Abstract
One week oral flurazepam (FZP) administration in rats results in reduced GABAA receptor-mediated synaptic transmission in CA1 pyramidal neurons associated with benzodiazepine tolerance in vivo and in vitro. Since voltage-gated calcium channel (VGCC) current density is enhanced 2-fold during chronic FZP treatment, the role of L-type VGCCs in regulating benzodiazepine-induced changes in CA1 neuron GABAA receptor-mediated function was evaluated. Nimodipine (10 mg/kg, i.p.) or vehicle (0.5 % Tween 80, 2 ml/kg) was injected 1 day after ending FZP treatment and 24 hours prior to hippocampal slice preparation for measurement of mIPSC characteristics and in vitro tolerance to zolpidem. The reduction in GABAA receptor-mediated mIPSC amplitude and estimated unitary channel conductance measured 2 days after drug removal was no longer observed following prior nimodipine injection. However, the single nimodipine injection failed to prevent in vitro tolerance to zolpidem's ability to prolong mIPSC decay in FZP-treated neurons, suggesting multiple mechanisms may be involved in regulating GABAA receptor-mediated synaptic transmission following chronic FZP administration. As reported previously in recombinant receptors, nimodipine inhibited synaptic GABAA receptor currents only at high concentrations (>30 μM), significantly greater than attained in vivo (1 μM) 45 min after a single antagonist injection. Thus, the effects of nimodipine were unlikely to be related to direct effects on GABAA receptors. As with nimodipine injection, buffering intracellular free [Ca2+] with BAPTA similarly prevented the effects on GABAA receptor-mediated synaptic transmission, suggesting intracellular Ca2+ homeostasis is important to maintain GABAA receptor function. The findings further support a role for activation of L-type VGCCs, and perhaps other Ca2+-mediated signaling pathways, in the modulation of GABAA receptor synaptic function following chronic benzodiazepine administration, independent of modulation of the allosteric interactions between benzodiazepine and GABA binding sites.
Keywords: L-type voltage-gated calcium channels, flurazepam, tolerance, zolpidem, unitary channel conductance, BAPTA
Introduction
Benzodiazepines, widely prescribed for their potent hypnotic, anxiolytic and anticonvulsant actions, exert their therapeutic effects by potentiating fast, γ-aminobutyric acid (GABA) type A receptor-mediated inhibitory neurotransmission (Wafford, 2005). Nevertheless, prolonged benzodiazepine administration results in functional tolerance, primarily to their sedative and anticonvulsant actions (for reviews; Bateson, 2002, Wafford, 2005).
GABAA receptors, the main locus of benzodiazepine actions, have been extensively studied to elucidate the neuroadaptive process underlying benzodiazepine tolerance. Native GABAA receptors are pentameric assemblies of subunit proteins (α1-6, β1-4, γ1-3, ρ1-3, σ, π, ε and θ) with an integral Cl- channel (Wafford, 2005). BZ tolerance appears to be mediated by at least two temporally separable GABAergic mechanisms. First, there is general agreement that consistent reductions in allosteric coupling, i.e. the functional linkage between GABA and benzodiazepine binding sites, mediates an initial decrease in BZ and GABA affinity associated with BZ tolerance both in vivo and in vitro. Dependent on the drug administration model employed, additional changes in GABA receptor structure, function, and pharmacology include region-specific decreases in the number of benzodiazepine binding sites, reduced GABA agonist potency, modulation of GABAA receptor subunit mRNA and protein expression, on the whole reflected in a reduction in GABAergic synaptic inhibition (Bateson, 2002; Wafford, 2005; Gravielle et al, 2005). In hippocampus, ‘uncoupling’ induced by chronic benzodiazepine treatment is manifest by the reduced ability of the GABAA receptor α1 subunit-preferring compound, zolpidem to prolong CA1 neuron miniature postsynaptic current (mIPSC) decay (Itier et al, 1996; Zeng and Tietz, 1999, Tietz et al, 1999). Second, a delayed, but marked reduction in GABAAR function is reflected in a >50% reduction in mIPSC amplitude, a progressive decrease in channel conductance and an absence of mIPSCs in 30%-80% of recorded neurons (Poisbeau et al, 1997; Zeng and Tietz, 1999, Tietz et al, 1999).
Although the neural mechanisms underlying the development of benzodiazepine tolerance remain incomplete, new evidence indicates a role for L-type voltage-gated calcium channels (VGCCs) in mediating benzodiazepine-induced modulation of GABAA receptor function. Benzodiazepines can directly modulate VGCC-mediated Ca2+ flux (Taft and DeLorenzo, 1984; Reuveny et al, 1993; Ishizawa et al, 1997). In addition, chronic benzodiazepine administration in vivo enhances high-voltage activated Ca2+ currents in dissociated hippocampal CA1 neurons (Xiang and Tietz, 2006) and increases L-type VGCC subunit expression in vitro (Katsura et al, 2007). Moreover, raising intracellular Ca2+ or activating Ca2+/calmodulin-dependent protein kinase or phosphatases has been shown to regulate GABAA receptor-mediated Cl- currents (De Koninck and Mody, 1996; Smart, 1997; Aguayo et al, 1998; Churn and DeLorenzo, 1998; Poisbeau et al, 1999; Stelzer et al, 1998; Sanchez et al). GABA-induced GABAA receptor downregulation was suggested to be the product of transcriptional repression of GABAA receptor subunit genes dependent on activation of L-type VGCC (Lyons et al, 2001; Gravielle et al, 2005) since nifedipine inhibited both GABA-induced increases in [Ca2+]i and GABAA receptor down-regulation without effects on the allosteric coupling between benzodiazepine and GABA binding sites (Lyons et al, 2001). Regulation of L-VGCCS was also implicated in the reduction in GABA function associated with hypoxia in cortical cultures (Wang and Greenfield, in press). It is unknown whether Ca2+ signaling mechanisms downstream of L-VGCC activation underlie GABAergic system dysfunction associated with chronic benzodiazepine administration.
The role of L-type VGCCs in mediating GABAA receptor functional plasticity was explored via pharmacological antagonism of L-VGCC activity. Whole-cell hippocampal slice-patch studies were carried out to evaluate the effect of prior systemic nimodipine administration on mIPSC amplitude, conductance and kinetics and on zolpidem tolerance in vitro. To rule out direct effects of systemic nimodpine on GABAA receptor function (Das et al, 2004), nimodipine's concentration-dependent effect on GABAA receptor-mediated mIPSCs was also investigated in vitro. In some experiments, the high affinity Ca2+ chelator BAPTA was used to evaluate the effects of lowering intracellular free Ca2+ on mIPSC characteristics. The effects of an L-type VGCC antagonist on multiple measures of GABA dysfunction provide additional insights into the role of Ca2+ influx in mediating differential aspects of benzodiazepine tolerance.
Methods
Experimental protocols involving the use of vertebrate animals were approved by the University of Toledo College of Medicine (formerly the Medical University of Ohio), Institutional Animal Care and Use Committee (IACUC) and conformed to National Institutes of Health ethical guidelines.
Drug Treatments
Chronic Flurazepam Administration
FZP treatment in rats was as previously described (Zeng and Tietz, 1999). In short, following a 2 day adaptation period when rats were offered only a 0.02% saccharin vehicle, male Sprague-Dawley rats (initial age PN22-25, Harlan, Indianapolis, IN) were offered FZP (flurazepam dihydrochloride, pH 5.8) for 1 week in saccharin solution as their only source of drinking water. The concentration of FZP was adjusted daily according to each rat's body weight and fluid consumption (100 mg/kg X 3 days and 150 mg/kg X 4 days) appropriate to flurazepam's relative potency, oral bioavailability and biotransformation resulting in brain concentrations of FZP and its active metabolites of 1.2 μM, equivalent to 0.6 μM diazepam (Xie and Tietz, 1992), a range similar to other chronic benzodiazepine treatment regimens (Rosenberg et al, 1985; Gallager et al, 1985). Only rats that consumed a criterion dose of an average >120 mg/kg/day were accepted for study. Saccharin water was again provided during the 2-day withdrawal period. Unlike in humans, residual FZP and metabolites rapidly decline over the first 24 hours after drug removal and are no longer detectable in hippocampus 2 days after 1-week FZP administration (Xie and Tietz, 1992). Pair-handled control rats receive saccharin water for the same length of time. Rats were euthanized for hippocampal slice preparation on PN35-40. The experimenter was not informed the rats' treatment histories until after the data analysis were completed.
Systemic antagonist injection
Two groups of control and FZP-treated rats were given a single intraperitoneal injection of L-type VGCC antagonist, nimodipine (10 mg/kg, i.p.) or the vehicle 0.5 % Tween 80 (2 ml/kg) 1 day after ending 1-week FZP treatment and 24 hours prior to hippocampal slice preparation. The dose of nimodipine (10 mg/kg, i.p.) was chosen based on dose-response studies of a variety of dihydropyridine calcium channel antagonists to protect against ethanol withdrawal hyperexcitability. Nimodipine (10 mg/kg, i.p.) had minimal effect on locomotion, did not produce ataxia and had no effect on seizure threshold, yet reversed behavioral signs of ethanol dependence (Watson and Little, 2002). Moreover, this dose also had a similar effect to reverse the enhanced CA1 neuron glutamatergic synaptic strength (Song et al, 2007; Das et al, in press) and withdrawal-anxiety (Xiang and Tietz, 2007) during FZP withdrawal. This dose of nimodipine has minimal effect on locomotion, does not produce ataxia and has no effect on seizure threshold, yet reverses behavioral signs of ethanol dependence (Watson and Little, 2002).
Measurement of peak nimodipine brain level in vivo
In order to assess the nimodipine concentration in the rat brain as a function of time after in vivo injection, a radioreceptor assay was carried out as previously described (Xie and Tietz, 1992) to evaluate the effect of brain extracts to displace high affinity dihydropyridine (DHP) binding to whole rat brain membranes. Briefly, brain extracts were made from rats injected with nimodipine (10 mg/kg, i.p.) and euthanized 15, 30, 45, 60 120 min or 24 hr later (n=2-3 rats time-point) and compared to extracts from a vehicle-injected and non-injected saccharin-treated control rats, as well as non-injected rats euthanized immediately after 1-week FPZ treatment (n=4). Whole brains (minus brain stem) were dissected, homogenized in 4 volumes of ethanol and centrifuged at 10,000 X g for 20 min. Ethanol extracts (40 μl) were used to displace 2 nM specific [3H]PN200-110 high-affinity (PerkinElmer, Boston, MA) binding to triple-washed crude synaptosomal (P2) membranes prepared from whole rat brain minus cerebellum (∼1 mg/protein/ml, using standard techniques (Xie and Tietz, 1992) except that during the first resuspension in 50 mM Tris buffer (pH 7.7) membranes were brought to 37°C for 30 min to remove ascorbic acid (Weiland and Oswald, 1985). Tubes (0.4 ml) were incubated for 90 min at room temperature in the dark and the reaction terminated by vacuum filtration followed by 3 X 0.5 ml buffer washes on #32 glass fiber filters (Schleicher and Schuell, Keene, New Hampshire). Non-specific binding was determined in the presence of 10 μM nitrendipine. Radioactivity on filters was counted 5 min in ScintiSafe 30% (Fisher Scientific, Pittsburgh, PA). Brain extracts were compared to a standard curve generated with 14 concentrations of nimodipine ranging from 0.01 nM to 10 mM nimodipine.
Electrophysiology
Hippocampal slice preparation
Hippocampal slices (400 μm) were prepared from rats as previously described (Van Sickle et al, 2004). Briefly, transverse dorsal hippocampal slices were cut on a Vibratome (Ted Pella, Inc.) in ice-cold, pre-gassed (95%O2 /5% CO2) artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl, 120; KCl, 2.5; CaCl2, 0.5; MgSO4 7.0; NaH2PO4 1.2; NaHCO3, 2; D-glucose, 20; ascorbate, 1.3, pH 7.4. Slices were maintained at room temperature (RT) for 15 min in gassed, low-calcium, high-magnesium ACSF, then transferred to normal ACSF containing (in mM): NaCl, 119; KCl, 2.5; CaCl2, 1.8; MgSO4 1.3; NaH2PO4 1.25; NaHCO3, 26; D-glucose, 10; pH 7.4. Slices were maintained at room temperature for ≥ 1 hr in ACSF. During recording, slices were superfused at a rate of 2.5 ml/min with gassed ACSF at room temperature.
GABAA receptor-mediated mIPSC recording
GABAA receptor-mediated mIPSCs were isolated from CA1 pyramidal neurons in the presence of 1 μM tetrodotoxin (TTX), 10 μM DNQX (6,7-dinitroquinoxaline-2,3-done) and 50 μM APV (DL-2-amino-5 phosphonovaleric acid) with or without 1 μM zolpidem. Patch pipettes for mIPSC recording were filled with (in mM): CsCl, 130; HEPES, 10; Ethylene glycol-bis(β-aminoethylether) N,N,N′,N′-tetraacetic acid (EGTA), 1; CaCl2, 0.5; MgCl2, 1; Mg-ATP, 2; QX-314, 2; pH 7.2 adjusted with CsOH. Cells were Cl- -loaded to minimize the possible contribution of intracellular Cl- accumulation to the reduction of GABA-mediated inhibition suggest by a shift in the EIPSC in previous experiments in FZP-treated neurons. Chloride-loading was demonstrated to reverse the use-dependent shift in ECl- due to prolonged GABA activation (Zeng and Tietz, 1999). QX-314 (2 mM), an intracellular sodium channel blocker, was also included to block the spontaneous firing of CA1 pyramidal neurons. Resting membrane potential (RMP) was measured immediately upon cell break-in. Neurons were voltage-clamped (VH = -70 mV) in continuous mode (cSEVC) using an Axoclamp 2A amplifier (Axon Instr., Union City, CA). Current output was low-pass filtered (10 kHz), DC-offset, amplified 10,000-fold and continuously monitored on-line (pCLAMP 8.0, Axon). The digitized signal (Digidata 1200A, Axon) was stored on disk for later off-line analysis. Cells in which the holding current changed by more than 20% or the seal degraded, were abandoned. mIPSC activity was recorded 5 min and analyzed with MiniAnalysis software (Synaptosoft Inc., Leonia, NJ). Peak mIPSC amplitude was measured from baseline. Decay kinetics and mIPSC amplitude were estimated using a single exponential function: [y(t)=a*exp(-t/τ)]. Whole-cell data were compared by repeated measures ANOVA with post-hoc analysis by the method of Scheffé.
Non-stationary fluctuation analysis
Non-stationary fluctuation analysis (NSFA) was used to extract single channel current information from macroscopic current decay. NSFA can be affected by passive electrophysiological parameters such as membrane capacitance, membrane resistance and access resistance, which in turn can be affected by electrode current compensation circuits. In addition estimates of unitary conductance can be affected by circuit noise and analysis parameters such as event selection bias, bin width, and quality of fit, yet NSFA can still provide an accurate estimate of changes in single-channel conductance at intact synapses (Ghavanini et al., 2006, Benke et al., 2001). mIPSC events from FZP-treated and control cells following vehicle or nimodipine injection were also analyzed using peak-scaled NSFA using MiniAnalysis software. The average of 100-150 mIPSCs was scaled to each individual event before computing the variance. Data were fitted with the equation σ2=iI-I2/N, where σ2 was the variance, I was the mean current, N was the number of channels activated at the peak of the mean current and, i was the single-channel current (Zeng and Tietz, 1999). Unitary channel conductance (γ) was derived from γ=i/V, where V was the driving force (VH = -70 mV, EREV = 0 mV).
Zolpidem effects on mIPSC amplitude and decay
In a subset of FZP-treated and control cells, mIPSC activity was recorded for 8 min in the presence of 1μM zolpidem after the 5 min baseline recording. The final 3 min segment in the presence of zolpidem was used for off-line analysis of mIPSC amplitude and decay kinetics. mIPSC decay phase was fitted with a single exponential function y(t)=a*exp(-t/τ). It was previously shown in a lager number of CA1 neurons that the proportion of control (65%) and FZP-treated (62%) neurons best fit with a mono-exponential versus bi-exponential decay were similar (Zeng and Tietz, 1999). The degree of zolpidem potentiation of mIPSC decay was expressed as a fraction of the control response.
Nimodipine concentration-response effects on mIPSC characteristics
To determine whether nimodipine had direct effects on GABAR-mediated function, nimodipine (0.1-100 μM) was superfused onto hippocampal slices during mIPSC recording. After recording baseline mIPSC activity for 5 min, nimodipine or vehicle (0.0001% to 0.1% DMSO in water) was added to the superfusate in increasing concentrations for 10 min each. mIPSC amplitude and kinetics were analyzed using the final 5 min recording period at each concentration.
High-affinity calcium buffering
In some experiments the effect of lowering intracellular calcium concentration on GABAA receptor channel function was evaluated by replacing 1 mM EGTA in the patch pipette recording solution with 10 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N- tetraacetic acid (BAPTA), a calcium chelator with a high buffering capacity. Following cell dialysis, free intracellular [Ca2+] contributed by the EGTA pipette solution was ∼190 nM vs. 15 nM in BAPTA-containing pipette solution (Webmaxc Standard, http://www.stanford.edu/∼cpatton/webmaxc/webmaxcS.html) Both BAPTA and EGTA were introduced into the pyramidal neurons by diffusion for at least 15 min before data collection.
Drug solutions
Drugs used for superfusion during whole-cell recording were dissolved at 100 times their final concentration and added to the superfusate with a syringe pump (Razel, World Precision Instruments, Inc., Sarasota, FL) at a rate of 25 to 75 μl/min to achieve their final concentrations. For in vivo injection, nimodipine was dissolved in 0.5 % Tween-80 solution and kept in a light-tight vial. For in vitro perfusion, nimodipine was dissolved in DMSO to make a 10 mM stock solution diluted to the final concentration as needed (from 0.1 to 100 μM). All other drugs were dissolved in dH2O. DNQX (6,7-dinitroquinoxaline-2,3-done), QX-314 (lidocaine N-ethyl bromide quaternary salt), APV (DL-2-amino-5 phosphonovaleric acid), FZP dihydrochloride, and nimodipine are all from Sigma-Aldrich Chemical Co. (St Louis, MO). Tetrodotoxin (TTX) was obtained from Alamone Laboratories (Jerusalem, Israel). Zolpidem was kindly provided by Synthélabo Recherche (Bagneux, France).
Results
Prior nimodipine injection prevents the decrease in mIPSC amplitude in CA1 neurons from FZP-treated rats
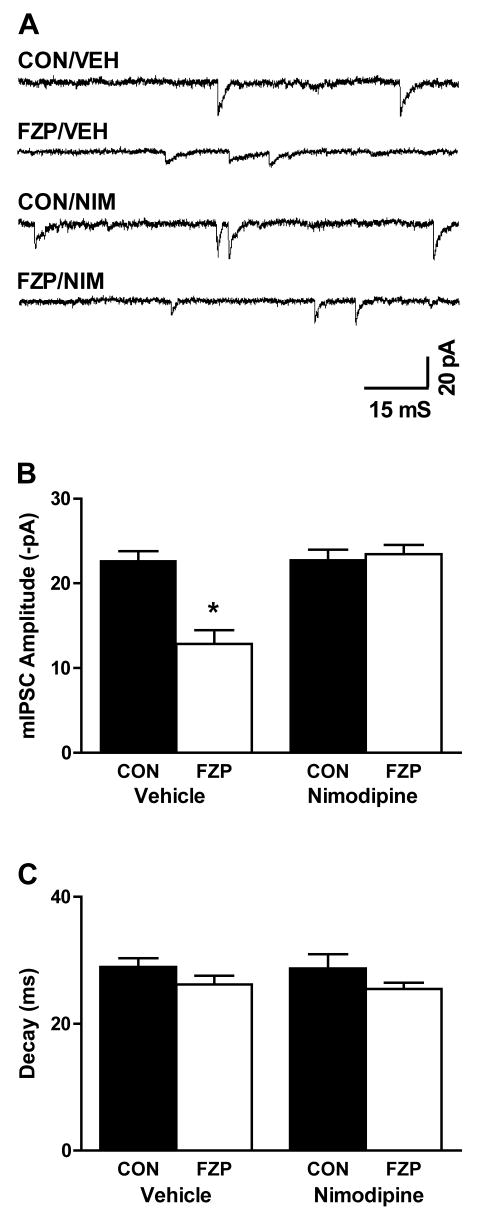
Since inhibition of L-type VGCCs can modulate GABAA receptor downregulation after prolonged GABA exposure (Lyons et al, 2001; Gravielle et al, 2005) and L-VGCC current density is doubled after chronic FZP administration (Xiang and Tietz, 2006), the potential role of L-type VGCC antagonists to modulate GABAA receptor dysfunction following chronic benzodiazepine administration was investigated. Following 1-week FZP administration, rats were injected systemically with the L-type VGCC antagonist nimodipine (10 mg/kg, i.p.) or vehicle (0.5% Tween-80, 2 ml/kg, i.p.) one day before hippocampal slice preparation. One day later, hippocampal slices were prepared from 2-day FZP-withdrawn rats. GABAA receptor-mediated mIPSCs were recorded in CA1 neurons in the presence of 1μM TTX, 10μM DNQX and 50 μM APV at a holding potential of -70 mV (Fig. 1A and Table 1). As previously reported (Poisbeau et al, 1997; Zeng and Tietz, 1999), there was a significant decrease in mIPSC amplitude (43.0%, p<0.05) in CA1 neurons from 2-day FZP-withdrawn rats (FZP/VEH: 12.9 ±1.6 pA, n=8) in comparison to those isolated from control rats (CON/VEH: 22.7 ± 1.1 pA, n=9). Prior vehicle injection had no effect on basal GABAA receptor mIPSC amplitude in control neurons or the decrease in mIPSC amplitude in 2-day FZP-withdrawn rats. However, following prior nimodipine injection the mean GABAA receptor mIPSC amplitude returned to control levels in neurons from 2-day FZP-withdrawn rats (Fig. 1B and Table 1, CON/NIM: 22.8 ± 1.2 pA, n=8; FZP/NIM: 23.5 ± 1.1, n=9; p>0.05). Neither vehicle nor nimodipine injection had an effect on mIPSC decay (Fig. 1C and Table 1). As reported previously (Poisbeau et al, 1997; Zeng and Tietz, 1999), there were also no significant differences (p>0.05) in resting membrane potential, mIPSC frequency or rise time in neurons from control vs. FZP-treated rats.
Fig 1. Prior nimodipine injection prevents the reduction in GABAAR-mediated mIPSCs in CA1 neurons in hippocampal slices from FZP-treated rats.
Rats were injected systemically with the L-type VGCC antagonist nimodipine (10 mg/kg, i.p.) or vehicle (0.5% Tween-80, 2 ml/kg, i.p.) 24 h before hippocampal slice preparation. 1 day later hippocampal slices were prepared from 2-day FZP-withdrawn rats followed by electrophysiological recording. A) Representative mIPSC current traces isolated from FZP-treated (FZP) and control neurons (CON) injected with nimpodipine (NIM) or vehicle (VEH). B) Average amplitudes of GABAA receptor-mediated mIPSCs (VH = -70 mV) in CA1 neurons isolated from control rats (solid bars) and FZP-treated rats (open bars) were compared following either vehicle or nimodipine injection. There was a significant decrease in mIPSC amplitude (43.0%, *p<0.05) in CA1 neurons from FZP-treated rats (n=8) in comparison to those in control neurons (n=9) without any changes in resting membrane potential, mIPSC frequency, rise-time or decay. For comparisons of decay, peak-scaled mIPSCs in the absence of zolpidem are shown in Fig. 3A. As shown in Table 1, there was no difference in mIPSC amplitude in CA1 neurons from FZP-treated rats (p>0.05, n=7) given a systemic nimodipine injection 24 hr prior to recording, compared to those from controls (n=8) and C) There were no differences in mIPSC decay in control (solid bars) or FZP-treated (open bars) CA1 neurons following either vehicle or nimodipine injection.
Table 1. Effects of systemic nimodipine on CA1 pyramidal neuron mIPSC characteristics with and without zolpidem.
| Group | RMP
(mV) |
mIPSC Characteristics | Group | Zolpidem Potentiation | ||||
|---|---|---|---|---|---|---|---|---|
| Frequency
(Hz) |
Rise Time
(ms) |
Amplitude
(-pA) |
τ
(ms) |
Amplitude
(%pA) |
τ
(%ms) |
|||
| CON-VEH
(n=9) |
-62.8 ± 1.1 | 0.8 ± 0.1 | 2.1 ± 0.2 | 22.7±1.1
*p=0.00 vs. CON-NIM |
29.0 ±1.4
p=1.00 vs. CON-NIM |
CON-VEH
(n=6) |
106.3±3.1
p=0.11 |
131.0 ± 5.4
*p=0.01 |
| FZP-VEH
(n=8) |
-62.4 ±0.8 | 0.5 ± 0.1 | 2.5 ± 0.2 | 12.9 ± 1.6
*p=0.00 vs. CON-VEH *p=0.00 vs. FZP-NIM |
26.2 ±1.4
p=0.67 vs. CON-VEH p=0.99 vs. FZP-NIM |
FZP-VEH
(n=7) |
105.1±2.7
p=0.52 |
104.0 ± 3.9
p=0.73 |
| CON-NIM
(n=8) |
-62.8 ± 1.2 | 0.6 ± 0.1 | 2.2 ± 0.5 | 22.8 ± 1.2 | 28.7±2.2 | CON-NIM
(n=6) |
110.4±6.4
p=0.38 |
130.2 ±3.0
*p=0.04 |
| FZP-NIM
(n=7) |
-62.3 ± 1.2 | 0.6 ± 0.1 | 2.1 ± 0.4 | 23.5 ± 1.1
p=0.98 vs. CON-NIM |
25.5±1.1
p=0.59 vs. CON-NIM |
FZP-NIM
(n=7) |
107.9±5.8
p=0.20 |
101.37±2.7
p=0.91 |
| ANOVA | P>0.05 | P>0.05 | P>0.05 | *p<0.001 | P>0.05 | p>0.05 | *p<0.001 | |
Values are means ± S.E.M; VEH: i.p. injection of vehicle; NIM: i.p. injection of nimodipine. RMP = resting membrane potential, p values indicated within rows represent post-hoc comparisons between injection groups by Scheffé. Zolpidem potentiation (%) is expressed as a percentage change in peak amplitude or decay in the presence vs. absence of zolpidem.
Effects of nimodipine injection on peak-scaled non-stationary variance analysis of mIPSCs recorded in CA1 neurons in hippocampal slices from FZP-treated rats
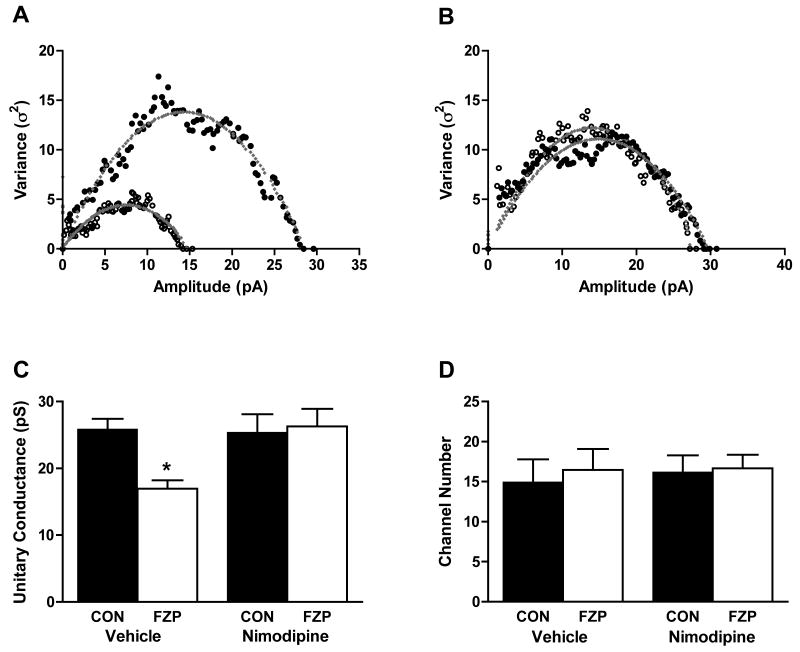
Previous studies have demonstrated a decrease in GABAA receptor single channel conductance, but not channel number, after chronic flurazepam treatment (Poisbeau et al, 1997; Zeng and Tietz, 1999). Therefore, the effect of systemic vehicle or nimodipine injection on GABAA receptor unitary channel conductance was analyzed using non-stationary fluctuation analysis (NSFA) in CA1 neurons from control (Fig. 2A) or FZP-treated rats (Fig. 2B).
Fig. 2. Prior nimodipine injection prevents the reduction in unitary conductance estimated by peak-scaled non-stationary variance analysis from CA1 neuron mIPSCs from FZP-treated rats.
Representative amplitude-variance plots following peak-scaled non-stationary variance analysis of mIPSCs in CA1 neurons from control or FZP-treated rats after systemic vehicle A) or nimodipine B) injection. Data were fitted with the equation σ2=iI-I2/N, where σ2 is the variance, I is the mean current, N is the number of channels activated at the peak of the mean current, i is the single-channel current. C) Prior nimodipine injection prevents the decrease in GABAA receptor single channel conductance in CA1 neurons in hippocampal slices from FZP-treated rats. Unitary channel conductance (γ) was derived from γ=i/V, where V is the driving force (VH = -70 mV, EREV = 0 mV). There was a significant decrease in GABAA receptor single channel conductance (∼38.0%, *p<0.05) in CA1 neurons from FZP-treated rats (n=9) in comparison to those recorded control slices (n=8). However, there was no difference in GABAA receptor single channel conductance in CA1 neurons from FZP-treated rats (p>0.05, n=7) given a systemic nimodipine injection 24 hr prior to recording, compared to those isolated from the matched control group (n=8). D) There was no significant difference in the mean channel number (N) between control and FZP-treated neurons. Nimodipine injection had no effect on channel number in either group (p>0.05).
As shown in Fig. 2C, there was a significant (∼38.0%) decrease GABAA receptor unitary channel conductance in CA1 neurons in hippocampal slices from 2-day FZP-withdrawn rats compared to control rats (CON-VEH: 25.9 ± 1.6 pS, n=9; FZP-VEH: 17.0 ± 1.3 pS, n=8, p< 0.05). Prior nimodipine injection returned the decrease in GABAA receptor unitary channel conductance to basal levels (CON-NIM: 25.4 ± 2.7 pS, n=8; FZP-NIM: 26.3 ± 2.6 pS, n=7, p>0.05). As shown in Fig. 2D, there was no significant difference in the mean channel number (N) between control (14.9 ± 2.9, n=9) and FZP-treated neurons (16.5 ± 2.6, n=8, p>0.05). Prior nimodipine injection had no effect on channel number (CON-NIM: 16.2 ± 2.1, n=8; FZP-NIM: 16.6 ± 1.7, n=7, p>0.05) or channel open probability in either group.
Prior nimodipine injection failed to prevent tolerance to zolpidem's ability to prolong decay of mIPSCs in CA1 neurons from FZP-treated rats
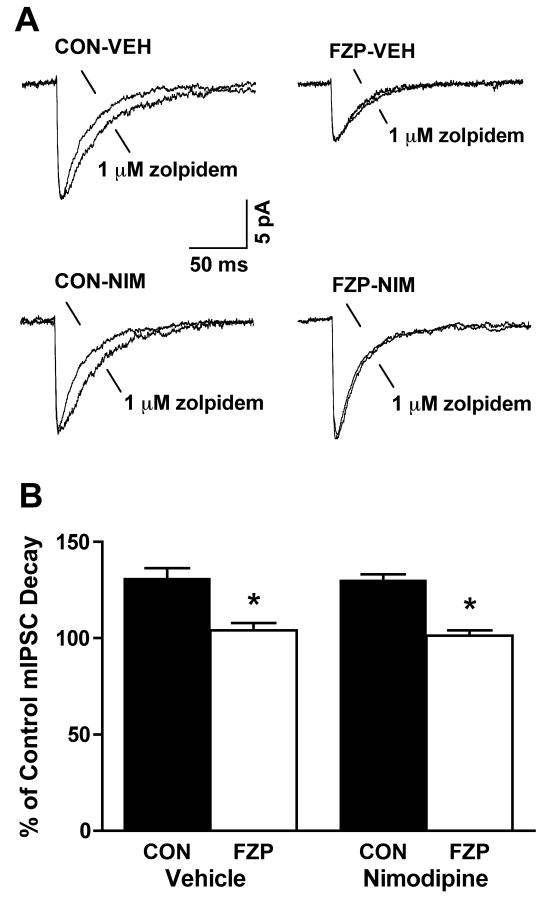
One way in which BZ tolerance can be assessed in vitro is by the shift in zolpidem's ability to prolong the decay of mIPSCs recorded in hippocampal CA1 neurons (Perrais and Ropert, 1999; Zeng and Tietz, 1999; Tietz et al, 1999). To investigate the role of L-type VGCC in mediating BZ tolerance in vitro, the ability of 1 μM zolpidem to modify mIPSC amplitude and decay was evaluated in CA1 neurons from control and FZP-treated rats injected with vehicle or nimodipine, 1 day before recording. Effects of zolpidem to prolong decay were expressed as the percentage increase in decay in the presence of zolpidem vs. the absence of zolpidem. As seen in Fig. 3 and Table 1, zolpidem tolerance was indicated by a significant reduction in the ability of zolpidem to enhance mIPSC decay in FZP-VEH neurons (104.0 ± 3.9%, p>0.05, n=7) in comparison to CON-VEH neurons (131.0 ± 5.4%, p<0.05, n=6). Prior systemic nimodipine injection failed to prevent zolpidem tolerance in FZP-NIM neurons (101.4 ± 2.7%, p>0.05, n=7) in comparison to CON-NIM neurons (130.2 ± 3.03%, p<0.05, n=6). The absence of an effect of nimodipine on in vitro tolerance to zolpidem in contrast to its effects to prevent the reduction in GABA mIPSC amplitude suggests that L-type VGCC-mediated Ca2+ influx might mediate some, but not other measures of GABA dysfunction in rats chronically administered benzodiazepines. As expected from previous recordings carried out at room temperature (Perrais and Ropert, 1999; Zeng and Tietz, 1999) 1 μM zolpidem also increased the amplitude of mIPSCs in control and FZP-treated cells from rats injected with both vehicle and nimodipine (CON-VEH: 106.3 ± 3.1%, n=7; FZP-VEH: 105.1 ± 2.7%, n=7; CON-NIM: 110.4 ± 6.4%, N=6; FZP-NIM: 107.9 ± 0.2%, N=7, p>0.05).
Fig 3. Prior nimodipine injection failed to prevent zolpidem's ability to prolong mIPSC decay in CA1 neurons from FZP-treated rats.
A) Representative mIPSC traces, averaged from 130 to 170 miniature events, before and after superfusion of 1 μM zolpidem. CA1 neurons were recorded from control (CON) and FZP-treated (FZP) rats systemically injected with vehicle or nimodipine 24 h before recording. The peak amplitude of averaged mIPSCs after zolpidem superfusion was normalized to the peak amplitude of averaged mIPSCs before zolpidem application for comparison. B) Effects of 1 μM zolpidem to prolong mIPSC decay in CA1 neurons was expressed as a percentage of the baseline average mIPSC decay recorded in the absence of zolpidem. Zolpidem tolerance was measured as a significant reduction in the ability of zolpidem to enhance mIPSC decay in FZP-VEH neurons (* p<0.05, n=7) in comparison to CON-VEH neurons (n=6). Prior systemic nimodipine injection failed to prevent zolpidem tolerance in FZP-NIM neurons (* p<0.05, n=7) in comparison to CON-NIM neurons (n=6), as shown in Table 1.
Nimodipine concentration in vivo is insufficient to modify GABA function in vitro
DHP antagonists were reported to inhibit recombinant GABAA receptor mediated currents in HEK293-T cells in the micromolar range (Das et al, 2004). To exclude the possibility that systemic nimodipine injection would directly interrupt GABAA receptor function, nimodipine brain levels in vivo were first estimated by radioreceptor assay. The effects of nimodipine on native GABAA receptors was then evaluated by its concentration-dependent profile to inhibit mIPSCs in hippocampal slices in vitro
Nimodipine peak concentration in brain (1.1 μM) was achieved 45 min after a single injection (10 mg/kg, i.p.), equivalent to 60.1% inhibition of 2 nM [3H]PN200-110 binding. Nimodipine levels were negligible (2.2% inhibition) within 2 hrs and somewhat lower than in extracts from rats 24 hr after nimodipine injection (8.6%±6.3) when rat hippocampal slices were prepared for mIPSC recording. The latter levels were not significantly different (F = 0.25, df = 2, p = 0.78) from vehicle or non-injected control rats (10.2±9.4%) or non-injected 0 hr FZP-withdrawn rats. (8.0±2.7%). This degree of dihydropyridine-like inhibition may reflect the level of ascorbic acid in rat brain at the time of euthanasia (Weiland and Oswald, 1985).
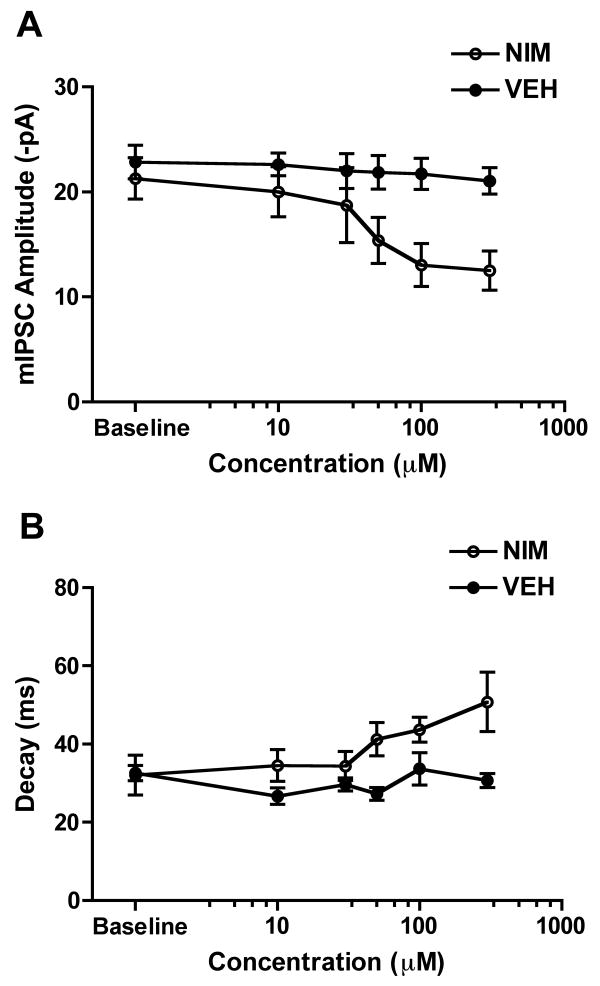
To evaluate the concentration-response profile of nimodipine, mIPSCs were recorded for 5 min in the absence of nimodipine during the baseline recording period, then in the presence of increasing concentrations ranging from 10 μM to 300 μM. As shown in Fig. 4A and consistent with studies in recombinant GABAA receptors (Das et al, 2004), mIPSC amplitude (VH = -70 mV) was decreased in a concentration-dependent manner during nimodipine superfusion (n=4) at concentrations greater than 30 μM in comparison to neurons superfused with vehicle (n=3). At the highest nimodipine concentration tested (300 μM) mIPSC amplitude was inhibited ∼40% in comparison to vehicle (VEH: 21.0 ± 1.3 pA; NIM: 12.5 ± 1.9 pA, p<0.05). There were no significant changes (p>0.05) in mIPSC resting membrane potential (VEH: -62.0 ± 1.1 mV; NIM: -64.7 ± 2.3 mV); rise time (VEH: 1.7 ± 0.2 ms; NIM: 1.5 ± 0.2 ms), or frequency (VEH: 0.7 ± 0.1 Hz, NIM: 0.7 ± 0.1 Hz) during nimodipine superfusion. Nimodipine also produced a concentration-dependent prolongation of mIPSC decay at concentrations greater than 30 μM. At 300 μM nimodipine, the highest concentration tested, there was a ∼58% prolongation of mIPSC decay (VEH: 32.0 ± 5.1 ms; 300 μM NIM: 50.8 ± 7.6 ms, p<0.05), while vehicle had no effects on mIPSC decay (VEH: 32.6 ± 2.0 ms; 300 μM VEH: 30.7 ± 1.8 ms, p>0.05). Since peak brain levels of nimodipine in vivo are in the low micromolar range (1.1 μM), far lower than the concentration that appeared to affect mIPSCs characteristics (> 30 μM) in vitro it is highly unlikely that nimodipine injection directly interrupts GABAA receptor-mediated function in vivo.
Fig 4. Effects of in vitro nimodipine superfusion on GABAA receptor-mediated mIPSCs.
Mean concentration-response profile for the effect of nimodipine on CA1 neuron GABAA receptor-mediated mIPSCs. A) Average GABAA receptor mIPSC amplitude (VH = -70 mV) during vehicle (close circles) or nimodipine (open circles) superfusion of hippocampal slices. Baseline mIPSC amplitude was recorded for 5 min without nimodipine. In comparison to vehicle superfusion (n=3), nimodipine decreased mIPSC amplitude in concentration-dependent superfusion (n=4) at concentrations ranging from 10 μM to 300 μM. There were no changes in mIPSC frequency or rise time during nimodipine perfusion. B) Nimodipine also produced a concentration-dependent prolongation of mIPSC decay at concentrations higher than 50 μM.
Effect of lowering [Ca2+]i on mIPSC characteristics
The potential Ca2+-dependence of the FZP treatment-induced modulation of mIPSC characteristics was assessed by varying the intracellular Ca2+ buffering conditions. Lowering intracellular free Ca2+ with BAPTA, had no significant effect on basal GABAA receptor mIPSC amplitude or decay in control neurons (Fig 5A. CON: 19.4 ± 3.1 pA; n=5, p>0.05) compared with use of EGTA-containing solution (Fig 1B and table 1). In contrast to the decreased mIPSC amplitude in CA1 neurons from 2-day FZP-withdrawn rats recorded using EGTA (Fig 1B and Table 1), a reduction in mIPSC amplitude was no longer observed using BAPTA (Fig 5A. FZP: 20.4 ± 2.6 pA, n=5, p>0.05). mIPSC decay was unaffected in either experimental group at either low (CON: 30.0 ± 2.3 ms, n=5; FZP: 28.2 ± 2.1 ms, n=5. p>0.05) or normal free [Ca2+]i (Fig 1B and Table 1). When compared between experimental groups or to neurons recorded with EGTA, increasing the Ca2+ buffering capacity also had no effect on resting membrane potential (CON: -66.4 ± 2.7 mV, FZP: -62.2 ±0.8 mV; CON/NIM: -62.8 ± 2.0 mV. p>0.05), rise time (CON: 2.2 ± 0.3 ms; FZP: 2.1 ± 0.2 ms. p>0.05) or mIPSC frequency (CON: 0.6 ± 0.2 Hz; FZP: 0.8 ± 0.2 Hz. p>0.05).
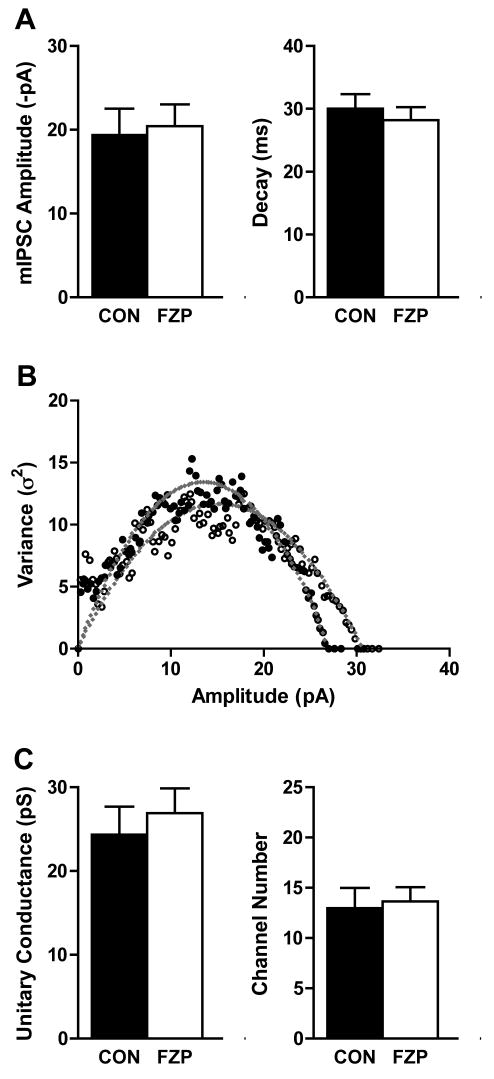
Fig 5. Effect of BAPTA on mIPSC characteristics in CA1 neurons from FZP-treated rats.
BAPTA (10mM) replaced EGTA (1 mM) in the pipette solution during mIPSC recordings to clamp free [Ca2+]ii at a >10-fold lower concentration. A) Average amplitudes of GABAA receptor-mediated mIPSCs (VH = -70 mV) in CA1 neurons isolated from control rats (solid bars) and 2-day FZP-treated rats (open bars) recorded in the BAPTA-containing pipette solution. There was a significant decrease in mIPSC amplitude (43.0%, *p<0.05) in CA1 neurons from 2-day FZP-treated rats (n=8) in comparison to those isolated from control rats (n=9). However, there was no difference in mIPSC amplitude in CA1 neurons from FZP treated rats (p>0.05, n=5) in BAPTA recording solution, compared to those isolated controls (n=5), as shown in Table 1. Likewise, there were no differences in mIPSCs decay in CA1 neurons isolated from control rats (solid bars) versus FZP-treated rats (open bars) using BAPTA-containing pipette solution. B) Representative amplitude-variance plots following peak-scaled non-stationary variance analysis of mIPSCs in CA1 neurons from control or FZP-withdrawn rats. C) There was no difference in GABAA receptor unitary channel conductance in CA1 neurons from FZP-withdrawn rats (p>0.05, n=5) when intracellular Ca2+ was buffered with BAPTA, compared to neurons isolated from the matched control group (n=5) suggesting intracellular Ca2+ homeostasis is important to maintain GABAA receptor function. There were also no significant differences in the mean channel number (N) between control and FZP-treated neurons.
The effects of lowering [Ca2+]i with BAPTA on GABAA receptor unitary channel conductance in CA1 neurons from control and FZP-withdrawn rats was also analyzed. Use of BAPTA had no effect on estimated channel conductance in control neurons (Fig 5D. CON: 24.3 ± 3.4 pS, n=5) compared to EGTA (Fig 2C and Table 1). However, no reduction in channel conductance was observed in CA1 neurons from 2-day FZP-withdrawn rats when recorded using BAPTA (Fig 5D. FZP: 26.9 ± 3.0 pS, n=5. p>0.05), in contrast to decreased conductance observed with EGTA solution (Fig 1B and Table 1). As with EGTA (Fig 3D and Table 1), there was no difference in the mean channel number (N) between control (13.0 ± 2.0, n=5) and FZP-withdrawn neurons (13.6 ± 1.4, n=5, p>0.05) when buffering with BAPTA.
Discussion
Both acute and prolonged benzodiazepine administration can modulate L-VGCC-mediated Ca2+ influx (Taft and DeLorenzo, 1984; Reuveny et al, 1993; Ishizawa et al, 1997; Xiang and Tietz, 2006, Katsura et al, 2007). In vitro exposure of cortical cultures to diazepam resulted in [45Ca2+] influx through L-VGCCs accompanied by an increase in L-VGCC subunit protein levels (Katsura et al, 2007). Notably, the density of high-voltage activated calcium currents more than doubled immediately and 2 days after ending FZP treatment in vivo (Xiang et al, 2006 and unpublished observations). The goal of this study was to explore whether activation of L-type VGCCs may play a role in mediating the reductions in GABAA receptor function associated with benzodiazepine chronic treatment and anticonvulsant tolerance in vivo (Poisbeau et al, 1997; Zeng and Tietz, 1999; Tietz et al, 1999). The observation that systemic nimodipine injection prevented the reduction in hippocampal CA1 neuron GABAA receptor-mediated synaptic current amplitude and estimated unitary conductance (Figs. 1 and 2) suggests that activation of L-type VGCCs may contribute to benzodiazepine-induced regulation of GABAA receptor dysfunction.
The acute effect of benzodiazepine site ligands (Itier et al, 1996; Zeng and Tietz, 1999; Tietz et al, 1999) to prolong CA1 neuron mIPSC decay is attributable to an increase in apparent GABA affinity related to a change in the conformation of the GABAA receptor to prolong current deactivation (Lavoie and Twyman, 1996; Bianchi et al, 2007). Zolpidem (30 nM – 100 μM), an α1 subunit-preferring ligand, prolongs mIPSC decay in a concentration-dependent manner, an effect significantly reduced in 1-week FZP treated neurons (Zeng and Tietz, 1999). Conversly, Ca2+ concentrations from 200-400 nM prolong mIPSC decay (DeKoninck and Mody, 1996) and micromolar concentrations of intracellular free Ca2+ can enhance GABA affinity in a calmodulin-dependent fashion (Majewska and Chuang, 1984). The failure of nimodipine injection to affect in vitro tolerance to zolpidem (1 μM) in the same CA1 neurons (Fig. 3 and Table 1) suggests that separable mechanisms underlie the dual chronic benzodiazepine-induced effects on GABAA receptor function observed. A similar dissociation was shown following persistent exposure of cortical neurons to GABA in vitro (Gravielle et al., 2005), whereas both post-hypoxic downregulation of GABA currents and the associated increase in zolpidem potentiation (Wang and Greenfield, in press) could be modulated by nifedipine preincubation. Importantly, systemic nimodipine effects were distinct from the systemic effects of the benzodiazepine antagonist, flumazenil, which prevented both the reduction in CA1 neuron mIPSC amplitude and in vitro zolpidem (1 μM) tolerance (Tietz et al, 1999). This suggests that nimodipine antagonism of GABAA receptor-mediated function does not occur via the benzodiazepine binding site, but more likely through interruption of L-type VGCC activation.
DHP antagonists block L-type Ca2+ currents in neuronal membranes with low nanomolar affinity, whereas a variety of non-L-type calcium channels and ionotropic receptors including recombinant α1β2γ2 GABAA receptors, the dominant hippocampal subtype, are inhibited in the micromolar to millimolar range (Das et al, 2004). An equivalent concentration of nimodipine (>30 μM, Fig. 4) was required to directly inhibit native hippocampal CA1 neuron GABAA receptor synaptic currents (Fig. 4). Therefore, CA1 GABAA receptors were not likely directly affected by residual nimodipine since the concentration of nimodipine and its metabolites reached a maximal concentration (∼1 μM) in brain 45 min after systemic injection and was not present during electrophysiological recordings, consistent with pharmacokinetic studies (Maruhn et al, 1985). Furthermore, since L-type VGCC Ca2+ channel density and nimodipine affinity are significantly higher on neurons than on cerebral vasculature (nM vs. μM to mM) (Hell et al, 1993; Ricci et al, 2002) interruption of a neuronal L-type VGCC mediated-signaling pathway during the benzodiazepine withdrawal period is the most reasonable explanation for nimodipine's effects to avert benzodiazepine-induced GABAA receptor dysfunction.
Calcium acts as a second messenger for a variety of cellular processes regulating a range of voltage-dependent and -independent conductances, including the GABAA receptor (Smart, 1997; Stelzer et al, 1998). Buffering [Ca2+]i to <10-8 M is necessary to maintain GABAA receptor currents in CA1 neurons, probably related to activation of the Ca2+/calmodulin-dependent phosphatase, calcineurin, whereas activation of kinases such as protein kinase C (PKC) increased GABA currents or had no effect (Houston and Smart, 1996; Poisbeau et al, 1999; Sanchez et al, 2005; Smart, 1997). Conversely, Ca2+/calmodulin-dependent kinase (CaMKII) potentiated GABA currents in mouse cortical neurons and in α1β3γ2 recombinant GABAA receptors (Aguayo et al, 1998; Houston et al, 2006). In the present study lowering Ca2+ levels greater than 10-fold (to <15 nM) by buffering with BAPTA had no effect on basal mIPSC characteristics (Fig 5A and B), yet prevented the benzodiazepine treatment-induced reduction of mIPSC amplitude and conductance, providing additional evidence that some aspects of GABAA receptor channel function are dependent on intracellular Ca2+ homeostasis (Smart, 1997; Stelzer et al, 1998).
While a link has not been established between the systemic actions of the L-VGCC antagonist, nimodipine, and the intracellular effects of the high affinity Ca2+ chelator, BAPTA, the rapidity of the effects of BAPTA suggest that GABAergic synaptic transmission may, at least in part be mediated by Ca2+-dependent phosphorylation/dephosphorylation processes. This hypothesis is supported by preliminary single-channel cell-attached patch studies exploring possible phosphorylation/dephosphorylation mechanisms underlying GABAA receptor dysfunction in CA1 neurons after chronic FZP-treatment, which suggest that Ca2+-mediated activation of CaMKII may reduce GABAA receptor conductance (Das et al., 2008). These initial single-channel studies also further validated the decrease in GABAA receptor-mediated chloride conductance, estimated by NSFA (Fig. 1 and 2, Zeng and Tietz, 1999). Nonetheless, it is uncertain whether such a mechanism results in non-functional membrane bound receptors, receptor internalization or a rapid switch in subunit composition related to a change in membrane conductance (De Koninck and Mody, 1996; Smart, 1997; Churn and DeLorenzo, 1998; Stelzer et al, 1998; Poisbeau et al, 1999; Sanchez et al, 2005; Houston et al, 2006).
Studies of the spatial and temporal dynamics of [Ca2+]i in synaptically-activated CA1 neurons indicate that widespread intracellular Ca2+ accumulation arises from a variety of spatially-localized Ca2+ channels, Ca2+ conductances and uptake and extrusion mechanisms (Regehr and Tank, 1992). Ca2+ influx through NMDA-type glutamate receptors plays a significant role in basal and high frequency synaptic transmission. Large, relatively slow Ca2+ transients through L-VGCCs, which represent ∼80% of the non-NMDA receptor component, are primarily localized to proximal-apical and basal dendrites and to a lesser extent somata, consistent with the distribution of L-VGCCs on pyramidal neurons (Hell et al, 1993). The amplitude of Ca2+ transients varies from 50-240 nM in CA1 neuron somata and dendrites (Regehr and Tank, 1992; Thibault et al, 2001) in the vicinity of a large fraction of GABAergic symmetric synapses (Megias et al, 2001).
Even so, both voltage-dependent and -independent Ca2+ channels may contribute to an influx of Ca2+ following 1-week chronic FZP treatment. Specifically, prior nimodipine injection also prevented the benzodiazepine-induced enhancement of inwardly rectifying AMPA receptor currents associated with withdrawal-anxiety (Xiang and Tietz, 2007), indicating that benzodiazepine-induced activation of L-VGCCs may also regulate synaptic membrane incorporation of Ca2+ permeable AMPA receptors (Das et al., in press). The delayed time-course of reductions in inhibitory synaptic currents (Poisbeau et al, 1997; Zeng and Tietz, 1999; Van Sickle et al, 2004) relative to enhancement of excitatory currents further supports the possibility that the drug-induced increase in Ca2+ influx through GluR1-containing AMPA receptors (Song et al, 2007, Das et al, in press) might also contribute to the decrease in GABAA receptor function. Correspondingly, Jensen and colleagues proposed that hypoxia-induced seizures in neonatal rats induced a dephosphorylation-dependent downregulation of GABAergic synaptic currents induced by activation of Ca2+-permeable AMPA receptors (Sanchez et al, 2005). The sequential regulation of voltage-dependent and -independent Ca2+ channels and subsequent changes in GABAergic synaptic transmission suggests that Ca2+ influx via L-VGCCs (Xiang and Tietz, 2006) and AMPA receptors (Song et al., 2007, Das et al, in press) may be additive. Thus it is conceivable that intracellular Ca2+ accumulates during drug withdrawal and ultimately affects GABAA receptor function. Collectively, the current and previous findings in this model suggest that downstream Ca2+ signaling may play a prominent role in mediating functional GABA system plasticity associated with use-dependent activation of GABAA receptors during prolonged benzodiazepine administration.
Acknowledgments
Portions of this work have appeared in abstract form: Soc. Neurosci. Abstr, 463.19, 2007. We thank William C. Ferencak, III, Krista Pettee, Brian Behrle for technical assistance. We thank Dr. L. John Greenfield, Jr. for helpful comments. This work was supported by NIDA grants: R01-DA04075 and R01-DA018342 (to E.I.T.), University of Toledo College of Medicine Drug Abuse Research Funds (to E.I.T.) and University of Toledo College of Medicine Predoctoral Fellowship (to K.X.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguayo LG, Espinosa F, Kunos G, Satin LS. Effects of intracellular calcium on GABAA receptors in mouse cortical neurons. Pflüger Arch. 1998;435:382–387. doi: 10.1007/s004240050527. [DOI] [PubMed] [Google Scholar]
- Bateson AN. Basic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Curr Pharm Des. 2002;8:5–21. doi: 10.2174/1381612023396681. [DOI] [PubMed] [Google Scholar]
- Benke TA, Lüthi A, Palmer MJ, Wikström MA, Anderson WW, Isaac JT, Collingridge GL. Mathematical modeling of non-stationary fluctuation analysis for studying channel properties of synaptic AMPA receptors. J Physiol. 2001;537:407–20. doi: 10.1111/j.1469-7793.2001.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Botzolakis EJ, Haas KF, Fisher JL, Macdonald RL. Microscopic kinetic determinants of macroscopic currents: insights from coupling and uncoupling of GABAA receptor desensitization and deactivation. J Physiol. 2007;584:769–787. doi: 10.1113/jphysiol.2007.142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churn SB, DeLorenzo RJ. Modulation of GABAergic receptor function is regulated by phosphorylation in acutely dissociated guinea pig hippocampal neurons. J Physiol (Lond) 1998;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Bell-Horner CL, Huang RQ, Raut A, Gonzales EB, Chen ZL, Covey DF, Dillon GH. Inhibition of type A GABA receptors by L-type calcium channel blockers. Neuroscience. 2004;124:195–206. doi: 10.1016/j.neuroscience.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Das P, Greenfield LJ, Jr, Tietz EI. Abstract Viewer/Itinerary Planner. Washington, DC: Soc for Neurosci.; 2008. CaMKII inhibition reverses benzodiazepine-induced reduction of GABA sensitivity at single GABAA receptor channels. Online. [Google Scholar]
- Das P, Lilly SM, Zerda R, Gunning WT, Alvarez FJ, Tietz EI. Increased AMPA receptor GluR1 subunit incorporation in rat hippocampal CA1 synapses during benzodiazepine withdrawal. J Comp Neurol. 2008 doi: 10.1002/cne.21866. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallager DW, Malcolm AB, Anderson SA, Gonsalves SF. Continuous release of diazepam: electrophysiological, biochemical and behavioral consequences. Brain Res. 1985;342:26–36. doi: 10.1016/0006-8993(85)91349-6. [DOI] [PubMed] [Google Scholar]
- Ghavanini AA, Isbasescu IM, Mathers DA, Puil E. Optimizing fluctuation analysis of GABAergic IPSCs for accurate unitary currents. J Neurosci Meth. 2006;158:150–156. doi: 10.1016/j.jneumeth.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Gravielle MC, Faris R, Russek SJ, Farb DH. GABA induces activity dependent delayed-onset uncoupling of GABA/Benzodiazepine site interactions in neocortical neurons. J Biol Chem. 2005;280:20954–20960. doi: 10.1074/jbc.M500131200. [DOI] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston CM, Lee HC, Hosie AM, Moss SJ, Smart TG. Identification of the sites for CaMK-II-dependent phosphorylation of GABA(A) receptors. J Biol Chem. 2006;282:17855–17866. doi: 10.1074/jbc.M611533200. [DOI] [PubMed] [Google Scholar]
- Houston CM, Smart TG. CaMK-II modulation of GABA(A) receptors expressed in HEK293, NG108-15 and rat cerebellar granule neurons. Eur J Neurosci. 24:2504–14. doi: 10.1111/j.1460-9568.2006.05145.x. [DOI] [PubMed] [Google Scholar]
- Itier V, Granger P, Perrault C, Depoortere H, Scatton B, Avenet P. Protracted treatment with diazepam reduces benzodiazepine receptor-mediated potentiation of γ-amino butyric acid-induced currents in dissociated rat hippocampal neurons. J Pharmacol Exp Ther. 1996;279:1092–1099. [PubMed] [Google Scholar]
- Katsura M, Shibasaki M, Kurokawa K, Tsujimura A, Ohkuma S. Up-regulation of L-type high voltage-gated calcium channel subunits by sustained exposure to 1,4- and 1,5-benzodiazepines in cerebrocortical neurons. J Neurochem. 2007;103:2518–2528. doi: 10.1111/j.1471-4159.2007.04984.x. [DOI] [PubMed] [Google Scholar]
- Lavoie AM, Twyman RE. Direct evidence for diazepam modulation of GABAA receptor microscopic affinity. Neuropharmacology. 1996;35(910):1383–1392. doi: 10.1016/s0028-3908(96)00077-9. [DOI] [PubMed] [Google Scholar]
- Lyons HR, Land MB, Gibbs TT, Farb DH. Distinct signal transduction pathways for GABA-induced GABA(A) receptor down-regulation and uncoupling in neuronal culture: a role for voltage-gated calcium channels. J Neurochem. 2001;78:1114–1126. doi: 10.1046/j.1471-4159.2001.00501.x. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Chuang DM. Modulation by calcium of γ-Aminobutyric acid (GABA) binding to GABAA and GABAB Recognition Sites in Rat Brain. Mol Pharm. 1984;25:352–359. [PubMed] [Google Scholar]
- Maruhn D, Siefert HM, Weber H, Rämsch K, Suwelack D. Pharmacokinetics of nimodipine. I. Communication: absorption, concentration in plasma and excretion after single administration of [14C]nimodipine in rat, dog and monkey. Arzneimittelforschung. 1985;35:1781–1786. [PubMed] [Google Scholar]
- Megias M, Emri Z, Freund TF, Gulyás AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- Perrais D, Ropert N. Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses. J Neurosci. 1999;19:578–588. doi: 10.1523/JNEUROSCI.19-02-00578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisbeau P, Williams SR, Mody I. Silent GABAA synapses during flurazepam withdrawal are region-specific in the hippocampal formation. J Neurosci. 1997;17:3467–3475. doi: 10.1523/JNEUROSCI.17-10-03467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisbeau P, Cheney MC, Browning MD, Mody I. Modulation of synaptic GABAA receptor function by PKA and PKC in adult hippocampal neurons. J Neurosci. 1999;19:674–683. doi: 10.1523/JNEUROSCI.19-02-00674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Tank DW. Calcium concentration dynamics produced by synaptic activation of CA1 hippocampal pyramidal cells. J Neurosci. 1992;12:4202–4023. doi: 10.1523/JNEUROSCI.12-11-04202.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny E, Twombly DA, Narahashi T. Chlordiazepoxide block of two types of calcium channels in neuroblastoma cells. J Pharmacol Exp Ther. 1993;264:22–28. [PubMed] [Google Scholar]
- Ricci A, Sabbatini M, Tomassoni D, Mignini F, Petrelli C, Amenta F. Neuronal populations of rat cerebral cortex and hippocampus expressed a higher density of L-type Ca2+ channel than corresponding cerebral vessels. Clin Exper Hyper. 2002;24:715–726. doi: 10.1081/ceh-120015347. [DOI] [PubMed] [Google Scholar]
- Rosenberg HC, Tietz EI, Chiu TH. Tolerance to the anticonvulsant action of benzodiazepines. Relationship to decreased receptor density. Neuropharmacology. 1985;24:639–44. doi: 10.1016/0028-3908(85)90106-6. [DOI] [PubMed] [Google Scholar]
- Sanchez RM, Dai W, Levada RE, Lippman JJ, Jensen FE. AMPA/kainate receptor-mediated downregulation of GABAergic synaptic transmission by calcineurin after seizures in the developing rat brain. J Neurosci. 2005;25:3442–3451. doi: 10.1523/JNEUROSCI.0204-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG. Regulation of excitatory and inhibitory neurotransmitter-gated ion channels by protein phosphorylation. Curr Opin Neurobiol. 1997;7:358–367. doi: 10.1016/s0959-4388(97)80063-3. [DOI] [PubMed] [Google Scholar]
- Song J, Shen G, Greenfield LJ, Jr, Tietz EI. Benzodiazepine withdrawal-induced glutamatergic plasticity involves up-regulation of GluR1-containing alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in hippocampal CA1 neurons. J Pharmacol Exp Ther. 2007;322:569–581. doi: 10.1124/jpet.107.121798. [DOI] [PubMed] [Google Scholar]
- Stelzer A, Kay AR, Wong RK. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1998;241:339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- Taft WC, DeLorenzo RJ. Micromolar-affinity benzodiazepine receptors regulate voltage-sensitive calcium channels in nerve terminal preparations. Proc Natl Acad Sci. 1984;81:3118–3122. doi: 10.1073/pnas.81.10.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Hadley R, Landfield PW. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity. J Neurosci. 2001;21:9744–9756. doi: 10.1523/JNEUROSCI.21-24-09744.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz EI, Zeng XJ, Chen S, Lilly SM, Rosenberg HC, Kometiani P. Antagonist-induced reversal of functional and structural measures of hippocampal benzodiazepine tolerance. J Pharmacol Exp Ther. 1999;291:932–942. [PubMed] [Google Scholar]
- Van Sickle B, Xiang K, Tietz EI. Transient plasticity of hippocampal CA1 neuron glutamate receptors contributes to benzodiazepine withdrawal anxiety. Neuropsychopharmacology. 2004;29:1994–2006. doi: 10.1038/sj.npp.1300531. [DOI] [PubMed] [Google Scholar]
- Wang L, Greenfield LJ., Jr Post-hypoxic changes in rat cortical neuron GABAA receptor function require L-type voltage-gated calcium channel activation. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.07.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA. GABAA receptor subtypes: any clues to the mechanism of benzodiazepine dependence? Curr Opin Pharmacol. 2005;5:47–52. doi: 10.1016/j.coph.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Watson WP, Little HJ. Selectivity of the protective effects of dihydropyridine calcium channel antagonist against the ethanol withdrawal syndrome. Brain Res. 2002;930:111–122. doi: 10.1016/s0006-8993(02)02236-9. [DOI] [PubMed] [Google Scholar]
- Weiland GA, Oswald RE. The mechanism of binding of dihydropyridine calcium channel blockers to rat brain membranes. J Biol Chem. 1985;260:8456–8464. [PubMed] [Google Scholar]
- Xiang K, Giovannucci DR, Greenfield LJ, Jr, Tietz EI. Abstract Viewer/Itinerary Planner. Atlanta, GA: Soc for Neurosci.; 2006. Enhanced calcium entry through L-type voltage-gated calcium channels contributes to AMPA receptor plasticity in hippocampal CA1 neurons after chronic benzodiazepine treatment. Online. Manuscript submitted. [Google Scholar]
- Xiang K, Tietz EI. Benzodiazepine-induced hippocampal CA1 neuron AMPA receptor plasticity linked to severity of withdrawal-anxiety: Differential role of voltage-gated calcium channels and NMDA receptors. Behav Pharmacol. 2007;18:447–460. doi: 10.1097/FBP.0b013e3282d28f2b. [DOI] [PubMed] [Google Scholar]
- Xie XH, Tietz EI. Reduction in potency of selective gamma-aminobutyric acid A agonists and diazepam in CA1 region of in vitro hippocampal slices from chronic flurazepam-treated rats. J Pharmacol Exp Ther. 1992;262:204–211. [PubMed] [Google Scholar]
- Zeng XJ, Tietz EI. Benzodiazepine tolerance at GABAergic synapses on hippocampal CA1 pyramidal cells. Synapse. 1999;31:263–277. doi: 10.1002/(SICI)1098-2396(19990315)31:4<263::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]