Abstract
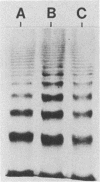
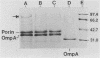
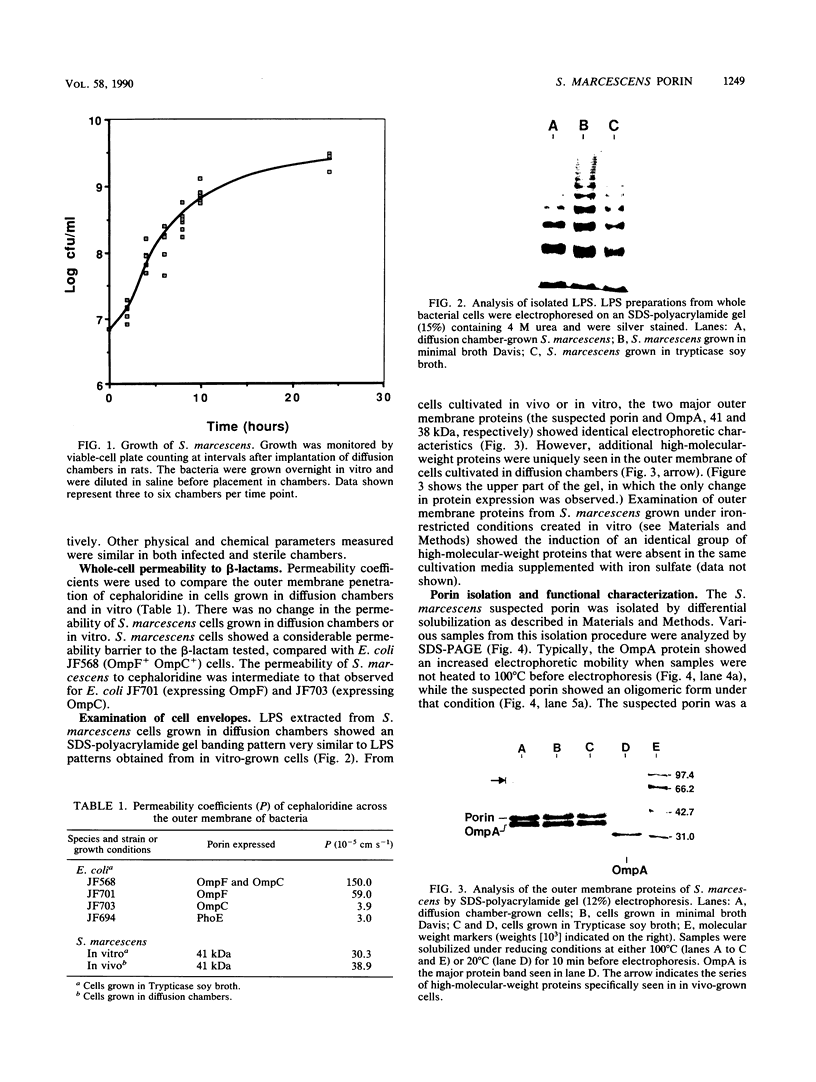
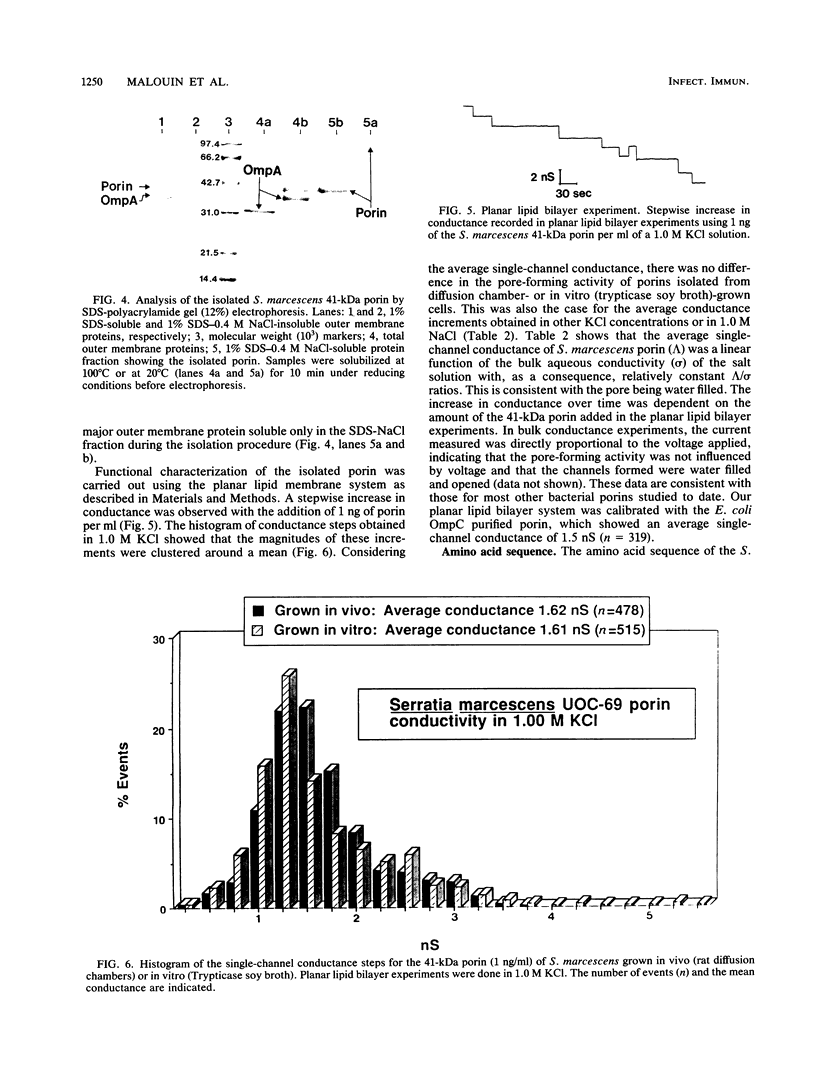
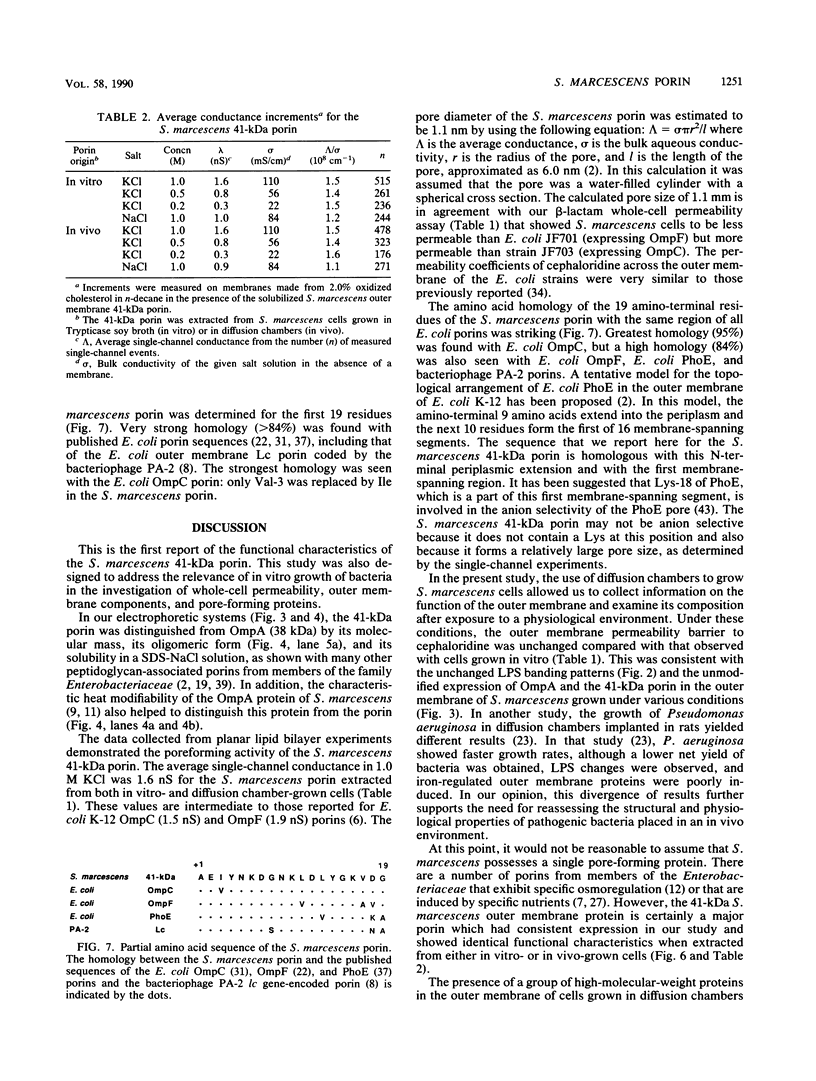
The composition and antibiotic permeability barrier of the outer membrane of Serratia marcescens were assessed in cells grown in vivo and in vitro. Intraperitoneal diffusion chambers implanted in rats were used for the in vivo cultivation of bacteria. Outer membranes isolated from log-phase bacterial cells recovered from these chambers were compared with membranes isolated from cells grown in vitro. Analysis revealed that the suspected 41-kilodalton porin and the OmpA protein were recovered on sodium dodecyl sulfate-polyacrylamide gels in equal quantities. Several high-molecular-weight proteins, thought to be iron starvation induced, appeared in the diffusion chamber-grown cells. The outer membrane permeability barriers to cephaloridine were similar in in vivo- and in vitro-grown cells based on permeability coefficient calculations. The permeability coefficient of cephaloridine in S. marcescens cells (30.3 x 10(-5) to 38.9 x 10(-5) cm s-1) was greater than that obtained for an Escherichia coli strain expressing only porin OmpC but smaller than those obtained for the E. coli wild type and a strain expressing only porin OmpF. Functional characterization of the suspected porin was performed by using the planar lipid bilayer technology. The sodium dodecyl sulfate-0.4 M NaCl-soluble porin from both in vitro- and in vivo-grown cells showed an average single-channel conductance in 1 M KCl of 1.6. A partial amino acid sequence (19 residues) was obtained for the S. marcescens porin. The sequence showed a very high homology to the E. coli OmpC porin. These data identified the S. marcescens outer membrane 41-kilodalton protein as a porin by both functional and amino acid analyses. Also, the methodology used allowed for efficient growth and recovery of diffusion chamber-grown bacterial cells and permitted identification of specific in vivo-induced changes in bacterial cell membrane composition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay R. The role of iron in infection. Med Lab Sci. 1985 Apr;42(2):166–177. [PubMed] [Google Scholar]
- Benz R., Hancock R. E. Properties of the large ion-permeable pores formed from protein F of Pseudomonas aeruginosa in lipid bilayer membranes. Biochim Biophys Acta. 1981 Aug 20;646(2):298–308. doi: 10.1016/0005-2736(81)90336-9. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Boos W., Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978 Aug 17;511(3):305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Läuger P. Ionic selectivity of pores formed by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1979 Mar 8;551(2):238–247. doi: 10.1016/0005-2736(89)90002-3. [DOI] [PubMed] [Google Scholar]
- Benz R., Schmid A., Hancock R. E. Ion selectivity of gram-negative bacterial porins. J Bacteriol. 1985 May;162(2):722–727. doi: 10.1128/jb.162.2.722-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Schmid A., Vos-Scheperkeuter G. H. Mechanism of sugar transport through the sugar-specific LamB channel of Escherichia coli outer membrane. J Membr Biol. 1987;100(1):21–29. doi: 10.1007/BF02209137. [DOI] [PubMed] [Google Scholar]
- Benz R. Structure and function of porins from gram-negative bacteria. Annu Rev Microbiol. 1988;42:359–393. doi: 10.1146/annurev.mi.42.100188.002043. [DOI] [PubMed] [Google Scholar]
- Blasband A. J., Marcotte W. R., Jr, Schnaitman C. A. Structure of the lc and nmpC outer membrane porin protein genes of lambdoid bacteriophage. J Biol Chem. 1986 Sep 25;261(27):12723–12732. [PubMed] [Google Scholar]
- Braun G., Cole S. T. DNA sequence analysis of the Serratia marcescens ompA gene: implications for the organisation of an enterobacterial outer membrane protein. Mol Gen Genet. 1984;195(1-2):321–328. doi: 10.1007/BF00332766. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Sonntag I., Henning U. Cloning and expression in Escherichia coli K-12 of the genes for major outer membrane protein OmpA from Shigella dysenteriae, Enterobacter aerogenes, and Serratia marcescens. J Bacteriol. 1982 Jan;149(1):145–150. doi: 10.1128/jb.149.1.145-150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang P., Gutmann L., Quentin C., Williamson R., Collatz E. Some properties of Serratia marcescens, Salmonella paratyphi A, and Enterobacter cloacae with non-enzyme-dependent multiple resistance to beta-lactam antibiotics, aminoglycosides, and quinolones. Rev Infect Dis. 1988 Jul-Aug;10(4):899–904. doi: 10.1093/clinids/10.4.899. [DOI] [PubMed] [Google Scholar]
- Day S. E., Vasli K. K., Russell R. J., Arbuthnott J. P. A simple method for the study in vivo of bacterial growth and accompanying host response. J Infect. 1980 Mar;2(1):39–51. doi: 10.1016/s0163-4453(80)91773-9. [DOI] [PubMed] [Google Scholar]
- Godfrey A. J., Hatlelid L., Bryan L. E. Correlation between lipopolysaccharide structure and permeability resistance in beta-lactam-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):181–186. doi: 10.1128/aac.26.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Chabbert Y. A. Different mechanisms of resistance to latamoxef (moxalactam) in Serratia marcescens. J Antimicrob Chemother. 1984 Jan;13(1):15–22. doi: 10.1093/jac/13.1.15. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Moreau N., Kitzis M. D., Collatz E., Acar J. F., Goldstein F. W. Cross-resistance to nalidixic acid, trimethoprim, and chloramphenicol associated with alterations in outer membrane proteins of Klebsiella, Enterobacter, and Serratia. J Infect Dis. 1985 Mar;151(3):501–507. doi: 10.1093/infdis/151.3.501. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Role of porins in outer membrane permeability. J Bacteriol. 1987 Mar;169(3):929–933. doi: 10.1128/jb.169.3.929-933.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi K., Mutoh N., Matsuyama S., Mizushima S. Primary structure of the ompF gene that codes for a major outer membrane protein of Escherichia coli K-12. Nucleic Acids Res. 1982 Nov 11;10(21):6957–6968. doi: 10.1093/nar/10.21.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly N. M., Bell A., Hancock R. E. Surface characteristics of Pseudomonas aeruginosa grown in a chamber implant model in mice and rats. Infect Immun. 1989 Feb;57(2):344–350. doi: 10.1128/iai.57.2.344-350.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Maier C., Bremer E., Schmid A., Benz R. Pore-forming activity of the Tsx protein from the outer membrane of Escherichia coli. Demonstration of a nucleoside-specific binding site. J Biol Chem. 1988 Feb 15;263(5):2493–2499. [PubMed] [Google Scholar]
- Markowitz S. M., Sibilla D. J. Comparative susceptibilities of clinical isolates of Serratia marcescens to newer cephalosporins, alone and in combination with various aminoglycosides. Antimicrob Agents Chemother. 1980 Nov;18(5):651–655. doi: 10.1128/aac.18.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W. Analytical isoelectric focusing of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1976 Feb;125(2):713–718. doi: 10.1128/jb.125.2.713-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983 Jun 10;258(11):6932–6940. [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olexy V. M., Bird T. J., Grieble H. G., Farrand S. K. Hospital isolates of Serratia marcescens transferring ampicillin, carbenicillin, and gentamicin resistance to other gram-negative bacteria including Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1979 Jan;15(1):93–100. doi: 10.1128/aac.15.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeeke N., Bergmans H., van Mansfeld F., Lugtenberg B. Complete nucleotide sequence of phoE, the structural gene for the phosphate limitation inducible outer membrane pore protein of Escherichia coli K12. J Mol Biol. 1983 Feb 5;163(4):513–532. doi: 10.1016/0022-2836(83)90110-9. [DOI] [PubMed] [Google Scholar]
- Tokunaga H., Tokunaga M., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 1. Chemical analysis. Eur J Biochem. 1979 Apr;95(3):433–439. doi: 10.1111/j.1432-1033.1979.tb12982.x. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Okajima Y., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 2. Physical properties of the functional oligomeric aggregates. Eur J Biochem. 1979 Apr;95(3):441–448. doi: 10.1111/j.1432-1033.1979.tb12983.x. [DOI] [PubMed] [Google Scholar]
- Traub W. H., Spohr M., Bauer D. Plasmid-independent resistance of 'gray' colony variants of a strain of Serratia marcescens resistant to amikacin, cefotaxime and lamoxactam. Chemotherapy. 1983;29(4):265–274. doi: 10.1159/000238208. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Yu V. L., Oakes C. A., Axnick K. J., Merigan T. C. Patient factors contributing to the emergence of gentamicin-resistant Serratia marcescens. Am J Med. 1979 Mar;66(3):468–472. doi: 10.1016/0002-9343(79)91074-x. [DOI] [PubMed] [Google Scholar]
- Yu V. L. Serratia marcescens: historical perspective and clinical review. N Engl J Med. 1979 Apr 19;300(16):887–893. doi: 10.1056/NEJM197904193001604. [DOI] [PubMed] [Google Scholar]
- Zimmermann L., Angerer A., Braun V. Mechanistically novel iron(III) transport system in Serratia marcescens. J Bacteriol. 1989 Jan;171(1):238–243. doi: 10.1128/jb.171.1.238-243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ley P., Burm P., Agterberg M., van Meersbergen J., Tommassen J. Analysis of structure-function relationships in Escherichia coli K12 outer membrane porins with the aid of ompC-phoE and phoE-ompC hybrid genes. Mol Gen Genet. 1987 Oct;209(3):585–591. doi: 10.1007/BF00331167. [DOI] [PubMed] [Google Scholar]