Abstract
The present study was designed to obtain further information on the nature of the corneal macromolecule(s) to which Pseudomonas aeruginosa adheres and how adherence might be prevented. Scarified adult mouse corneas in organ culture were treated with trypsin or lipase to determine whether the receptor molecule(s) was protein or lipid in nature. Trypsin (20 micrograms/ml) treatment of the cornea for 5 min had no significant effect on bacterial adherence, and longer periods of enzyme exposure resulted in extensive surface cell lysis. In contrast, lipase treatment (50,000 U/ml) for 1 h caused little visible cell lysis and significantly reduced bacterial adherence. To test further the lipid nature of the receptor, a highly purified monosialoganglioside (GM1) preparation (500 micrograms/ml) was used to preincubate (1 h) the cornea prior to bacterial application, and this also inhibited bacterial adherence. Similar corneal treatment with gangliotetraosylceramide (asialo GM1) (500 micrograms/ml) had little effect on ocular bacterial binding. Premixing of the bacterial inoculum with GM1 prior to corneal application had no significant effect on inhibiting bacterial binding, but similarly premixing the bacterial inoculum with asialo GM1 transiently decreased adherence. Lastly, premixing of the bacterial inoculum or preincubation of corneas with fibronectin (500 micrograms/ml for 1 h) both decreased bacterial adherence. These findings provide evidence that the receptor-adhesin interactions of P. aeruginosa at the ocular surface in organ culture are complex, involve a glycolipid moiety, and may be blocked by a ganglioside containing at least one sialosyl residue or by fibronectin, which may bind to membrane-associated gangliosides.
Full text
PDF
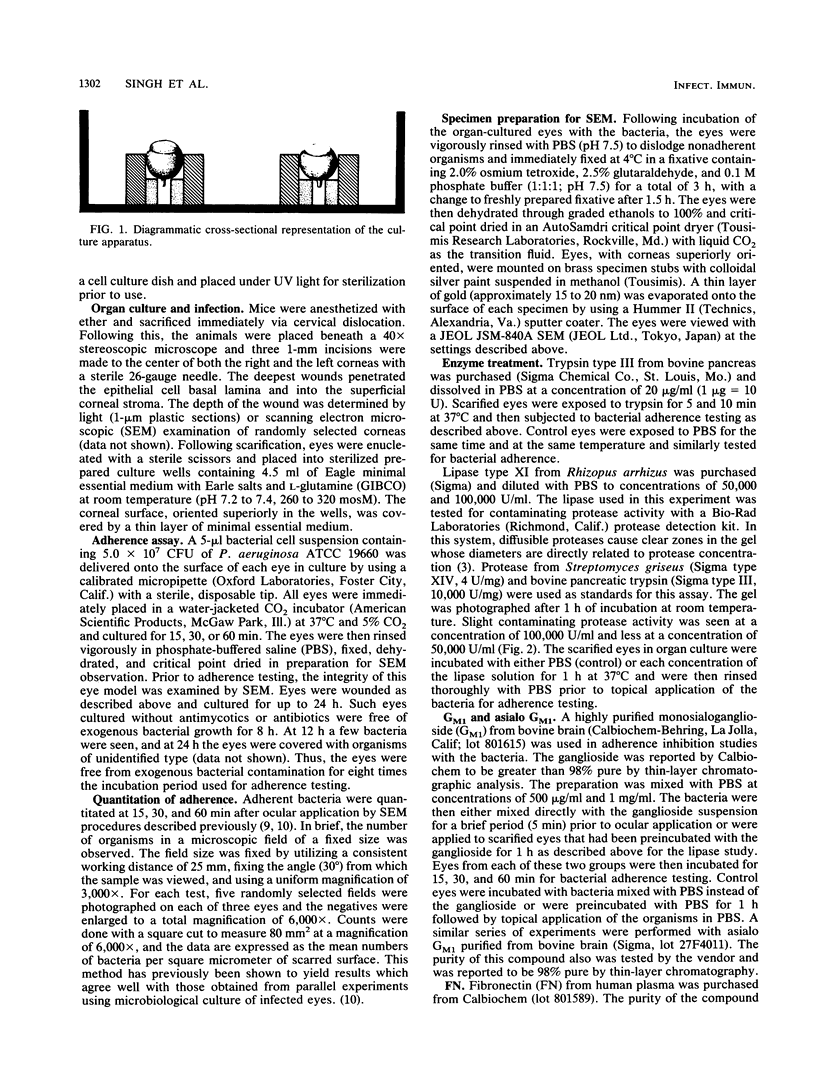

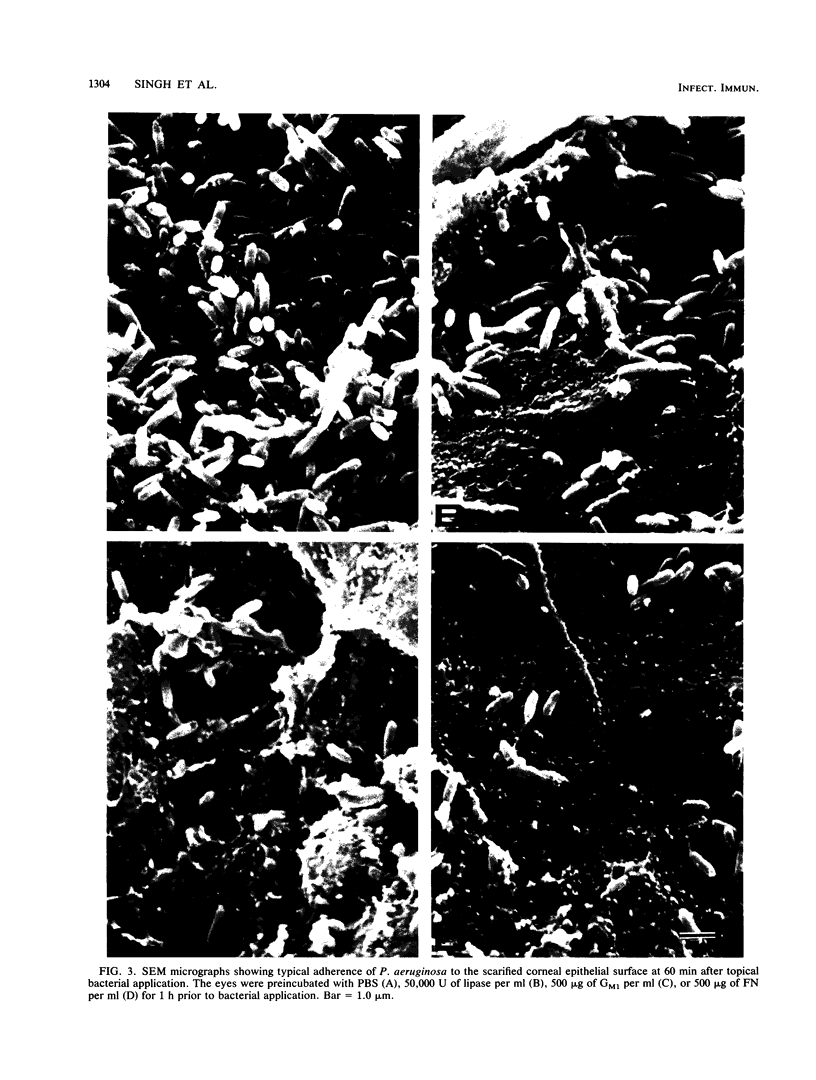


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS R. P., RHODES D. H., Jr Pseudomonas eye infection as a cause of death in premature infants. Arch Ophthalmol. 1961 Apr;65:517–525. doi: 10.1001/archopht.1961.01840020519010. [DOI] [PubMed] [Google Scholar]
- Berk R. S., Beisel K., Hazlett L. D. Genetic studies of the murine corneal response to Pseudomonas aeruginosa. Infect Immun. 1981 Oct;34(1):1–5. doi: 10.1128/iai.34.1.1-5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk R. S., Leon M. A., Hazlett L. D. Genetic control of the murine corneal response to Pseudomonas aeruginosa. Infect Immun. 1979 Dec;26(3):1221–1223. doi: 10.1128/iai.26.3.1221-1223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney H. S., Ofek I., Simpson W. A., Hasty D. L., Beachey E. H. Binding of Streptococcus pyogenes to soluble and insoluble fibronectin. Infect Immun. 1986 Sep;53(3):454–459. doi: 10.1128/iai.53.3.454-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke J. R., Magliocco M. V. Experimental Pseudomonas aeruginosa Infection of the Mouse Cornea. Infect Immun. 1971 Feb;3(2):209–216. doi: 10.1128/iai.3.2.209-216.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa-Garber N. Pseudomonas aeruginosa lectins. Methods Enzymol. 1982;83:378–385. doi: 10.1016/0076-6879(82)83034-6. [DOI] [PubMed] [Google Scholar]
- Glick J., Garber N., Shohet D. Surface haemagglutinating activity of Pseudomonas aeruginosa. Microbios. 1987;50(203):69–80. [PubMed] [Google Scholar]
- Hazlett L. D., Moon M. M., Strejc M., Berk R. S. Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Invest Ophthalmol Vis Sci. 1987 Dec;28(12):1978–1985. [PubMed] [Google Scholar]
- Hazlett L. D., Moon M., Berk R. S. In vivo identification of sialic acid as the ocular receptor for Pseudomonas aeruginosa. Infect Immun. 1986 Feb;51(2):687–689. doi: 10.1128/iai.51.2.687-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett L. D., Rosen D. D., Berk R. S. Age-related susceptibility to Pseudomonas aeruginosa ocular infections in mice. Infect Immun. 1978 Apr;20(1):25–29. doi: 10.1128/iai.20.1.25-29.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida T., Katoh M., Matsuo Y. Adherence of Pseudomonas aeruginosa to a rabbit cornea cell line (SIRC) cells. Hiroshima J Med Sci. 1982 Dec;31(4):225–232. [PubMed] [Google Scholar]
- Krivan H. C., Ginsburg V., Roberts D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch Biochem Biophys. 1988 Jan;260(1):493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Roberts D. D., Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laibson P. R. Cornea and sclera. Arch Ophthalmol. 1972 Nov;88(5):553–574. doi: 10.1001/archopht.1972.01000030555018. [DOI] [PubMed] [Google Scholar]
- Leffler H., Svanborg-Edén C. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect Immun. 1981 Dec;34(3):920–929. doi: 10.1128/iai.34.3.920-929.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre E. B., Kuusela P. Binding of human fibronectin to group A, C, and G streptococci. Infect Immun. 1983 Apr;40(1):29–34. doi: 10.1128/iai.40.1.29-34.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjwani N., Zaidi T. S., Gigstad J. E., Jungalwala F. B., Barza M., Baum J. Binding of Pseudomonas aeruginosa to neutral glycosphingolipids of rabbit corneal epithelium. Infect Immun. 1990 Jan;58(1):114–118. doi: 10.1128/iai.58.1.114-118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Pyle M. Further characterization of the tracheal receptor for Pseudomonas aeruginosa. Eur J Clin Microbiol. 1985 Apr;4(2):160–162. doi: 10.1007/BF02013590. [DOI] [PubMed] [Google Scholar]
- Simpson W. A., Courtney H. S., Ofek I. Interactions of fibronectin with streptococci: the role of fibronectin as a receptor for Streptococcus pyogenes. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S351–S359. doi: 10.1093/clinids/9.supplement_4.s351. [DOI] [PubMed] [Google Scholar]
- Spurr-Michaud S. J., Barza M., Gipson I. K. An organ culture system for study of adherence of Pseudomonas aeruginosa to normal and wounded corneas. Invest Ophthalmol Vis Sci. 1988 Mar;29(3):379–386. [PubMed] [Google Scholar]
- Stanislawski L., Simpson W. A., Hasty D., Sharon N., Beachey E. H., Ofek I. Role of fibronectin in attachment of Streptococcus pyogenes and Escherichia coli to human cell lines and isolated oral epithelial cells. Infect Immun. 1985 Apr;48(1):257–259. doi: 10.1128/iai.48.1.257-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern G. A., Weitzenkorn D., Valenti J. Adherence of Pseudomonas aeruginosa to the mouse cornea. Epithelial v stromal adherence. Arch Ophthalmol. 1982 Dec;100(12):1956–1958. doi: 10.1001/archopht.1982.01030040936014. [DOI] [PubMed] [Google Scholar]
- Takasaki S., Yamashita K., Suzuki K., Kobata A. Structural studies of the sugar chains of cold-insoluble globulin isolated from human plasma. J Biochem. 1980 Dec;88(6):1587–1594. doi: 10.1093/oxfordjournals.jbchem.a133133. [DOI] [PubMed] [Google Scholar]
- Thompson L. K., Horowitz P. M., Bentley K. L., Thomas D. D., Alderete J. F., Klebe R. J. Localization of the ganglioside-binding site of fibronectin. J Biol Chem. 1986 Apr 15;261(11):5209–5214. [PubMed] [Google Scholar]
- Van de Water L., Destree A. T., Hynes R. O. Fibronectin binds to some bacteria but does not promote their uptake by phagocytic cells. Science. 1983 Apr 8;220(4593):201–204. doi: 10.1126/science.6338594. [DOI] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- Woods D. E. Role of fibronectin in the pathogenesis of gram-negative bacillary pneumonia. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S386–S390. doi: 10.1093/clinids/9.supplement_4.s386. [DOI] [PubMed] [Google Scholar]