Abstract
Most schizophrenia patients do not inhibit their P50 auditory evoked potential to the second of duplicate auditory stimuli, reflecting a failure to inhibit responses to irrelevant sensory input. Typical antipsychotic drugs do not improve this deficit while some atypical antipsychotics do. A previous study using an animal model, deficient P20-N40 (which corresponds to the human P50) inhibitory processing in DBA/2 mice found that sensory inhibition was improved by clozapine, the prototypical atypical antipsychotic, but not by haloperidol, a typical antipsychotic. The improvement after clozapine was mediated by α7 nicotinic receptors. The present study addresses whether another atypical antipsychotic, olanzapine, will also improve sensory inhibition deficits in the mouse model. In vivo electrophysiological recordings of the P20-N40 auditory evoked potential in anesthetized DBA/2 mice, which spontaneously exhibit a schizophrenia-like inhibitory processing deficit, were obtained after olanzapine alone (0.01, 0.033, 0.1, 0.33 mg/kg, IP) and the efficacious dose of olanzapine (0.033 mg/kg, IP) in combination with either the α7 nicotinic receptor antagonist α-bungarotoxin or the α4β2 nicotinic receptor antagonist di-hydro-β-erythroidine. All doses of olanzapine produced improved P20-N40 inhibitory processing in DBA/2 mice. The normalization observed after the 0.033 mg/kg dose of olanzapine was due to a selective decrease in response to the second auditory stimulus indicating an increase in inhibitory processing. This improvement was blocked by pre-administration of α-bungarotoxin but not di-hydro-β-erythroidine. Like clozapine, olanzapine acts via α7 nicotinic receptors to elicit improved inhibitory processing of auditory stimuli.
Keywords: P50 gating, schizophrenia, hippocampal auditory evoked response, atypical antipsychotic, α7 nicotinic cholinergic receptor
Introduction
Deficient inhibitory processing of information has long been associated with schizophrenia. Specifically, inhibition of the P50 auditory evoked potential (AEP) to repeated stimuli is reduced in schizophrenia patients and is not improved by treatment with typical antipsychotic drugs (Adler et al 1985; 1990b; 2004; Arango et al 2003; Baker et al 1987; Freedman et al 1983; Myles-Worsley 2002; Nagamoto et al 1989; 1991; 1993; Waldo et al 1992; Yee et al 1998). Typically, the human P50 response to the second of closely paired (500 msec) identical auditory stimuli as reduced compared to the first in normal individuals. However, schizophrenia patients fail to inhibit the response to the second stimulus, often showing similar magnitude responses to both stimuli (Baker et al 1987). Typical antipsychotics generally increase the response amplitude to both stimuli without affecting the ratio of the second to the first response, which is the “TC ratio” (ratio of the test amplitude/conditioning amplitude). This is the measure used to assess level of inhibition. When the test response is reduced, the TC ratio is less than 1. Interestingly, the deficit is improved by nicotine, whether from cigarette smoking or nicotine gum (Adler et al 1992; 1993).
The rodent P20-N40 models the human P50. Studies in rodent models of deficient P20-N40 were the first to suggest involvement of a specific subtype of nicotinic receptors, the α7 (Luntz-Leybman et al 1992). Later studies correlated numbers of this receptor in the hippocampus to level of inhibition, i.e. strains with fewer receptors such as the DBA/2 mice have poorer inhibition (Stevens et al 1996), and direct agonist studies confirmed that selective stimulation of the α7 nicotinic receptor produced improvement in deficient sensory inhibition in DBA/2 mice (Simosky et al 2001; Stevens et al 1998; Stevens and Wear 1997).
Although typical antipsychotic drugs fail to improve the deficient sensory inhibition in schizophrenia patients (Adler et al 1985; Baker et al 1987), several studies report improvement associated with administration of clozapine or other atypical antipsychotics (Adler et al 2004; Light et al 2000; Nagamoto et al 1996; 1999; Yee et al 1998). However, not all atypical antipsychotics are as reliable as clozapine in producing improved sensory inhibition. For example, the results from studies of olanzapine are less clear in that some investigators observe improvement in sensory inhibition (Light et al 2000) while others fail to observe improvement (Adler et al 2004; Arango et al 2003).
A recent rodent study suggested a possible mechanism for the improvement in sensory inhibition seen in schizophrenia patients on clozapine. DBA/2 mice, a model of deficient sensory inhibition observed in schizophrenia (Stevens et al 1996), showed an improvement after clozapine which was blocked by pre-administration of the selective α7 nicotinic receptor antagonist α-bungarotoxin (Simosky et al 2003). This data suggested that activity at α7 nicotinic receptors underlies improved sensory inhibition, though clozapine does not bind nicotinic cholinergic receptors (Arnt 1998; Ashby et al 1989).
Because the ability of olanzapine, another atypical antipsychotic, to normalize inhibition of the P50 auditory evoked potential in schizophrenics has been called into question by recent data (Adler et al 2004; Arango et al 2003), the present experiments explored the effects of olanzapine on processing of the P20-N40 auditory evoked potential in the DBA/2 mouse model. We generated a dose-response curve and assessed the ability of a selective antagonist of either high-affinity (α4β2) or low-affinity nicotinic receptors (α7) to block an olanzapine-induced improvement in sensory inhibition. We also compared the results with olanzapine to previously published results with clozapine and haloperidol.
Results
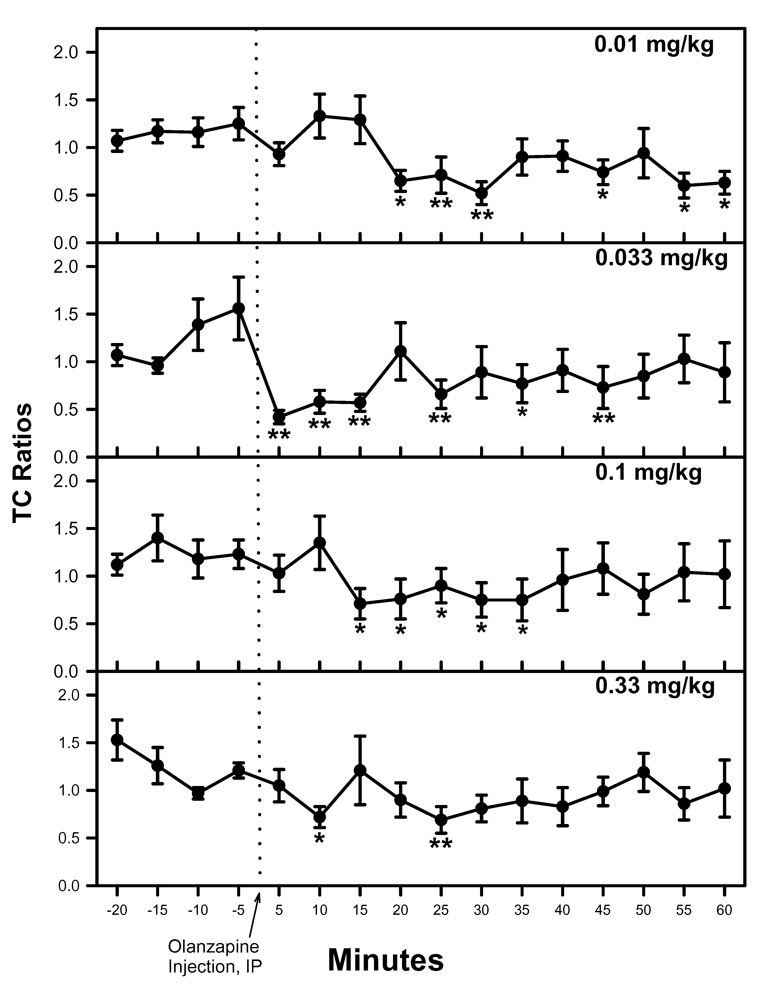
DBA/2 mice showed a deficit in inhibitory processing of the P20-N40 auditory evoked potential under baseline conditions before administration of olanzapine (Figure 1). Administration of olanzapine (0.01, 0.033, 0.10, or 0.33 mg/kg, IP) produced improvements in the inhibitory processing of the P20-N40 auditory evoked potential (Figure 1). Repeated measures MANOVA did not show a significant effect of time or dose nor time-by-dose interaction. However, all of the a priori contrast analyses for TC ratio after the various doses of olanzapine were significant [0.01 mg/kg: F(1, 28) = 13.563, p = 0.001; 0.033 mg/kg: F(1, 28) = 28.193, p < 0.001; 0.10 mg/kg: F(1, 28) = 12.280, p = 0.002, 0.33 mg/kg: F(1, 28) = 12.763, p = 0.001]. These a priori contrast results indicate that there were differences in TC ratio when pre-olanzapine values were compared to post-olanzapine values. At many of the individual time points post-olanzapine administration, the TC ratio was significantly decreased relative to the average of the pre-drug measurements, as determined by Fisher’s PLSD a posteriori analysis following the a priori contrast analyses (Figure 1).
1.
The improved inhibitory processing after olanzapine was most evident after the 0.033 mg/kg dose, though improved TC ratios were also seen after 0.01, 0.1 and 0.33 mg/kg. Asterisks mark post-olanzapine time points at which the TC ratio was significantly different from the average of the baseline TC ratio, as determined by Fisher’s LSD (*p<0.05, **p<0.01). Data are mean ± SEM. For all doses, n = 8.
A repeated measures MANOVA of the conditioning amplitude was not significant for time or dose or time-by-dose interaction. However, two of the a priori contrast analyses for dose were significant [0.01 mg/kg: F(1, 28) = 5.536, p = 0.026; 0.33 mg/kg: F(1, 28) = 6.155, p = 0.019] (Figure 2A). Similarly, a repeated measures MANOVA for test amplitude did not achieve significance for time or dose or time-by-dose interaction, while one of the a priori contrast analyses was significant [0.033 mg/kg: F(1, 28) = 12.068, p = 0.002] (Figure 2B).
2.
Amplitudes of the conditioning (A) and test (B) response before and after olanzapine administration after the four doses (0.01, 0.033, 0.1, or 0.33 mg/kg, IP). The 0.01 and 0.33 mg/kg doses produced significant increases in conditioning response amplitude (A). The test response amplitude (B) was significantly reduced only by the 0.033 mg/kg dose. Asterisks mark those post-olanzapine time points at which the conditioning or test amplitude was significantly different from the average of the baseline conditioning or test amplitude, as determined by Fisher’s LSD (*p<0.05, **p<0.01). Data are mean ± SEM. For all doses, n = 8.
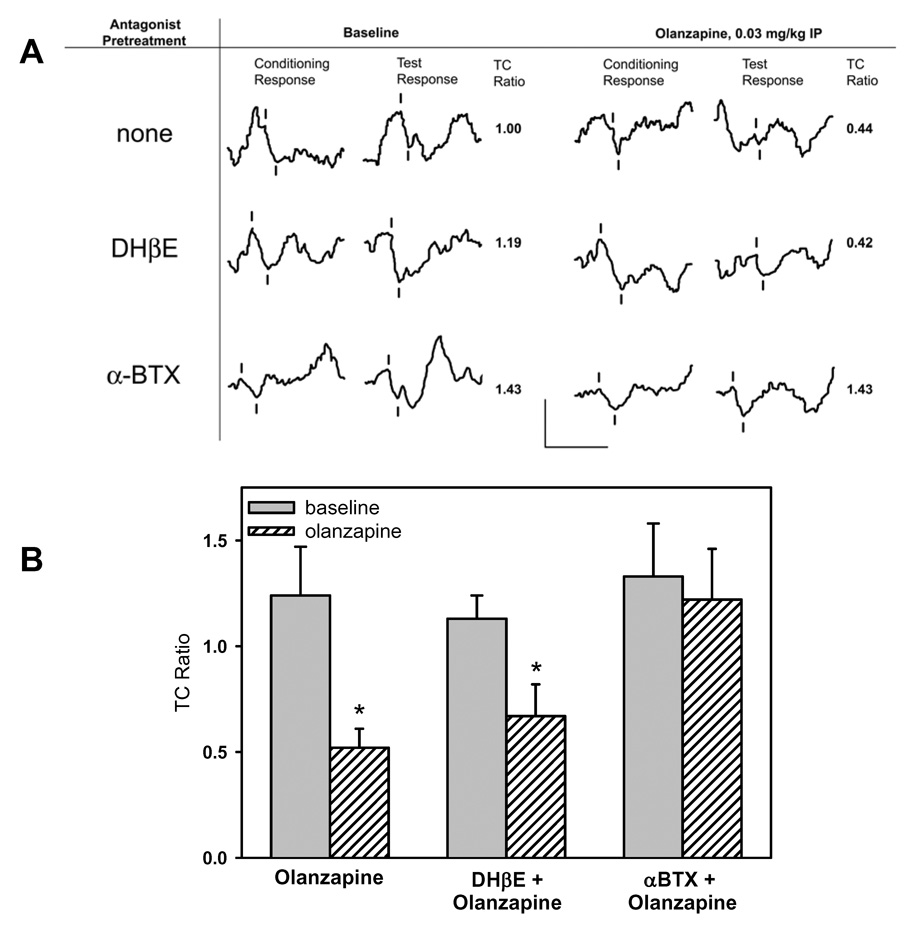
Selective antagonist studies (1 µl of either 1.25 nM α-bungarotoxin or 30 nM DHβE) were conducted with the olanzapine dose that selectively increased P20-N40 inhibition (0.03 mg/kg), as indicated by a selective decrease in test amplitude (Figure 3). The repeated-measures MANOVA for TC ratio showed a significant effect of time (F(15, 225) = 2.198, p = 0.007), but not for a drug or time-by-drug interaction. The a priori contrast analyses for olanzapine and olanzapine plus DHβE were significant [for olanzapine F(1, 15) = 25.765, p < 0.001; for olanzapine plus DHβE F(1, 15) = 6.970, p = 0.019]. However, the contrast for olanzapine plus α-bungarotoxin was not significant. Decreased TC ratios did not occur when olanzapine administration followed α-bungarotoxin administration. As was seen with olanzapine alone, decreased TC ratios did occur when olanzapine administration followed DHβE administration.
3.
Effect of selective nicotinic antagonists on olanzapine-induced changes in sensory inhibition. The effect of olanzapine was assessed alone and after preadministration of either α-bungarotoxin (α-BTX, an α7 nicotinic receptor antagonist) or di-hydro-β-erythroidine (DHβE, an α4β2 nicotinic receptor antagonist). (A) Representative waveforms from 3 DBA/2 mice show that pretreatment with α-BTX blocked the olanzapine-induced normalization of the deficient sensory inhibition of the P20-N40 AEP while administration of DHβE did not. Tick marks note the P20-N40 waveform. Calibration: 50 ms by 25 µV. (B) Comparison of the average TC ratios from the 15 minutes before and after olanzapine administration shows a significant decrease in TC ratio only after olanzapine or olanzapine plus DHβE. Administration of the α7 nicotinic receptor antagonist α-BTX prevents the olanzapine-induced improvement. n=8 for olanzapine, n=4 for DHβE, n=7 for α-BTX. *p<0.05, Student’s t-test.
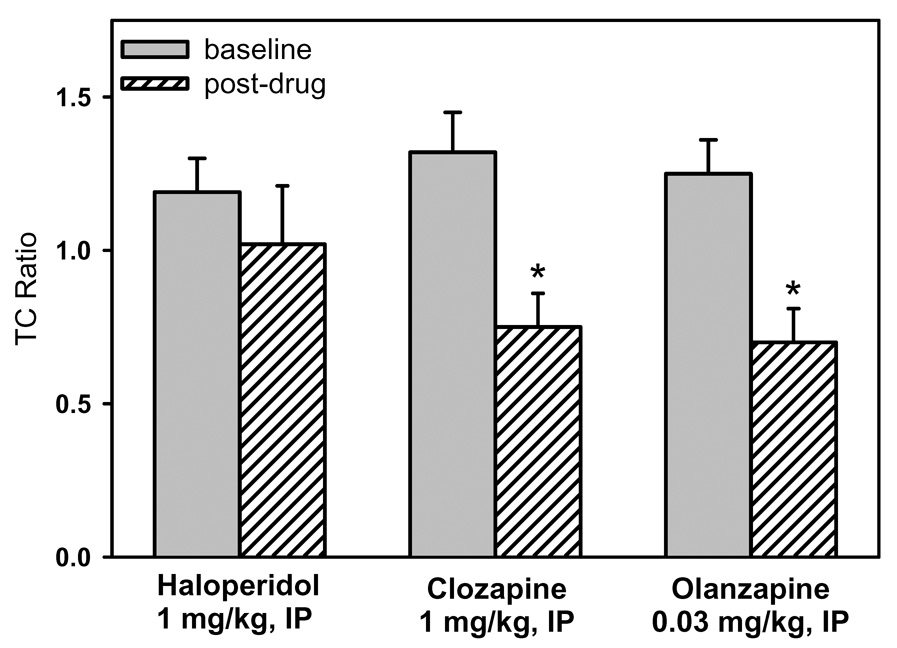
To place the results with olanzapine into context, we compared the effects of olanzapine to previously published data (Simosky et al 2003) on the effects of clozapine and haloperidol on the deficient P20-N40 inhibitory processing in DBA/2 mice (Figure 4). A repeated measures MANOVA showed a significant effect of drug [F(2, 27) = 4.954, p = 0.015], but not a significant effect of time or a significant drug-by-time interaction. The a priori contrast analyses for clozapine and olanzapine were also significant [Clozapine: F(1, 27) = 25.435, p < 0.001; Olanzapine: F(1, 27) = 12.231, p = 0.002]. Fisher’s PLSD a posteriori analysis by drug indicated that both clozapine and olanzapine were significantly different from haloperidol but not from each other.
4.
Comparison of the effects of olanzapine on deficient sensory inhibition with the effects of clozapine and haloperidol (Simosky et al 2003). Shown is the average TC ratio for before and after administration of each antipsychotic. In DBA/2 mice, as in schizophrenia patients, only atypical antipsychotics are associated with improved inhibitory sensory processing. Data are mean ± SEM, *p<0.05, Student’s t-test, n=14 for haloperidol, n=8 for clozapine, n=8 for olanzapine.
Discussion
These studies demonstrate that olanzapine, like clozapine (Simosky et al 2003), improves inhibitory sensory processing in DBA/2 mice. However, the efficacy of olanzapine against deficient inhibition of the P20-N40 auditory response does not appear to be as robust as the previously observed efficacy of clozapine. Significantly improved TC ratios after olanzapine were only detectable when using an a priori contrast analysis, not when using a repeated measures MANOVA. The repeated measures MANOVA was able to detect improved TC ratios in previous studies with clozapine, despite the potential confound of repeated measurements (Simosky et al 2003). The 0.033 mg/kg dose of olanzapine (IP) produced decreased TC ratios via a selective decrease in test amplitude, indicating a selective increase in response inhibition. Decreased TC ratios were also seen after the 0.01, 0.1, and 0.3 doses of olanzapine, though the relative effects on the test versus the conditioning amplitudes varied. For the 0.033 mg/kg dose, the ability to induce improved P20-N40 inhibitory processing was blocked by the pre-administration of the α7-selective antagonist α-bungarotoxin but not the α4β2 antagonist DHβE. Thus, like 1 mg/kg clozapine, olanzapine 0.033 mg/kg appears to improve deficient P20-N40 inhibitory processing via stimulation of α7 nicotinic cholinergic receptors.
The initial doses of olanzapine tested in this study were based on olanzapine’s clinical potency relative to clozapine. Olanzapine is about 10-fold more potent than clozapine (Woods 2003), and thus the initial olanzapine dose tested was 0.1 mg/kg, which is 10% of the clozapine dose that was most efficacious against deficient P20-N40 inhibitory processing in DBA/2 mice (Simosky et al 2003). Interestingly, the efficacious doses of olanzapine in the present study are generally lower than doses of olanzapine reported as efficacious elsewhere in the literature. Studies which tested olanzapine’s ability to block psychostimulant-induced motor changes used doses ranging from 0.03 to 10 mg/kg (Fu et al 2000; Moore et al 1992; Ninan and Kulkarni 1999b; Noda et al 2000; Rasmussen et al 2001), with one study finding that 0.033 mg/kg olanzapine was able to reverse the effects of phencyclidine (Gleason and Shannon 1997). The requirement for higher doses of olanzapine to reverse stimulant-induced locomotor changes, relative to the low dose to improve sensory inhibition is probably related to the differences in neurotransmitter systems involved with the 2 paradigms. Alterations in locomotor activity are generally attributed to the dopaminergic system (for review see Swerdlow et al 1986) while both the whole catecholaminergic and the nicotinic acetylcholine systems modulate alterations in inhibitory sensory processing (Adler et al 1990a; 1998; Stevens et al 1991; 1994). Another method of assessing potential antipsychotic ability of a drug is the ability to improve a conditioned avoidance response. In this paradigm, which does not require a psychotomimetic, olanzapine had an ED50 of 2.72 mg/kg, PO (Fu et al 2000). A companion paradigm to sensory inhibition is prepulse-startle inhibition (PPI) which also measures inhibitory processes. A study using a slightly different substrain of DBA/2 mice (DBA/2NCrl) found improvement in deficient PPI with doses of olanzapine ranging from 1–6 mg/kg (Flood et al 2007). A study which assessed olanzapine’s ability to reverse murine cognitive deficits found efficacy as low as 0.063 mg/kg (Ninan and Kulkarni 1999a; 1999b; 1999c), a dose more in line with the present findings.
While olanzapine’s efficacy against P50 inhibitory processing deficits in schizophrenia patients does not appear to be as robust as clozapine’s (Adler et al 2004; Arango et al 2003; Light et al 2000; Nagamoto et al 1996; 1999), it is possible that both atypical antipsychotic drugs increase acetylcholine release, resulting in indirect agonism of the α7 nicotinic receptor. This is supported by both human studies in which atypical antipsychotics appear to result in spontaneous decreases in smoking behavior by schizophrenia patients (Buckley 1998; Combs and Advocat 2000; McEvoy et al 1995; 1999; Procyshyn et al 2001; ; 2002; Young et al 1997) and by rodent microdialysis studies in which atypical antipsychotics increased acetylcholine levels in prefrontal cortex (Ichikawa et al 2000a; 2002b; 2002c; 2002d) and in hippocampus (Shirazi-Southall et al 2002). Interestingly, this last study showed a far greater increase in acetylcholine after olanzapine (10–15 fold) than after clozapine (5-fold). A possible explanation for olanzapine’s apparently reduced reliability in improving rodent or human information processing deficits is that olanzapine produces a faster and higher increase in acetylcholine levels, which may only temporarily activate the α7 nicotinic receptors before driving them into a desensitized state. It is possible that patients on olanzapine are receiving a dose efficacious against their most apparent schizophrenia symptoms, but that dose is too high to effectively normalize their P50 inhibitory processing deficits. Studies in rodents with full agonists such as nicotine show an inverted U dose response curve with low doses ineffective, mid-range doses improving inhibition and high doses again ineffective (Stevens and Wear, 1997) probably due to receptor desensitization.
In summary, the present study found that olanzapine, like clozapine, was effective in improving deficient sensory inhibition in DBA/2 mice, a model of deficient inhibitory processing in schizophrenia. The improvement was blocked by pre-administration of a selective α7 nicotinic antagonist but not a selective α4β2 nicotinic antagonist, suggesting modulation through α7 nicotinic receptors.
Experimental Procedure
Animals used in experimental protocols
Male DBA/2 mice (20–25 gm) from Harlan SD (Indianapolis, IN) were group housed until recorded. Food (Purina Rodent Chow) and water were available ad libitum and lighting was cycled at 12-hour intervals (lights on at 6 AM). “Principles of Laboratory Animal Care” (NIH Publication No. 85-23, revised 1985) were followed. The Institutional Animal Care and Use Committee of the Denver Veterans Affairs Medical Center and/or the University of Colorado Denver-School of Medicine approved the experimental protocols.
Surgery
On the day of the experiment, drug-naïve mice were anesthetized by intraperitoneal (IP) injection of chloral hydrate (400 mg/kg) and pyrazole (400 mg/kg, to slow chloral hydrate metabolism). Anesthesia was supplemented as necessary to maintain a surgical plane of anesthesia. For all mice, a tracheotomy was performed, with insertion of polyethylene tubing (1.22 mm, outer diameter) to decrease the risk of anoxia while under anesthesia without oxygen support. The anesthetized animal was placed in a mouse adapter (Neuroprobe, Cabin John, MD) for a Kopf stereotaxic instrument (Kopf Instruments, Tujunga, CA). Hollow ear bars, attached to miniature earphones, were placed adjacent to the externalization of the aural canal. A stable body temperature was maintained throughout the experiment with a heating pad coupled to a rectal probe. The scalp was incised and a burr hole opened over the CA3 region of hippocampus [−1.8 anterior-posterior, +2.70 medial-lateral (Franklin and Paxinos 1997)]. A Teflon-coated stainless steel cut-wire electrode (0.127 mm diameter) was inserted into the CA3 pyramidal cell layer of the hippocampus (1.65 – 1.70 mm dorsal to the brain surface). Final electrode location was further identified by the presence of complex action potentials typical of hippocampal pyramidal neurons (Miller et al 1992). A second identical cut-wire electrode was placed on contralateral anterior cortex to serve as reference.
Electrophysiological Recordings
The electrical activity of the CA3 region of the hippocampus was amplified 1000 times with bandpass 1–500 Hz (Miller et al 1992) and led to an analog-to-digital converter (RC Electronics, Bakersfield, CA) for averaging by computer. Auditory stimuli (3000 HZ, 10 msec duration, 70 dB SPL generated as a sine wave) were presented in pairs, with a 500-millisecond interval between the paired tones and a 10 second interval between pairs. Although DBA/2 mice suffer hearing loss as they age, these tones were within the audible range for the mice (Willott et al 1982). For each record, responses to 16 pairs of tones were averaged and digitally filtered by computer, with an allowed bandpass between 10 and 250 Hz. The maximum negativity between 20 and 60 msec after each stimulus was selected as the N40 wave and measured relative to the preceding positivity, a P20 wave. This P20-N40 wave complex has greater reproducibility for repeated measurements than either component alone (Cook et al 1968; Hashimoto et al 2005). The ratio of the amplitudes of response to the second (test) stimulus and the first (conditioning) stimulus provides a measure of the inhibition of the auditory response: significant decreases in this “TC ratio” (relative to baseline) indicate improved inhibition of the auditory response. The amplitude of the conditioning response and the amplitude of the test response were also used to characterize the P20-N40 response.
Four baseline records were recorded prior to drug administration. Olanzapine (0.01, 0.033, 0.10, 0.33 mg/kg; n = 8 per dose) was administered in a volume of 100 µl per every 20 grams body weight. Olanzapine was initially dissolved in small volume of saline acidified with HCl, after which the pH was titrated back to physiological pH with NaOH and additional saline. After drug administration, records were collected at 5-minute intervals for 60 minutes.
For the antagonist experiments, an additional burr hole was opened over the anterior lateral ventricle [+0.1 anterior-posterior, +0.8 medial-lateral (Franklin and Paxinos 1997)], ipsilateral to the recording electrode. A 26-gauge cannula was inserted (2.0 mm below dura) for the intracerebroventricular (ICV) administration of antagonist. For all antagonists, 0.5–1 µl of antagonist was injected directly into the anterior lateral ventricle over a 30 sec period (Stevens and Wear 1997). At the end of each experiment, cannula placement into the lateral ventricle was histologically verified along with verification of no ventricular enlargement due to the ICV injection. Five minutes following antagonist administration, one recording was taken, after which the test drug was administered. Records were then obtained at 5-minute intervals for the next 60 minutes. In these studies 1.25 nmol α-bungarotoxin [α7 antagonist; (Schoepfer et al 1990); n = 6] or 30 nmol dihydro-β-erythroidine [α4β2 antagonist; (Harvey and Luetje 1996; Harvey et al 1996); n = 4] was administered before IP injection of 0.033 mg/kg olanzapine.
Chemicals
Chemicals obtained from Sigma RBI included the anesthetics (chloral hydrate and pyrazole) and the nicotinic receptor antagonists [α-bungarotoxin (α-BTX), and dihydro-β-erythroidine (DHβE)]. The olanzapine was provided by Eli Lilly and Company (Indianapolis, IN).
Statistical Analyses
The following parameters were analyzed: conditioning amplitude (response to the first stimulus); test amplitude (response to the second stimulus) and TC ratio (test amplitude / conditioning amplitude). Data were analyzed by repeated measures MANOVA (over time and nested by dose) after the use of a priori contrast analyses for each dose to compare the mean of the “baseline” (pre-drug) auditory evoked potential measures and the mean of the “treatment” (post-drug) AEP measures. Fisher’s PLSD a posteriori tests were performed where appropriate. Similar to the clinical studies with clozapine (Nagamoto et al 1996), a maximum TC ratio of 3 was used to prevent outliers from disproportionately affecting group means. In a previous report, the effects of haloperidol administration on P20-N40 inhibitory processing was compared to the results after clozapine using a repeated measures MANOVA (Simosky et al 2003). In the results presented in this article, the analysis was rerun after the addition of 0.033 mg/kg olanzapine as a third drug condition.
Acknowledgements
This work was supported by an Eli Lilly Investigator-Initiated Trials research award and an NRSA Predoctoral fellowhip (JKS), and a USPHS grant MH58680 (KES).
Abbreviations
- α-BTX
α–bungarotoxin
- DHβE
di-hydro-β-erythroidan
- AEP
Auditory evoked potentials
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler LE, Gerhardt GA, Franks R, Baker N, Nagamoto HT, Drebing C, Freedman R. Sensory physiology and catecholamines in schizophrenia and mania. Psychiatry Res. 1990a;31:297–309. doi: 10.1016/0165-1781(90)90099-q. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am. J. Psychiat. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LD, Griffith J, Waldo MC, Freedman R. Normalization by nicotine of deficient sensory gating in the relatives of schizophrenics. Biol Psychiatry. 1992;3:607–616. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- Adler LE, Olincy A, Cawthra EM, McRae KA, Harris JG, Nagamoto HT, Waldo MC, Hall MH, Bowles A, Woodward L, Ross RG, Freedman R. The varied effects of atypical neuroleptics on P50 auditory gating in schizophrenia patients. Am. J. Psychiatry. 2004;161:1822–1828. doi: 10.1176/ajp.161.10.1822. [DOI] [PubMed] [Google Scholar]
- Adler LE, Waldo MC, Freedman R. Neurophysiologic studies of sensory gating in schizophrenia: comparison of auditory and visual responses. Biol. Psychiatry. 1985;20:1284–1296. doi: 10.1016/0006-3223(85)90113-1. [DOI] [PubMed] [Google Scholar]
- Adler LE, Waldo MC, Tatcher A, Cawthra EM, Baker N, Freedman R. Lack of relationship of auditory gating defects to negative symptoms in schizophrenia. Schizophrenia Res. 1990b;3:131–138. doi: 10.1016/0920-9964(90)90046-a. [DOI] [PubMed] [Google Scholar]
- Arango CR, Summerfelt A, Buchanan RW. Olanzapine effects on auditory sensory gating in schizophrenia. Am. J. Psychiatry. 2003;160:2066–2068. doi: 10.1176/appi.ajp.160.11.2066. [DOI] [PubMed] [Google Scholar]
- Arnt J. Pharmacological differentiation of classical and novel antipsychotics. Intl. Clin. Psychopharmacol. 1998;13:S7–S14. doi: 10.1097/00004850-199803003-00002. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Edwards E, Harkins K, Wang RY. Differential effect of typical and atypical antipsychotic drugs on the suppressant action of 2-methylserotonin on medial prefrontal cortical cells: a microiontophoretic study. Eur. J. Pharmacol. 1989;166:583–584. doi: 10.1016/0014-2999(89)90382-8. [DOI] [PubMed] [Google Scholar]
- Baker NJ, Adler LE, Franks RD, Waldo MC, Berry S, Nagamoto HT, Muckle A, Freedman R. Neurophysiological assessment of sensory gating in psychiatric inpatients: comparison between schizophrenia and other diagnoses. Biol. Psychiatry. 1987;22:603–617. doi: 10.1016/0006-3223(87)90188-0. [DOI] [PubMed] [Google Scholar]
- Buckley PF. Novel antipsychotic medications and the treatment of comorbid substance abuse in schizophrenia. J. Substance Abuse Treatment. 1998;15:113–116. doi: 10.1016/s0740-5472(97)00134-7. [DOI] [PubMed] [Google Scholar]
- Combs DR, Advocat C. Antipsychotic medication and smoking in acutely hospitalized patients with chronic schizophrenia. Schizophrenia Res. 2000;46:129–137. doi: 10.1016/s0920-9964(00)00026-8. [DOI] [PubMed] [Google Scholar]
- Cook JD, Ellinwood EH, Jr, Wilson WP. Auditory habituation at primary cortex as a function of stimulus rate. Exp. Neurobiol. 1968;21:167–175. doi: 10.1016/0014-4886(68)90135-0. [DOI] [PubMed] [Google Scholar]
- Flood DG, Gasior M, Marino MJ. Variables affecting prepulse inhibition of the startle reflex and the response to antipsychotics in DBA/2NCrl mice. Psychopharmacol. 2007;195:203–211. doi: 10.1007/s00213-007-0894-9. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Freedman R, Adler LE, Waldo MC, Pachtman E, Franks RD. Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patients. Biol. Psychiatry. 1983;18:537–551. [PubMed] [Google Scholar]
- Fu Y, Zhu ZT, Chen LJ, Yu LD, Jin G. Behavioral characteristics of olanzapine: an atypical neuroleptic. Acta Pharmacol. Sin. 2000;21:329–334. [PubMed] [Google Scholar]
- Gleason SD, Shannon HE. Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacol. 1997;129:79–84. doi: 10.1007/s002130050165. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Luetje CW. Determinants of competitive antagonist selectivity on neuronal nicotinic receptor beta subunits. J. Neurosci. 1996;16:3798–3806. doi: 10.1523/JNEUROSCI.16-12-03798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Maddox FN, Luetje CW. Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Iyo M, Freedman R, Stevens KE. Tropisetron improved deficient inhibitory auditory processing in DBA/2 mice: Role of α7 nicotinic acetylcholine receptors. Psychopharmacol. 2005;183:13–19. doi: 10.1007/s00213-005-0142-0. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Dai J, Meltzer HY. Acetylcholinesterase inhibitors are neither necessary nor desirable for microdialysis studies of brain acetylcholine. Curr. Sep. 2000a;19:37–43. [Google Scholar]
- Ichikawa J, Dai J, Meltzer HY. 5-HT1A and 5-HT2A receptors minimally contribute to clozapine-induced acetylcholine release in rat medial prefrontal cortex. Brain Res. 2002b;939:34–42. doi: 10.1016/s0006-8993(02)02544-1. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Dai J, O'Laughlin IA, Fowler WL, Meltzer HY. Atypical, but not typical, antipsychotic drugs increase cortical acetylcholine release without an effect in the nucleus accumbens or striatum. Neuropsychopharmacol. 2002c;26:325–339. doi: 10.1016/S0893-133X(01)00312-8. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Li Z, Dai J, Meltzer HY. Atypical antipsychotic drugs, quetiapine, iloperidone and melperone, preferentially increase dopamine and acetylcholine release in rat medial prefrontal cortex: role of 5-HT1A receptor agonism. Brain Res. 2002d;965:349–357. doi: 10.1016/s0006-8993(02)03570-9. [DOI] [PubMed] [Google Scholar]
- Light GA, Geyer MA, Clementz BA, Cadenhead KS, Braff DL. Normal P50 suppression in schizophrenia patients treated with atypical antipsychotic medications. Am. J. Psychiatry. 2000;157:767–771. doi: 10.1176/appi.ajp.157.5.767. [DOI] [PubMed] [Google Scholar]
- Luntz-Leybman V, Bickford PC, Freedman R. Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res. 1992;587:130–136. doi: 10.1016/0006-8993(92)91437-j. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Freudenreich O, McGee M, VanderZwagg C, Levin E, Rose J. Clozapine decreases smoking in patients with chronic schizophrenia. Biol. Psychiatry. 1995;37:550–552. doi: 10.1016/0006-3223(94)00365-A. [DOI] [PubMed] [Google Scholar]
- McEvoy J, Freudenreich O, Wilson WH. Smoking and therapeutic response to clozapine in patients with schizophrenia. Biol. Psychiatry. 1999;46:125–129. doi: 10.1016/s0006-3223(98)00377-1. [DOI] [PubMed] [Google Scholar]
- Miller CL, Bickford PC, Luntz-Leybman V, Adler LE, Gerhardt GA, Freedman R. Phenycyclidine and auditory sensory gating in the hippocampus of the rat. Neuropharmacol. 1992;31:1041–1048. doi: 10.1016/0028-3908(92)90106-y. [DOI] [PubMed] [Google Scholar]
- Moore NA, Tye NC, Axton MS, Risius FC. The behavioral pharmacology of olanzapine, a novel "atypical" antipsychotic agent. J. Pharmacol. Exp. Ther. 1992;262:545–551. [PubMed] [Google Scholar]
- Myles-Worsley M. P50 sensory gating in multiplex schizophrenia families from a Pacific island isolate. Am. J. Psychiatry. 2002;159:2007–2012. doi: 10.1176/appi.ajp.159.12.2007. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Hea RA, Griffith JM, McRae KA, Freedman R. Gating of auditory P50 in schizophrenics: unique effects of clozapine. Biol. Psychiatry. 1996;40:181–188. doi: 10.1016/0006-3223(95)00371-1. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Hea RA, Hoffer LD, Freedman R. Gating of auditory P50 in schizophrenia: Trait and state related phenomena. Biol. Psychiatry. 1993;33:120A. [Google Scholar]
- Nagamoto HT, Adler LE, McRae KA, Huettl P, Cawthra EM, Gerhardt GA, Hea RA, Griffith J. Auditory P50 in schizophrenics on clozapine: improved gating parallels clinical improvement and changes in plasma 3-methoxy-4-hydroxyphenylglycol. Neuropsychobiol. 1999;39:10–17. doi: 10.1159/000026553. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Waldo MC, Freedman R. Sensory gating in schizophrenics and normal controls: Effects of changing stimulation interval. Biol. Psychiatry. 1989;25:549–561. doi: 10.1016/0006-3223(89)90215-1. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Waldo MC, Griffith J, Freedman R. Gating of the auditory response in schizophrenics and normal controls: Effects of recording site and stimulus interval on the P50 wave. Schizophrenia Res. 1991;4:31–40. doi: 10.1016/0920-9964(91)90007-e. [DOI] [PubMed] [Google Scholar]
- Ninan I, Kulkarni S. Differential effects of olanzapine at dopamine D1 and D2 receptors in dopamine depleted animals. Psychopharmacol. 1999a;142:175–181. doi: 10.1007/s002130050877. [DOI] [PubMed] [Google Scholar]
- Ninan I, Kulkarni S. Effect of olanzapine on behavioral changes induced by FG 7142 and dizocilpine on active avoidance and plus maze tasks. Brain Res. 1999b;830:337–344. doi: 10.1016/s0006-8993(99)01422-5. [DOI] [PubMed] [Google Scholar]
- Ninan I, Kulkarni S. Preferential inhibition of dizocilpine-induced hyperlocomotion by olanzapine. Eur. J. Pharmacol. 1999c;368:1–7. doi: 10.1016/s0014-2999(98)00982-0. [DOI] [PubMed] [Google Scholar]
- Noda Y, Kamei H, Mamiya T, Furukawa H, Nabeshima T. Repeated phencyclidine treatment induces negative symptom-like behavior in forced swimming test in mice: imbalance of prefrontal serotonergic and dopaminergic functions. Neuropsychopharmacol. 2000;23:375–387. doi: 10.1016/S0893-133X(00)00138-X. [DOI] [PubMed] [Google Scholar]
- Procyshyn RM, Ihsan N, Thompson D. A comparison of smoking behaviors between patients treated with clozapine and depot neuroleptics. Int. Clin. Psychopharmacol. 2001;16:291–294. doi: 10.1097/00004850-200109000-00007. [DOI] [PubMed] [Google Scholar]
- Procyshyn RM, Tse G, Sin O, Flynn S. Concomitant clozapine reduces smoking in patients treated with risperidone. Eur. Neuropsychopharmacol. 2002;12:77–80. doi: 10.1016/s0924-977x(01)00130-4. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Fink-Jensen A, Sauerberg P, Swedberg MD, Thomsen C, Sheardown MJ, Jeppsen L, Calligaro DO, DeLapp NW, Whitesitt C, Ward JS, Shannon HE, Bymaster FP. The muscarinic receptor agonist BuTAC, a novel potential antipsychotic, does not impair learning and memory in mouse passive avoidance. Schizophrenia Res. 2001;49:193–201. doi: 10.1016/s0920-9964(00)00129-8. [DOI] [PubMed] [Google Scholar]
- Schoepfer R, Conroy WG, Whiting P, Gore M, Lindstrom J. Brain α-bungarotoxin binding protein cDNAs and mAbs reveal subtypes of this branch of the ligand gated ion channel superfamily. Neuron. 1990;5:35–48. doi: 10.1016/0896-6273(90)90031-a. [DOI] [PubMed] [Google Scholar]
- Shirazi-Southall S, Rodriguez DE, Nomikos GG. Effects of typical and atypical antipsychotics and receptor selective compounds on acetylcholine efflux in the hippocampus of the rat. Neuropsychopharmacol. 2002;26:583–594. doi: 10.1016/S0893-133X(01)00400-6. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Adler LE, Freedman R. Clozapine improves deficient inhibitory auditory processing in DBA/2 mice, via a nicotinic cholinergic mechanism. Psychopharmacol. 2003;165:386–396. doi: 10.1007/s00213-002-1285-x. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Kem WR, Freedman R. Intragastric DMXB-A, an a7 nicotinic agonist, improves deficient sensory inhibition in DBA/2 mice. Biol. Psychiatry. 2001;50:493–500. doi: 10.1016/s0006-3223(01)01093-9. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, Rose GM. Genetic correlation of inhibitory gating of hippocampal auditory response and a-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacol. 1996;15:152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Fuller LL, Rose GM. Dopaminergic and noradrenergic modulation of amphetamine-induced changes in auditory gating. Brain Res. 1991;555:91–98. doi: 10.1016/0006-8993(91)90864-r. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective a7 nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacol. 1998;136:320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- Stevens KR, Wear KD. Normalizing effects of nicotine and a novel nicotinic agonist on hippocampal auditory gating in two animal models. Pharmacol. Biochem. Behav. 1997;57:869–874. doi: 10.1016/s0091-3057(96)00466-2. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Vaccarino FJ, Amalric M, Koob GF. The neural substrates for the motor-activating properties of psychostimulants: a review of recent findings. Pharmacol Biochem Behav. 1986;25:233–248. doi: 10.1016/0091-3057(86)90261-3. [DOI] [PubMed] [Google Scholar]
- Waldo MC, Gerhardt GA, Baker NJ, Drebing C, Adler LE, Freedman R. Auditory sensory gating and catecholamine metabolism in schizophrenic and normal subjects. Psychiatry Res. 1992;44:21–32. doi: 10.1016/0165-1781(92)90066-c. [DOI] [PubMed] [Google Scholar]
- Willott JF, Demuth RM, Lu SM, Van Bergem P. Abnormal tonotopic organization in the ventral cochlear nucleus of the hearing-impaired DBA/2 mouse. Neurosci. Lett. 1982;34:13–17. doi: 10.1016/0304-3940(82)90085-4. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Yee CR, Nuechterlein KH, Morris SE, White PM. P50 suppression in recentonset schizophrenia: clinical correlates and risperidone effects. J. Abnorm. Psychol. 1998;107:691–698. doi: 10.1037//0021-843x.107.4.691. [DOI] [PubMed] [Google Scholar]
- Young CR, Longhurst JG, Bowers BM, Jr, Mazure CM. The expanding indications for clozapine. Exp. Clin. Psychopharmacol. 1997;5:216–234. doi: 10.1037//1064-1297.5.3.216. [DOI] [PubMed] [Google Scholar]