Abstract
Background
We performed a study to better characterize the natural history of genital human papillomavirus (HPV) infection in a cohort of closely followed adolescent women.
Methods
A cohort of 60 adolescent women was followed over a 2.2-year period, on average. A median of 41.5 self-collected vaginal and clinician-obtained cervical swabs were obtained from each subject
Results
HPV was detected in 45.3% of all adequate specimens, by use of a polymerase chain reaction/reverse blot strip assay. Oncogenic—or high-risk (HR)—HPV types were detected in 38.6% of specimens, and nononcogenic—or low-risk (LR)—types were detected in 19.6% of specimens. During the entire study period, 49 of 60 subjects tested positive for HPV (cumulative prevalence, 81.7%). The most frequently detected HR types were HPV types 52, 16, and 59. Infections with multiple HPV types were common. The median duration of persistence of a specific HPV type was 168 days, and HR types were more persistent than LR types. Abnormal cervical cytological results occurred in 37% of the adolescent women and were significantly associated with HR HPV infection.
Conclusions
The cumulative prevalence of HPV infection in sexually active adolescent women is extremely high, involves numerous HPV types, and frequently results in cervical dysplasia.
The marked upsurge in coital activity, pregnancy, and sexually transmitted infections (STIs) among middle-adolescent women justifies specific focus on this demographic group. Adolescents (10–19 years of age) and young adults (20–24 years of age) account for >65% of all reported STIs [1]. Human papillomavirus (HPV) is an STI of particular interest, because of its high prevalence rates and causal association with cervical malignancy. Our understanding of the epidemiology of HPV infection has grown and shifted immensely during the past 2 decades. It is well understood that infection of the genital tract with HPV leads to a range of pathologic states, including asymptomatic carriage of the virus, genital warts, cervical dysplasia, and cervical carcinoma [2]. Approximately one-third of the 100 known HPV types regularly infect the genital tract. Certain HPV types, including HPV 16, 18, and others, are associated with an increased risk of cervical malignancy and are known as oncogenic or high-risk (HR) types. Other types, such as HPV 6, cause genital warts and low-grade cervical dysplastic lesions and are known as nononcogenic or low-risk (LR) types, since they are rarely found as solitary isolates in genital-tract malignancy.
Although nearly all cervical cancer is caused by HR HPV, this complication occurs in a small percentage of infected women. Most HPV infections result in no signs or symptoms, or they may result in a minimal degree of cytologic abnormality. Women with persistent HPV infection are at increased risk for dysplasia and cervical malignancy, but the factors influencing persistence are not fully understood [3, 4].
The epidemiology and pathogenesis of HPV infections have been redefined on multiple occasions as techniques to improve sensitivity and specificity of viral detection have been developed, and cross-sectional studies have progressed to longitudinal ones. The current study was conducted to gain insight into HPV incidence, prevalence, type distribution, persistence, and associated dysplasia, in a group of closely followed adolescent women. These adolescent women underwent very frequent HPV testing, careful HPV typing, and prolonged follow-up, thus providing a unique look at HPV natural history.
PATIENTS, MATERIALS, AND METHODS
Patients and specimens
A longitudinal study of sexual behavior and STIs in 250 adolescent women is currently under way. The current analysis was performed using data collected from the first 60 subjects who have completed at least 2 full years of testing. All patients were evaluated under the main protocol, which was approved by the local institutional review board. Adolescent women, aged 14–17 years and attending 1 of 3 primary-care clinics in Indianapolis, were enrolled in a 27-month longitudinal study. Inclusion criteria for the larger study, which were not modified for this analysis, included that the adolescent be between the ages of 14 and 17 years, be able to understand English and give written consent, not have any serious psychiatric disturbances or mental handicaps, and have a parent who was able to give permission for their participation in the study. The adolescent did not have to be sexually active to participate but could not be recruited if she were pregnant. As each subject was enrolled, informed consent and parental consent were obtained. All participants received financial remuneration for their time and efforts. Human-experimentation guidelines of Indiana University School of Medicine were followed in the conduct of this research.
All subjects participated in quarterly visits (every 3 months) at their clinic site and in as many as 5 diary collection periods of 3 months during the study. At the quarterly visits, subjects participated in face-to-face interviews and underwent a pelvic examination for STI screening, including a cervical cotton swab for HPV testing. Diary collection periods consisted of daily reporting of behaviors and weekly self-collected vaginal (cotton) swabbing for STIs, again including HPV. Dry cervical swabs were delivered to the central laboratory for the ongoing study of STIs in adolescent women. Swabs were vortexed in a tube containing 1 mL of sterile water, and the water was stored at −20°C. All vaginal swabs were obtained at the subject’s home when study personnel arrived weekly. Dry vaginal swabs were delivered to the same central laboratory for processing in a manner similar to that of the cervical swabs. An aliquot of each specimen was delivered to the HPV laboratory for further testing. Specimens were not processed in any particular order, and specimens from individual women were not processed together but rather in the order in which they were obtained.
Cervical cytological results were obtained at intervals of 6–12 months and were determined by standard cytologic methods. Cervical cytological testing was not performed on 3 subjects who did not report engaging in sexual intercourse.
Detection of specific HPV types by polymerase chain reaction (PCR)/reverse blot strip assay
DNA was extracted using the High Viral Pure Kit (Roche Molecular Diagnostics). The Roche PCR/reverse blot strip assay was used to detect specific HPV types in the cervical and vaginal swab specimens [5, 6]. This assay uses nondegenerate biotinylated primer pairs (5 upstream primers, designated “PGMY 11,” and 13 downstream primers, designated “PGMY 09”) to amplify 27 individual genital HPV types, including types 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, 66, 68, 73, 82, 83, and 84. To determine specimen adequacy, the GH20 and PC04 human β-globin targets were coamplified. Reactions were amplified in a Perkin Elmer TC9600 Thermal Cycler (Perkin Elmer), as described elsewhere [7]. DNA isolated from a condyloma acuminata lesion was used as a positive control. A separate PCR containing no added DNA was included in each assay as a negative control, thus assuring that no contamination had occurred either from another subject specimen or from the technician performing the assay. Samples were included in the analysis for HPV if human β-globin sequences were detected by reverse blot strip assay and if the “no added DNA” control was negative for both β-globin and HPV.
The reverse blot strip assay that contains 29 probe lines plus 1 reference line was used, detecting the 27 individual HPV genotypes mentioned above and 2 concentrations of the β-globin control probe [5]. Bovine serum albumin–conjugated probes for each HPV type are deposited in a single line. Hybridization and detection of PCR products bound to immobilized probes was performed as described elsewhere [7].
Statistical measures and analysis
The presence and persistence of each individual HPV type were determined by use of a combination of quarterly cervical swabs and weekly vaginal swabs. The persistence of HPV was assessed by the number of days that a viral type remained at a detectable level. The series of days on which a specific type of HPV had been present was referred to as a “run” [8]. A run was defined as at least 2 occurrences of the same HPV type. The run came to an end when the HPV became undetectable for at least 2 consecutive weeks. Practically, a run could last until the end of the study, meaning that the virus maintained a detectable presence in the subject until the end (or within 2 weeks of the end) of the observation. When this happened, we would not be able to observe the exact length of the run; therefore, we would consider the length censored. By our definition, runs were HPV type–specific. One individual could contribute multiple runs if she had >1 type of HPV. In this study, we used run length to describe the persistence of type-specific HPV in the study subjects.
First, we reported statistical summaries of runs of HR or LR HPV and HPV persistence in subjects with and without cervical dysplasia. We categorized the HPV types as a binary variable indicating either HR or LR. Similarly, Pap smear results from within the time period between enrollment and 6 months after the last specimen had been collected were categorized as either normal or abnormal. Abnormal smears were classified as atypical squamous cells of uncertain significance (ASCUS), low-grade squamous intraepithelial lesions (LGSILs), or high-grade squamous intraepithelial lesions (HGSILs). We used Kaplan-Meier curves to depict the survival functions of time to clearance for HR and LR HPV runs, as well as those for the runs associated with or without abnormal Pap smears. To formally test the effect of the HPV types on the viral persistence (run length), we used a Cox proportional hazard model with random subject effect (also known as the “frailty model”); the random subject effect was introduced to accommodate the correlations among the runs contributed by the same subject [9].
RESULTS
Population demographics
The median age of the cohort at enrollment was 15.0 years (mean, 15.3 years; SD, 0.9 years; range, 14–17 years); 85.0% of subjects were African-American, 11.7% were white, and 3.3% were Hispanic. The median follow-up period for the 60 subjects was 2.2 years (mean, 2.2 years; SD, 34 days). Three subjects did not report ever having sexual intercourse; the remaining 57 subjects were sexually active. The median number of lifetime sex partners for these 57 subjects was 2.0 (21.1%, 15.8%, and 49.1% had reported 1, 2, and ≥3 lifetime sex partners, respectively).
Analysis of swab specimens for HPV
A total of 2458 swab specimens were collected (mean, 41.0 swabs/subject; SD, 9.3 swabs/subject; median, 41.5 swabs/subject; range, 18–64 swabs/subject). Of these swabs, 353 were from the cervix, and 2105 were self-obtained vaginal swabs. Swabs that yielded a clear positive band on the strip assay for β-globin for both the high -and low-abundance controls were considered adequate for HPV analysis (table 1). Overall, 2107 (85.7%) of 2458 swabs were adequate for analysis of HPV. Vaginal swabs yielded a higher percentage of β-globin–positive specimens than did cervical swabs (86.4% vs. 81.9%; p = .027). The 14.3% of swabs that did not contain adequate cellular material, as determined by negative amplification of β-globin, may have been a result of poor collection technique on the part of the subject or the nurse. Alternatively, DNA may have been lost during the purification procedure prior to performance of the PCR/reverse blot strip assay.
Table 1.
Cervical and vaginal swab positivity for β-globin, human papillomavirus (HPV), high-risk (HR) HPV, and low-risk (LR) HPV.
| Positive swabs, no. (%)
|
||||
|---|---|---|---|---|
| Swab type | β-globin | HPV | HR HPV | LR HPV |
| Cervical (n = 353) | 289 (81.9) | 116 (40.1) | 104 (36.0) | 42 (14.5) |
| Vaginal (n = 2105) | 1818 (86.4) | 838 (46.1) | 710 (39.1) | 372 (20.5) |
| All (n = 2458) | 2107 (85.7) | 954 (45.3) | 814 (38.6) | 414 (19.6) |
Overall, 45.3% of adequate swabs were positive for HPV. Vaginal swabs were more likely to be positive for HPV than were cervical swabs (46.1% vs. 40.1%; p = .003). However, the higher overall HPV prevalence in vaginal swabs was driven by a higher prevalence of LR types. Testing the significance of the proportion differences in the HR/LR strata showed a significant value for LR types (p = .02) but not for HR types (p = .31).
HR HPV types (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 55, 56, 58, 59, 68, 73, 82, 83, and 84) were detected in 38.6% of all adequate swab specimens (table 1). LR HPV types (6, 11, 40, 42, 53, 54, 57, and 66) were detected in 19.6% (table 1).
HPV 52 was the most frequently detected type, found in 13.6% of all adequate swab specimens (figure 1). HPV 16 was found in 11.7% of all specimens, and HPV 59 was found in 6.6% of all specimens. The most frequently detected LR types were HPV 66 (6.1% of adequate specimens) and HPV 6 (5.6%).
Figure 1.
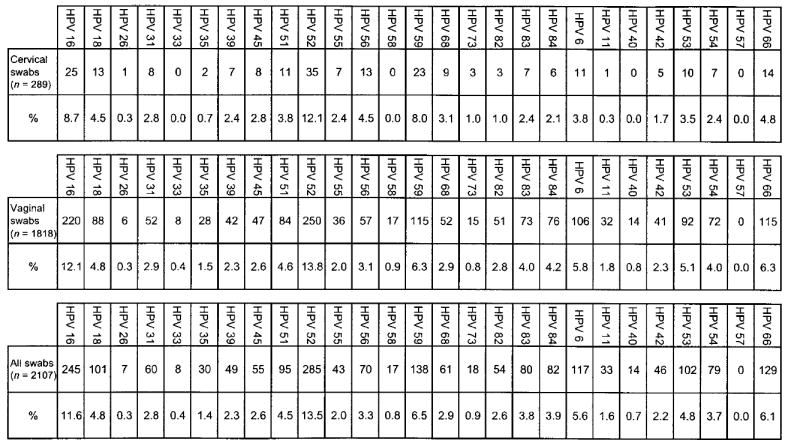
Distribution of specific human papillomavirus (HPV) types in cervical swabs, vaginal swabs, and all swabs. The upper rows show the no. of swabs that were positive for each HPV type, and the lower rows show the percentage of swabs that were positive for each type.
Several types were detected in 3%–5% of specimens, including HR HPV types 18, 51, 56, 73, and 84 and LR HPV types 53 and 54 (figure 1). Other types were detected in <3% of all swabs.
In general, most HPV types were detected in a higher percentage of vaginal swabs than cervical swabs (figure 1). HPV types 56, 59, and 68 were exceptions, being detected more often in cervical swabs than in vaginal swabs.
Detection of multiple HPV types in swab specimens
Of all 2107 adequate swab specimens, the number of HPV types detected per specimen ranged from 0 to 8, with 54.7% of the specimens testing negative for HPV. Of the 1818 adequate weekly vaginal swab specimens, the number of HPV types detected was between 0 and 6, with 53.9% testing negative. There were 289 adequate quarterly cervical swabs. The number of HPV types detected per cervical swab ranged from 0 to 8, with 59.9% of cervical swabs testing negative. The mean number of HPV types detected per vaginal swab was 0.99 (SD, 1.43), significantly higher than the mean of 0.79 (SD, 1.21) detected in cervical swabs (p = .047). A single HPV type was detected in 44.1% of all the HPV-positive swabs, and multiple HPV types were detected in the remaining 55.9%. Overall, a similar percentage of cervical swabs contained multiple types compared with vaginal swabs (56.9% of cervical swabs; 55.7% of vaginal swabs). However, ≥4 HPV types were detected in 12 (10.3%) of 116 of HPV-positive cervical swabs, compared with 132 (15.8%) of 838 of HPV-positive vaginal swabs.
High cumulative prevalence of HPV infection in adolescent women
We next considered how often adolescent women (in contrast to all subjects) had detectable infection with a specific HPV type during the study (table 2). Three subjects’ specimens all tested negative for HPV (all 3 reported never having sexual intercourse). Eight subjects had only a single swab specimen that tested positive for any HPV; therefore, these subjects were not considered to be infected by our definition. These single positive specimens were excluded because they may have represented laboratory error or deposition of HPV by a sex partner rather than true infection. Therefore, 11 of the adolescent women were considered to be HPV negative throughout the entire study period, and the remaining 49 subjects were considered to be HPV positive.
Table 2.
Point (enrollment and last study visit) and cumulative prevalence of all human papillomavirus (HPV) detection and detection of high-risk (HR) and low-risk (LR) HPV.
| Positive swabs, no. (%)
|
|||
|---|---|---|---|
| Time point(s) | HPV | HR HPV | LR HPV |
| Enrollment (n = 60) | 17 (28.3) | 13 (21.7) | 7 (11.7) |
| Last visit (n = 60) | 24 (40.0) | 22 (36.7) | 11 (18.3) |
| All (n = 60) | 49 (81.7) | 46 (76.7) | 34 (56.7) |
The first and last specimens collected for each subject were analyzed for HPV, and the results were compared with the cumulative detection for every swab specimen collected for each individual subject. HPV positivity increased numerically during the study period, for both HR and LR HPV types (table 2). The cumulative prevalence of all HPV detection was 81.7% (table 2).
Figure 2 depicts the cumulative HPV type distribution among all 60 adolescent women in the study. HPV 52 was detected at some point in 38.3% of all subjects, and HPV 16, the second-most-frequent type, was detected in 31.3% of subjects. Other frequently detected HR types included HPV 59 (found in 23.3% of all subjects), HPV 84 (21.7%), and HPV 18 (20.0%). Frequently detected LR types included HPV 66 (28.3% of all subjects), HPV 6 (25.0%), and HPV 53 (20.0%).
Figure 2.
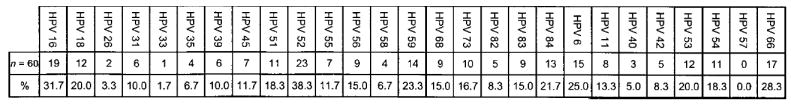
Human papillomavirus (HPV) type distribution among 60 adolescent women. The upper row shows the no. of swabs that were positive for each HPV type, and the lower row shows the percentage of subjects in whom each type was detected.
Frequent detection of multiple HPV types in adolescent women
The mean number of HPV types per HPV-positive subject (n = 49) was 4.9 (SD, 3.3 types; median, 5.0 types; range, 1–14 types). A single HPV type was detected in specimens from 18.4% of these individuals, whereas multiple types were detected in 81.6% who were HPV-positive. Adolescent women with abnormal Pap smears had a mean of 6.0 different HPV types (SD, 3.4 types), compared with a mean of 4.4 types (SD, 2.9 types) in adolescents who had normal Pap smears.
Persistence of HPV
Lengths of type-specific HPV runs were analyzed to gain insight into HPV persistence. The mean number of days of follow-up for subjects with HPV infections was 794 (median, 793 days; SD, 34 days; range, 712–869 days). Subjects who were HPV positive contributed 241 HPV “runs.” Of these subjects, 47 had Pap smear results and thus were included in the survival analysis. The median length of all 239 HPV runs was 168 days. Among all the runs, 166 were of HR HPV types, with a median length of 188 days. There were 73 LR HPV runs, with median length of 89 days. Figure 3 shows Kaplan-Meier estimates of HR and LR HPV persistence (runs). When the impact of the censored HPV runs was taken into account, Kaplan-Meier estimates of the median clearance time were 226 and 170 days, in HR HPV and LR HPV, respectively. Using a Cox regression model with random subject effect, we found that HPV type was significantly associated with run length (p = .034). The risk ratio associated with HR HPV was 0.683. This implied that there was a 31.7% reduction in the probability that an HPV run became undetectable if the run was of an HR HPV. Persistence of any HPV was marginally associated with abnormal Pap smear results (p = .053). Figure 4 shows Kaplan-Meier estimates of runs of HPV in subjects with and without abnormal Pap smear results. The Kaplan-Meier estimates of the median clearance time were 255 and 170 days, in HPV runs with and without abnormal Pap smear results, respectively. The risk ratio associated with an abnormal Pap smear was 0.597, suggesting that the likelihood of an HPV run accompanied by abnormal Pap smear results becoming undetectable was 59.7% of that in a run without an abnormal Pap smear. In other words, HPV runs were more persistent in subjects with abnormal Pap smears. We did not have enough observations to calculate Kaplan-Meier estimates for abnormal/normal Pap smears associated with either HR or LR HPV.
Figure 3.
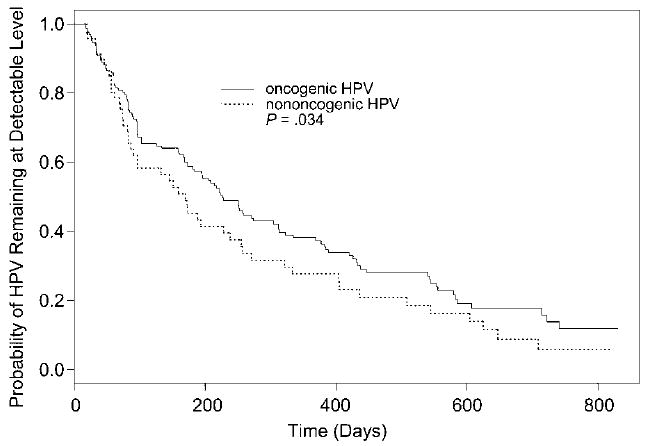
Kaplan-Meier estimates of human papillomavirus (HPV) clearance time in high-risk (HR; oncogenic) and low-risk (LR; nononcogenic) runs. The estimated median clearance time for HR HPV was 226 days, and the estimated median clearance time for LR HPV was 170 days.
Figure 4.
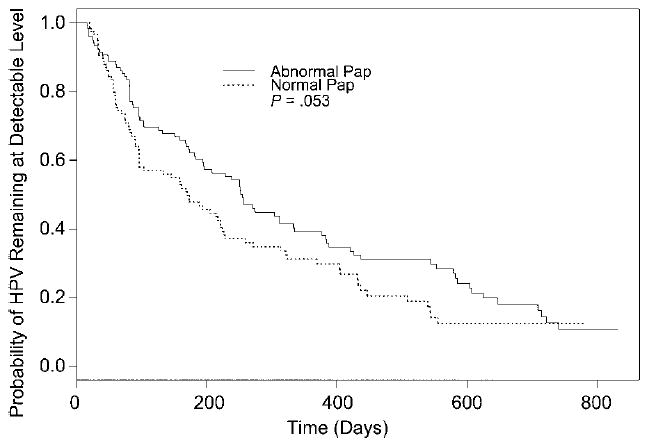
Kaplan-Meier estimates of human papillomavirus (HPV) persistence in individual subjects with or without abnormal Pap smear results. The Kaplan-Meier estimates of the median clearance time were 255 and 170 days, in HPV runs with and without abnormal pap smear results, respectively.
High frequency of abnormal cervical cytologic tests in adolescent women
Three subjects who did not report any past or present sexual intercourse did not undergo cytologic testing. Pap smears were available for 54 of the remaining 57 adolescent women in the study. The mean number of Pap smears acquired per subject was 3.0 (SD, 1.6; range, 1–8). Among the 161 Pap smears obtained, results were obtained for 158 (98.1%). Of these, 69.6% were normal, and 30.4% were abnormal (with ASCUS, LGSILs, or HGSILs) (table 3). ASCUS was the most common abnormality, occurring in 28 Pap smears. LGSILs occurred in 19 Pap smears, and HGSILs occurred in 1. Among the 54 subjects with at least 1 Pap smear, 37% had at least 1 abnormal test, and 63% had entirely normal tests (table 3).
Table 3.
Distribution of all Pap smear results in 54 subjects and the most severe Pap smear result during the study period for each individual subject.
| Pap smear result | All subjects, no. (%)
(n = 158 smears) |
Subjects in whom Pap result was most severe, no. (%)
(n = 54 subjects) |
|---|---|---|
| Normal | 110 (69.6) | 34 (63.0) |
| ASCUS | 28 (17.7) | 9 (16.7) |
| LGSILs | 19 (12.0) | 10 (18.5) |
| HGSILs | 1 (0.6) | 1 (1.9) |
NOTE. ASCUS, atypical squamous cells of uncertain significance; LGSILs, low-grade squamous intraepithelial lesions; HGSILs, high-grade squamous intraepithelial lesions.
The most severe grade of Pap smear abnormality for each of the 54 adolescent women was compared with HR HPV positivity. As expected, Pap smear abnormalities were associated with detection of HR HPV. All 20 subjects with abnormal Pap smears had HR HPV detected at some point during the study period, compared with 24 of 34 subjects with normal Pap smears (p = .009; Fisher’s exact test). The proportions of HR HPV– and LR HPV–positive specimens within the abnormal Pap smear categories were not determined; this was because we used a subject-level summary definition for Pap abnormality, whether or not any HPV-positive specimen was ever of the HR category. The tests for HPV and Pap were different, and dates of specimen acquisition did not always coincide. Therefore, a subject may have had 1 to several Pap tests with different results, as well as many HPV tests with variable results.
Analysis of individual adolescent women
The complete analysis of individual subjects illustrated the acquisition and persistence of specific HPV types during the study. The analysis for 4 adolescent women is shown in figure 5.
Figure 5.
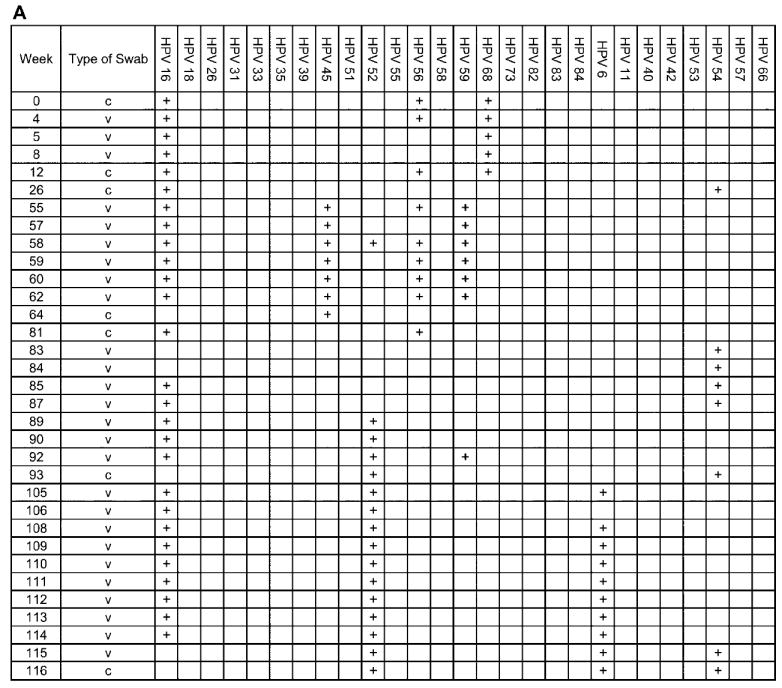
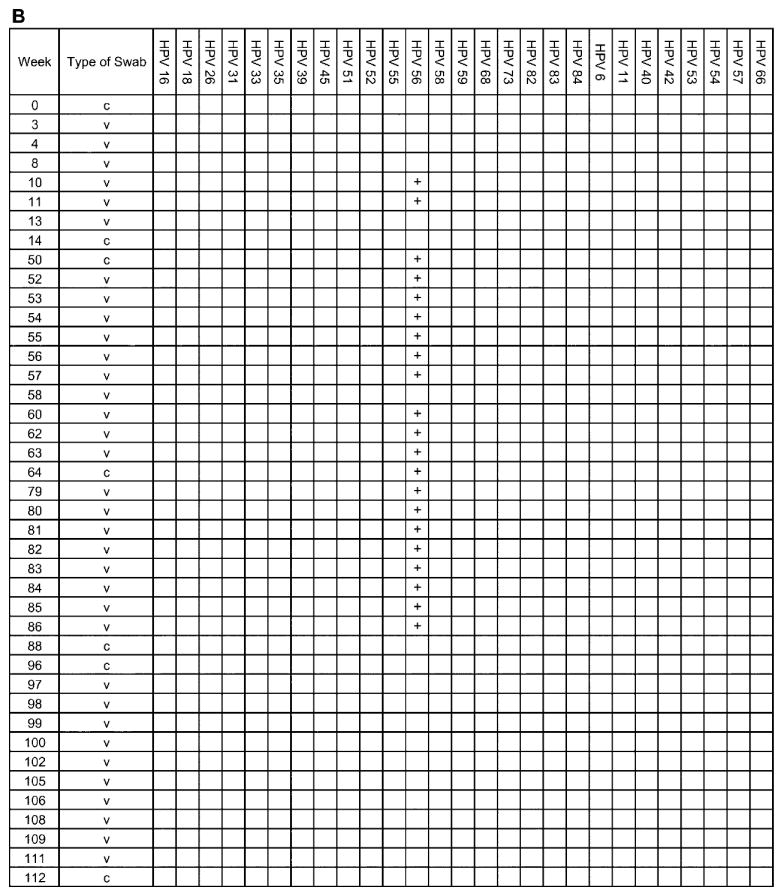
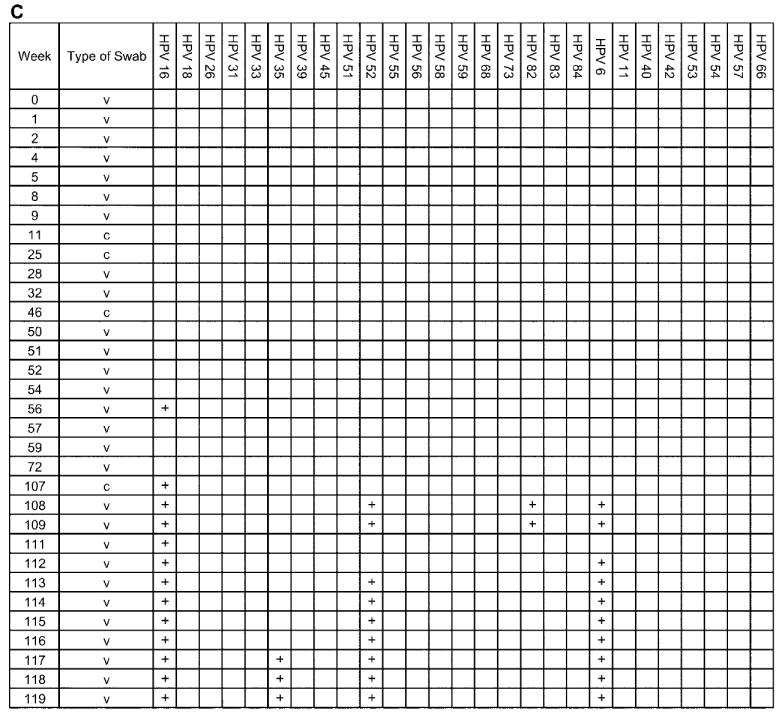
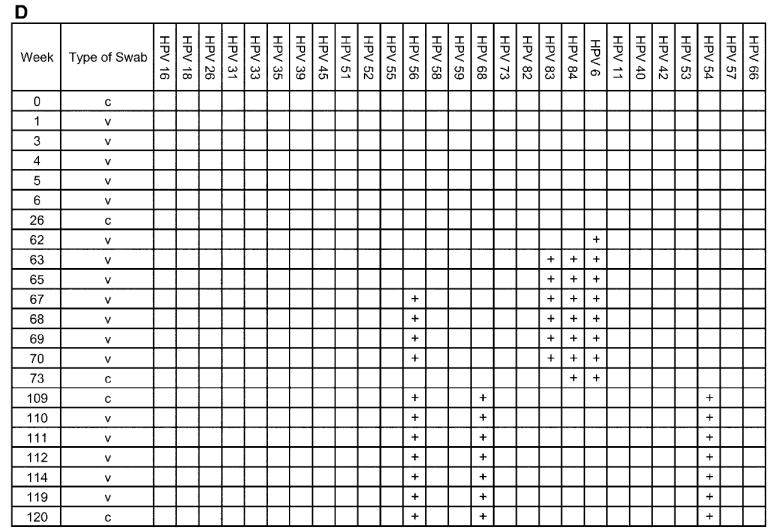
The complete analysis of 4 individual adolescent woman (subjects A, B, C, and D), shown to illustrate the acquisition and persistence of specific human papillomavirus (HPV) types during the study period. The week in which the specimen was acquired is shown in the left-most column. HPV types are indicated at the top of the figure. c, clinician-obtained cervical swab; v, self-collected vaginal swab. Plus (+) signs indicate positive results for the specimen.
Subject A was infected with numerous HPV types (figure 5A). HPV 16 was detected in the swab obtained from her first visit and persisted throughout most of the study period. HPV types 45, 52, 56, 59, and 68 (all HR types) were repeatedly detected. HPV types 6 and 54 (LR types) were also detected. Her most severe cytologic abnormality was LGSILs.
In subject B, HPV 56 was the only type detected, persisting for 9 months (figure 5B). Cytologic testing revealed HGSILs as her most severe abnormality.
Subject C had no HPV types detected for the first 14 months of the study (figure 5C) but then became infected with numerous types that persisted throughout the study. Cytologic testing showed no abnormalities.
Subject D had no HPV types detected for the first 12 months of the study but then became infected with HPV types 83, 84, and 6, all of which were undetectable after several months (figure 5D). She then became infected with HPV types 56, 68, and 54, which persisted throughout the remainder of the study. Cytologic testing revealed LGSILs as her most severe abnormality.
DISCUSSION
A complete understanding of the epidemiology of HPV infection in adolescent women is imperative for effective targeted interventions. This is exemplified by HPV vaccine trials, in which it is important to understand the significance of infection with specific HPV types and multiple types. The intent of the present study was to more accurately characterize the natural history of genital tract HPV infections early in life, at the point when such interventions may be most effective.
As with previous studies, we found that HPV infection is extremely common in sexually active adolescent women. The point prevalence of detectable HPV infection in our study was in the range of 25%–40%. The cumulative prevalence in our longitudinal analysis of adolescent women was >80%, a figure that exceeds that found in other studies.
Moscicki et al. found prevalence rates of 54.5% for all genital HPV types and 29.1% for HR HPV types in a cross-sectional study of adolescent females [10]. The youngest women in a longitudinal study of college-aged women by Ho et al. had the highest prevalence of HPV infection. Sampling for HPV was performed every 6 months for 3 years. When women who were positive for HPV at baseline were included, 60% were infected with HPV at some point during the 3-year study period [11]. In a cross-sectional study by Tarkowski et al., HPV was detected in 64% of 312 adolescent women; more than half of these women had multiple HPV types detected [12].
The very high cumulative prevalence in our study was most likely a result of the large number of specimens obtained from each subject. Many infections were detectable for only a few weeks and would have been missed if specimens were obtained at 4- or 6-month intervals. In addition, we used an assay that detects more HPV types than did the assays used in some previous studies, thus contributing to the higher HPV prevalence. Some of the differences in prevalence may be related to behaviors of the cohort (to be described in a forthcoming article). Thus, in part, the high HPV prevalence may be reflective of the at-risk population served at the clinics used for this study. The young women were recruited from a population in which the initiation of sexual activity occurred at a mean age of 14 years [13]. Close to 14% of adolescent females who received reproductive health care in these clinics had Chlamydia trachomatis infections, and 8.9% were infected with Neisseria gonorrhoeae, rates above the reported national averages for this population.
Self-collected vaginal swabs potentially offer an inexpensive method of collecting genital secretions for HPV testing. In our study, self collection of vaginal swabs was acceptable and reliable for HPV detection. In other studies, results of HPV testing of self-collected vaginal swabs also approximated those of clinician-obtained specimens from the cervix [14-16]. In the study by Gravitt et al., self-collected vaginal swab samples were compared with clinician-obtained cervical swab samples from 268 women participating in a study of cervical cancer [15]. The overall agreement between test results from the clinician- and self-collected swabs was excellent.
Overall, ~85% of swabs in the present study contained adequate cellular material, on the basis of β-globin amplification. Sampling errors or loss of DNA in the purification process could account for the 15% of swabs that did not contain adequate cellular material. Interestingly, we found that vaginal swabs were more likely than cervical swabs to contain adequate cellular material. In addition, HPV detection in vaginal swabs exceeded that in cervical swabs. One explanation is that cervical swabs, obtained by direct visualization and cotton swab sampling of the squamo-columnar junction, would have yielded more cellular material if a cytobrush had been used. The higher percentage of vaginal swabs containing detectable HPV could be due to the larger surface area of genital mucosa sampled by vaginal swabs compared with cervical swabs.
We identified a large range of HPV types in the adolescent women. Overall, HPV types 52, 16, and 59 were the most frequently detected HR types. We have no obvious explanation for the high prevalence of HPV 52. In a cohort of older women in Indianapolis of the same racial distribution, we previously found that HPV 52 was detected much less frequently than other HR types [17]. Thus, it is possible that HPV 52 preferentially infects younger women, but additional studies are needed to test this hypothesis.
The most frequently detected LR types were HPV 66 (6.1% of adequate specimens) and HPV 6 (5.6%). HPV 66 is a type detected with variable prevalence in cervical swab or lavage specimens from women with normal or abnormal cervical cytological results [12, 16-20]. Some experts have classified HPV 66 as an HR type, whereas others include this type in the LR category. In a study of an urban population similar to the one in the present study, HPV 66 was detected in cervical specimens from 8.0% of adolescent women who were sampled only once during the study [12]. HPV 66 was the third-most frequently detected type in that study, after HPV types 16 and 59.
Natural history studies indicate that most HPV infections “clear,” meaning that the specific HPV type is no longer detectable by current methods [18, 21-25]. Our study is consistent with this observation. However, the inability to detect HPV infection in swabs by use of PCR is not proof that clearance has occurred. It is possible that infection persists at very low levels and can potentially reactivate later in life. As additional longitudinal studies are performed and improved detection methods are developed, we believe that the issue of HPV clearance will need to be reconsidered.
We found a significant difference in persistence of HR and LR HPV types on the basis of survival analysis, which is consistent with findings in previous studies. For example, Londesborough et al. studied women infected with HPV and found that HPV 16 persisted longer than other types [26]. Giuliano et al. found that the median time to clearance of HPV infection was 9.8 months for HR HPV types, compared with 4.3 months for LR types [19]. Franco et al. showed that the mean infection durations were 13.5 and 8.2 months for HR and LR HPV types, respectively [18]. In the study by Moscicki et al., HR HPV types were less likely to regress than were LR types [24]. HR HPV types may therefore possess a unique ability to persist longer than LR types, and it is the persistence of HR HPV that is associated with cervical dysplasia.
We found that 37.0% of the cohort of young adolescent women had a least 1 abnormal cervical cytologic examination during the study period. One of the participants developed an HGSIL abnormality during the study period. This rate of cytologic abnormalities is within range of those from other studies of adolescent women. Moscicki et al. reported a combined incidence and prevalence rate of 33.5% for LGSILs in young women infected with HPV [24]. Interestingly, Moscicki found 11 women (1.8% of the total number of women) who developed HGSILs in the 40-month observation period, and the authors suggested that dysplasia in adolescents may progress more quickly than dysplasia in older women. In the above-mentioned cross-sectional study by Tarkowski et al., cytologic abnormalities were detected in 38% of 312 adolescent women.
In summary, we found that a high proportion of adolescent females were infected with HPV at any given time, and, over the study period, >80% of the subjects had evidence of HPV infection. A high percentage of these subjects had cervical cytologic abnormalities. One defining step in the epidemiology of HPV infection will be to determine whether infections at a very young age are those that resurge and are detected later in life, versus acquisition of a new infection. Finally, if infections do persist at very low levels from young adulthood, it is important to determine the factors associated with the progression from HPV infection to dysplasia and malignancy.
Acknowledgments
We thank Raymond J. Apple and Amanda Shepard (Roche Molecular Systems, Alameda, CA), for providing the polymerase chain reaction/reverse blot assay, and Patti Gravitt (Johns Hopkins Bloomberg School of Public Health, Baltimore, MD), for critical reading of the manuscript and for her helpful suggestions.
Financial support: National Institutes of Health, National Institute of Allergy and Infectious Disease (grant U19 AI43924); National Institute of Child Health and Human Development (grant RO1HD42404-01, for the development of statistical models used in this research).
Footnotes
Presented in part: 21st International Papillomavirus Conference, 20–27 February 2004, Mexico City, Mexico (abstract 223).
References
- 1.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance, 1995. Atlanta: US Department of Health and Human Services, Public Health Service; 1996. [Google Scholar]
- 2.Schiffman MH, Burk RD. Human papillomaviruses. In: Kaslow RA, editor. Viral infections of humans. New York: Plenum Medical Book; 1997. pp. 983–1022. [Google Scholar]
- 3.Asato T, Nakajima Y, Nagamine M, et al. Correlation between the progression of cervical dysplasia and the prevalence of human papillomavirus. J Infect Dis. 1994;169:940–1. doi: 10.1093/infdis/169.4.940. [DOI] [PubMed] [Google Scholar]
- 4.Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3–8. doi: 10.1016/s0002-9343(97)00177-0. [DOI] [PubMed] [Google Scholar]
- 5.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 HPV types from L1 consensus PCR products using a single hybridization, reverse line-blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DR, Schroeder JM, Bryan JT, Stoler MH, Fife KH. Detection of multiple human papillomavirus types in condylomata acuminata lesions from otherwise healthy and immunosuppressed patients. J Clin Microbiol. 1999;37:3316–22. doi: 10.1128/jcm.37.10.3316-3322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz B, Fortenberry J, Tu W, Harezlak J, Orr D. Sexual behavior among adolescent women at high risk for sexually transmitted infection. Sex Transm Dis. 2001;28:247–51. doi: 10.1097/00007435-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Thereau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer; 2002. [Google Scholar]
- 10.Moscicki AB, Ellenberg JH, Vermund SH, et al. Prevalence of and risks for cervical human papillomavirus infection and squamous intraepithelial lesions in adolescent girls: impact of infection with human immunodeficiency virus. Arch Pediatr Adolesc Med. 2000;154:127–34. doi: 10.1001/archpedi.154.2.127. [DOI] [PubMed] [Google Scholar]
- 11.Ho GYF, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 12.Tarkowski T, Koumans E, Sawyer M, et al. Epidemiology of human papillomavirus infection and abnormal cytologic test results in an urban adolescent population. J Infect Dis. 2004;189:46–50. doi: 10.1086/380466. [DOI] [PubMed] [Google Scholar]
- 13.Orr DP, Fortenberry JD, Blythe MJ. Validity of self-reported sexual behaviors in adolescent women using biomarker outcomes. Sex Transm Dis. 1997;24:261–6. doi: 10.1097/00007435-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Wright TC, Denny L, Kuhn L, Pollack A, Lorincz A. HPV DNA testing of self-collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA. 2000;283:81–6. doi: 10.1001/jama.283.1.81. [DOI] [PubMed] [Google Scholar]
- 15.Gravitt P, Lacey J, Brinton L, et al. Evaluation of self-collected cervicovaginal cell samples for human papillomavirus testing by polymerase chain reaction. Cancer Epidemiol Biomarkers Prev. 2001;10:95–100. [PubMed] [Google Scholar]
- 16.Shah K, Daniel R, Tennant M, et al. Diagnosis of human papillomavirus infection by dry vaginal swabs in military women. Sex Transm Infect. 2001;77:260–4. doi: 10.1136/sti.77.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown D, Legge D, Qadadri B. Distribution of human papillomavirus types in cervicovaginal washings from women evaluated in a sexually transmitted diseases clinic. Sex Transm Dis. 2002;29:763–8. doi: 10.1097/00007435-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Franco EL, Villa LL, Sobrinho JP, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999;180:1415–23. doi: 10.1086/315086. [DOI] [PubMed] [Google Scholar]
- 19.Giuliano A, Harris R, Sedjo R, et al. Incidence, prevalence, and clearance of type-specific human papillomavirus infections: The Young Women’s Health Study. J Infect Dis. 2002;186:462–9. doi: 10.1086/341782. [DOI] [PubMed] [Google Scholar]
- 20.Sotlar K, Diemer D, Dethleffs A, et al. Detection and typing of human papillomavirus by E6 nested multiplex PCR. J Clin Microbiol. 2004;42:3176–84. doi: 10.1128/JCM.42.7.3176-3184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moscicki AB, Palefsky J, Smith G, Siboshski S, Schoolnik G. Variability of human papillomavirus DNA testing in a longitudinal cohort of young women. Obstet Gynecol. 1993;82:578–85. [PubMed] [Google Scholar]
- 22.Evander M, Edlund K, Gustafsson A, et al. Human papillomavirus infection is transient in young women: a population-based cohort study. J Infect Dis. 1995;171:1026–30. doi: 10.1093/infdis/171.4.1026. [DOI] [PubMed] [Google Scholar]
- 23.Brisson J, Bairati I, Morin C, et al. Determinants of persistent detection of human papillomavirus DNA in the uterine cervix. J Infect Dis. 1996;173:794–9. doi: 10.1093/infdis/173.4.794. [DOI] [PubMed] [Google Scholar]
- 24.Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277–84. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 25.Sellors J, Karwalajtys T, Kaczorowski J, et al. Incidence, clearance and predictors of human papillomavirus infection in women. CMAJ. 2003;168:421–5. [PMC free article] [PubMed] [Google Scholar]
- 26.Londesborough P, Ho L, Terry G, Cuzick J, Wheeler C, Singer A. Human papillomavirus genotype as a predictor of persistence and development of high-grade lesions in women with minor cervical abnormalities. Int J Cancer. 1996;69:364–8. doi: 10.1002/(SICI)1097-0215(19961021)69:5<364::AID-IJC2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]


