Abstract
An international collaborative study was conducted of a high-performance liquid chromatographic (HPLC)-UV method for the determination of coenzyme Q10 (CoQ10, ubidecarenone) in raw materials and dietary supplements. Ten collaborating laboratories determined the total CoQ10 content in 8 blind duplicate samples. Sample materials included CoQ10 raw material and 4 finished product dietary supplements representing softgels, hardshell gelatin capsules, and chewable wafers. In addition, collaborating laboratories received a negative control and negative control spiked with CoQ10 at low and high levels to determine recovery. Materials were extracted with an acetonitrile–tetrahydrofuran–water mixture. Ferric chloride was added to the test solutions to ensure all CoQ10 was in the oxidized form. The HPLC analyses were performed on a C18 column using UV detection at 275 nm. Repeatability relative standard deviations (RSDr) ranged from 0.94 to 5.05%. Reproducibility relative standard deviations (RSDR) ranged from 3.08 to 17.1%, with HorRat values ranging from 1.26 to 5.17. Recoveries ranged from 74.0 to 115%. Based on these results, the method is recommended for Official First Action for determination of CoQ10 in raw materials and dietary supplement finished products containing CoQ10 at a concentration of >100 mg CoQ10/g test material.
Coenzyme Q10 (CoQ10; ubidecarenone) is a biologically active compound that is similar in chemical structure to menaquinones (vitamin K2). Part of a family of quinone compounds known as coenzyme Q, CoQ10 is characterized by a quinone ring attached to a repeating series of side-chain isoprene units (Figure 1). The number of isoprene units is denoted by the coenzyme-X designation. In the case of CoQ10, there are 10 repeating isoprene units.
Figure 1.
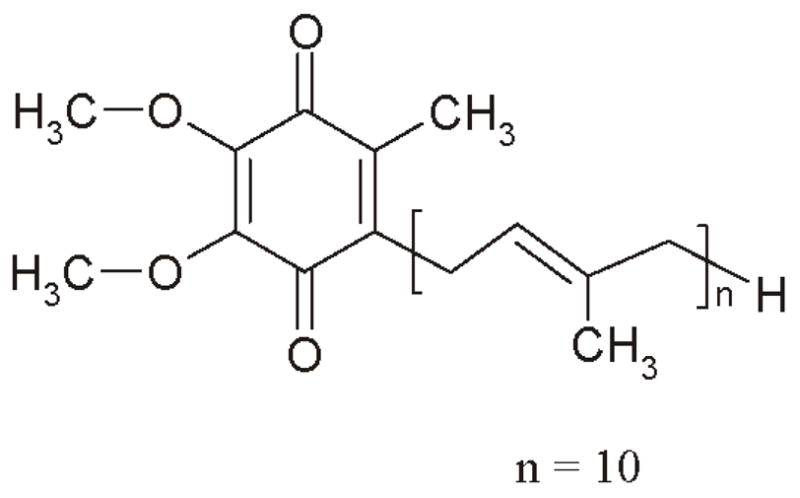
Chemical structure of CoQ10.
Coenzyme Q was first discovered by researchers at the University of Wisconsin in 1957 (1). Later, Wolf et al. (2) reported the chemical structure of the compound. The reduced biologically active forms of CoQ10, QH, and QH2 are the result of protonation at the carbonyl moieties of the quinone ring (Figure 2). CoQ10 is lipophilic and highly water-insoluble.
Figure 2.
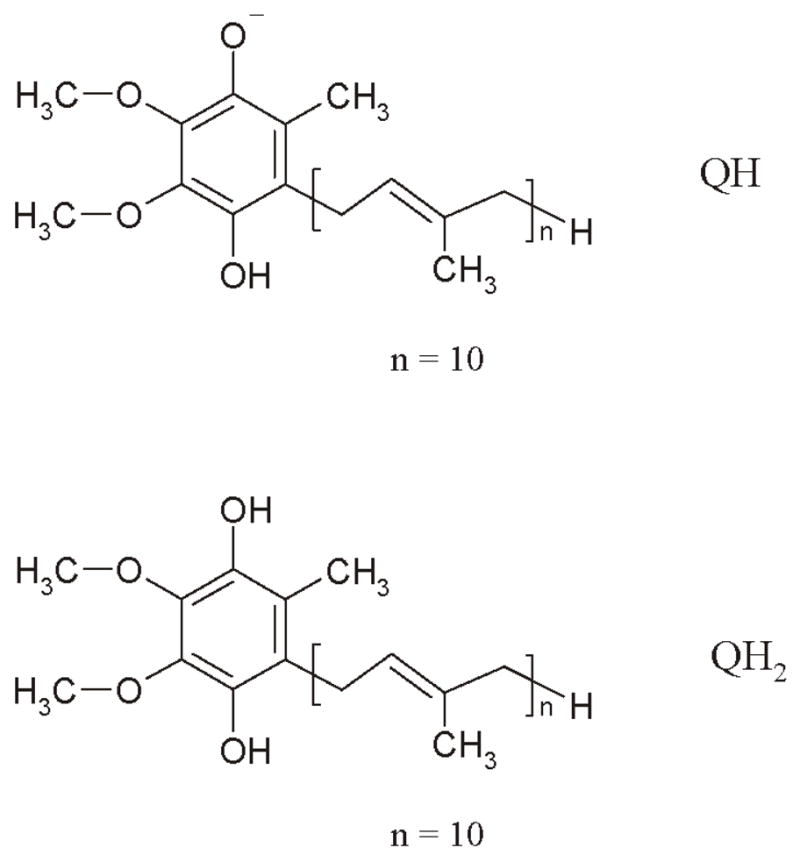
Chemical structure of reduced CoQ10.
CoQ10 is found in numerous cellular structures within the body, including the endoplasmic reticulum, lysosomes, other vesicles, and mitochondria, where it is an important part of the electron transport chain. Other purported beneficial effects of CoQ10 include the prevention of lipid peroxidation initiation in plasma membranes (3), prevention of low-density lipoprotein oxidation (4), antihypertensive functions (5), migraine headache treatment (6), neurodegenerative disease treatment (Parkinson’s Disease; 7), and cardiovascular disease (8). There are no known toxicity factors.
A large number of dietary supplements containing CoQ10 are currently on the market. These products include softgels, 2-piece hardshell capsules, and chewable tablets. CoQ10 may be present as a single entity or in combination with other active ingredients, such as fish oil, vitamins, or botanicals.
In support of a National Institutes of Health-Office of Dietary Supplements (NIH-ODS) and U.S. Food and Drug Administration (FDA) contract with AOAC, an interlaboratory study of a high-performance liquid chromatographic (HPLC) method for the determination of CoQ10 in raw materials and dietary supplement finished products was conducted. The method was selected by an expert review panel (ERP), and a single-laboratory validation (SLV) was performed (9). The interlaboratory study involved 8 materials submitted as blind duplicates to 11 laboratories in 4 countries.
Single-Laboratory Validation
A complete description of the SLV study has been published (9). Results of the SLV are summarized here.
Concentration range
A 5-point calibration curve covering from 0.025 to 0.125 mg/mL CoQ10 demonstrated that the method is linear over this range with a determination coefficient, r2, of 0.999939.
Accuracy
The method exhibited acceptable accuracy upon spiking at 50, 100, and 200% of the target CoQ10 level. The average assay for all levels was 97.3%, with a relative standard deviation (RSD) of 0.5%.
Repeatability
The repeatability RSDs ranged from about 2.2% for a raw material to about 5.0% for a softgel finished product dosage form, with HorRat values ranging from 1.1 to 1.9. Table 1 summarizes the repeatability data.
Table 1.
Single-laboratory validation repeatability data
| Materiala | nb | Average result mg/g | SD, mg/g | RSD, % | PRSDc | HorRat |
|---|---|---|---|---|---|---|
| A | 15 | 996 | 2.34 | 2.35 | 2.00 | 1.18 |
| B | 15 | 1001 | 2.31 | 2.30 | 2.00 | 1.15 |
| C | 23 | 985 | 2.12 | 2.15 | 2.00 | 1.08 |
| D | 15 | 54.8 | 1.39 | 4.70 | 3.09 | 1.52 |
| E | 15 | 41.6 | 2.45 | 2.51 | 3.22 | 0.78 |
| F | 15 | 183.9 | 4.61 | 4.97 | 2.58 | 1.93 |
| G | 15 | 176.1 | 2.36 | 5.00 | 2.60 | 1.92 |
| H | 15 | 256.2 | 4.55 | 4.38 | 2.45 | 1.79 |
A = Raw material; B = raw material; C = raw material; D = softgel, 30 mg CoQ10/unit; E = chewable tablet with 100 mg CoQ10/tablet; F = softgel with 100 mg CoQ10, 250 mg L-carnitine tartarate, 30 mg pycnogenol; G = softgel with 50 mg CoQ10, 30 IU d-alpha tocopheryl acetate, 70 μg selenium; H = 2-piece hardshell with 100 mg CoQ10.
n = Number of replicates.
PRSD = Predicted relative standard deviation, calculated as 2C−0.1505.
Limit of detection (LOD)/limit of quantitation (LOQ)
The LOD for the method was determined to be 3 (μg/mL. The corresponding LOQ for the method was found to be 9 (μg/mL. This corresponds roughly to 3 and 9 mg/g, respectively; however, since sample solutions are prepared based upon CoQ10 concentration in the matrix, the LOD and LOQ will vary depending upon the material.
Collaborative Study
Study Design
The HPLC-UV method was provided to 11 laboratories participating in the collaborative study. Each laboratory was sent 8 materials as blind duplicates, for a total of 16 samples. A description of the test materials is as follows: CoQ10 raw material, 98+%; CoQ10 softgels, 50 mg CoQ10/softgel; CoQ10 hardshell capsules, 100 mg CoQ10/capsule; CoQ10 softgels, 30 mg CoQ10/softgel; CoQ10 chewable tablets, 100 mg/tablet; powdered negative control material containing magnesium stearate, dibasic calcium phosphate, α-tocopherol acetate, and β-carotene; negative control containing 40 mg/g CoQ10; negative control containing 900 mg/g CoQ10. Random identification numbers were assigned to each sample, which was blinded in terms of composition and concentration of CoQ10; however, a dilution scheme was provided to the laboratories based upon the material, as the nature of the material is important to the sample preparation procedure.
Collaborators
Eleven laboratories originally agreed to participate in the collaborative study and received materials to conduct the study. Ten laboratories completed the study in the allotted time. One participating laboratory was not able to complete the work because of other obligations and withdrew from the study. Of the 10 laboratories that completed the study, 7 were from the United States, one was from Japan, one was from Germany, and one was from Italy. The laboratories represented CoQ10 raw material, dietary supplement, and pharmaceutical finished product manufacturers, and contract analytical laboratories.
Test Sample Preparation
Raw material samples, negative controls, and spiked negative controls were tested as-is. For tablet and hardshell capsule finished product dosage forms, a minimum of 20 dosage units were combined and powdered by the participating laboratories to reduce variations due to sample inhomogeneity. For softgel samples, the contents of a minimum of 20 capsules were combined by the laboratories. A dilution scheme for the samples was provided to the laboratories to assist analysts in preparing working sample solutions at the appropriate concentration (Table 2). Spiked negative controls were prepared by the originating laboratory by blending known amounts of CoQ10 and negative control.
Table 2.
Sample dilution scheme
| Sample code No.a | Volume of stock sample solution pipetted, mL |
|---|---|
| CQ001, CQ002, CQ005, CQ013 | 2.00 |
| CQ010, CQ012, CQ014, CQ015 | 5.00 |
| CQ003, CQ004, CQ006, CQ007, CQ008, CQ009, CQ011, CQ016 | 8.00 |
CQ001, CQ005 = Chewable tablets; CQ002, CQ013 = CoQ10 hardshell capsules, 100 mg/capsule; CQ010, CQ014 = CoQ10 softgels, 30mg/softgel; CQ012, CQ015 = CoQ10 softgels, 50 mg/softgel; CQ003, CQ007 = negative control; CQ004, CQ009 = high spike; CQ006, CQ011 = raw material; CQ008, CQ016 = low spike.
Standard
CoQ10 reference standard was provided to each of the collaborating laboratories. The reference standard was obtained from Sigma-Aldrich (St. Louis, MO; P/N C9538, Lot No. 026K1667) and the purity was determined by HPLC analysis against a USP reference standard. An assigned purity of 98.6% was used for this standard for the study.
Test Material Homogeneity
Raw material samples were mixed well prior to shipment; however, no additional homogeneity testing was performed as these materials were already of high purity. Multiple bottles of each of the same lot of the tablet, hardshell capsule, and softgel finished product dosage forms were combined, and subsamples of each of these composites were distributed to the laboratories.
For the spiked negative controls, triplicate portions of each spiked material were sampled and tested by the originating laboratory to ensure homogeneity. Results of the homogeneity testing are presented in Table 3.
Table 3.
Homogeneity data
| Material | Sample | Result, % (w/w) | Average | RSD, % |
|---|---|---|---|---|
| Low spike | 1 | 3.62 | 3.59 | 1.3 |
| 2 | 3.53 | |||
| 3 | 3.60 | |||
| High spike | 1 | 84.7 | 85.2 | 0.58 |
| 2 | 85.7 | |||
| 3 | 85.3 |
Sample Preparation and Shipment
Sixteen test materials were shipped to each of the collaborating laboratories. A sufficient amount of each finished product test material was packaged in suitable sized high-density polyethylene or polyethylene terephthalate bottles by the Study Director. CoQ10 raw material, negative control, and spiked negative control materials were packaged in 4 mL amber glass vials. The bottles/vials were labeled with random identification codes. Test materials were shipped overnight at ambient temperature to the collaborating laboratories. Upon receipt, the laboratories were instructed to store the test materials at refrigerated temperature (4°C) until use. Reference standard was stored frozen (−20°C) until use.
AOAC Official Method 2008.07 Coenzyme Q10 Content in Raw Materials and Dietary Supplements
High-Performance Liquid Chromatography-UV First Action 2008
[Applicable for the determination of coenzyme Q10 (CoQ10) content in CoQ10 raw materials and dietary supplements containing CoQ10.]
See Table 2008.07A for the results of the interlaboratory study supporting acceptance of the method.
A. Principle
CoQ10 is extracted from the matrixes with a mixture of acetonitrile, tetrahydrofuran (THF), and water (55 + 40 + 5, v/v/v), and diluted with 0.1% ferric chloride in ethanol to convert any reduced CoQ10 into the oxidized form. The solution is subjected to reversed-phase high-performance liquid chromatographic (HPLC) analysis using a C18 column and UV detection. Quantitation is performed using a CoQ10 external standard and a 5-point calibration curve.
B. Apparatus
Note: Equivalent apparatus may be substituted. All volumetric pipets and volumetric flasks are Class A.
LC system.—Equipped with pump, autosampler, and UV detector. HPLC operating conditions: column temperature, ambient; mobile phase flow rate, 1.0 mL/min (isocratic); injection volume, 20 μL; detection, 275 nm.
LC column.—Hypersil ODS, 4.0 × 125 mm, 5 μm particle size (Thermo Electron Corp., Waltham, MA: www.thermo.com), or Phenomenex Prodigy ODS-3, 4.6 × 150 mm, 5 μm particle size (Phenomenex, Torrance, CA: www.phenomenex.com).
Analytical balance.—Readability, ±0.01 mg.
Ultrasonication bath.
Low actinic glass (LAG) volumetric flasks.—50, 100, and 200 mL.
Graduated cylinders.—100, 500, and 1000 mL.
Volumetric pipets.—2–5, 8, and 10 mL.
Syringe filters.—PTFE, 0.45 μm pore.
LC injection vials.—2 mL, with Teflon-coated caps.
Syringe.—Luer-Lok, 3 or 10 mL.
C, Reagents
Note: Chemicals from other suppliers meeting specifications may also be used. Acetonitrile, stabilized THF, reagent alcohol, and hexane are flammable and should be stored away from heat and flames.
Solvents.—Acetonitrile, HPLC grade; water, HPLC grade; THF, stabilized, HPLC grade; reagent alcohol (90% ethanol:5% 2-propanol-5% methanol), ACS grade; hexanes, ACS grade.
Ferric chloride.—Minimum 98%.
Mobile phase.—Prepare sufficient quantity for use as both mobile phase and preparation solvent. For every 1000 mL mobile phase, mix 550 mL acetonitrile, 400 mL THF, and 50 mL water. Mix well and degas.
0.1% FeCl3 in alcohol.—Weigh 100.0 ± 10.0 mg ferric chloride and transfer into a 100 mL volumetric flask. Add approximately 50 mL reagent alcohol and sonicate for 30 min. Dilute to volume with reagent alcohol, and mix well.
Reference standards.—Ubidecarenone, USP current lot (U.S. Pharmacopeia, Rockville, MD), or suitably qualified secondary standard.
D. Preparation of Test Solutions
(a) Standard solutions
Accurately weigh about 125 mg (±5 mg) ubidecarenone reference standard and transfer into a 100 mL LAG volumetric flask. Add approximately 50 mL mobile phase, C(c), and sonicate for 30 min. Dilute to volume with mobile phase and mix thoroughly. This is the stock standard solution, with a CoQ10 concentration of about 1.25 mg/mL. Prepare linearity dilutions as shown in Table 2008.07B by pipetting the indicated amount of stock standard solution into the indicated size volume flask and diluting to volume with mobile phase.
(b) Sample test solutions
(1) Raw materials
Accurately weigh about 125 mg (±5 mg) CoQ10 raw material into a 100 mL LAG volumetric flask. Add about 50 mL mobile phase, and sonicate for 30 min. Dilute to volume with mobile phase and mix well. Pipet 8.0 mL of this solution into a 100 mL LAG volumetric flask. Add 10.0 mL 0.1% FeCl3 in alcohol to the flask. Dilute to volume with mobile phase and mix thoroughly. Filter a portion through a 0.45 μm PTFE syringe filter into an HPLC autosampler vial. Label as Test Solution.
(2) Two-piece hardshell capsules (split shell, dry contents)
Determine the average content weight of the capsule. Composite the contents of 20 split capsules into a glass container. Transfer the composite to a mortar and pestle (or suitable grinder) and homogenize. Accurately weigh 5 times the average content weight ±10% into a 100 mL low actinic volumetric flask. Add approximately 50 mL mobile phase and sonicate for 30 ± 5 min. Dilute to volume with mobile phase, invert, and swirl to mix.
Note: If the amount of CoQ10 in one capsule is >100 mg, weigh 2½ times the average content weight ±10%. The concentration of this solution should not be >5.0 mg/mL.
Quantitatively transfer via pipet enough of this solution to make a 0.1 mg/mL CoQ10 final concentration in a 100 mL low actinic volumetric flask. For a 5.0 mg/mL solution, transfer 2.0 mL. To this, add 10.0 mL ferric chloride working solution. Dilute to volume with mobile phase, invert, and swirl to mix. Filter through a syringe filter.
(3) Tablets
Determine the average weight of the tablet. Composite 20 tablets into a glass container. Transfer the composite to a mortar and pestle (or suitable grinder) and homogenize. Accurately weigh 5 times the average content weight ±10% into a 100 mL low actinic volumetric flask. Add approximately 50 mL mobile phase and sonicate for 30 ± 5 min. Dilute to volume with mobile phase, invert, and swirl to mix.
Note: If the amount of CoQ10 in one tablet is >100 mg, weigh 2½ times the average content weight ±10%. The concentration of this solution should not be >5.0 mg/mL.
Quantitatively transfer via pipet enough of this solution to make a 0.1 mg/mL CoQ10 final concentration in a 100 mL low actinic volumetric flask. For a 5.0 mg/mL solution, transfer 2.0 mL. To this, add 10.0 mL ferric chloride working solution. Dilute to volume with mobile phase, invert, and swirl to mix. Filter through a syringe filter.
(4) Softgel capsules
Determine the average capsule content weight by weighing 20 capsules. Record the weight. Express the fill material from the capsules and combine the capsule contents. Thoroughly clean the capsule shells by rinsing with hexane and allow to dry. Weigh and record the weight of the empty capsule shells.
where C = total weight of 20 capsules and S = total weight of 20 capsule shells.
Accurately weigh 10 times the average content weight ±10% into a 200 mL low actinic volumetric flask. Add approximately 100 mL mobile phase and sonicate for 30 ± 5 min. Dilute to volume with mobile phase, invert, and swirl to mix.
Note: If the amount of CoQ10 in one softgel is > 100 mg, weigh 5 times the average content weight ±10%. The concentration of this solution should not be >5.0 mg/mL.
Quantitatively transfer via pipet enough of the solution to make a 0.1 mg/mL CoQ10 final concentration in a 100 mL low actinic volumetric flask. For a 5.0 mg/mL solution, transfer 2.0 mL. Add 10.0 mL ferric chloride working solution. Dilute to volume with mobile phase, invert, and swirl to mix. Filter through a syringe filter.
E. Determination
(a) System suitability test
Equilibrate the LC system with the mobile phase for at least 10 min until a stable baseline is obtained. Make 5 replicate 20 μL injections of Linearity Standard Solution 1. The system is considered suitable if the retention times of the standard peak do not deviate more than 0.5 min, and the RSD of the peak area is ≤2.0%.
(b) Calibration
Make single 20 μL injections of each linearity standard solution. Calculate the slope, y-intercept, and r2 value for the calibration curve. The r2 value should be ≥0.995.
(c) Injection
Make single 20 μL injections of each test solution.
F. Calculations
The amount of CoQ10 in the test material, in mg/g, is calculated as follows:
where P0 = peak area of CoQ10 in sample chromatogram; b0 = y-intercept of calibration curve for CoQ10; m0 = slope of calibration curve for CoQ10; V = volume of Test Solution 1 in mL; W = sample weight, in g; D = dilution factor.
Percent (w/w) is calculated from mg/g as follows:
Milligrams per tablet (mg/tab) is calculated from mg/g as follows:
where TW = average tablet weight in grams.
Milligrams per capsule (mg/cap) is calculated from mg/g as follows:
where FW = average capsule fill weight in grams. Reference: J. AOAC Int. 91, 702(2008).
Results and Discussion
Eleven laboratories agreed to participate in the collaborative study. Ten laboratories were able to submit the data before the deadline. The remaining laboratory was not able to finish the study because of lack of time.
Results, in mg CoQ10/g material, for each of the 8 blind replicates are presented in Table 4. Test samples were given random codes prior to shipment to the collaborators, and then decoded when the results were returned. Table 2008.07A presents a statistical summary of the results. Statistical analysis to determine repeatability and reproducibility was performed using the AOAC Interlaboratory Study Workbook for Blind (Unpaired) Replicates, v. 2.0 (10). Repeatability standard deviations (sr), reproducibility standard deviations (SR), repeatability relative standard deviations (RSDr), reproducibility relative standard deviations (RSDR), and number of statistical outliers are presented. HorRat values are also presented in these tables, and are calculated as RSDR (observed)/RSDR (predicted), where RSDR (predicted) is calculated using the equation RSDR = 2C−0.1505, where C is the measured analyte concentration in decimal mass units (11). Cochran, Grubbs, and double Grubbs tests were used to remove statistical outliers where appropriate. Recovery data for the low-spike and high-spike negative controls are presented in Table 5, and coefficients of determination for all calibration curves are presented in Table 6.
Table 4.
Results for blind replicates
| Results, mg/g, by Lab No.
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Material | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Raw material | 950 | 1010 | 1000 | 1010 | 1020 | 1030 | 989 | 972 | 916 | 1000 |
| 928 | 998 | 994 | 1010 | 1020 | 1060 | 989 | 947 | 941 | 1000 | |
| Negative control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Low-spike negative control | 29.6 | 36.0 | NRa | 26.9 | 40.4 | 39.8 | 33.3 | 36.9 | 45.0 | NR |
| 29.4 | 35.8 | NR | 26.2 | 42.2 | 39.3 | 34.4 | 36.4 | 45.6 | NR | |
| High-spike negative control | 937 | 891 | 873 | 864 | 917 | 872 | 869 | 851 | 852 | 883 |
| 842 | 897 | 891 | 893 | 904 | 941 | 873 | 845 | 856 | 880 | |
| Hardshell capsules | 250 | 269 | 259 | 262 | 264 | 260 | 261 | 253 | 244 | 261 |
| 246 | 264 | 258 | 264 | 269 | 257 | 263 | 249 | 245 | 265 | |
| Softgels, 50 mg | 176 | 187 | 181 | 183 | 189 | 194 | 183 | 171 | 172 | 181 |
| 172 | 187 | 179 | 183 | 185 | 194 | 182 | 176 | 177 | 184 | |
| Softgels, 30 mg | 46.9 | 50.9 | 54.7 | 51.3 | 52.2 | 53.5 | 45.3 | 50.9 | 58.8 | 42.0 |
| 46.7 | 47.2 | 54.2 | 50.2 | 52.5 | 56.6 | 45.2 | 51.0 | 60.5 | 42.5 | |
| Chewable wafers | 41.0 | 43.6 | 45.4 | 45.1b | 42.3 | 45.9 | 44.9 | 41.1 | 51.9 | 43.2 |
| 41.6 | 43.1 | 46.1 | 35.4b | 42.6 | 45.4 | 44.1 | 41.5 | 51.4 | 42.0 | |
NR- Not reported.
Cochran outlier.
Table 5.
Recovery results
| Lab No.
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Material | Target, mg/g | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Low spike | 35.9 | Found, mg/g | 29.5 | 35.9 | NRa | 26.6 | 41.8 | 39.5 | 33.8 | 36.6 | 45.3 | NR |
| 35.9 | Recovery, % | 82.2 | 100 | —b | 74.0 | 116 | 100 | 94.2 | 92.7 | 115 | — | |
| High spike | 852 | Found, mg/g | 890 | 894 | 882 | 878 | 910 | 906 | 871 | 848 | 854 | 882 |
| 852 | Recovery, % | 104 | 105 | 104 | 103 | 107 | 106 | 102 | 99.5 | 100 | 104 | |
NR = Not reported.
— = Not applicable.
Table 6.
Average coefficients of determination (r2)
| Lab | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| r2 | 0.9998 | 0.9999 | 0.9988 | 0.9997 | 0.9993 | 0.9999 | 1.000 | 1.000 | 1.000 | 0.9998 |
Collaborators’ Comments
Laboratory 1 noted that the peak retention time was temperature dependent and shifted as the room became warmer. Laboratory 1 also noted that the retention time of the CoQ10 was consistently longer than that specified in the method. Several laboratories noted that the results for the low spike fell below the calibration range of the instrument. No other comments were received.
Performance Characteristics
The repeatability (RSDr) and reproducibility (RSDR) for all materials were acceptable, with HorRat values ranging from 1.0 to 2.0 with the exception of the low spike negative control, chewable tablets, and the 30 mg softgels. One Cochran outlier was identified for the chewable tablets material, and this data pair was removed before the statistical analysis. No other outliers (Cochran or Grubbs) were identified for any material; therefore, all remaining data were used. The 30 mg softgel material yielded a HorRat value of 3.3; the 50 mg softgel material, however, yielded a HorRat of 1.4. The reason for the significant difference in the reproducibility between these 2 samples of the same type has not been conclusively identified, but may be dependent upon the CoQ10 concentration in the test material.
The average recovery of CoQ10 from the low-spike negative control was 98.3%; however, this material yielded a HorRat value of 5.1, with individual recoveries ranging from 73.0 to 127%. Laboratories 3 and 10 did not report any results as the data obtained fell below the calibration curve. The average recovery of the CoQ10 from the high-spike negative control was 103%, with aHorRat value of 1.6. No CoQ10 was detected by any laboratory in the negative control material.
It may be notable that all materials yielding high HorRat values (30 mg softgels, chewable tablets, and low-spike negative control) all had CoQ10 concentrations of <100mg/g, while all materials yielding acceptable HorRat values contained CoQ10 at concentrations >100 mg/g.
Recommendations
On the basis of the results of this study, it is recommended that the method be adopted for Official First Action for the determination of CoQ10 in raw materials and finished product dietary supplements containing CoQ10 at a concentration of >100 mg/g. Currently, no data are available to support recommending the method for testing products containing CoQ10 at concentrations <100 mg/g. Use of a thermostatted column oven to maintain constant temperature over the course of the analysis is also recommended.
Table 2008.07A.
Interlaboratory study results for CoQ10 content in raw materials and dietary supplements Table 2008.07A.
| Samplea | Average, mg/g | sr | RSDr, % | sR | RSDR, % | HorRat | Outlier labs | No. labs used |
|---|---|---|---|---|---|---|---|---|
| A | 989 | 11.9 | 1.2 | 37.4 | 3.78 | 1.89 | 0 | 10 |
| B | 36.1 | 0.606 | 1.68 | 6.15 | 17.1 | 5.17 | 0 | 8 |
| C | 882 | 27.6 | 3.13 | 28.1 | 3.19 | 1.56 | 0 | 10 |
| D | 258 | 2.42 | 0.94 | 7.95 | 3.08 | 1.26 | 0 | 10 |
| E | 50.7 | 1.18 | 2.34 | 5.18 | 10.2 | 3.26 | 0 | 10 |
| F | 182 | 2.18 | 1.2 | 6.77 | 3.72 | 1.44 | 0 | 10 |
| G | 44.5 | 0.395 | 0.89 | 3.39 | 7.62 | 2.39 | 1 | 9 |
| H | 0 | NAb | NA | NA | NA | NA | NA | 10 |
A = Raw material; B = low-spike negative control; C = high-spike negative control; D = 2-piece hardshell with 100 mg CoQ10; E = softgel, 30 mg CoQ10/unit; F = softgel with 50 mg CoQ10, 30 IU d-alpha tocopheryl acetate, 70 μg selenium; G = chewable tablet with 100 mg CoQ10/tablet; H = negative control.
NA = Not applicable.
Table 2008.07B.
Preparation of linearity dilutions Table 2008.07B.
| Linearity No. | Volume stock, mL | Flask volume, mL | Approximate concn, mg/mL |
|---|---|---|---|
| 1 | 5 | 50 | 0.125 |
| 2 | 4 | 50 | 0.100 |
| 3 | 3 | 50 | 0.075 |
| 4 | 2 | 50 | 0.050 |
| 5 | 2 | 100 | 0.025 |
Acknowledgments
We acknowledge Eurofins Scientific for performing the SLV of the method, and Kaneka, Pharmavite, NOW Foods, and Compound Solutions for providing test materials for the study. We also acknowledge the following collaborators for participation in the study: Amitabh Chandrah, Access Business Group, Ada, MI
Tadahiro Edamura, Kaneka, Hyogo, Japan
Takako Honda, Shaklee Corp., Hayward, CA
Kerri LeVanseler, NSF International, Ann Arbor, MI
Mythili Nagarajan, Enzymatic Therapy, Green Bay, WI
Nila Patel, Leiner Health Products, Garden Grove, CA
Klaus Reif, Phytolab GmbH & Co., Vestenbergsgreuth, Germany
Brian Schaneberg, ChromaDex, Boulder, CO
Darryl Sullivan, Covance Laboratories, Madison, WI
Luigi Trussardo, LUPI-Lundbeck Pharmaceuticals Italy, Padova, Italy
Footnotes
The recommendation was approved by the Methods Committee on Dietary Supplements as First Action. See “Official Methods Program Actions,” (2008) Inside Laboratory Management, May/June issue.
Contributor Information
Steven Lunetta, Pharmavite LLC, 1150 Aviation P1, San Fernando, CA 91340.
Mark Roman, Tampa Bay Analytical Research, PO Box 931, Safety Harbor, FL 34695-0931.
References
- 1.Crane FL, Hatefi Y, Lester RI, Widmer C. Biochim Biophys Acta. 1957;25:220–221. doi: 10.1016/0006-3002(57)90457-2. [DOI] [PubMed] [Google Scholar]
- 2.Wolf DE, Hoffman CH, Trenner NR, Arison BH, Shunk CH, Linn BD, McPherson JF, Folkers K. J Am Chem Soc. 1958;80:4750–4752. [Google Scholar]
- 3.Alleva R, Scaraarmucci A, Mantera F, Bompandre S, Leoni L, Linarro GP. Mol Aspects Med. 1997;18:221–228. doi: 10.1016/s0098-2997(97)00040-x. [DOI] [PubMed] [Google Scholar]
- 4.Raitakari OT, McCredie RJ, Witting P, Griffiths KA, Letter J, Sullivan D, Stocker R, Celermajer DS. Free Radic Biol Med. 2000;28:1100–1105. doi: 10.1016/s0891-5849(00)00201-x. [DOI] [PubMed] [Google Scholar]
- 5.Langsjoen P, Langsjoen A, Willis R, Folkers K. Mol Aspects Med. 1994;15:s265–s272. doi: 10.1016/0098-2997(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 6.Rozen TD. Cephalalgia. 2002;22:137–141. doi: 10.1046/j.1468-2982.2002.00335.x. [DOI] [PubMed] [Google Scholar]
- 7.Muller T, Buttner T, Gholipour AF, Kuhn W. Neurosci Lett. 2003;341:201–204. doi: 10.1016/s0304-3940(03)00185-x. [DOI] [PubMed] [Google Scholar]
- 8.Kendler BS. Prog Cardiovasc Nurs. 1997;12:3–23. [PubMed] [Google Scholar]
- 9.Orozco D, Skamarack J, Reins K, Titlow B, Lunetta S, Li F, Roman M. J AOAC Int. 2007;90:1227–1236. [PMC free article] [PubMed] [Google Scholar]
- 10.AOAC Interlaboratory Study Workbook Blind (Unpaired) Replicates. AOAC INTERNATIONAL; Gaithersburg, MD: 2006. Version 2.0. [Google Scholar]
- 11.Horwitz W, Kamps LR, Boyer KW. J Assoc Off Anal Chem. 1980;63:1344–1354. [PubMed] [Google Scholar]


