Abstract
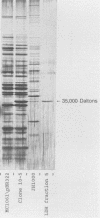
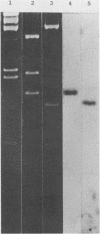
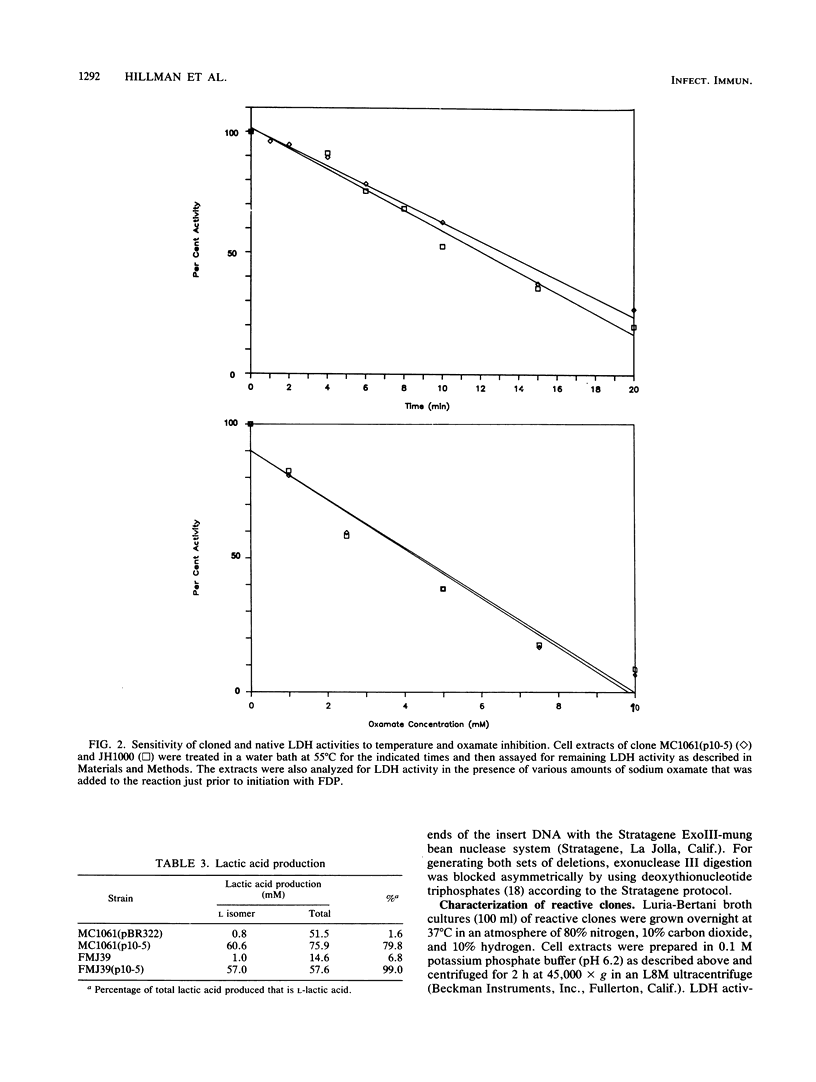
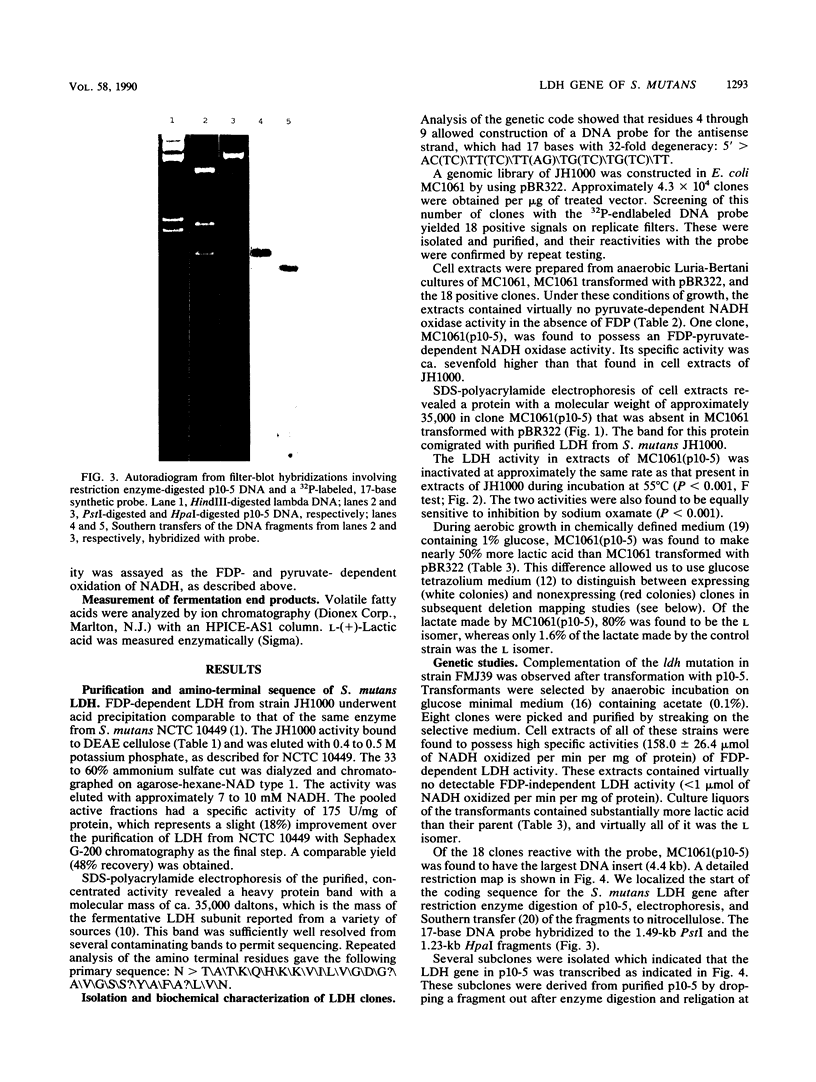
The fructose-1,6-diphosphate-dependent lactate dehydrogenase from Streptococcus mutans JH1000 was purified by a modification of published methods. The sequence of 27 amino-terminal amino acids was determined, which allowed us to construct a 17-base DNA probe that had 32-fold degeneracy. The probe was used to screen a genomic library in pBR322. Of 18 reactive clones, 1 was found that expressed lactate dehydrogenase (LDH) activity identical to that of S. mutans with regard to dependence on fructose-1,6-diphosphate, thermal inactivation profile, and inhibition by sodium oxamate. Extracts of this clone possessed a protein band that comigrated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis with purified LDH from JH1000. Compared with controls, the clone was shown to produce elevated amounts of L-(+)-lactic acid during growth in the presence of glucose, thereby indicating that the activity was expressed in vivo. This result was substantiated by demonstrating that the activity could complement a mutation in the fermentative D-(-)-LDH of Escherichia coli. Subcloning showed that the S. mutans LDH subunit is encoded by a 1.2-kilobase gene. Our ability to clone this gene is expected to have great practical significance in the construction of an effector strain for use in the replacement therapy of dental caries.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. T., Wittenberger C. L. Fructose-1,6-diphosphate-dependent lactate dehydrogenase from a cariogenic streptococcus: purification and regulatory properties. J Bacteriol. 1972 May;110(2):604–615. doi: 10.1128/jb.110.2.604-615.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Genetic analysis of Streptococcus mutans virulence. Curr Top Microbiol Immunol. 1985;118:253–277. doi: 10.1007/978-3-642-70586-1_14. [DOI] [PubMed] [Google Scholar]
- Dewhirst F. E., Stashenko P. P., Mole J. E., Tsurumachi T. Purification and partial sequence of human osteoclast-activating factor: identity with interleukin 1 beta. J Immunol. 1985 Oct;135(4):2562–2568. [PubMed] [Google Scholar]
- Hillman J. D., Dzuback A. L., Andrews S. W. Colonization of the human oral cavity by a Streptococcus mutans mutant producing increased bacteriocin. J Dent Res. 1987 Jun;66(6):1092–1094. doi: 10.1177/00220345870660060101. [DOI] [PubMed] [Google Scholar]
- Hillman J. D., Johnson K. P., Yaphe B. I. Isolation of a Streptococcus mutans strain producing a novel bacteriocin. Infect Immun. 1984 Apr;44(1):141–144. doi: 10.1128/iai.44.1.141-144.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman J. D., Socransky S. S. Replacement therapy of the prevention of dental disease. Adv Dent Res. 1987 Oct;1(1):119–125. doi: 10.1177/08959374870010010301. [DOI] [PubMed] [Google Scholar]
- Hillman J. D., Yaphe B. I., Johnson K. P. Colonization of the human oral cavity by a strain of Streptococcus mutans. J Dent Res. 1985 Nov;64(11):1272–1274. doi: 10.1177/00220345850640110301. [DOI] [PubMed] [Google Scholar]
- Jagusztyn-Krynicka E. K., Smorawinska M., Curtiss R., 3rd Expression of Streptococcus mutans aspartate-semialdehyde dehydrogenase gene cloned into plasmid pBR322. J Gen Microbiol. 1982 May;128(5):1135–1145. doi: 10.1099/00221287-128-5-1135. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederberg J. Detection of Fermentative Variants with Tetrazolium. J Bacteriol. 1948 Nov;56(5):695–695. doi: 10.1128/jb.56.5.695-695.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mat-Jan F., Alam K. Y., Clark D. P. Mutants of Escherichia coli deficient in the fermentative lactate dehydrogenase. J Bacteriol. 1989 Jan;171(1):342–348. doi: 10.1128/jb.171.1.342-348.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Putney S. D., Benkovic S. J., Schimmel P. R. A DNA fragment with an alpha-phosphorothioate nucleotide at one end is asymmetrically blocked from digestion by exonuclease III and can be replicated in vivo. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7350–7354. doi: 10.1073/pnas.78.12.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S., Dzink J. L., Smith C. M. Chemically defined medium for oral microorganisms. J Clin Microbiol. 1985 Aug;22(2):303–305. doi: 10.1128/jcm.22.2.303-305.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Chemical characterization of D-lactate dehydrogenase from Escherichia coli B. J Biol Chem. 1968 May 25;243(10):2579–2586. [PubMed] [Google Scholar]
- Womble D. D., Taylor D. P., Rownd R. H. Method for obtaining more-accurate covalently closed circular plasmid-to-chromosome ratios from bacterial lysates by dye-buoyant density centrifugation. J Bacteriol. 1977 Apr;130(1):148–153. doi: 10.1128/jb.130.1.148-153.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]