Abstract
Activation of the Gq-coupled P2Y6 receptor heterologously expressed in astrocytes significantly attenuates apoptosis induced by tumor necrosis factor α (TNFα). We have extended the analysis of P2Y6 receptor-induced cytoprotection to mouse skeletal muscle cells endogenously expressing this receptor. The endogenous P2Y6 receptor agonist UDP and synthetic agonist MRS2693 protected C2C12 skeletal muscle cells against apoptosis in a concentration-dependent manner (0.1−10 nM) as determined by propidium iodide staining, histochemical analysis using hematoxylin and Hoechst 33258, and DNA fragmentation. The insurmountable P2Y6 receptor antagonist MRS2578 blocked the protection. TNFα-induced apoptosis in C2C12 cells correlated with activation of the transcription factor NF-κB. The NF-κB activation was attenuated by 10 nM MRS2693, which activated the antiapoptic ERK1/2 pathway. In an in vivo mouse hindlimb model, MRS2693 protected against skeletal muscle ischemia/reperfusion injury. The P2Y6 receptor is a novel cytoprotective receptor that deserves further exploration in ameliorating skeletal muscle injury.
Keywords: ischemia, uracil nucleotide, G protein-coupled receptor, tumor necrosis factor, skeletal muscle, UDP
Introduction
The P2Y receptors comprise eight subtypes of G protein-coupled receptors (GPCRs) that respond to extracellular purine and pyrimidine nucleotides [1]. There is increasing interest in the therapeutic potential of compounds that act at these receptors for treating a wide range of diseases, and novel, selective agonists and antagonists have been introduced for some of the P2Y subtypes [2,3]. The P2Y6 receptor is activated by uridine-5′-diphosphate (UDP) and is distributed throughout various tissues, including the lungs, heart, skeletal muscle, thymus, aorta, bone, spleen, digestive tract, placenta and brain. This receptor has been implicated in protection against apoptosis induced by the tumor necrosis factor α (TNFα) [4,5]. Other effects associated with the P2Y6 receptor are the enhancement of osteoclast survival through NF-κB activation [6], the regulation of electrolyte transport in the airways [7], the production of proinflammatory cytokines and chemokines [8,9], and the growth and contraction of vascular muscles [10].
We have reported the antiapoptotic effect of P2Y6 receptor activation and currently extend the analysis to protection of skeletal muscle. Most of the studies on P2Y receptors and cell death have used brain cells as a model [11]. We have already explored this relationship with particular focus on P2Y6 and P2Y12 receptors in stably-transfected 1321N1 astrocytoma cells [4,5,12,13]. Activation of either receptor by the appropriate nucleoside 5′-diphosphate can attenuate the proapoptotic effects of exposure to TNFα. Apoptosis has been described in developing and, recently, in adult skeletal muscle. The cellular and molecular aspects of apoptosis in myoblasts and myofibers are increasingly recognized as important in muscle disease [14]. Alterations in the pathways that regulate myoblast profileration/differentiation can induce apoptosis during myogenesis both in vitro and in vivo. The aim of our study was to investigate the protective effects of P2Y6 receptor agonists in skeletal muscle, both in cell culture and in vivo. We activated the receptor using the native agonist of the P2Y6 receptor, UDP, and novel selective and potent agonist, 5-iodouridine-5′-diphosphate (MRS2693) [2].
2. Materials and Methods
2.1. Materials
Mouse skeletal C2C12 myoblasts were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Life Technologies (Rockville, MD). Plastic collagen-coated cellware was purchased from Becton Dickinson (Bedford, MA). Horseradish peroxidase (HRP)-linked anti-rabbit IgG, HRP-linked anti-mouse IgG antibodies, and antibodies to ERK1, ERK2, NF-κB, β -actin and α, β and θ isoforms of protein kinase C (PKC) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies to the phosphorylated forms of ERK were also supplied by Santa Cruz Biotechnology. TNFα was purchased from Biosource International (Camarillo, CA). The rabbit polyclonal antibodies for the P2Y1, P2Y2, P2Y4, P2Y6, P2Y11 and P2Y12 receptors were purchased from Alomone Labs, Ltd. (Jerusalem, Israel). APOBrdU TUNEL Assay Kit and Hoechst 33258 (2′-[4-hydroxyphenyl]-5-[4-methyl-1-piperazinyl]-2,5′-bis-1H-benzimidazole trihydrochloride pentahydrate) were purchased from Molecular Probes (Invitrogen Detection Technologies, Carlsbad, CA). Calcium Mobilization Assay Kit was purchased from Molecular Devices (Sunnyvale, CA).
MRS2365 (2-methylthio-(N)-methanocarba-adenosine-5′-diphosphate) and MRS2578 (1,4-di-[(3-isothiocyanatophenyl)-thioureido]butane) were obtained from Tocris (Ellisville, MO). MRS2693 was synthesized as reported [2]. Hematoxylin solution, cycloheximide, and all other chemical reagents were purchased from Sigma (St. Louis, MO).
2.2. Cell culture
Mouse skeletal C2C12 myoblasts (ATCC, Rockville, MD, USA) were differentiated to myotubes following transfer of the cells from high (DMEM/F-12 medium, 1:1 supplemented with 10% FBS, 100 units/ml penicillin, 100 mg/ml streptomycin, and 2 mM L-glutamine) to low (5% horse serum + DMEM) serum growth medium at 37°C in a humidified incubator with 5% CO2/95% air. The myoblasts differentiated to myotubes 7 days after the serum was changed.
Cells were plated in 6-well collagen-coated plates at an original seeding density of 200,000−500,000 cells per well and cultured to ∼70% confluence for the apoptosis experiments.
2.3. Induction and detection of apoptosis
TNFα was used to induce apoptosis in C2C12 cells (5, 7 and 12 days old). After washing of the cells, the medium was replaced with fresh medium containing 5 μg/ml cycloheximide, which was present during the entire subsequent incubation to promote apoptosis as previously discussed [5,13]. In cases of coadminstration of the P2Y6 receptor antagonist MRS2578, this was the next reagent to be added. A freshly prepared DMSO solution of MRS2578 (1 mM) was added to the incubation medium to reach a final concentration of 10 μM for an optional initial incubation of 20 min. The next reagent added to the cells was either a P2Y6 receptor agonist (MRS2693 or UDP), when present, or TNFα (10 ng/ml). When P2Y6 receptor agonists were used, TNFα was added 10 min after the agonist. The cells remained in the presence of TNFα and other agents for 4 h. The medium was then changed and the culture was left in the presence of cycloheximide for 16 h. Cell death was observed 20 h after the first exposure to TNFα.
DNA fragmentation of apoptotic cells was detected using the standard Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) method. Cell death or apoptosis was indicated by PI positive cells and by fluorescence of termini of DNA fragments labeled with 5-bromo-2′-deoxyuridine 5′-triphosphate (BrdUTP). The presence of live cells was detected by fluorescent labeling of DNA with the cell-permeant dye Hoechst 33258 for staining nuclear chromatin. For microscopic applications, the cells were deposited onto slides.
2.4. Histochemical staining and cell viability
After treatment to induce apoptosis, cells detached by trypsin-EDTA were combined and centrifuged for the cell viability assay. The cells were washed with PBS (phosphate-buffered saline) and resuspended to 2−5×105/ml in PBS. The cells were then treated with PI solution (final concentration: 2 μg/ml). After incubating for 10 min at room temperature in the dark, the PI-positive cell fraction was analyzed by flow cytometry (BD FacsCalibur, Becton Dickinson).
Myotubes were grown on collagen-coated 6-well plates. For histochemical staining, the medium was removed from the wells, and the plates were rinsed three times for 5 min with PBS, fixed with methanol for 10 min, and washed again as above. The cells were incubated for 3 min in hematoxylin solution (35.2 g/l aluminum sulfate, 0.4 g/l sodium iodate). After three washes for 5 min each in PBS, the plates were allowed to air dry before glycerol was added to them. The image was visualized with a Zeiss (Thornwood, NY) wide-field microscope.
2.5. Western blotting
Cells were washed with PBS and were lysed with RIPA lysis buffer (Santa Cruz Biotechnology) (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS) containing X1 broad-spectrum protease inhibitor cocktail (Protease inhibitor cocktail I; Calbiochem) at 4°C and centrifuged at 14,000 × g for 10 min at 4°C. The proteins in the supernatants were denatured with SDS lysis buffer containing dithiothreitol for 5 min at 95°C. The cell lysates (40 μg) were separated on 10% SDS-PAGE gel electrophoresis, then transfered to a nitrocellulose membrane (Invitrogen), and incubated with primary antibodies at the appropriate dilution to: NF-κB (1:1000); PKC θ, α, and βI (1:1000); and β-actin (1:7000).
For analysis of ERK activation, Western blots were generated as described above but developed with an affinity-purified mouse monoclonal antibody that specifically recognizes the dually Thr202/Tyr204-phosphorylated, active form of ERK (anti-phospho-ERK; 1:1000; Santa Cruz Biotechnology). The Western blots shown are representative of three separate experiments, and each panel is taken from a single immunoblot. The quantitative analysis of the data form Western blots was done using the ImageJ software, provided at the NIH website (http://rsb.info.nih.gov/ij/index.html).
2.6. Statistical analysis of in vitro data
Pharmacological parameters were analyzed with the GraphPAD Prism software (version 4.0, GraphPAD Prism, San Diego, CA). Data were expressed as mean ± standard error. Statistical significance was calculated using the Student's t-test. P values less than 0.05 (P<0.05) were considered to be statistically significant. Where applicable, treatment mean comparisons were made by employing the Tukey-Kramer HSD test using a significance level of P<0.05 [15].
2.7. Mouse hindlimb ischemia and reperfusion model
A previously described mouse model of hindlimb ischemia and reperfusion was used [16]. Wild-type mice of the C57BL6 strain (2.5- to 3-months-old), each weighing ∼25 g, were sedated with the anesthetic pentobarbital (50 mg/kg) by intraperitoneal (i.p.) injection. A right or left hindlimb (chosen randomly) of each mouse was elevated briefly to minimize retained blood before being subjected to ischemia. Ischemia was induced by placement of a constrictor band (Latex O-Rings, Miltex Instruments, York, PA) above the greater trochanter with a McGiveney Hemorrhoidal Ligator (7 in long; Miltex) according to a modification of the previously described method [15,16]. After 90 min of warm ischemia at 37°C, the constrictor was removed to allow reperfusion for 24 h. The mice were continuously maintained on a 37°C warming pad (Physitemp Instruments, Inc., Clifton, NJ) during the reperfusion.
After the mice were killed by anesthetic overdose, the gastrocnemius muscle was quickly frozen, cut into three pieces, and embedded in Shandon Cryomatrix (polyvinyl alcohol 10%, polyethylene glycol 4%; Anatomical Pathology U.S.A., Pittsburgh, PA). Gastrocnemius was used because of its high proportion of fast-twitch muscle, which is prone to ischemia and reperfusion injury [16]. Each piece was processed as one 10-μm slice on a Thermo Electron/Shandon Cryotome (Anatomical Pathology), fixed in ice-cold acetone, air dried, and washed in PBS.
2.8. Quantification of skeletal muscle injury
The serum level of creatine kinase (CK) activity in the mice provided a circulating index of the extent of skeletal muscle injury. CK activity was measured with a previously described procedure [19].
Histological examination of mouse skeletal muscle tissue was performed to assess the degree of ischemic damage. Each 10-μm slice was then stained with rabbit polyclonal anti–skeletal muscle actin antibodies (ab15265; Abcam, Inc., Cambridge, MA) and goat polyclonal anti–rabbit IgG conjugated with fluorescein isothiocyanate. Evans Blue dye [EBD], the only dye that has been used as an established method for quantifying skeletal muscle injury in vivo, was the histochemical dye of choice in this model. Slices were mounted, and their cross sections were viewed with a fluorescent microscope (EBD-positive cells via a DM580 band pass filter 510−560 nm with emission of 590 nm; fluorescein-labeled cells via a DM510 filter of 450−490 nm with emission at 520 nm). Seven fields per slice were viewed and counted at 20 X magnification.
The same slices were viewed and their images captured through the two filters for quantification of muscle injury as follows. Images were acquired and stored as JPEG files with a Macrofire camera (Macrofire 1.0, Optronics, Goleta, CA). EBD-positive cells were quantified (ImageProPlus, version 5.0, Media Cybernetics, Inc., Silver Spring, MD) [17,18]. The percentage of EBD-positive areas was calculated by dividing the area of EBD staining by the total area of the muscle cells, a quantity defined as the sum of all areas stained by anti–skeletal muscle actin. The fraction of skeletal muscle that stained positive for EBD represented a direct determination of the muscle that was injured. Both EBD staining and CK measurements (above) are established and accepted methods to quantify skeletal muscle injury [16,19].
2.9. Protocol for in vivo administration of P2Y6 receptor agonist
The P2Y6 receptor agonist MRS2693 (300 μM) or vehicle alone (0.1% DMSO in PBS) was administered in a sterile 0.1-ml volume by i.p. injection 2 h before the induction of ischemia. This protocol was used in the previous study of adenosine receptor agonists and antagonists in the same model [16].
EBD (1% wt/vol solution to yield 1 mg of EBD/10 g body weight) was also given via a separate i.p. injection 2.5 h before the induction of ischemia. Student's t test was used to analyze the statistical significance of differences in more than two groups. P < 0.05 was considered statistically significant.
3. Results
3.1. Detection of the P2Y receptors subtypes by specific anti-rabbit antibodies
The presence of P2Y receptors in the C2C12 cells was probed immunologically. Expression of the P2Y6 receptor in the C2C12 cell line was confirmed using specific anti-rabbit antibodies (against human P2Y6). Western blot analysis also demonstrated the presence of P2Y1, P2Y4, and P2Y11 receptors, but not P2Y2 or P2Y12 receptors, in the C2C12 cell line (Figure 1A).
Figure 1.
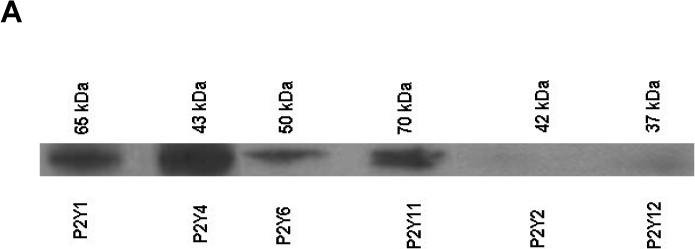
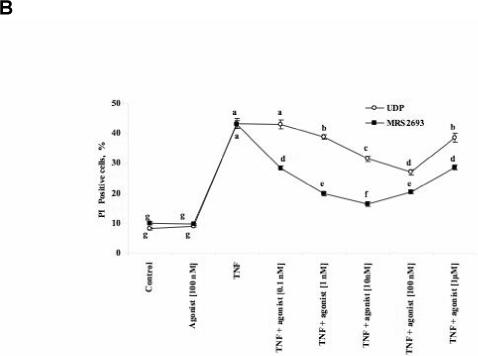
(A) Western blots indicated the presence of P2Y1, P2Y4, P2Y6, and P2Y11 receptors in the C2C12 cell line. (B) Induction by TNFα of cell death in 12 day-old C2C12 cell cultures and its modulation by P2Y6 receptor agonists, the classical agonist UDP (0.1 nM – 1 μM) and the selective P2Y6 receptor agonist MRS2693 (0.1 nM - 1 μM). Data shown are mean ± SD from three independent experiments in triplicate. Treatment means were compared by used of the Tukey-Kramer HSD test using a significance level of P<0.05. The points labeled with same letter are not significantly different at 0.05 levels.
3.2. Effects of P2Y receptor ligands on in vitro apoptosis
To study apoptosis using a quantifiable model in C2C12 cells, the cell culture (5 − 12 days old) was treated for 4 h with TNFα (10 ng/ml) and cycloheximide (5 ng/ml). The medium was then changed, and the culture was left in the presence of cycloheximide for 16 h. Cell death was observed 20 h after the first exposure to TNFα. Apoptosis was induced by this treatment, as indicated by an increase in the percent of PI-positive cells from <10% to 30−40%. Activation of the P2Y6 receptor by the native agonist UDP partially protected the 12 days old cells (Figure 1B) from TNFα-induced cell death. The protection was concentration-dependent over a range of 0.1 to 100 nM; however, at 1 μM UDP the degree of protection was reduced. The synthetic, selective P2Y6 receptor agonist MRS2693 [2] protected the C2C12 cell line against TNFα-induced apoptosis. Between 0.1 and 10 nM MRS2693 there was a concentration-dependent protection; however, at MRS2693 concentrations of 10 and 100 nM, protection was diminished. The protection induced by MRS2693 was more pronounced at the maximal degree of protection than that provided by UDP under similar conditions. There was no effect on cell death by administration of either P2Y6 receptor agonist in the absence of TNFα. Several concentrations of MRS2693 (10 and 100 nM) were also examined in C2C12 cell cultures at an earlier stage (5 and 7 days old). Both concentrations antagonized TNFα-induced cell death to the degree of 50 − 60% protection (data not shown). Other P2Y receptor agonists (UTP, ADP, MeSADP, the P2Y1 receptor selective agonist MRS2365) at 100 nM did not protect against TNFα-induced apoptosis in the C2C12 cell line (data not shown).
We examined the effects of the insurmountable P2Y6 receptor antagonist MRS2578 in blocking the nucleotide-induced protection as indicated by staining with PI [4]. The protective effects of 10 nM MRS2693 against TNFα-induced apoptosis were completely antagonized by a 20 min pretreatment of the C2C12 cells with 10 μM MRS2578 (data not shown). There was no effect of MRS2578 on cell death in the absence of TNFα.
3.3. Morphological and histochemical analysis of C2C12 cells
The C2C12 cells subjected to apoptotic conditions were examined for morphological changes and analyzed by various histochemical methods. PI staining was used in conjunction with the TUNEL assay to indicate the difference between apoptotic and dead cells. The stain Hoechst 33258, which labels DNA in order to visualize nuclei and mitochondria, was used as a marker of living cells.
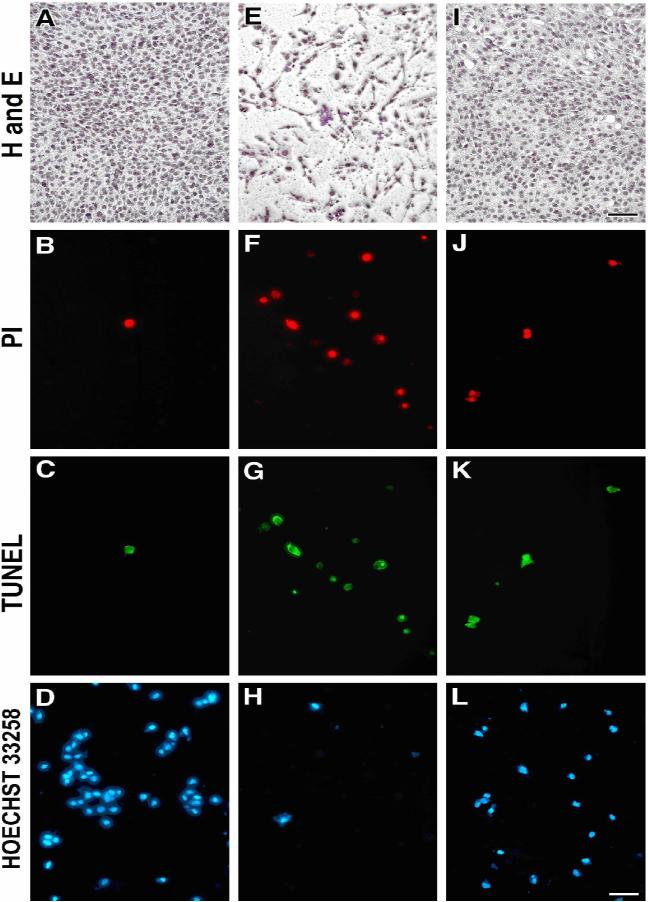
Histochemical staining with hematoxylin, application of Hoechst 33258, and the TUNEL assay of apoptosis clearly showed that activation of the P2Y6 receptor by the potent agonist MRS2693 protected the cells from TNFα-induced apoptosis (Figures 2 A- L). Treatment of the cells with TNFα and MRS2693, followed by fixation in methanol and hematoxylin staining, demonstrated that activation of the P2Y6 receptor by this agonist protected the cells from TNFα-induced apoptosis (Figures 2 A, E, I). Cells also were treated and stained with Hoechst 33258. An increase in the blue-stained cells also provided evidence of the protective effect of MRS2693 (Figures 2 D, H, L). Similarly, a DNA fragmentation assay also provided evidence of the protective effect of MRS2693. The corresponding images (Figures 2 F & G) illustrate apoptotic cells visualized by the TUNEL method in comparison to control cells (Figures 2 B & C). The protective effect of MRS2693 can be seen in Figures 2 J & K.
Figure 2.
Histochemical staining by hematoxylin, Hoechst 33258 and TUNEL in C2C12 cells subjected to proapoptotic conditions. Morphological changes of C2C12 cells were observed 16 h after treatment as indicated in Materials and Methods. Light microscopic images following hematoxylin staining in C2C12 cells: (A) nontreated control cells; (E) TNFα-treated cells; (I) TNFα + MRS2693-treated cells. Fluorescence micrographs of TUNEL-assayed cells. Other panels represent: Nontreated control cells stained with PI (B), negative TUNEL-stained (C), and (D) control cells stained with the dye Hoechst 33258; TNFα-treated cells (F) cells stained by PI, (G) positive TUNEL-stained cells, (H) cells stained with Hoechst 33258; TNFα + MRS2693-treated cells (protected cells) stained with PI (J), negative TUNEL-stained (K), and (L) control cells stained with Hoechst 33258. The concentration of MRS2693 was 10 nM. Pictures were taken using a Zeiss wide-field microscope. Magnification of the figures was either 63× (TUNEL and Hoechst 33258) or 20× (hematoxylin). Bars = 10 μm. Each image is representative of three experiments.
3.4. Mechanistic probing of the in vitro antiapoptotic effects in C2C12 cells
In an effort to probe mechanistically the protective effects in C2C12 cells, the time course of activation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) and other intracellular kinases by MRS2693 was explored. As shown previously for UDP in the astrocytoma cells and in C2C12 cells [21], P2Y6 receptor activation by MRS2693 increased the level of phosphorylated ERK1/2 by 3.4-fold in this C2C12 cell culture (Figure 3).
Figure 3.
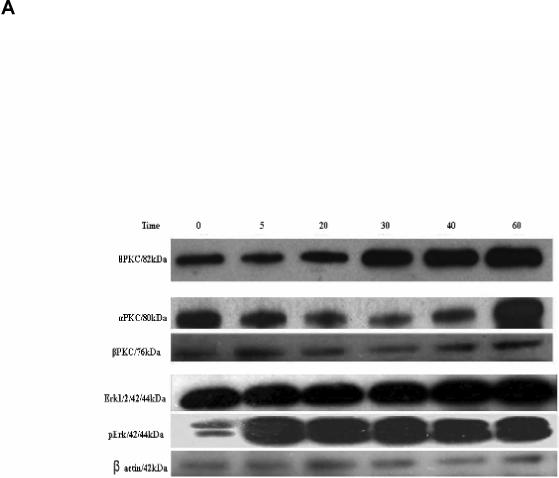
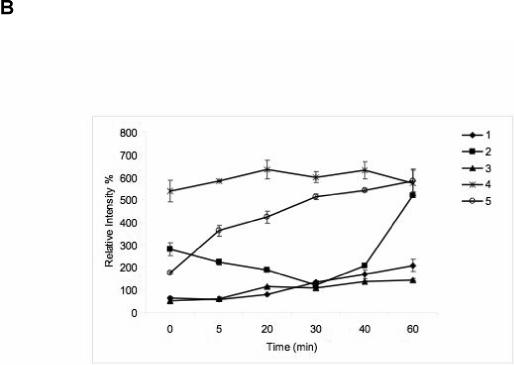
(A) Effect on the expression of PKC α, β, θ and ERK1/2, after incubation of C2C12 cells with the agonist MRS2693 (10 nM) for a period ranging from 5 to 60 min. A total of 40 μg of protein containing an actin standard was applied to each lane. (B) The quantitative analysis of the data in (A), done using the ImageJ software. 100% is defined as the average intensity of the actin standard. The proteins were numbered as 1, PKCθ; 2, PKCα; 3, PKCβ; 4, Erk1/2; 5, pErk1/2.
Activation of protein kinase C (PKC) isozymes following P2Y6 receptor activation in C2C12 cells was also probed. As shown in Figure 4, activation of the P2Y6 receptor with MRS2693 (10 nM) increased the expression level of PKC θ after 20 min. However, the expression level of PKCβ was essentially unchanged during an incubation of 60 min. PKCα shows an initial decline and a sudden increase toward the end of the 60 min period.
Figure 4.

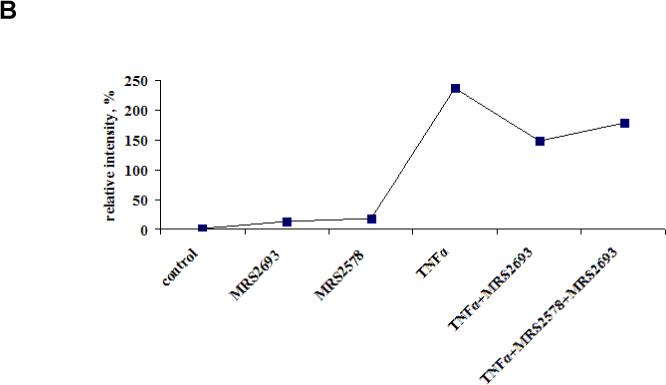
(A) Detection of NF-κB in C2C12 cells after treatment with TNFα, MRS2693 (10 nM) and MRS2578 (10 μM). The incubation time was 4 h. β-Actin was included as a standard to demonstrate consistency of protein loading. For all Western blots, proteins were extracted and applied to immunoblotting as described in Materials and Methods. A total of 40 μg of protein was applied to each lane. The Western blots are representative of two separate transfections, and each panel was taken from a single immunoblot, following separation by 10% polyacrylamide gel electrophoresis and transfer onto nitrocellulose. The uniformity of protein sample loading in each lane was confirmed by Ponceau Red membrane staining following the blotting (data not shown). (B) The quantitative analysis of the data in (A), done using the ImageJ software. 100% is defined as the average intensity of the actin standard.
We examined the effects of P2Y6 receptor signaling on the transcription factor NF-κB [25]. Western Blot results showed the link between TNFα and NF-κB (Figure 4). There was a markedly increased expression of NF-κB upon 4 h exposure of the cells to TNFα. This increase was attenuated following activation of the P2Y6 receptor by MRS2693, and this attenuation was partly antagonized by MRS2578. MRS2693 alone slightly increased the level of NF-κB.
3.5. Cytoprotection in an in vivo model of mouse skeletal muscle ischemia/reperfusion
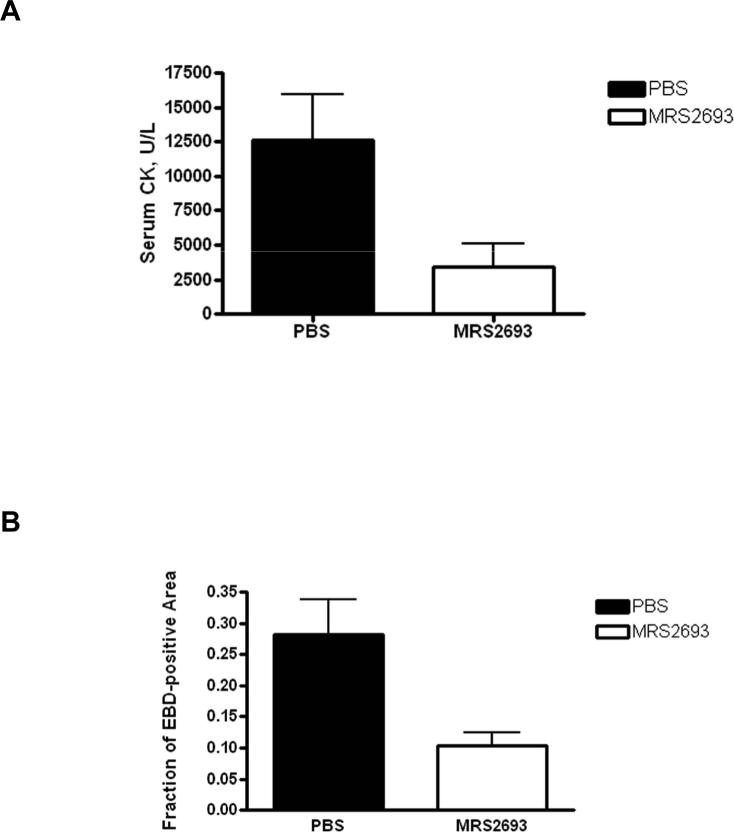
We used a mouse hindlimb ischemia/reperfusion model to test the in vivo protective ability of the selective P2Y6 agonist MRS2693. As demonstrated in a previous study of adenosine receptor-induced protection in the same model [16], ischemia induced by an external constrictor (90 min) followed by reperfusion (24 h) resulted in significant skeletal muscle injury in PBS vehicle–treated mice. The extent of injury was quantified by an increase in the staining of the skeletal myocytes with EBD, which binds to albumin and enters only damaged cells, and by a higher level of serum creatine kinase. Administration of MRS2693 (1 mg/kg, i.p. administration) prior to ischemia and reperfusion caused a significant reduction in the extent of injury (Figure 5). The compound reduced skeletal muscle injury with a significant decrease in serum CK level (3450 U/L ± 1660 U/L, n = 10, SE, vs. vehicle-treated 12,600 U/L ± 3300 U/L, n = 14, P = 0.037). Similarly, the percent EBD-stained area was also significantly reduced by MRS2693 (10.4% ± 2.0%, SE, n = 10 vs. vehicle-treated 28.3% ± 5.6%, n = 7, P=0.0038). The myocytes stained with EBD in treated and untreated mice in sections are shown in Figure 6.
Figure 5.
Cytoprotective action of MRS2693 in a quantitative model of ischemia and reperfusion (I/R) injury in the mouse hindlimb. Adult wild-type mice were injected with MRS2693 (1 mg/kg, i.p. n = 10 mice) or vehicle (PBS containing 0.1% DMSO, n = 7 mice), subjected to I/R injury, and their skeletal muscle injuries quantified as described in the Methods. (A) MRS2693 reduced the serum CK value, (p = 0.037 vs. PBS treatment). (B) The % EBD-stained cells was also reduced significantly by prior treatment with MRS2693 (p = 0.0038 vs. PBS-treatment).
Figure 6.
Cytoprotective action of a P2Y6 agonist in a quantitative model of ischemia and reperfusion (I/R) injury model (corresponding to data in Figure 5). After I/R injury, EBD-staining is shown in a representative hindlimb skeletal muscle section of adult mice treated with (A) vehicle (0.1% DMSO in phosphate-buffered saline, pH 7.4, n = 10 mice) or injected with (B) MRS2693 (1 mg/kg, i.p. , n = 7 mice) as described in the Methods.
4. Discussion
The effects of extracellular nucleotides acting at P2Y receptors in primary myotube cultures and in C2C12 mouse skeletal myoblasts have been studied, but not extensively under ischemic and apoptotic conditions [20,21]. We previously described the antiapoptotic effects of human P2Y6 and P2Y12 receptor activation in modified astrocytoma cells [4,5,12,13]. We now extend this approach to protection of skeletal muscle by nucleotides acting at P2Y receptors, as shown here in C2C12 cells and in vivo in mice. We have not studied the effects of extracellular nucleotides acting at P2Y6 receptors in primary myotube cultures.
The kinases ERK1/2 were previously implicated in the protection against TNFα-induced apoptosis in P2Y receptor-expressing astrocytoma cells [12]. Activation of the Gq-coupled P2Y6 receptor heterologously expressed in astrocytes was shown to significantly attenuate TNFα-induced apoptosis, and the protection was mediated by activation of ERK1/2, but not by calcium mobilization. P2Y6 receptor-mediated protection against TNFα-induced apoptosis occurred at very low concentrations of the agonist. Activation of the Gi-coupled P2Y12 receptor by the agonist MeSADP, at the protective concentration of 100 pM, involved only phosphorylation of ERK1/2, but not Akt or JNK or calcium mobilization. Thus, the activation of ERK1/2 is hypothesized to be a major mechanism for the protective effects induced by both P2Y6 and P2Y12 receptor activation.
C2C12 cells are mouse myoblasts, which are satellite cells that proliferate, differentiate and fuse to regenerate myotubes in damaged skeletal muscle. In a study by Banachewicz et al. [21], expression of the P2Y6 receptor and other P2 receptors in C2C12 cells was demonstrated by RT-PCR. Activation of ERK1/2 in response to various nucleotides, including UDP, was demonstrated. From experiments in myotubes [26], prolonged exposure to TNFα is implicated in a complex signaling pathway resulting in muscle wasting.
For the investigation of the role of the P2Y6 receptor in skeletal muscle cell injury, we have chosen to use a novel, potent synthetic agonist, MRS2693. MRS2693 can be considered more selective for this subtype because: 1) MRS2693 itself has no activity at other P2Y subtypes and 2) the corresponding 5′-triphosphate derivative is a much weaker agonist than UTP at other P2Y receptor subtypes [2]. We have demonstrated that this derivative is cytoprotective toward skeletal muscle cells in the mouse both in vitro and in vivo.
Activation of the endogenous P2Y6 receptor in C2C12 cells significantly attenuated TNFα-induced apoptosis. The protection occurred at very low agonist concentrations and correlated with the activation of ERK1/2. The potent antiapoptotic protection by the novel P2Y6 receptor agonist MRS2693 in the C2C12 cell line was concentration-dependent between 0.1 and 10 nM. Similar results were obtained in astrocytoma cells, in which the protection by the appropriate P2Y agonist was clearly P2Y6 receptor-dependent and was absent in untransfected control cells or cells expressing the P2Y4 receptor [5,12].
Members of the protein kinase C (PKC) family of serine/threonine protein kinases are involved in many cellular responses across a wide range of cell types. Each PKC isoenzyme may be involved in specific regulatory processes. Various PKC isoenzymes exhibit differences in tissue distribution, intracellular localization, and cofactor requirements, suggesting that they are freely regulated in response to discrete ligands, and that they may act on distinct protein substrates [23,24]. Our results showed that activation of the P2Y6 receptor by MRS2693 increased the expression level of PKC θ, which might control stimulation of ERK1/2 activation [29]. ERK1/2 is a contributing pathway in the protection by MRS2693 against TNFα-induced cell death in C2C12 cells. Thus, the protection in skeletal muscle cells mechanistically resembles protection in the astrocytoma cells.
There are no competitive antagonists that can be used as pharmacological probes of the P2Y6 receptor, so we used the diisothiocyanate derivative MRS2578 as P2Y6 receptor antagonist [4]. This antagonist blocked the protection provided by MRS2693 against apoptosis in the C2C12 cell line. The reduced protection at higher concentrations of the agonists might be a result of interaction with other extracellular nucleotide binding sites or enzymes that act on nucleotides.
TNFα-induced apoptosis in C2C12 cells correlated with NF-κB activation. This was consistent with numerous previous reports in which TNFα has been noted to activate NF-κB in various systems [26]. NF-κB elevation was also noted to induce damage accompanied by protein degradation in cultured rat skeletal muscle cells [27]. Curiously, P2Y6 receptor activation was previously reported to induce translocation of NF-κB to the nucleus in certain cell types, including osteoblasts [6,28]. In our study, P2Y6 receptor activation alone had little effect on NF-κB, yet substantially reduced the dramatic elevation of NF-κB induced by TNFα.
In an in vivo mouse model of hindlimb skeletal muscle ischemia/reperfusion injury, MRS2693 was also able to exert a potent cytoprotective effect. The same model was previously applied to study the protective effect of adenosine receptor agonists [16]. We have not yet elucidated the mechanism of the P2Y6 receptor-induced protection in vivo.
In conclusion, the P2Y6 receptor is a novel cytoprotective receptor that warrants exploration for ameliorating skeletal muscle injury. This effort will be aided with the development of even more potent, selective, and stable agonists of the P2Y6 receptor.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases. We thank Cara Heller (NIDDK) for proofreading this manuscript.
Abbreviations
- BrdUTP
5-bromo-2′-deoxyuridine 5′-triphosphate
- ERK1/2
extracellular signal-regulated protein kinases 1 and 2
- FBS
fetal bovine serum
- HRP
horseradish peroxidase
- MAPK
mitogen-activated protein kinase
- MeSADP
2-methylthio-adenosine-5′-diphosphate
- MRS2365
2-methylthio-(N)-methanocarbaadenosine-5′-diphosphate
- MRS2693
5-iodo-uridine-5′-diphosphate
- PBS
phosphate buffered saline
- PI
propidium iodide
- MRS2578
1,4-di-[(3-isothiocyanatophenyl)-thioureido]butane
- PKC
protein kinase C
- NF-κB
Nuclear factor-κB
- TNF
tumor necrosis factor
- TUNEL
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling
- FACS
fluorescence-activated cell sorting
- Hoechst 33258
2′-[4-hydroxyphenyl]-5-[4-methyl-1-piperazinyl]-2,5′-bis-1H-benzimidazole trihydrochloride pentahydrate
- DMEM
Dulbecco's modified Eagle's medium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Fumagalli M, King BF, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII. Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besada P, Shin DH, Costanzi S, Ko HJ, Mathé C, Gagneron J, Gosselin G, Maddileti S, Harden TK, Jacobson KA. Structure activity relationship of uridine 5′-diphosphate analogues at the human P2Y6 receptor. J Med Chem. 2006;49:5532–5543. doi: 10.1021/jm060485n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Tayeb A, Qi A, Müller CE. Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors. J Med Chem. 2006;49:7076–7087. doi: 10.1021/jm060848j. [DOI] [PubMed] [Google Scholar]
- 4.Mamedova L, Joshi BV, Gao ZG, von Kügelgen I, Jacobson KA. Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol. 2004;67:1763–1770. doi: 10.1016/j.bcp.2004.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SG, Soltysiak KA, Gao ZG, Chang TS, Chung E, Jacobson KA. Tumor necrosis factor α-induced apoptosis in astrocytes is prevented by the activation of P2Y6, but not P2Y4 nucleotide receptors. Biochem Pharmacol. 2003;65:923–931. doi: 10.1016/s0006-2952(02)01614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korcok J, Raimundo LN, Du X, Sims SM, Dixon SJ. P2Y6 nucleotide receptors activate NF-κB and increase survival of osteoclasts. J Biol Chem. 2005;280:16909–16915. doi: 10.1074/jbc.M410764200. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber R, Kunzelmann K. Purinergic P2Y6 receptors induce Ca2+ and CFTR dependent Cl− secretion in mouse trachea. Cell Physiol Biochem. 2005;16:99–108. doi: 10.1159/000087736. [DOI] [PubMed] [Google Scholar]
- 8.Cox MA, Gomes B, Palmer K, Du K, Wiekowski M, Wilburn B, Petro M, Chou CC, Desquitado C, Schwarz M, Lunn C, Lundell D, Narula SK, Zavodny PJ, Jenh CH. The pyrimidinergic P2Y6 receptor mediates a novel release of proinflammatory cytokines and chemokines in monocytic cells stimulated with UDP. Biochem Biophys Res Commun. 2005;330:467–473. doi: 10.1016/j.bbrc.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Khine AA, Del Sorbo L, Vaschetto R, Voglis S, Tullis E, Slutsky AS, Downey GP, Zhang H. Human neutrophil peptides induce interleukin-8 production via P2Y6 signaling pathway. Blood. 2006;107:2936–2942. doi: 10.1182/blood-2005-06-2314. [DOI] [PubMed] [Google Scholar]
- 10.Hou M, Harden TK, Kuhn CM, Baldetorp B, Lazarowski E, Pendergast W, Möller S, Edvinsson L, Erlinge D. UDP acts as a growth factor for vascular smooth muscle cells by activation of P2Y6 receptors. Am J Physiol Heart Circ Physiol. 2002;282:H784–H792. doi: 10.1152/ajpheart.00997.2000. [DOI] [PubMed] [Google Scholar]
- 11.Franke H, Krugel U, Illes P. P2 receptors and neuronal injury. Pflugers Arch. 2006;452:622–44. doi: 10.1007/s00424-006-0071-8. [DOI] [PubMed] [Google Scholar]
- 12.Kim SG, Gao ZG, Soltysiak KA, Chang TS, Brodie C, Jacobson KA. P2Y6 nucleotide receptor activates PKC to protect 1321N1 astrocytoma cells against tumor necrosis factor-induced apoptosis. Cell Mol Neurobiol. 2003;23:410–418. doi: 10.1023/a:1023696806609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mamedova LK, Gao ZG, Jacobson KA. Regulation of death and survival in astrocytes by ADP acting at P2Y1 and P2Y12 receptors. Biochem Pharmacol. 2006;72:1031–1041. doi: 10.1016/j.bcp.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandri M, Carraro U. Apoptosis of skeletal muscles during development and disease. Int J Biochem Cell Biol. 1999;31:1373–1390. doi: 10.1016/s1357-2725(99)00063-1. [DOI] [PubMed] [Google Scholar]
- 15.SAS Institute . SAS guide for personal computers - Version 6.07. SAS Institute; Cary, NC: 1995. [Google Scholar]
- 16.Zheng J, Wang R, Zambraski E, Wu D, Jacobson KA, Liang BT. A novel protective action of adenosine A3 receptors: Attenuation of skeletal muscle ischemia and reperfusion injury. Am J Physiol. 2007;293:3685–3691. doi: 10.1152/ajpheart.00819.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durbeej M, Sawatzki SM, Barresi R, Schmainda KM, Allamand V, Michele DE, Campbell KP. Gene transfer establishes primacy of striated vs. smooth muscle sarcoglycan complex in limb-girdle muscular dystrophy. Proc Natl Acad Sci USA. 2003;100:8910–8915. doi: 10.1073/pnas.1537554100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen JB, Cronin C, Sonin D, Joshi BV, Nieto MG, Harrison D, Jacobson KA, Liang BT. P2X purinergic receptor-mediated ionic current in cardiac myocytes of calsequestrin model of cardiomyopathy. Implications for the treatment of heart failure. Am J Physiol Heart Circ Physiol. 2007;292:1077–1084. doi: 10.1152/ajpheart.00515.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duclos F, Straub V, Moore SA, Venzke DP, Hrstka RF, Crosbie RH, Durbeej M, Lebakken CS, Ettinger AJ, van der Meulen J, Holt KH, Lim LE, Sanes JR, Davidson BL, Faulkner JA, Williamson R, Campbell KP. Progressive muscular dystrophy in α-sarcoglycan-deficient mice. J Cell Biol. 1998;142:1461–1471. doi: 10.1083/jcb.142.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmet J, Järlebark L, Hammarberg T, van Galen PJM, Jacobson KA, Heilbronn E. Synthesis and biological activity of novel 2-thio derivatives of ATP. Nucleosides and Nucleotides. 1993;12:1–20. doi: 10.1080/07328319308016190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banachewicz W, Suplat D, Krzeminski P, Pomorski P, Barfanska J. P2 nucelotide receptors on C2C12 satellite cells. Purinergic Signalling. 2005;1:249–257. doi: 10.1007/s11302-005-6311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourdon DM, Boyer JL, Mahanty S, Jacobson KA, Harden TK. (N)-methanocarba-2MeSADP (MRS2365) is a subtype-specific agonist that induces rapid desensitization of the P2Y1 receptor of human platelets. J Thromb Haemost. 2006;4:861–868. doi: 10.1111/j.1538-7836.2006.01866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stabel S, Parker PJ. Protein kinase C. Pharmacol Ther. 1991;51:71–95. doi: 10.1016/0163-7258(91)90042-k. [DOI] [PubMed] [Google Scholar]
- 24.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 25.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 26.Stewart CEH, Newcomb PV, Holly JMP. Multifaceted roles of TNF-κ in myoblast destruction: A multitude of signal transduction pathways. J Cell Physiol. 2004;198:237–247. doi: 10.1002/jcp.10387. [DOI] [PubMed] [Google Scholar]
- 27.Bar-Shai M, Reznick AZ. Reactive nitrogen species induce nuclear factor-kappaB-mediated protein degradation in skeletal muscle cells. Free Radic Biol Med. 2006;40:2112–2125. doi: 10.1016/j.freeradbiomed.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong S, Korcok J, Sims SM, Dixon SJ. Activation of transcription factors by extracellular nucleotides in immune and related cell types. Purinergic Signalling. 2007;3:59–69. doi: 10.1007/s11302-006-9037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]