Abstract
Although β-adrenergic stimuli are essential for myocardial contractility, β-blockers have a proven beneficial effect on the treatment of heart failure, but the mechanism is not fully understood. The stimulatory G protein α-subunit (Gsα) couples the β-adrenoreceptor to adenylyl cyclase and the intracellular cAMP response. In a mouse model of conditional Gsα deficiency in the cardiac muscle (Gsα-DF), we demonstrated heart failure phenotypes accompanied by increases in the level of a truncated cardiac troponin I (cTnI-ND) from restricted removal of the cTnI-specific N-terminal extension. To investigate the functional significance of the increase of cTnI-ND in Gsα-DF cardiac muscle, we generated double transgenic mice to overexpress cTnI-ND in Gsα-DF hearts. The overexpression of cTnI-ND in Gsα-DF failing hearts increased relaxation velocity and left ventricular end diastolic volume to produce higher left ventricle maximum pressure and stroke volume. Supporting the hypothesis that up-regulation of cTnI-ND is a compensatory rather than a destructive myocardial response to impaired β-adrenergic signaling, the aberrant expression of β-myosin heavy chain in adult Gsα-DF but not control mouse hearts was reversed by cTnI overexpression. These data indicate that the up-regulation of cTnI-ND may partially compensate for the cardiac inefficiency in impaired β-adrenergic signaling.
The β-adrenoreceptor (β-AR)3 signaling pathway plays an important role in the regulation of heart function (1–3). Activation of β-AR by catecholamines stimulates adenylyl cyclase and cAMP production via coupling to stimulatory G-protein (Gs), which in turn leads to activation of cAMP-dependent protein kinase (PKA) and phosphorylation of a multitude of intracellular substrates in the Ca2+ handling system (sarcolemmal L-type Ca2+ channels (4), the ryanodine receptor (5), and phospholamban in sarcoplasmic reticulum (6), and myofilament proteins (cardiac troponin I (cTnI) (7) and myosin-binding protein C (8)).
In heart failure patients, the level of plasma norepinephrine is enhanced, resulting from activation of sympathetic nervous system, which induces chronic stimulation of cardiac β-adrenergic receptors (9). Although acute β-stimulation enhances cardiac function to adapt to systemic needs, chronic stimulation of the β1-adrenergic receptor is detrimental and contributes to cardiomyocyte hypertrophy, cell death, and progression of heart failure (10–12). Transgenic mice overexpressing β1-adrenoreceptor (13) or the Gs subunit α (Gsα) (14) in the heart developed heart failure with increased level of apoptosis and fibrotic degeneration similar to that observed in dilated cardiomyopathy in humans. Overexpression of the catalytic subunit of PKA also produced cardiomyocyte hypertrophy, fibrosis, and a progressive decline in cardiac function, resulting in heart failure (15). On the other hand, failing hearts respond to the chronically elevated norepinephrine concentrations by desensitizing their response to β-adrenergic stimulation (11, 16).
Cardiac TnI is the inhibitory subunit of the troponin complex and plays an essential role in Ca2+ regulation of cardiac muscle contraction. Cardiac TnI is a substrate of PKA and is phosphorylated upon β-adrenergic stimulation (7). The PKA phosphorylation sites are two adjacent serine residues, Ser23/Ser24 (rat/mouse residue numbers), located in the cardiac specific N-terminal extension of cTnI. β-Adrenergic stimulated phosphorylation of cTnI Ser23/Ser24 reduces myofilament Ca2+ sensitivity (7, 17) and increases the rate of cardiac muscle relaxation (18–21). PKA-dependent cTnI phosphorylation is decreased in heart failure (22, 23).
A restricted N-terminal truncation of cTnI occurs at low levels in normal heart and increases in adaptation to hemodynamic changes in the tail suspension rat model of simulated microgravity (24). Peptide sequencing showed that this posttranslational modification preserves the core structure of TnI but selectively removes the cTnI-specific N-terminal extension, including the PKA phosphorylation sites Ser23 and Ser24. Transgenic mouse hearts overexpressing the N-terminal truncated cTnI (cTnI-ND) demonstrated increased myocardial relaxation and improved ventricular filling for a better utilization of the Frank-Starling mechanism, mimicking the effect of PKA phosphorylation (25). These results suggest that the β-adrenergic signaling pathway affects cTnI function by both phosphorylation and proteolytic modulation to regulate myocardial contraction.
In the present study, we showed that impaired β-adrenergic signaling resulting from myocardial Gsα deficiency (Gsα-DF) leads to impaired cardiac function accompanied by an up-regulation of cTnI-ND. Overexpression of cTnI-ND in Gsα-DF hearts increased relaxation velocity and left ventricular end diastolic volume to produce higher left ventricle maximum pressure and stroke volume. Supporting the hypothesis that the up-regulation of cTnI-ND is a compensatory rather than a destructive myocardial response to impaired β-adrenergic signaling, cTnI-ND overexpression reversed the aberrant expression of β-myosin heavy chain (β-MHC) in Gsα-DF hearts. These data indicate that up-regulation of cTnI-ND may partially compensate for the cardiac inefficiency in impaired β-adrenergic signaling, suggesting a potential therapeutic target for the treatment of heart failure.
EXPERIMENTAL PROCEDURES
Production of Gsα-DF in Mouse Cardiac Muscle—Mice with floxed Gsα exon 1 allele (E1fl) (26) were bred with the muscle creatine kinase (MCK)-cre mice (Taconic, Hudson, NY) to induce striated muscle-specific disruption of the Gsα gene (MCK-cre,E1fl/fl;MGsKO). The E1fl allele has no effect on Gsα expression or phenotype (27), and therefore both MCK-cre-minus E1fl and E1+/+ littermates were used as controls. Gsα genotyping to distinguish wild type (E1+) and E1fl alleles was performed by PCR using primers flanking the downstream loxP site (27). The presence or absence of the MCK-cre transgene was determined by PCR using cre-specific primers (27). Animals were maintained on a 12-h light/12-h dark cycle (6:00 a.m./6:00 p.m.) and standard pellet diet. Mice age 3–5 months of both sexes were used for experiments unless noted in the figure legend. The protocols were approved by the Institutional Animal Care and Use Committees and were conducted in accordance with the Guiding Principles in the Care and Use of Animals, as approved by the Council of the American Physiological Society.
Overexpression of cTnI-ND in Gsα-DF Cardiac Muscle—We have previously developed transgenic mouse lines overexpressing cTnI-ND in the cardiac muscle under the control of α-MHC promoter (25). Double transgenic mice were generated by mating cTnI-ND transgenic mice with MGsKO mice for cardiac functional studies. The genotype screening was done by PCR on genomic DNA isolated from tail biopsies, and all hearts used for functional studies were confirmed by Western blot for the knockdown of Gsα (27) and the overexpression of cTnI-ND (25).
SDS-PAGE and Immunoblot Analysis—Gsα protein expression in the cardiac muscle was measured on total tissue protein extracts by SDS-PAGE and Western immunoblotting using a Gsα-specific antibody (27). Cardiac TnI and cTnI-ND were examined by Western blotting using a monoclonal antibody TnI-1 (28) and a rabbit antiserum 7428 raised against the N-terminal peptide of cTnI.
Pro-Q Diamond Phosphoprotein Staining—To detect phosphorylated protein bands in SDS-gel, the manufacturer's protocol (Invitrogen) was applied with a few modifications. Briefly, SDS-polyacrylamide gels were prefixed in 50% methanol, 10% acetic acid for 45 min and transferred to fresh fixer overnight. After washing 3 times in deionized water for 10 min each, the gels were stained for 90 min in a dark container with Pro-Q Diamond stain freshly equilibrated to room temperature by vigorously shaking. After destaining three times in 20% acetonitrile, 50 mm sodium acetate, pH 4.0, for 30 min each, the gels were washed twice for 5 min each in deionized water and then scanned on a Typhoon 9410 fluorescence imager (GE Healthcare) using fluorescence mode (600 V, high sensitivity, green laser 532 nm for excitation and 560 nm long pass for emission).
Identification and Quantification of MHC Isoforms—Cardiac myosin heavy chain isoforms were separated by glycerol-SDS-PAGE and quantified as previously described (29). Total protein was extracted from cardiac muscle by direct homogenization in SDS-PAGE sample buffer. Myosin heavy chain isoforms were resolved using 8% polyacrylamide gel with a 50:1 ratio of acrylamide/bisacrylamide containing 30% glycerol prepared in 200 mm Tris-HCl, 100 mm glycine (pH 8.8), and 0.4% SDS. The upper running buffer was composed of 100 mm Tris base, 150 mm glycine, and 0.1% SDS containing 10 mm β-ME. The lower running buffer was 50 mm Tris base, 75 mm glycine, and 0.05% SDS. After running at 100 V in an icebox for 24 h, resolved protein bands were visualized by staining with Coomassie Blue R250.
Isolated Working Mouse Heart Preparation and Functional Measurements—Cardiac function of transgenic and wild type mice was measured in isolated working heart preparations (25, 30, 31). Thirty minutes after intraperitoneal injection of 100 units of heparin, the mice were anesthetized with pentobarbital sodium (100 mg/kg body weight, intraperitoneally), and the heart was rapidly isolated.
A modified 18-gauge needle 6 mm long with a thinned wall to reduce the outside diameter was used as the aortic cannula. A pin with a pointed end was placed inside of the needle as a guide to facilitate the cannulation. After establishing retrograde perfusion, a modified 16-gauge needle was used to cannulate the pulmonary vein for antegrade perfusion through the left atrium. A beveled PE-50 tubing was used to cannulate the pulmonary artery to collect coronary flow.
After all cannulations were established, the heart was switched to working mode by opening the left atrial perfusion. In all experiments, the hearts were perfused with Krebs-Hensileit buffer aerated with 95% O2, 5% CO2 at 37 °C without recycling to exclude the effects of metabolic chemicals and hormones. The buffer contents were modified as follows: 118 mm NaCl, 4.7 mm KCl, 2.25 mm CaCl2, 2.25 mm MgSO4, 1.2 mm KH2PO4, 0.32 mm EGTA, 25 mm NaHCO3, 15 mm d-glucose, and 2 mm sodium pyruvate, pH 7.4, adjusted at 37 °C. The high concentration of d-glucose (32) and sodium pyruvate effectively prevents contractile cycling (cyclic fluctuations) (33) caused by insufficient metabolic substrates for ex vivo working mouse heart (34), allowing the hearts to be functionally stable in working mode for over 2 h.
A 30-gauge needle was used to puncture the left ventricle wall from the apex to make a path for the insertion of a 1.2 French pressure-volume (P-V) catheter (model 898B; Scisense, London, Ontario, Canada; calibrated for pressure and volume at 37 °C). Aortic pressure was measured using an MLT844 pressure transducer (Capto, Horten, Norway). An air bubble was placed in the compliance chamber to mimic in vivo arterial compliance. The size of the bubble affects the aortic pressure trace and the shape of the left ventricular P-V loop. Based on conditions established by previous studies, we kept the bubble size at 0.5 ml in all experiments for isolated working mouse heart, which resembled P-V loops similar to that recorded in vivo (35).
Heart rate was controlled at 480 or 420 beats/min by supraventricular pacing using an isolated stimulator (A365; World Precision Instruments) with two microplatinum electrodes touching on the right atrium. Cardiac outputs were measured by the actual aortic and pulmonary artery flows recorded in real time by calibrated drop counting using a pair of copper electrodes (one was attached to an iron clip to produce a different potential from the other) feeding to computer software (Chart 5; AD Instruments) via a Powerlab/16 SP digital data archiving system (AD Instruments). Base-line function of the isolated working hearts was measured for the intraventricular pressure, the maximum rate of left ventricular pressure development (±dP/dt max), and left ventricular volume measurements. Stroke volume (μl/mg heart tissue) was calculated from the sum of aortic flow and coronary effluent, normalized to heart rate.
Cardiac Function at Various Pressure Loads—During stabilization of the mouse working heart preparations and the measurement of base-line functions, the preload pressure was 10 mm Hg, and the afterload was at 55 mm Hg (25, 30, 31). To evaluate the effect of Gsα-DF on cardiac function against pressure load ventricular performance was measured while increasing the afterload from 55 to 90 mm Hg.
Data Analysis—Densitometry analysis of SDS-gel and Western immunoblot was performed on images scanned at 600 dots/inch using NIH Image 1.61 software. All cardiac functional analyses were performed in a blinded setting. Quantitative data were documented as mean ± S.E. The statistical significance of differences between the mean values was analyzed by two-tail unpaired Student's t test unless noted in the table or figure legends.
RESULTS
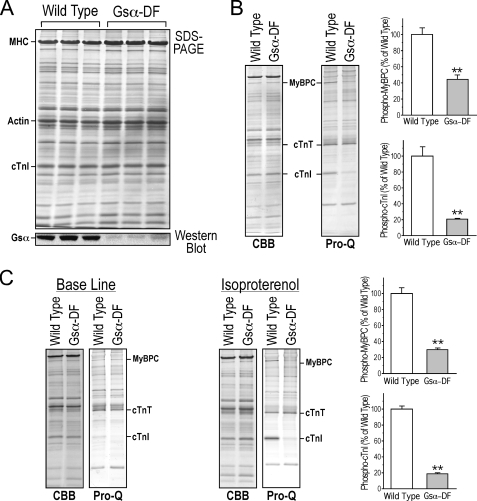
Gsα Deficiency in Cardiac Muscle Results in Heart Failure Phenotypes with Diminished β-Adrenergic Response—Immunoblots of ventricular muscle homogenates demonstrated that the level of Gsα protein was significantly diminished in the MCK-cre,E1fl/fl (MGsKO) mouse hearts as compared with that in wild type controls (Fig. 1A). The weak residual level of Gsα detected by immunoblot in the total heart tissue samples may be from noncardiac muscle tissues, such as the vasculature, nerves, and fibroblasts.
FIGURE 1.
Diminished Gsα-protein expression and β-adrenergic signaling in Gsα-DF mouse hearts. A, SDS-PAGE and immunoblots of total protein extracted from wild type and Gsα-DF hearts using a Gsα-specific antibody showed Gsα deficiency in Gsα-DF hearts. B, Coomassie Brilliant Blue (CBB) and Pro-Q Diamond phosphoprotein staining of SDS-polyacrylamide gels of total protein extracts from freshly isolated hearts and densitometric quantification showed that the in vivo phosphorylation of cTnI and myosin-binding protein C (MyBPC) is significantly lower in the Gsα-DF (n = 3) than that of wild type (n = 4) mouse hearts (**, p < 0.01). In contrast, the non-PKA-based phosphorylation of cardiac troponin T (cTnT) had no apparent change. C, although ex vivo perfusion of isolated working hearts significantly reduced the phosphorylation of cTnI and MyBPC, 10 nm isoproterenol treatment increased the phosphorylation of cTnI and MyBPC in wild type (n = 5) but not Gsα-DF (n = 4) hearts (**, p < 0.01). Since the SDS-gel samples were prepared by homogenization of the cardiac muscle, the isoproterenol-treated and control samples were from different working hearts.
Cardiac TnI and myosin-binding protein C are two major substrates of PKA downstream of the Gsα signaling pathway. Phosphoprotein staining showed decreased phosphorylation level of the two proteins in vivo in Gsα-DF hearts, whereas the non-PKA-based phosphorylation of cardiac troponin T had no apparent change (Fig. 1B). Although ex vivo perfusion of isolated working hearts significantly reduced the phosphorylation of cTnI and myosin-binding protein C, reflecting the removal of sympathetic nervous tone, isoproterenol treatment increased their phosphorylation levels in wild type hearts but had no effect on that in Gsα-DF hearts (Fig. 1C). Altogether, the diminished PKA phosphorylation confirmed that myocardial β-adrenergic signaling was impaired in this model.
Function of the Gsα-DF hearts was examined ex vivo in isolated working heart preparations. The heart rate was controlled in the physiological range from 420 to 480 beats/min by supraventricular pacing. Summarized in Table 1, the Gsα-DF hearts showed hypertrophy (increased heart/body weight ratio) without significant dilation, since there was no change in the left ventricular end diastolic volume. Cardiac function analysis at 10 mm Hg preload and 55 mm Hg afterload demonstrated, among multiple parameters measured, decreased contractile and relaxation velocities (±dP/dt), left ventricle maximum pressure, time to 50% peak pressure, mean aortic pressure (diastolic pressure + ⅓ pulse pressure; Fig. 2A), stroke volume, and ejection fraction, clearly indicating decreased cardiac function.
TABLE 1.
Base-line function of ex vivo working hearts Functional studies were performed ex vivo in working heart preparations at 10 mm Hg preload and 55 mm Hg afterload. Heart rates were controlled by supraventricular pacing. The Gsα-deficient hearts showed hypertrophy with no change in left ventricle end diastolic volume. The functional parameters of Gsα-deficient hearts demonstrate decreased myocardial contractility. LVPmax, left ventricular maximum pressure; LVPmin, left ventricular minimum pressure; LVEDV, left ventricular end diastolic volume; TP50, time to reach 50% peak pressure; RT75, time to 75% relaxation.
| Wild type (n = 8) | Gsα-DF (n = 7) | |
|---|---|---|
| Body weight (BW) (g) | 29.97 ± 1.70 | 26.27 ± 1.29 |
| Heart weight (HW) (mg) | 141.59 ± 5.41 | 152.49 ± 6.78 |
| HW/BW (mg/g) | 4.77 ± 0.16 | 5.81 ± 0.08a |
| +dP/dt (mm Hg/s) | 3745.88 ± 210.56 | 2134.57 ± 141.48a |
| –dP/dt (mm Hg/s) | 2912.63 ± 106.75 | 2046.71 ± 101.12a |
| LVPmax (mm Hg) | 70.96 ± 1.67 | 62.66 ± 1.84a |
| LVPmin (mm Hg) | 0.92 ± 0.48 | 0.97 ± 0.81 |
| LVEDV (μl) | 35.89 ± 1.58 | 36.40 ± 3.01 |
| Stroke volume (μl/mg) | 0.111 ± 0.007 | 0.043 ± 0.001a |
| Ejection fraction (%) | 44.65 ± 4.19 | 18.81 ± 2.35a |
| TP50 (ms) | 18.94 ± 0.57 | 22.86 ± 0.68a |
| RT75 (ms) | 47.75 ± 1.11 | 48.57 ± 5.54 |
p < 0.01 versus the wild type control
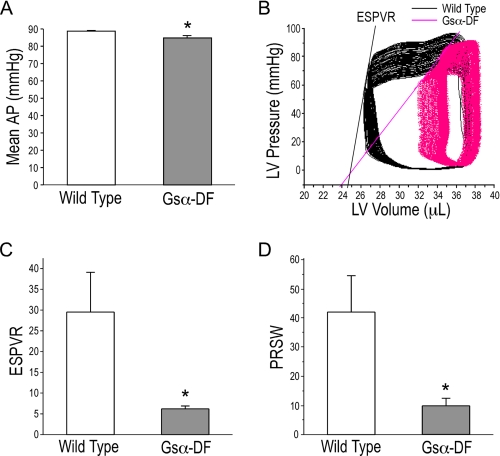
FIGURE 2.
Inability of Gsα-DF hearts to overcome pressure overload. A, mean aortic pressure (AP) at an afterload of 90 mm Hg was lower in Gsα-DF than that in wild type hearts. B, P-V loops of wild type and Gsα-DF ex vivo working hearts during the increasing of afterloads from 55 to 90 mm Hg. Lines indicate the end systolic pressure volume relationship (ESPVR), and their slopes are shown in C. D, pressure recruitment of stroke work (PRSW) in wild type and Gsα-DF hearts was derived from P-V loop measurements during the increasing of afterloads from 55 to 90 mm Hg and showed decreased stroke work in Gsα-DF hearts. *, p < 0.05 versus wild type; n = 8 for wild type and n = 7 for Gsα-DF groups. LV, left ventricular.
The failing phenotype of Gsα-DF hearts was further demonstrated by the change of pressure-volume loop when increasing the afterload from 55 to 90 mm Hg. After raising the afterload hydrostatic column, wild type mouse hearts were able to reach 90 mm Hg afterload and produce aortic output. In contrast, not all Gsα-DF hearts were able to reach 90 mm Hg afterload. Functional analysis of eight wild type and five Gsα-DF hearts that were able to overcome 90 mm Hg afterload further showed that Gsα deficiency resulted in more clear decrease in cardiac function at the increased afterload (the parameters measured are shown in Table 2), consistent with a decreased myocardial potential. Representative P-V loops during the increase in afterload are shown in Fig. 2B. End systolic pressure volume relationship for the slope of changing end systolic pressure volume values quantitatively demonstrated the significantly lower myocardial contractility of Gsα-DF hearts (Fig. 2C). Pressure recruitment of stroke work derived from P-V loop measurement also showed decreased stroke work in Gsα-DF hearts during the increase in afterload (Fig. 2D).
TABLE 2.
Isolated working heart function at increased afterload In comparison with the base-line function measured at 55 mm Hg afterload (Table 1), cardiac function in response to pressure load was examined by increasing the afterload to 90 mm Hg. Although all wild type hearts were able to reach 90 mm Hg afterload to produce aortic output, only five of seven Gsα-DF hearts tested were able to overcome 90 mm Hg afterload with significantly decreased function. LVPmax, left ventricular maximum pressure; LVPmin, left ventricular minimum pressure; LVEDV, left ventricular end diastolic volume.
| Wild type (n = 8) | Gsα-DF (n = 5) | |
|---|---|---|
| +dP/dt (mm Hg/s) | 4605 ± 257 | 3029 ± 280a |
| –dP/dt (mm Hg/s) | 4294 ± 160 | 3016 ± 173a |
| LVPmax (mm Hg) | 99.13 ± 2.08 | 89.42 ± 2.45b |
| LVPmin (mm Hg) | 0.46 ± 0.53 | 0.77 ± 1.08 |
| LVEDV (μl) | 35.86 ± 1.62 | 40.05 ± 4.31 |
| Stroke volume (μl/mg) | 0.094 ± 0.008 | 0.032 ± 0.002a |
| Ejection fraction (%) | 37.95 ± 4.02 | 12.89 ± 1.05a |
p < 0.01 versus the wild type control
p < 0.05 versus the wild type control
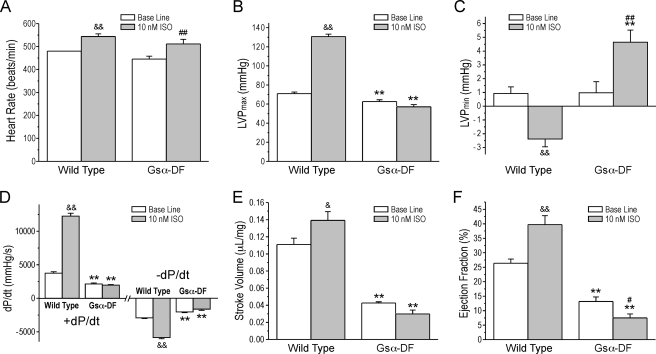
Isoproterenol treatment of the working heart further confirmed impaired β-adrenergic signaling in the Gsα-DF hearts (Fig. 3). The intrinsic heart rate of Gsα-DF hearts was responsive to the perfusion of 10 nm isoproterenol, similar to that of wild type hearts, indicating unaffected β-adrenergic signaling in the pacemaker cells (Fig. 3A). Isoproterenol produced significantly positive inotropic and lusitropic effects in wild type working hearts with increased contractile velocity (+dP/dt), relaxation velocity (-dP/dt), left ventricular maximal pressure, stroke volume, and ejection fraction and reduced left ventricular minimal pressure. In contrast, isoproterenol had no effects or opposite effects on those functional parameters in the Gsα-DF hearts (Fig. 3, B–F).
FIGURE 3.
Impaired β-adrenergic responsiveness in Gsα-DF hearts. The data summarize intrinsic heart rate (A), maximal left ventricular pressure (LVP) (B), minimal left ventricular pressure (C), contractile (+dP/dt) and relaxation (-dP/dt) velocities (D), stroke volume (E), and ejection fraction (F) in wild type and Gsα-DF hearts in the absence (Base Line) or presence of 10 nm isoproterenol (ISO). *, p < 0.05; **, p < 0.01 versus the wild type control; &, p < 0.05; &&, p < 0.01 isoproterenol versus base line in wild type hearts; #, p < 0.05; ##, p < 0.01 isoproterenol versus base line in Gsα-DF hearts; n = 8 for wild type and n = 7 for Gsα-DF groups.
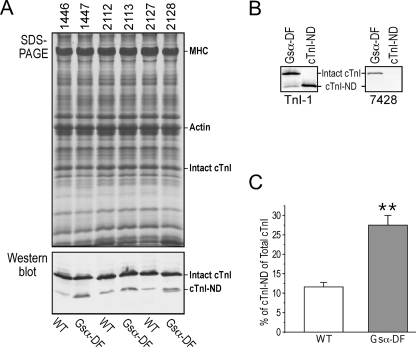
Gsα-DF Mouse Hearts Show an Increased Production of cTnI-ND—An interesting change in the myofilament proteins of Gsα-DF hearts was an increase of a cTnI fragment (Fig. 4A). Using monoclonal antibody TnI-1 against the C terminus of TnI and an anti-cardiac TnI N-terminal peptide rabbit polyclonal antibody 7428, immunoblots showed that this cTnI fragment lacked the N-terminal segment, whereas it retained an intact C terminus (Fig. 4B), indicating that the cTnI fragment was produced by restricted N-terminal truncation, similar to the cTnI fragment produced in rat hearts during hemodynamic adaptation to simulated microgravity (24). Quantification of the immunoblots showed that impaired β-adrenergic signaling increased the level of cTnI from 11.6 to 27.5% of total cTnI (Fig. 4C). This restricted proteolysis selectively removes the cTnI-specific N-terminal extension, including the PKA phosphorylation sites Ser23 and Ser24, retaining the functional core structure (24).
FIGURE 4.
Increased levels of cTnI-ND in Gsα-DF hearts. A, ventricular muscle of wild type (WT) and Gsα-DF mice was examined by SDS-PAGE (top) and immunoblot using monoclonal antibody TnI-1 against the C terminus of TnI (bottom). The positions of intact cTnI and cTnI-ND are indicated. B, immunoblots using monoclonal antibody TnI-1 or polyclonal antibody 7428 against the N-terminal peptide of cTnI on Gsα-DF heart tissue and engineered cTnI-ND control protein expressed in E. coli. The nonreactivity of the up-regulated cTnI fragment in Gsα-DF hearts to antibody 7428 indicates an N-terminal truncation. C, densitometry quantification of cTnI-ND versus total cTnI in wild type and Gsα-DF hearts (**, p < 0.01; n = 3 in each group).
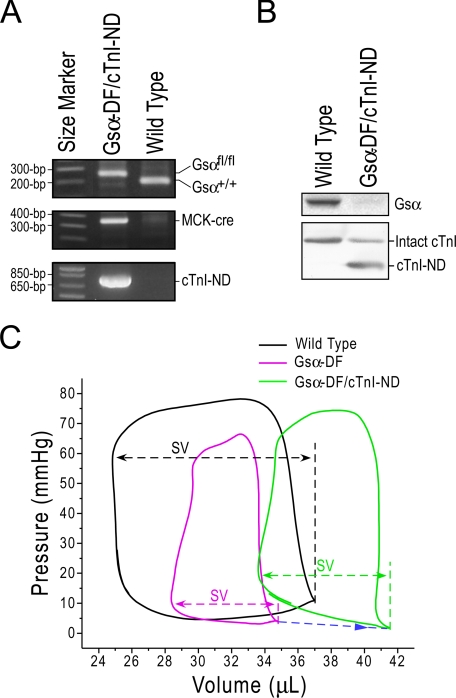
Overexpression of cTnI-ND Positively Affects the Function of Gsα-DF Failing Hearts—We have previously created transgenic mice overexpressing cTnI-ND in the heart to demonstrate effects on increasing cardiac muscle relaxation and improving ventricular filling (25). To investigate the functional significance of the increased cTnI-ND in Gsα-DF failing hearts, we generated double transgenic mice that overexpress cTnI-ND in the Gsα-DF hearts (Fig. 5A). Segregation of the cTnI-ND, Gsα-floxed, and MCK-cre alleles showed independent Mendelian patterns, indicating their locations on different chromosomes. Immunoblots confirmed the diminished Gsα protein and overexpression of cTnI-ND in the cardiac muscle of the double transgenic mice (Fig. 5B).
FIGURE 5.
Overexpression of cTnI-ND in Gsα-DF mouse hearts and functional effects. A, PCR genotyping using Gsα-specific (top), cre-specific (middle), and cTnI-ND-specific (bottom) primers demonstrated the generation of double transgenic mice to overexpress cTnI-ND in Gsα-DF cardiac muscle. B, immunoblots with Gsα-specific (top) and cTnI-specific (bottom) antibodies confirmed the loss of Gsα and overexpression of cTnI-ND in Gsα-DF/cTnI-ND hearts. C, representative left ventricular P-V loops showed reduced stroke volume (SV) in Gsα-DF hearts in comparison with the wild type controls. Overexpression of cTnI-ND in the Gsα-DF hearts partially resumed the stroke volume together with increased end diastolic volume (indicated by the blue arrow).
Functional characterization using isolated working heart preparations showed that overexpression of cTnI-ND in Gsα-DF mouse hearts did not significantly alter ventricular contractile velocity (+dP/dt) and minimal left ventricle pressure but increased ventricular relaxation velocity (-dP/dt), maximum left ventricle pressure, maximum aortic pressure, left ventricular end diastolic volume, and stroke volume, all consistent with the established role of cTnI-ND in better utilization of the Frank-Starling mechanism (25) (Table 3). The overexpression of cTnI-ND produced a trend of myocardial hypertrophy (Table 3), suggesting a consequence of enhanced contractility. The representative PV-loops shown in Fig. 5C demonstrate the decreased maximum left ventricle pressure and stroke volume, reflecting heart failure in the Gsα-DF hearts and the partial reversal of these parameters as well as left ventricular end diastolic volume by overexpression of cTnI-ND. These results show that cTnI-ND overexpression partially corrected the heart failure in Gsα-DF mice and support the hypothesis that the increase in cTnI-ND expression in Gsα-DF hearts is a compensatory adaptation against the heart failure phenotype originating from impaired β-adrenergic signaling.
TABLE 3.
Ex vivo function of Gsα-DF/cTnI-ND mouse hearts Function of Gsα-DF/cTnI-ND hearts was compared with that of Gsα-DF hearts for the effect of cTnI-ND overexpression. Hearts of 3–5-month-old mice were tested in ex vivo working heart preparations. The Gsα-DF/cTnI-ND hearts showed hypertrophic adaptations with increased relaxation velocity, left ventricular end diastolic volume, left ventricular maximum pressure, and stroke volume. The result demonstrated that overexpression of cTnI-ND in Gsα-DF hearts partially corrected the failing phenotype of β-adrenergic deficiency. LVPmax, left ventricular maximum pressure; LVPmin, left ventricular minimum pressure; LVEDV, left ventricular end diastolic volume; APmax, aortic maximum pressure.
| Gsα-DF (n = 4) | Gsα-DF/cTnI-ND (n = 5) | |
|---|---|---|
| Heart weight/body weight (mg/g) | 6.42 ± 0.19 | 8.44 ± 0.72a,b |
| Paced heart rate/min | 420 | 420 |
| +dP/dt (mm Hg/s) | 2374.67 ± 68.23 | 2496.40 ± 218.94 |
| –dP/dt (mm Hg/s) | 2166.67 ± 139.17 | 2692.80 ± 46.47c |
| LVPmax (mm Hg) | 68.76 ± 1.47 | 77.10 ± 0.78c |
| LVPmin (mm Hg) | 3.38 ± 1.68 | 2.71 ± 0.69 |
| APmax (mm Hg) | 65.93 ± 0.90 | 68.68 ± 0.26c |
| Stroke volume (μl/mg) | 0.045 ± 0.004 | 0.071 ± 0.006a |
| LVEDV (μl) | 34.67 ± 1.19 | 42.53 ± 2.59d |
p < 0.05 versus Gsα-DF controls by two-tailed unpaired Student's t test
n = 7 for Gsα-DF and n = 9 for Gsα-DF/cTnI-ND
p < 0.01 versus Gsα-DF controls by two-tailed unpaired Student's t test
p < 0.05 versus Gsα-DF by one-tailed unpaired Student's t test
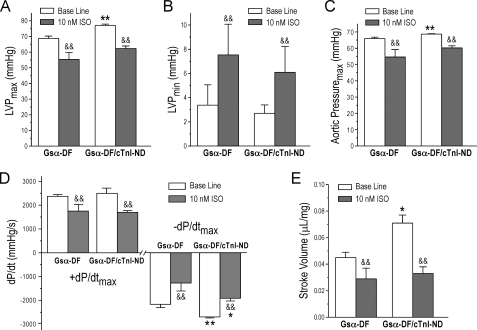
The Gsα-DF/cTnI-ND hearts also showed diminished or negative responses to the treatment of 10 nm isoproterenol, despite the improved base-line function in comparison with that of Gsα-DF hearts (Fig. 6). The results suggest that cTnI-ND compensates for cardiac function independent of β-adrenergic signaling.
FIGURE 6.
β-Adrenergic responsiveness of Gsα-DF/cTnI-ND double transgenic mouse hearts. The effect of 10 nm isoproterenol (ISO) on the function of Gsα-DF/cTnI-ND hearts was compared with that of Gsα-DF hearts. The functional parameters measured on ex vivo working hearts were as follows: maximal left ventricular pressure (LVP) (A), minimal left ventricular pressure (B), maximum aortic pressure (C), contractile (+dP/dt) and relaxation (-dP/dt) velocities (D), and stroke volume (E). The results showed that Gsα-DF/cTnI-ND hearts responded to 10 nm ISO treatment similar to that of Gsα-DF hearts. *, p < 0.05; **, p < 0.01 versus Gsα-DF; &&, p < 0.01 versus base line; n = 4 for Gsα-DF and n = 5 for Gsα-DF/cTnI-ND groups.
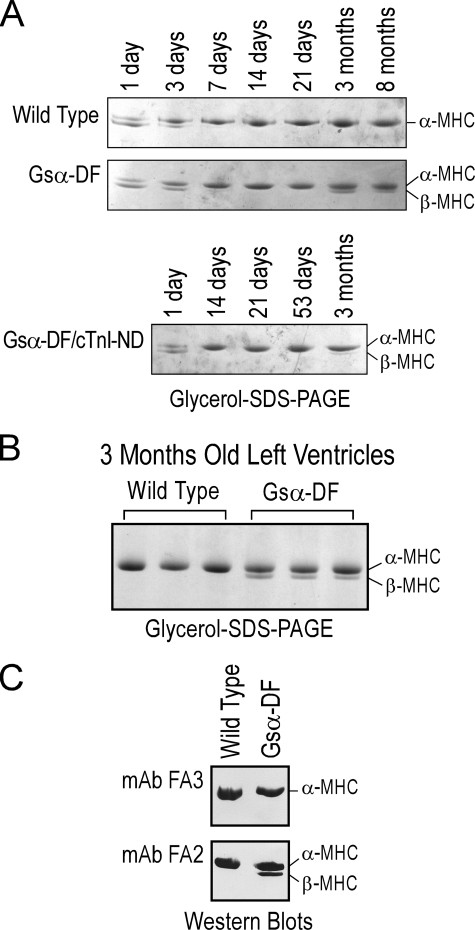
Overexpression of cTnI-ND Reverses the Aberrant Expression of the β-MHC in Gsα-DF Hearts—Results of glycerol SDS-PAGE showed that ventricular muscle from wild type mice expresses both α- and β-isoforms of MHC until 3 days of age and thereafter only expresses the α-isoform (Fig. 7, A and B). Similar developmental transitions were seen in neonatal Gsα-DF and Gsα-DF/cTnI-ND mouse hearts. The identification of cardiac MHC isoforms in glycerol SDS-PAGE was confirmed by Western blots using monoclonal antibodies FA3 and FA2 for α-MHC and α/β-MHC, respectively (36) (Fig. 7C). Although only α-MHC was expressed in wild type adult mouse hearts, β-MHC reappeared in adult Gsα-DF hearts by 3 months of age (Fig. 7, A and B). This developmental pattern indicates that the expression of β-MHC was not a primary phenotype of Gsα deficiency but a secondary response to the development of heart failure. This hypothesis is consistent with the observation that abnormal expression of β-MHC in the hypertrophied adult ventricular muscle is thought to be a compensatory response for mechanical (37) and energetic (38) efficiency.
FIGURE 7.
Adaptive reexpression of β-MHC in the hearts of adult Gsα-DF mice. A, the presence of α- and β-MHC isoforms was examined in wild type, Gsα-DF, and Gsα-DF/cTnI-ND mouse hearts at various ages by glycerol-SDS-PAGE. B, glycerol-SDS-PAGE demonstrating that only the α-MHC isoform is expressed in wild type adult mouse ventricular muscle, whereas β-MHC is re-expressed in the failing adult Gsα-DF hearts. C, immunoblotting of wild type and Gsα-DF hearts using monoclonal antibodies (mAb) FA3 (top) and FA2 (bottom) against α-MHC and α-/β-MHC, respectively, confirmed the different gel mobility of α- and β-MHC isoforms in glycerol-SDS-PAGE.
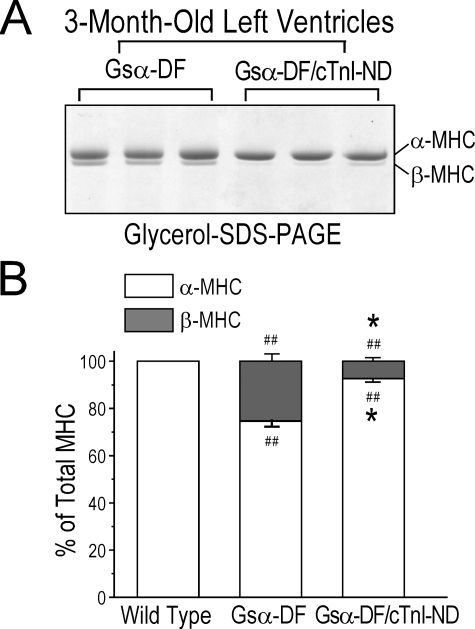
The expression of β-MHC in adult Gsα-ND hearts was diminished by the overexpression of cTnI-ND (Fig. 8A). These results are shown quantitatively in Fig. 8B. Together with the improved cardiac function, this reduced demand for β-MHC compensation supports the role of cTnI-ND in compensating for myocardial contractility under impaired β-adrenergic signaling.
FIGURE 8.
Minimized β-MHC expression in adult Gsα-DF mouse hearts overexpressing cTnI-ND. A, glycerol-SDS-PAGE demonstrating minimized expression of β-MHC in adult Gsα-DF/cTnI-ND double transgenic mouse hearts versus that in Gsα-DF hearts. B, densitometry analysis of immunoblots showing the percentage of α-MHC and β-MHC in adult wild type, Gsα-DF, and Gsα-DF/cTnI-ND mouse hearts confirms the compensatory effect of cTnI-ND in β-adrenergic deficient hearts (##, p < 0.01 versus wild type; *, p < 0.05 versus Gsα-DF; n = 3 in each group).
DISCUSSION
Gsα Deficiency Results in Heart Failure—Previous studies have shown that overexpression of signaling molecules in the β1-adrenergic pathway causes heart failure due to the detrimental effects of myocardial hypertrophy, apoptosis, and fibrosis degeneration (10–12). β1-AR knock-out mice survive to adulthood and appear normal but lack the chronotropic and inotopic responses to isopreterenol (39). Consistently, our present study showed that the diminished expression of Gsα protein in mouse cardiac muscle, which blocks β1-adrenergic signaling, produced depressed contractile function and heart failure phenotypes. The Gsα-DF failing hearts showed decreased heart functional parameters at base line and failed to overcome pressure overload.
Gsα-DF hearts also lacked β-adrenergic responsiveness. Phosphoprotein staining detected significantly decreased in vivo phosphorylation of cTnI and myosin-binding protein C (Fig. 1B). Although ex vivo perfusion of the working hearts significantly reduced the phosphorylation levels of cTnI and myosin-binding protein C, adding β-adrenergic agonist isopreterenol in the perfusion media very effectively restored the phosphorylation of cTnI and myosin-binding protein C in wild type hearts but had no such effect on the Gsα-DF hearts (Fig. 1C), indicating a lack of PKA activation. The loss of PKA activity in Gsα-DF cardiomyocytes is predicted to diminish the phosphorylation of proteins that regulate the Ca2+ handling system, including sarcolemmal L-Ca2+ channel, the ryanodine receptor, and phospholamban in sarcoplasmic reticulum, to negatively affect [Ca2+]i of cardiac myocyte (40), which will result in decreased systolic and diastolic function in the Gsα-DF hearts. It has been reported that β-adrenergic stimulation activates Ca2+/calmodulin-dependent protein kinase II in a non-cAMP manner to enhance cardiac muscle relaxation through phosphorylation of phospholamban (41). Therefore, the role of this signaling pathway in Gsα-DF hearts remains to be investigated. Although the Gsα-DF mouse heart is not a perfect clinical heart failure model, it provides a valuable experimental system to investigate the in vivo myocardial adaptation to β-adrenergic deficiency.
Increased cTnI-ND in Gsα-DF Failing Hearts— cTnI-ND is produced at low levels in normal cardiac muscle and up-regulated in physiological adaptation (24). An interesting finding in the present study is that the myocardial Gsα deficiency-induced heart failure was accompanied by an increased level of N-terminal truncated cTnI. The PKA phosphorylation sites in cTnI are two adjacent N-terminal serine residues, Ser23/Ser24 (rat/mouse residue numbers), in the N-terminal extension. A previous study demonstrated that PKA phosphorylation increases the resistance of cardiac TnI to calpain I proteolysis (42). Consistent with the diminished PKA activity due to Gsα deficiency, the level of cTnI phosphorylation was diminished in Gsα-DF hearts. Therefore, it is logical that diminished PKA phosphorylation in the Gsα-DF cardiac muscle would facilitate proteolytic modification of cTnI.
The N-terminal truncation of cTnI, which was first found in myocardial adaptation to hemodynamic changes in simulated microgravity, is a restricted proteolytic modification that preserves the core structure of TnI (24). In contrast to the proteolytic C-terminal truncation of cTnI, which removes a highly conserved segment of TnI and causes myocardial stunning (43), overexpression of cTnI-ND in transgenic mouse hearts resulted in a nondestructive cardiac phenotype with increased myocardial relaxation and improved ventricular filling for a better utilization of the Frank-Starling mechanism (25).
Proteolytic Modification of cTnI to Selectively Utilize a Downstream Mechanism of β-Adrenergic Signaling—It has been extensively demonstrated that PKA phosphorylation of the two N-terminal serines in cTnI reduces myofilament Ca2+ sensitivity (7, 17) and increases the rate of myocardial relaxation (18–20). The phosphorylation of cTnI is a part of the positive myocardial responses to β-adrenergic stimulation (21). Diminished PKA phosphorylation of cTnI would be predicted to decrease ventricular relaxation in the Gsα-DF hearts.
To test the hypothesis that cTnI-ND is a compensatory rather than a destructive response to impaired β-adrenergic signaling, we overexpressed cTnI-ND in the Gsα-DF hearts with a transgene and demonstrated that cTnI-ND overexpression improved cardiac function, with increased relaxation velocity, left ventricular maximum pressure, and stroke volume. The results indicate that cTnI-ND could partially compensate for the decreases in myocardial relaxation velocity caused by Gsα deficiency, mimicking the effect of phosphorylation of intact cTnI.
The effect of proteolytic removal of the N-terminal extension of cTnI on enhancing cardiac muscle relaxation mimics that of PKA phosphorylation of Ser23/Ser24. In contrast to the broad effects of PKA phosphorylation on cardiac muscle function, the restricted N-terminal truncation of cTnI selectively utilizes a downstream mechanism of β-adrenergic signaling to enhance cardiac function without many of the side effects that precluded the use of β-agonists in the treatment of heart failure.
N-terminal Truncation of cTnI as a Potential Target for the Treatment of Heart Failure—Although normal adult human ventricular muscle mainly expresses β-MHC, the low level expression of α-MHC is further diminished in failing adult ventricular muscle. The proportion of α-MHC mRNA of total MHC mRNA was reduced from ∼30% in nonfailing hearts to ∼2% in end stage failing hearts, corresponding to a switch of 7% α-MHC protein to 100% β-MHC (44, 45). Therefore, the up-regulation of β-MHC in adult mouse hearts seen in our study serves as an indication of heart failure.
Adult ventricular muscle of small mammals expresses mainly α-MHC. When heart failure was induced in Dahl salt-sensitive rats, MHC isoforms switched from ∼10% β-MHC to ∼45% β-MHC (46). β-MHC is expressed in embryonic mouse heart (47), and we detected it in neonatal cardiac muscle (Fig. 7A). The expression of β-MHC in mouse heart is rapidly down-regulated after birth, and adult mice normally express 100% α-MHC in cardiac muscle. The significant reappearance of β-MHC in the 3-month-old Gsα-KD failing hearts, therefore, indicates an adaptive response to heart failure.
β-MHC is known to hydrolyze ATP at approximately one-half the rate of α-MHC, thus producing a lower myofibrillar ATPase activity (48). Consistently, shortening and relengthening velocities are slower in hypothyroid rat myocytes or cardiac muscle expressing increased levels of β-MHC (49, 50). A previous study demonstrated that a shift from α-MHC to β-MHC isozymes in the mouse heart increased energy efficiency (51), suggesting a compensatory role in the case of chronic heart failure.
The level of β-MHC in Gsα-DF/cTnI-ND hearts was significantly lower than that in Gsα-DF hearts at 3 months of age. This effect of cTnI-ND overexpression further supports the functional compensation of overexpressing cTnI-ND in Gsα-DF failing hearts, which minimized the need for the β-MHC compensatory response. The reduced level of β-MHC in the ventricular muscle would be predicted to enhance the function, such as the maximum left ventricle pressure, of Gsα-DF/cTnI-ND hearts.
Since the modification of cTnI function is only a minor portion of the β-adrenergic mechanism in cardiac muscle contractility, the transgenic expression of cTnI-ND did not fully rescue the function of Gsα-DF hearts. It is also worth noting that the improved stroke volume of the Gsα-DF/cTnI-ND hearts was accompanied by a significant increase in ventricular end diastolic volume, suggesting significant left ventricular remodeling. Therefore, the long term effect on causing dilated cardiomyopathy remains to be investigated. Nonetheless, the significantly improved function of Gsα-DF/cTnI-ND hearts clearly demonstrated the value of increased cTnI-ND in Gsα-DF hearts as an endogenous adaptive response to heart failure.
In summary, the present study using genetically modified mouse models demonstrated a posttranslational modification of the thin filament regulation in cardiac muscle that compensates for the decreased cardiac function resulting from impaired β-adrenergic signaling. The restricted N-terminal truncation of cTnI forms a physiological mechanism in myocardial adaptation.
Acknowledgments
We thank Dr. Jeffrey Robbins for providing the cloned mouse cardiac α-MHC gene promoter and Dr. Yingru Lu for assisting the genotyping of transgenic mice.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-078773 and AR-048816 (to J.-P. J.) and by the Intramural Research Program of the National Institutes of Health, NIDDK. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: β-AR, β-adrenoceptor; Gsα, G protein α subunit; Gsα-DF, stimulatory G protein α subunit deficiency; cTnI, cardiac troponin I; cTnI-ND, N-terminal truncated cardiac troponin I; MHC, myosin heavy chain; PKA, protein kinase A; P-V, pressure-volume; MCK, muscle creatine kinase.
References
- 1.Bristow, M. R., Hershberger, R. E., Port, J. D., Gilbert, E. M., Sandoval, A., Rasmussen, R., Cates, A. E., and Feldman, A. M. (1990) Circulation 82 I12-I25 [PubMed] [Google Scholar]
- 2.Homcy, C. J., Vatner, S. F., and Vatner, D. E. (1991) Annu. Rev. Physiol. 53 137-159 [DOI] [PubMed] [Google Scholar]
- 3.Steinberg, S. F., and Brunton, L. L. (2001) Annu. Rev. Pharmacol. Toxicol. 41 751-773 [DOI] [PubMed] [Google Scholar]
- 4.Tsien, R. W., Bean, B. P., Hess, P., Lansman, J. B., Nilius, B., and Nowycky, M. C. (1986) J. Mol. Cell Cardiol. 18 691-710 [DOI] [PubMed] [Google Scholar]
- 5.Marx, S. O., Reiken, S., Hisamatsu, Y., Jayaraman, T., Burkhoff, D., Rosemblit, N., and Marks, A. R. (2000) Cell 101 365-376 [DOI] [PubMed] [Google Scholar]
- 6.Rapundalo, S. T., Solaro, R. J., and Kranias, E. G. (1989) Circ. Res. 64 104-111 [DOI] [PubMed] [Google Scholar]
- 7.Kranias, E. G., and Solaro, R. J. (1982) Nature 298 182-184 [DOI] [PubMed] [Google Scholar]
- 8.Hartzell, H. C., and Glass, D. B. (1984) J. Biol. Chem. 259 15587-15596 [PubMed] [Google Scholar]
- 9.Cohn, J. N., Levine, T. B., Olivari, M. T., Garberg, V., Lura, D., Francis, G. S., Simon, A. B., and Rector, T. (1984) N. Engl. J. Med. 311 819-823 [DOI] [PubMed] [Google Scholar]
- 10.Bristow, M. R. (2000) Circulation 101 558-569 [DOI] [PubMed] [Google Scholar]
- 11.Engelhardt, S. (2005) Heart Fail. Clin. 1 183-191 [DOI] [PubMed] [Google Scholar]
- 12.Lohse, M. J., Engelhardt, S., and Eschenhagen, T. (2003) Circ. Res. 93 896-906 [DOI] [PubMed] [Google Scholar]
- 13.Engelhardt, S., Hein, L., Wiesmann, F., and Lohse, M. J. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 7059-7064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vatner, S. F., Vatner, D. E., and Homcy, C. J. (2000) Circ. Res. 86 502-506 [DOI] [PubMed] [Google Scholar]
- 15.Antos, C. L., Frey, N., Marx, S. O., Reiken, S., Gaburjakova, M., Richardson, J. A., Marks, A. R., and Olson, E. N. (2001) Circ. Res. 89 997-1004 [DOI] [PubMed] [Google Scholar]
- 16.Bristow, M. R., Ginsburg, R., Minobe, W., Cubicciotti, R. S., Sageman, W. S., Lurie, K., Billingham, M. E., Harrison, D. C., and Stinson, E. B. (1982) N. Engl. J. Med. 307 205-211 [DOI] [PubMed] [Google Scholar]
- 17.Zhang, R., Zhao, J., and Potter, J. D. (1995) J. Biol. Chem. 270 30773-30780 [DOI] [PubMed] [Google Scholar]
- 18.Robertson, S. P., Johnson, J. D., Holroyde, M. J., Kranias, E. G., Potter, J. D., and Solaro, R. J. (1982) J. Biol. Chem. 257 260-263 [PubMed] [Google Scholar]
- 19.Zhang, R., Zhao, J., Mandveno, A., and Potter, J. D. (1995) Circ. Res. 76 1028-1035 [DOI] [PubMed] [Google Scholar]
- 20.Kentish, J. C., McCloskey, D. T., Layland, J., Palmer, S., Leiden, J. M., Martin, A. F., and Solaro, R. J. (2001) Circ. Res. 88 1059-1065 [DOI] [PubMed] [Google Scholar]
- 21.Wolska, B. M., Arteaga, G. M., Pena, J. R., Nowak, G., Phillips, R. M., Sahai, S., de Tombe, P. P., Martin, A. F., Kranias, E. G., and Solaro, R. J. (2002) Circ. Res. 90 882-888 [DOI] [PubMed] [Google Scholar]
- 22.McConnell, B. K., Moravec, C. S., and Bond, M. (1998) Am. J. Physiol. 274 H385-H396 [DOI] [PubMed] [Google Scholar]
- 23.Zakhary, D. R., Moravec, C. S., Stewart, R. W., and Bond, M. (1999) Circulation 99 505-510 [DOI] [PubMed] [Google Scholar]
- 24.Yu, Z. B., Zhang, L. F., and Jin, J.-P. (2001) J. Biol. Chem. 276 15753-15760 [DOI] [PubMed] [Google Scholar]
- 25.Barbato, J. C., Huang, Q. Q., Hossain, M. M., Bond, M., and Jin, J.-P. (2005) J. Biol. Chem. 280 6602-6609 [DOI] [PubMed] [Google Scholar]
- 26.Chen, M., Gavrilova, O., Liu, J., Xie, T., Deng, C., Nguyen, A. T., Nackers, L. M., Lorenzo, J., Shen, L., and Weinstein, L. S. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 7386-7391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen, M., Gavrilova, O., Zhao, W.Q., Nguyen, A., Lorenzo, J., Shen, L., Nackers, L., Pack, S., Jou, W., and Weinstein, L.S. (2005) J. Clin. Invest. 115 3217-3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin, J.-P., Yang, F. W., Yu, Z. B., Ruse, C. I., Bond, M., and Chen, A. (2001) Biochemistry 40 2623-2631 [DOI] [PubMed] [Google Scholar]
- 29.Yu, Z. B., Gao, F., Feng, H.-Z., and Jin, J.-P. (2007) Am. J. Physiol. 292 C1192-C1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang, Q.-Q., Feng, H.-Z., Liu, J., Du, J., Stull, L. B., Moravec, C., Huang, X., and Jin, J.-P. (2007) Am. J. Physiol. 294 C213-C222 [DOI] [PubMed] [Google Scholar]
- 31.Feng, H.-Z., Biesiadecki, B. J., Yu, Z.-B., Hossain, M. M., and Jin, J.-P. (2008) J. Physiol. (Lond.) 586 3537-3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauthier, N. S., Matherne, G. P., Morrison, R. R., and Headrick, J. P. (1998) J. Mol. Cell Cardiol. 30 453-461 [DOI] [PubMed] [Google Scholar]
- 33.Wang, Q. D., Tokuno, S., Valen, G., Sjoquist, P. O., and Thoren, P. (2002) Acta Physiol. Scand. 175 279-287 [DOI] [PubMed] [Google Scholar]
- 34.Sutherland, F. J., Baker, K. E., Shattock, M. J., and Hearse, D. J. (2003) Clin. Exp. Pharmacol. Physiol. 30 879-884 [DOI] [PubMed] [Google Scholar]
- 35.How, O. J., Aasum, E., Kunnathu, S., Severson, D. L., Myhre, E. S., and Larsen, T. S. (2005) Am. J. Physiol. 288 H2979-H2985 [DOI] [PubMed] [Google Scholar]
- 36.Jin, J.-P., Malik, M. L., and Lin, J. J.-C. (1990) Hybridoma 9 597-608 [DOI] [PubMed] [Google Scholar]
- 37.Katz, A. M. (1973) Circulation 47 1076-1079 [DOI] [PubMed] [Google Scholar]
- 38.Alpert, N. R., and Mulieri, L. (1983) Myocardial Hypertrophy and Failure, pp. 619-630, Raven Press Publishers, New York
- 39.Rohrer, D. K., Desai, K. H., Jasper, J. R., Stevens, M. E., Regula, D. P., Jr., Barsh, G. S., Bernstein, D., and Kobilka, B. K. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 7375-7380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao, A., Kohmoto, O., Oyama, T., Sugishita, Y., Shimizu, T., Harada, K., Matsui, H., Komuro, I., Nagai, R., Matsuo, H., Serizawa, T., Maruyama, T., and Takahashi, T. (2003) Circ. J. 67 83-90 [DOI] [PubMed] [Google Scholar]
- 41.Yurukova, S., Kilić, A., Všlker, K., Leineweber, K., Dybkova, N., Maier, L. S., Brodde, O. E., and Kuhn, M. (2007) Cardiovasc. Res. 73 678-688 [DOI] [PubMed] [Google Scholar]
- 42.Di Lisa, F., De Tullio, R., Salamino, F., Barbato, R., Melloni, E., Siliprandi, N., Schiaffino, S., and Pontremoli, S. (1995) Biochem. J. 308 57-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy, A. M., Kogler, H., Georgakopoulos, D., McDonough, J. L., Kass, D. A., Van Eyk, J. E., and Marban, E. (2000) Science 287 488-491 [DOI] [PubMed] [Google Scholar]
- 44.Miyata, S., Minobe, W., Bristow, M. R., and Leinwand, L. A. (2000) Circ. Res. 86 386-390 [DOI] [PubMed] [Google Scholar]
- 45.Nakao, K., Minobe, W., Roden, R., Bristow, M. R., and Leinwand, L. A. (1997) J. Clin. Invest. 100 2362-2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kameyama, T., Chen, Z., Bell, S. P., VanBuren, P., Maughan, D., and LeWinter, M. M. (1998) Circulation 98 2919-2929 [DOI] [PubMed] [Google Scholar]
- 47.Lompre, A., Mercadier, J., Wisnewsky, C., Bouveret, P., Pantaloni, C., D'Albis, A., and Schwartz, K. (1981) Dev. Biol. 84 286-290 [DOI] [PubMed] [Google Scholar]
- 48.VanBuren, P., Harris, D. E., Alpert, N. R., and Warshaw, D. M. (1995) Circ. Res. 77 439-444 [DOI] [PubMed] [Google Scholar]
- 49.Fitzsimons, D. P., Patel, J. R., and Moss, R. L. (1998) J. Physiol. (Lond.) 513 171-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cappelli, V., Bottinelli, R., Poggesi, C., Moggio, R., and Reggiani, C. (1989) Circ. Res. 65 446-457 [DOI] [PubMed] [Google Scholar]
- 51.Hoyer, K., Krenz, M., Robbins, J., and Ingwall, J. S. (2007) J. Mol. Cell Cardiol. 42 214-221 [DOI] [PMC free article] [PubMed] [Google Scholar]