β-carotene contents (mg/g or mg/dose); Atrans, A9-cis, A13-cis, A15-cis are the peak areas of all-trans-, 9-cis-, 13-cis-, 15-cis-β-carotene, respectively, in area units (AU); AX-cis is the sum of the peak area of other cis-isomers of β -carotene (AU); 1.2 and 1.4 are relative response factors (correction factors to compensate for lower specific absorption of 13-cis- and 15-cis-β-carotene compared to all-trans-β-carotene at the given wavelength); WD is the mean weight of a dose (g); m is the test portion amount (g); n is the number of tablets or capsules used as amount of test portion; RFtrans is the response factor of all-trans-β-carotene (AU × L/mg); V is the theoretical volume in which the test portion is dissolved (L); V1 is the volume of the flask used for extraction with dichloromethane-ethanol (L); V2 is the volume aliquot which is diluted or evaporated (L); V3 is the volume to which aliquot V2 is diluted or in which the residue after evaporation of aliquot V2 is dissolved (L). W1 and W2 apply only to extraction (d). W1 is the weight of the aqueous alcohol suspension in the first 100 mL volumetric flask, and W2 is the weight of the aliquot of the aqueous alcohol suspension transferred to the second 100 mL flask.
Procedures Used for Optimization and Validation
(a) Selectivity
The selectivity of the used LC systems was studied by chromatography of various synthetic carotenoid isomers (all-trans-lutein, all-trans-zeaxanthin, all-trans-β-cryptoxanthin, all- trans -lycopene, all- trans -α-carotene, all-trans-β-carotene, 9-cis-, 13-cis-, 15-cis-, 9,9′-di-cis, 9,15-di-cis-, 13,15-di-cis-β-carotene) and of iodine-isomerized α- and β-carotene solutions. Peak identification was done by co-chromatography with the mentioned compounds, by comparison of diode array detector spectra with published data (22) and by comparison with published elution profiles (23). In LC system A, peaks of various cis-isomers of α-carotene were additionally identified by injecting unidentified peak fractions collected from the eluate of LC system B. For iodine isomerization, 10 mg crystalline all-trans-α-carotene, 10 mg all-trans-β-carotene, and 1000 mg BHT were dissolved in 5 mL dichloromethane. The solution was diluted with cyclohexane to 100 mL. A 10 mL volume of this stock solution was combined in a 100 mL volumetric flask with 40 mL cyclohexane and 2 mL freshly prepared solution of 10 ppm iodine in cyclohexane (w/v). The mixture was incubated in a water bath at ca 65°C. After 15 min, the solution was quickly cooled to ambient temperature and diluted to volume with cyclohexane. A 50 mL volume of the solution was evaporated at 50°C under vacuum with a rotary evaporator. The dry residue was dissolved in 5 mL dichloromethane-ethanol (1 + 1) and filled into LC- vials.
(b) Linearity
The linearity of the response of LC systems A and B was examined. Dilutions from a solution of heat-isomerized β-carotene were prepared as described above in Calibration, with a total β-carotene range of ca 0.005–100 μg/mL. All dilutions were prepared and measured in duplicate. From the resulting acceptable chromatograms, the total peak areas of all detected β-carotene isomers as well as the peak area of all-trans-β-carotene were determined and averaged for the duplicates. The relative response of the analyte, measured as mean peak areas of all-trans-β-carotene and of total β-carotene, was plotted against the respective concentrations. Curves were constructed using the least-squares linear regression method.
(c) Precision
On 5 different days, 2 test portions from each of the 8 test materials were analyzed according to the method. One technician performed the work on the first 2 days, another on Days 3–5. The test portions consisted of 3 tablets or capsules (test materials Nos. 1–7) or 100 mg (test material No. 8). The extraction variants and LC systems used are shown in Table 2. The precision of extraction variant a was not examined as it should be equal or better than variant b.
Table 2.
Method precision for measurement of total and all-trans-β-carotene in test materials Nos. 1–8a
| Total β-carotene, mg/g | All-trans-β-carotene, mg/g | |||||
|---|---|---|---|---|---|---|
| Test material No. | Extraction variant | LC system | Mean value, n = 10 | RSD, % | Mean value, n = 10 | RSD, % |
| Multivitamin tablets (1) | c,2 | A | 0.494 | 2.4 | 0.428 | 3.5 |
| Softgels with β-carotene in soybean oil (2) | c,3 | A | 47.1 | 1.5 | 45.2 | 1.4 |
| Capsules with β-carotene in fish oil (3) | c,1b | B | 0.0520 | 3.1 | 0.0310 | 4.6 |
| Softgels with β-carotene from carrot oil extract (4) | c,3 | B | 9.64 | 2.2 | 6.62 | 2.4 |
| Tablets with β-carotene (5) | d,3b | A | 49.3 | 2.6 | 39.2 | 3.0 |
| Capsules with β-carotene (6a) | d,2b | A | 23.7 | 10.4 | 22.8 | 10.4 |
| Capsules with β-carotene (6b) | c,3 | A | 26.2 | 1.2 | 25.1 | 1.2 |
| Capsules with β-carotene (7) | c,3 | B | 32.2 | 4.4 | 23 | 5.4 |
| Beadlets raw material with pure β-carotene (8) | b | A | 201 | 1.3 | 160 | 2.0 |
Analyses were performed in duplicate by 2 technicians on 5 different days.
Only for validation purpose (analytical method prescribes other variants for these test materials).
On the first day of the precision tests, we realized that the aqueously suspended capsule shells of test material No. 6 agglutinated when ethanol was added. The clumps could not be dispersed with a homogenizer (Polytron), and it was not possible to take a homogeneous aliquot from this suspension for further extraction, as prescribed in extraction variant d (No. 6a). It seemed very probable that this variant could not work with this test material, and we decided to extract this material following variant c (No. 6b).
(d) Recovery
Negative control materials were fortified, in triplicate, with a mixture of β-carotene isomers generated by heat isomerization and analyzed according to the method. For a positive control, the spiking solutions used to spike the negative control materials were diluted, in triplicate, with dichloromethane–ethanol (1 + 1) and analyzed directly by LC. In addition, triplicate unfortified controls were analyzed. Only LC system A was used in this experiment.
For preparation of spiking solutions, 2.5 g β-carotene reference material was dissolved in ca 70 mL chloroform. The solution was enclosed in a pressure-resistant glass vessel and heated in a water bath at 80°C. After 2 h, the solution was cooled to room temperature and diluted with chloroform to 250 mL (spiking solution 1, 10 000 μg/mL). Spiking solution 2 (100 μg/mL) was prepared by pipetting 5 mL spiking solution 1 into 500 mL volumetric flask and diluting to volume with dichloromethane. Spiking solution 3 was obtained by diluting 5 mL spiking solution 2 to 500 mL with dichloromethane–ethanol (1 + 1). The concentration of spiking solution 3 was observed to be unstable in pure dichloromethane.
Negative control material No. 1 (placebo tablets) was spiked, in test portions of 3 tablets (for variant c) and 6 tablets (for variant d) at total β-carotene concentrations of ca 0.0l (= lower range limit of the present method), and 100 mg per test portion (= ca 130% of highest β-carotene concentration in test materials of this study). Negative control material No. 2 (placebo beadlets) was fortified at total β-carotene concentrations of ca 0.2% (= lower range limit of the attached method) and 40% (= ca 130% of highest β-carotene concentration in commercial product forms). The recovery of extraction variant a was not examined, as it should be equal to or better than variant b.
Results and Discussion
Optimization
(a) Extraction efficacy
In the present method, β-carotene is released from water-dispersible formulations such as powders and emulsions, or tablets and capsules, by enzymatic digestion with protease followed by extraction with a mixture of ethanol and dichloromethane. This procedure has been routinely used for more than 2 decades in the laboratories of Roche Vitamins Ltd. (now DSM Nutritional Products) for extraction of stabilized carotenoids and fat-soluble vitamins from formulations based on gelatin, pectin, gummy arabicum, poly- and oligosaccharides. In order to demonstrate its efficacy, an artificially cross-linked gelatin formulation (β-Carotene 10%B, DSM Nutritional Products, Product Code 04 3483 3) was extracted following the prescribed procedure. The filtered residue of this extraction was then washed from cortically adhering β-carotene with ethanol and re-extracted using a 3-fold concentration of enzyme and the stronger but more toxic solvent chloroform instead of dichloromethane. The second extraction released <0.01% β-carotene found after routine extraction of the sample. Thus, the prescribed extraction can be regarded as quantitative. Note: This extraction procedure does not quantitatively extract carotenoids from plant cells having thick cellulose walls. For such matrixes, extraction by saponification would be preferable.
(b) Isomerization and oxidation during the analysis
Carotenoids are susceptible to light- or heat-induced geometrical isomerization (24). Thus, β-carotene could be isomerized during the analytical procedure. However, as shown in Table 3, the isomeric ratios in a heat-treated solution of β-carotene and in an almost pure all-trans-β-carotene were barely affected when the solutions were subjected to the extraction procedure and the following steps of the proposed method (extraction variant b). The conditions of the assay were appropriate to retain the original isomeric ratio present in a sample to a high degree. Further, the procedure obviously did not give rise to oxidation of β-carotene as the analysis of various test samples performed in absence and presence of BHT gave well corresponding results. Nevertheless, BHT was prescribed in the present method to prevent any potential attack by oxidants possibly present in other samples. Similarly, the autosampler temperature was set to 15°C even though no significant degradation or isomerization of β-carotene was observed, when standard solutions, heat-isomerized control solutions, or extracts of test materials stood for 20 h at 23°C in the autosampler rack.
Table 3.
Isomerization of β-carotene during extraction procedure
| Percentage of (β-carotene isomers %a |
||||||||
|---|---|---|---|---|---|---|---|---|
| Direct
|
After extraction
|
|||||||
| Sample | All-trans | 9-cis | 13-cis | 15-cis | All-trans | 9-cis | 13-cis | 15-cis |
| Almost pure all-trans-β-carotene | 97.2 | 0.7 | 2.2 | – | 97.4 | 0.8 | 1.9 | – |
| Heat-isomerization (β-carotene | 73.0 | 1.1 | 23.4 | 2.5 | 72.6 | 1.6 | 24.0 | 1.8 |
Total β-carotene = 100%.
(c) Selectivity
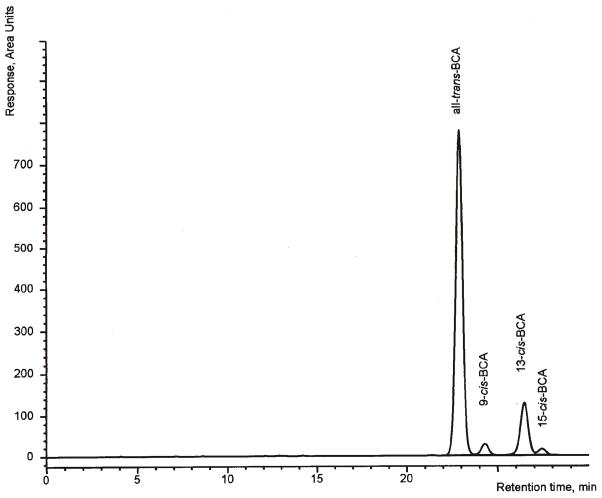
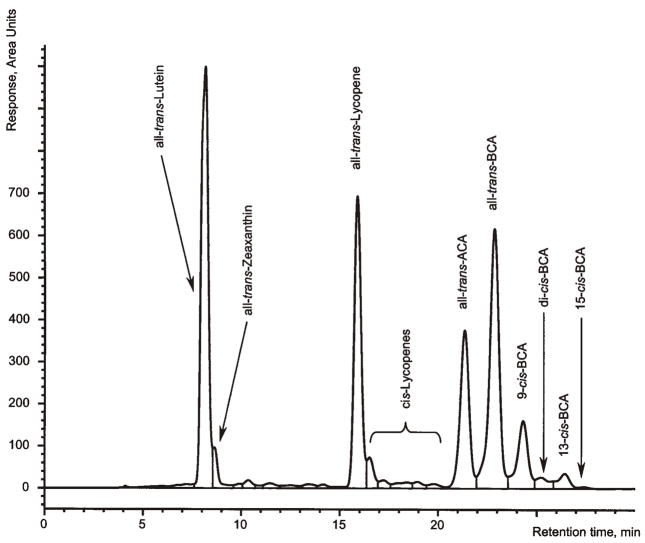
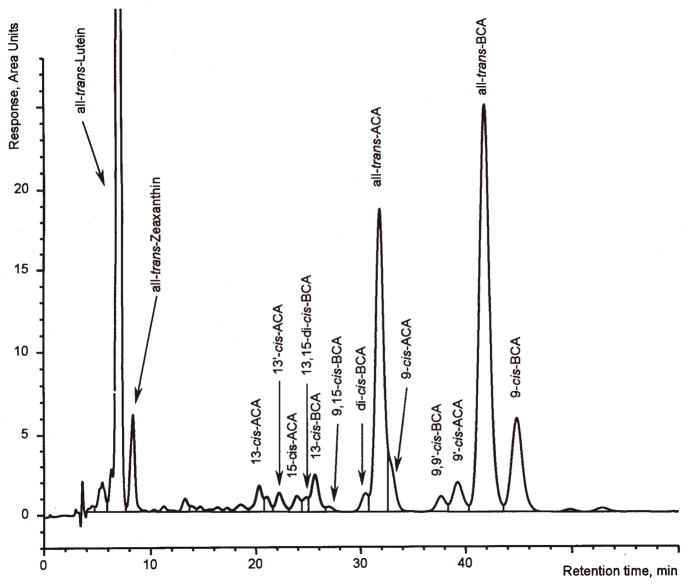
LC system A of the present method has been used for many years in our laboratory for the routine analysis of β-carotene in food (18). The system is able to separate the mono-cis-isomers of β-carotene from each other and from all-trans-β-carotene as well as the β-carotene isomers from other carotenoids as lutein, zeaxanthin, β-cryptoxanthin, and lycopene (Figures 2 and 3). The present optimization revealed that LC system A also separated all-trans- and 9′-cis-α-carotene from all the β-carotene isomers detected in an iodine-isomerized preparation, but 9-cis-, 13-cis-, and 13′-cis-α-carotene co-eluted with the β-carotene isomers. This interference may be negligible for supplements and raw materials containing low total amounts of α-carotene or only trans-α-carotene but not for materials with high amounts of isomerized α-carotene, e.g., palm or carrot oil. For such samples, the more selective LC system B was used, which provided a good separation of many cis-isomers of α-carotene from those of β-carotene (Figure 4). This system was optimized starting from the conditions described by Emenhiser et al. (23) by increasing the flow rate to 0.9 mL/min and by introducing a flush step to remove the late eluting lycopene isomers. Thereby, the run time was reduced to 1 h or less without compromising the quality of the separations. However, because of the complicated identification of the various geometrical isomers and the longer run time, this system may complement but not replace LC system A for the general routine analysis of β-carotene in supplements and raw materials. Therefore, we recommend analysis of unknown samples first with LC system A. If the result of this analysis indicates that all-trans-α-carotene exceeds a portion of 5% of the total-β-carotene (on peak area basis) the sample should be analyzed by LC system B (see Chromatography, above).
Figure 2.
LC system A chromatogram of an isomeric mixture of β-carotene as present in many supplements. BCA: β-carotene.
Figure 3.
Chromatogram of test material No. 4 (Nature’s Plus, Octa-carotene) obtained with LC system A. ACA: α-carotene; BCA: β-carotene.
Figure 4.
Chromatogram of test sample No. 4 (Nature’s Plus, Octa-carotene) obtained with LC system B. ACA: α-carotene; BCA: β-carotene.
Total and all-trans-β-carotene were analyzed in the test materials with both, LC system A and the more selective LC system B. As shown in Table 4, the only significant difference between the results from LC systems A and B was observed for test material No. 4, which is due to the high content of isomerized α-carotene. It is obvious from the good agreement of the results from the other test materials that the selectivity of LC system A was sufficient to accurately quantify total and all-trans-β-carotene.
Table 4.
Total and all-trans-β-carotene in test materials Nos. 1–8 measured by LC systems A and B
| Total-β-carotene, mg/dose
|
All-trans-β-carotene, mg/dose
|
|||||
|---|---|---|---|---|---|---|
| Test material (No.) | LC system A | LC system B | Difference, %a | LC system A | LC system B | Difference, %a |
| Multivitamin tablets (1) | 0.725 | 0.743 | 2.5 | 0.625 | 0.629 | 0.5 |
| Softgels with β-carotene in soybean oil (2) | 15.9 | 16.00 | 0.8 | 15.2 | 15.3 | 0.9 |
| Capsules with β-carotene in fish oil (3) | 0.0599 | 0.0599 | 0.0 | 0.0370 | 0.0364 | −1.5 |
| Softgels with β-carotene from carrot oil extract (4) | 6.83 | 6.31 | −7.7 | 4.85 | 4.36 | −10.0 |
| Tablets with β-carotene (5) | 24.6 | 25.1 | 2.4 | 19.5 | 19.5 | −0.4 |
| Capsules with β-carotene (6) | 6.82 | 6.81 | −0.0 | 6.53 | 6.53 | 0.0 |
| Capsules with β-carotene, claim 25 mg β-carotene (7) | 20.7 | 20.9 | 1.2 | 14.9 | 15.0 | 0.6 |
| Beadlets raw material with pure β-carotene (8)b | 199 | 205 | 3.2 | 157 | 158 | 0.7 |
Results for LC system A = 100%.
Unit is g/kg instead of mg/dose.
In both LC systems of the present method, fatty acid esters of xanthophylls, e.g., present in nonsaponified preparations of tagetes (mainly esters of lutein and zeaxanthin) or paprika (mainly esters of capsanthin, lutein, and zeaxanthin), can interfere with the analysis of β-carotene. These esters are usually less polar than the carotenes and can therefore elute as relatively broad peaks in subsequent chromatograms. The interference from such peaks can be avoided by implementation of flush steps in the run sequence or by saponification of the sample material. The latter, however, could alter the cis/trans-isomer ratios of the carotenoids present in the samples.
(d) Column-to-column variation
A comparison of Suplex pKb-100 columns from 4 different batches revealed that the retention times of all-trans-β-carotene varied from 19.9 to 22.6 min and that the relative retention times of various cis-isomers were very constant. In contrast, the 2 batches of YMC C30 columns studied gave absolute retention times of β-carotene isomers varying from 42.2 to 53.6 min, and the relative retention times of certain isomers, e.g., 9-cis-β-carotene were not constant from column to column. However, a sufficient separation of the relevant isomers was always achieved.
(e) Quantification of cis-isomers
In an earlier work, relative response factors had been established for the quantification of the mono-cis-isomers of β-carotene by a reversed-phase LC system that was calibrated with all-trans-β-carotene (25). In that system, the detection wavelength was set to 448 nm because at this wavelength the specific response of 2 of the predominant isomers, 9-cis- and all-trans- β-carotene, were congruent. Because the eluents of the present LC systems A and B differ from that of the former system, solutions of synthesized all-trans-, 9-cis-, 13-cis-, and 15-cis-β-carotene were chromatographed with the eluents of the original and of the present LC systems at varying detection wavelengths. The peak area ratios obtained (Table 5) were unaltered if the detection wavelengths were set to 448 nm (LC system A) or 445 nm (LC system B). This allowed the transfer of the original relative response factors to the LC systems of the present method.
Table 5.
Peak area ratios of β-carotene isomers in eluents of LC-systems A and B of the present method compared with earlier LC system (25)
| Eluent of LC system published earliera 445 nm, 0.8 mL/min
|
Eluent of LC system A, 448 nm, 0.6 mL/min
|
|||
|---|---|---|---|---|
| Sample | Peak areasb | cis/trans ratio | Peak areasb | cis/trans ratio |
| All-trans-β-carotene | 1209 ± 21 | – | 902 ± 15 | – |
| 9-cis-β-carotene | 1241 ± 4 | 1.03 | 925 ± 7 | 1.03 |
| 13-cis-β-carotene | 375 ± 19 | 0.31 | 281 ± 9 | 0.31 |
| 15-cis-β-carotene | 774 ± 25 | 0.64 | 573 ± 8 | 0.64 |
| Eluent of LC system published earliera 445 nm, 0.8 mL/min
|
Eluent of LC system B, 445 nm 0.4 mL/minc
|
|||
| Peak areasb | cis/trans ratio | Peak areasb | cis/trans ratio | |
| All-trans-β-carotene | 546 ± 2 | – | 1083 ± 3 | – |
| 9-cis-β-carotene | 560 ± 4 | 1.03 | 1115 ± 1 | 1.03 |
| 13-cis-β-carotene | 447 ± 1 | 0.82 | 881 ± 4 | 0.81 |
| 15-cis-β-carotene | 390 ± 2 | 0.71 | 774 ± 1 | 0.72 |
Methanol–THF(99 + 1), 100 ppm vitamin C (25).
Mean and standard deviation from 4 injections.
Flow rate was sent to a 0.4 mL/min for better separation of isomers.
Validation
Seven dietary supplements containing β-carotene and one β-carotene beadlet raw material were analyzed. The dietary supplements included multivitamin tablets, fish oil capsules, softgels, and tablets containing β-carotene as the main ingredient. The label claims ranged from no β-carotene for the fish oil capsules, 0.6–25 mg β-carotene/tablet or capsule for the dietary supplements, and 20% β-carotene for the beadlet raw material. The sources of β-carotene included synthetic material, carrot oil, and Dunaliella salina algae extract.
The LC systems A and B were calibrated and the response factors RFtrans were determined. The apparent E1%,1cm of the standard measuring solution was 2514, the chromatographic purities for all-trans-β-carotene were 96.8% (LC system A) and 97.2% (LC system B) based on peak area comparison. These results met the method requirements (E1%,1cm > 2375, LC purity > 95%). The response of both LC systems was controlled during the validation study with solutions of heat-isomerized β-carotene. The β-carotene concentrations of these control solutions never deviated by more than ±2% from the total and all-trans-β-carotene concentrations measured on the day of calibration (= 100%).
In the range of 0.1–50 μg/mL, the regression line of the response of LC systems A and B showed determination coefficients (R2) > 0.999 for total β-carotene as well as for all-trans- β-carotene and thus met the requirement (R2 > 0.995). The instrumental detection limit, estimated as the concentration for which the peak signal of all-trans- β-carotene is approximately three times the baseline noise, was ca 0.001 μg/mL for LC system A and ca 0.01 μg/mL for LC system B.
The results of the precision experiment (Table 2) clearly show that the capsules of test material No. 6 could not be extracted reproducibly by use of extraction variant d (No. 6a). The high SD of 10.4% was due to the extraction problem mentioned above. Therefore, extraction variant d should only be used for large tablets that are dispersible in water (e.g., effervescent tablets).
The relative standard deviations (RSD%) obtained for the total and all-trans-β-carotene contents of test materials Nos. 1–4, and 6(b) met the requirements for repeatability precision (RSDr) recommended by AOAC (see AOAC requirements for single laboratory validation of chemical methods for dietary supplements and other materials, Draft 2002-11 -24). In the case of test material No. 8 (beadlets raw material), the fluctuation of total-β-carotene contents (RSD% = 1.3%) was within the recommended limit (RSDr = 1.5%), but the measured all-trans-β-carotene contents varied slightly more (2.0%).
In contrast, the variation of the total and all-trans-β-carotene values obtained for test materials Nos. 5 and 7 clearly exceeded the repeatability precision limit recommended for this range of concentration (1.5–2%). In each of these cases, one outlier could be identified using the Grubbs test. Exclusion of these outliers shifted the coefficients of variation calculated for test material No. 5 into the recommended range, whereas those obtained for test material No. 7 remained above. However, it is apparent from the results in Table 2 that the extraction variant used for the softgels of test material No. 7 gave acceptable results when applied to other similar as well as different test materials. Compare data for Nos. 2 and 4 (softgels) and No. 6b, (capsules). This suggests that test material inhomogeneity may also contribute to the relatively high variation found for test material No. 7.
The present precision test was not performed under distinct repeatability conditions, as 2 technicians and 5 working days were involved. For this reason, the relatively low limits for repeatability precision may not be fully adequate to evaluate the present results.
As shown in Table 6, recoveries of 99.7 and 99.5% were found for the analysis of total β-carotene in beadlet raw materials spiked at β-carotene concentrations of 0.2 and 40%, respectively. The recoveries obtained for the analysis of β-carotene in supplements varied between 97.5 and 102.1% independently of the spiked β-carotene concentrations (10 μg–100 mg/test portion) and of the assay variants. β-carotene was not detected in the extracts of unfortified control materials.
Table 6.
Recovery of different extraction variants
| Spiked total β-carotene, a mg/g
|
Recovered total β-carotene, mg/g
|
|||||
|---|---|---|---|---|---|---|
| Extraction variant | Negative control material No. | Mean | RSD, % (n = 3) | Mean | RSD, % (n = 3) | Recovery, %b |
| b | 2 | 2.05 | 0.5 | 2.04 | 1.5 | 99.7 |
| b | 2 | 398 | 0.5 | 396 | 0.3 | 99.5 |
| c,1 | 1 | 0.00876 | 2.1 | 0.00854 | 7.6 | 97.5 |
| c,2 | 1 | 1.023 | 0.5 | 0.999 | 1.2 | 97.6 |
| c,3 | 1 | 99.5 | 0.5 | 100.5 | 1.1 | 101.1 |
| d,1 | 1 | 0.00983 | 0.8 | 0.00996 | 4.3 | 101.3 |
| d,2 | 1 | 0.995 | 0.7 | 0.983 | 2.3 | 98.8 |
| d,3 | 1 | 99.5 | 0.5 | 101.6 | 2.0 | 102.1 |
Concentration determined after appropriate dilution of spiking solution.
Relative to amount of spiked total-β-carotene (= 100%).
Further Potential of the Method
The present method may also be suitable for the tentative quantification of α-carotene in the presence of other carotenes and xanthophylls. LC system B should be used for samples containing isomer mixtures of α-carotene (e.g., palm oil, carrot oil, or heat-treated preparations). LC system A would be applicable if α-carotene is present in the all-trans configuration. The calibration of α-carotene could be done with reference material of all-trans-β-carotene by use of a relative response factor of ca 0.95 to compensate for the higher specific absorption of all-trans-α-carotene compared to all-trans-β-carotene at the detection wavelength used. This factor was obtained by co-chromatography of solutions of all-trans-α-carotene and all-trans-β-catotene reference materials. The concentrations of these standard solutions were determined by spectrophotometry using E1%/1 cm values of 2710 in n-hexane for all-trans-α-caxotene (26) and of 2500 for all-trans-β-carotene in cyclohexane. However, the quantification of α-carotene is less correct than that of β-carotene because relative response factors are not available for the cis-isomers of α-carotene. In addition, no specific validation was conducted for α-carotene.
For several years, LC system A of the present method has been used in our laboratory for the routine analysis of lycopene in feed, food, and pharmaceutical preparations. The system is able to separate the most relevant isomers of lycopene from those of α- and β-carotene as well as from xanthophylls, such as all-trans-lutein, alWraTu-zeaxanthin, and all-trans-β-cryptoxanthin. To our knowledge, only a cis-isomer of β-cryptoxanthin co-elutes with all-trans-lycopene. This interference can be neglected in most supplements and raw materials, because β-cryptoxanthin is usually present in low amounts compared to lycopene. Calibration can be performed by use of reference material of all-trans-lycopene. Relative response factors for the cis-isomers of lycopene are not established because the specific absorbance of the quantitatively most important cis-isomer of lycopene, 5 -cis-lycopene, corresponds well with that of the all-trans-isomer. Whereas all-trans-, 9-cis-, and 13-cis-lycopene are well resolved from each other, 5-cis-lycopene usually occurs as a shoulder on the peak of all-trans-lycopene.
Both LC systems of the present method separated all-trans-lutein from all-trans-zeaxanthin, and both of these xanthophylls from many other carotenols and carotenes. However, with LC system A, a main cis-fraction of lutein co-elutes with all-trans-zeaxanthin. Such an interference also probably occurs in LC systemB because of the relatively short retention times, but this has to be experimentally confirmed. Some commercial raw materials contain isomeric mixtures of lutein and zeaxanthin. Therefore, based on our current knowledge, we would recommend combination of the sample preparation procedure of the present method with an LC system showing a higher selectivity for the cis-/trans- isomers of lutein and zeaxanthin (e.g., an isocratic normal-phase system as used at DSM Nutritional Products and other suppliers for this purpose). Calibration could be done with only all-trans-zeaxanthin using relative response factors for all-trans-lutein and the cis-isomers of both xanthophylls. Pure reference material of all-trans-lutein is very difficult to obtain.
Acknowledgments
We thank Christelle Mary and Jean-David Klipfel for their skillful assistance, our colleagues from the Research and Development chemical department of DSM Nutritional Products for standard substances of β-carotene cis-isomers, and AOAC INTERNATIONAL for editorial support and test materials.
This study was performed to provide the FDA-NIH/ODS, as well as other government agencies and the dietary supplements industry, with AOAC® Official Methods, applicable to commercially available dietary supplements and their raw materials.
References
- 1.Mathews-Roth MM. Biochemie. 1986;68:875–884. doi: 10.1016/s0300-9084(86)80104-3. [DOI] [PubMed] [Google Scholar]
- 2.Olson JA. J Nutr. 1986;166:1127–1130. doi: 10.1093/jn/116.6.1127. [DOI] [PubMed] [Google Scholar]
- 3.Temple NJ, Basu TK. Nutr Res. 1988;8:685–701. [Google Scholar]
- 4.Schierle J, Schellenberger T, Fizet C, Betz R. Eur Food Res Technol. 2002;215:268–274. [Google Scholar]
- 5.Sweeny JP, Marsh AC. J Assoc Off Anal Chem. 1970;53:937–940. [Google Scholar]
- 6.Panalaks CA, Warthesen JJ, Taoukis PS. J Inst Can Technol Aliment. 1990;3:145–151. [Google Scholar]
- 7.Sweeny JP, Marsh AC. J Am Diet Assoc. 1971;59:238–243. [PubMed] [Google Scholar]
- 8.Tsukida K, Saiki K, Sugiura M. J Nutr Sci Vitaminal. 1981;27:551–561. doi: 10.3177/jnsv.27.551. [DOI] [PubMed] [Google Scholar]
- 9.Bushway RJ. J Liq Chromatogr. 1985;8:1527–1547. [Google Scholar]
- 10.Chandler LA, Schwartz SJ. J Food Sci. 1987;52:669–672. [Google Scholar]
- 11.Quackenbusch FW. J Liq Chromatogr. 1987;10:643–653. [Google Scholar]
- 12.Godoy HT, Rodriguez-Amaya DB. J Agric Food Chem. 1994;42:1306–1313. [Google Scholar]
- 13.Marty C, Berset C. J Agric Food Chem. 1990;38:1063–1067. [Google Scholar]
- 14.Tsukida K, Saiki K, Takii T, Koyama Y. J Chromatogr. 1982;245:359–364. [Google Scholar]
- 15.Vecchi M, Englert G, Maurer R, Meduna V. Helv Chim Acta. 1981;64:2746–2758. [Google Scholar]
- 16.Quackenbusch FW, Smallidge RI. J Assoc Off Anal Chem. 1986;69:767–771. [PubMed] [Google Scholar]
- 17.Ben-Amotz A, Lers A, Avron M. Plant Physiol. 1988;86:1286–1291. doi: 10.1104/pp.86.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schüep W, Schierle J. J AOAC Int. 1997;80:1057–1064. [Google Scholar]
- 19.Sundaresan PR. J AOAC Int. 2002;85:1127–1135. [PubMed] [Google Scholar]
- 20.Eitenmiller RR, Landen WO. Vitamin Analysis for the Health and Food Sciences. CRC Press; Boca Raton, FL: 1999. pp. 3–75. [Google Scholar]
- 21.WHO/FAO. Report of a Joint FAO/WHO expert consultation, Bangkok, Thailand. FAO/WHO; Rome, Italy: 2002. Human Vitamin and Mineral Requirements, Chapter 7: Vitamin A; pp. 87–107. [Google Scholar]
- 22.Zechmeister L, Polgar A. J Am Chem Soc. 1944;66:137–144. [Google Scholar]
- 23.Emenhiser C, Sander LC, Schwartz SJ. J Agric Food Chem. 1996;44:3887–3893. [Google Scholar]
- 24.Mortensen A, Skibsted LH. J Agric Food Chem. 2000;48:279–286. doi: 10.1021/jf9904620. [DOI] [PubMed] [Google Scholar]
- 25.Schierle J, Härdi W, Faccin N, Bühler I. In: Carotenoids, Volume 1A, Isolation and Analysis. Britton G, Liaaen-Jensen S, Pfander H, editors. Birkhäuser Verlag; Basel, Switzerland: 1995. pp. 265–272. [Google Scholar]
- 26.Britton G. In: Carotenoids, Volume 1B, Spectroscopy. Britton G, Liaaen-Jensen S, Pfander H, editors. Birkhäuser Verlag; Basel, Switzerland: 1995. pp. 13–62. [Google Scholar]